Recent Advances on Topical Application of Ceramides to Restore Barrier Function of Skin
Abstract
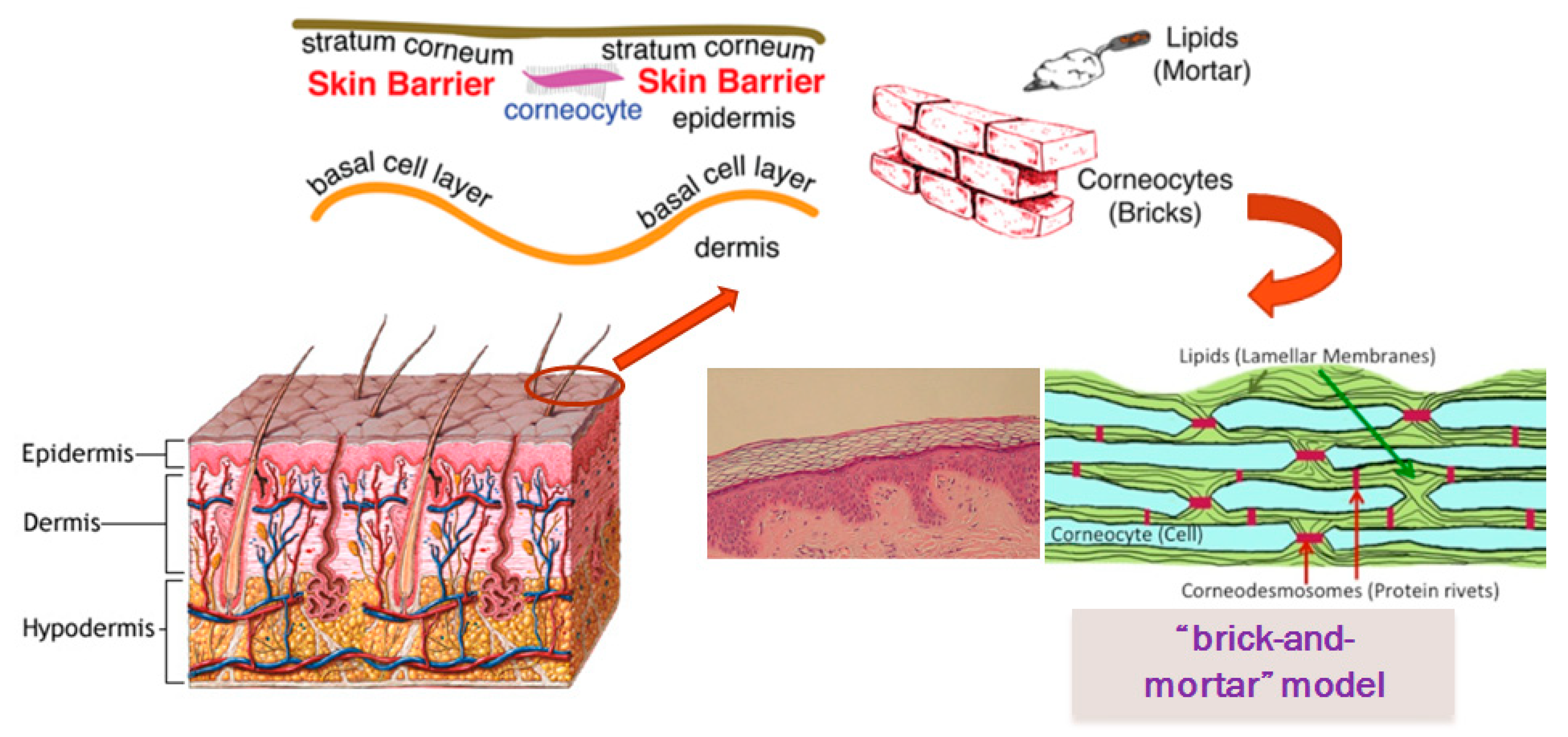
1. Skin Barrier
- Basal layer (stratum germinativum)
- Prickle layer (stratum spinosum)
- Granular layer (stratum granulosum)
- Stratum lucidum
- Stratumcorneum.
1.1. Barrier Function of Stratum Corneum
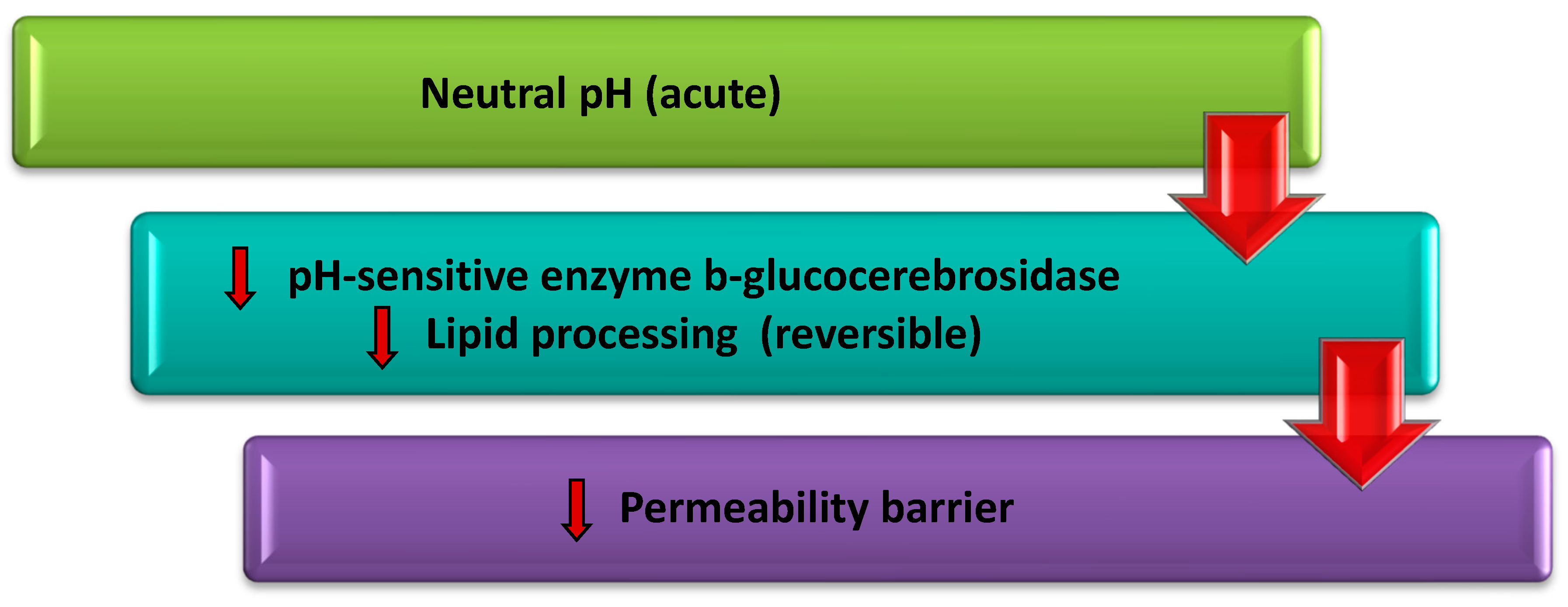
1.2. Relationship of Skin pH, Transepidermal Water Loss (TEWL) and Stratum Corneum Barrier
1.3. Major Lipids of Stratum Corneum
1.3.1. Cholesterol
1.3.2. Free Fatty Acids
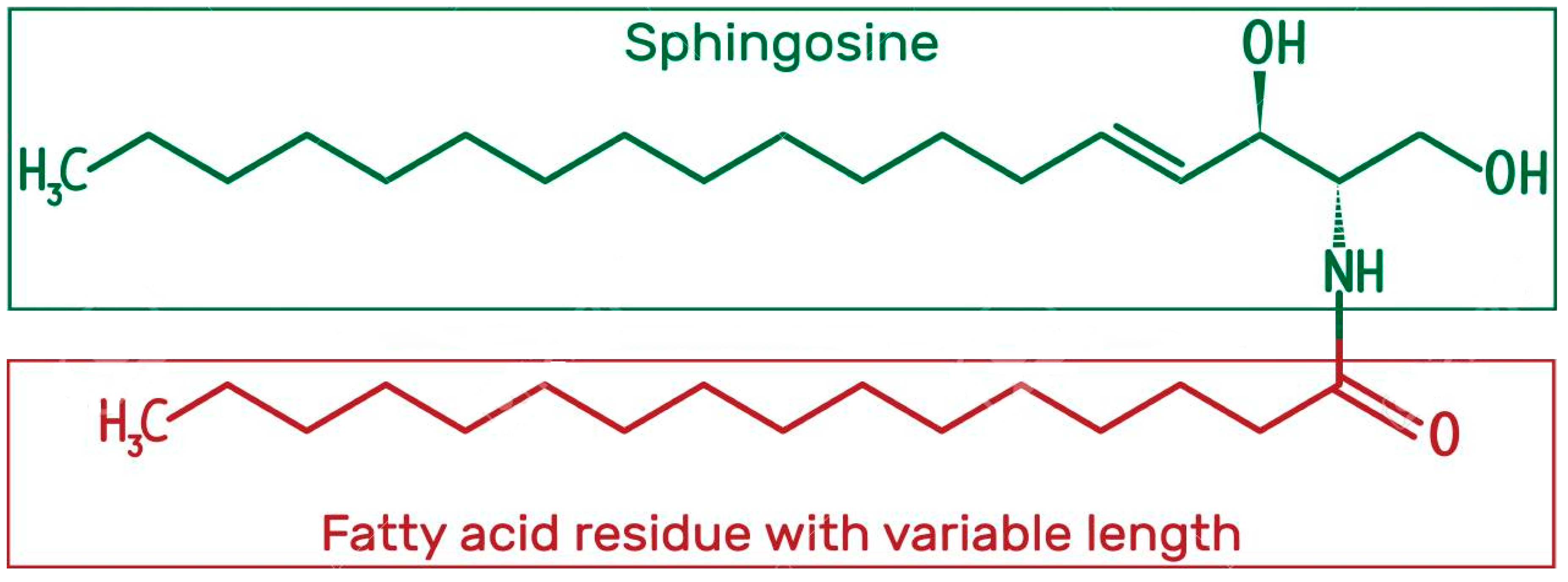
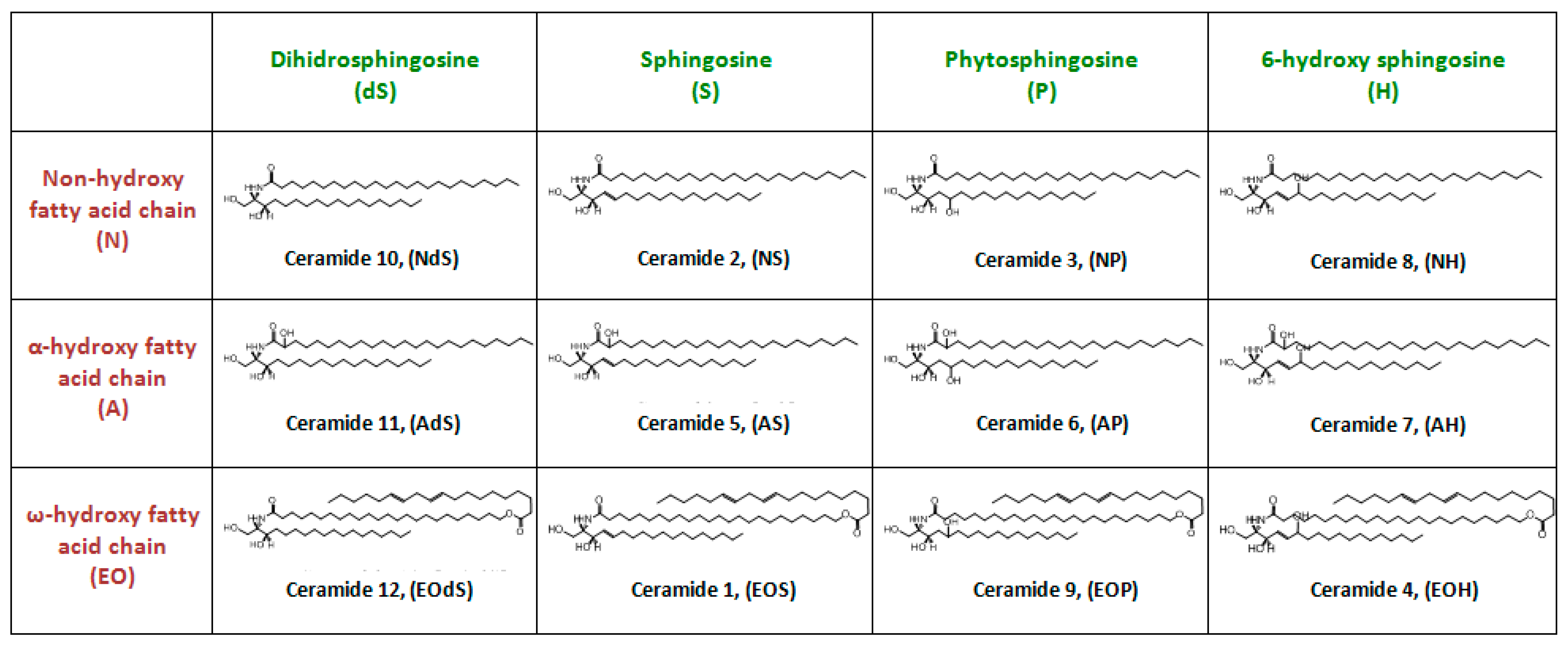
1.3.3. Ceramides
Structure and Physicochemical Characteristics of Ceramides
Biosynthesis of Ceramides in the Skin
Location and Position of Ceramides in the Skin
Role of Ceramides in the Skin Barrier and Skin Disorders
2. Topical Applications of Ceramides
2.1. Conventional Carrier Systems
2.2. Novel Carrier Systems
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aoki, M.; Ogai, K.; Kobayashi, M.; Minematsu, T.; Nakatani, T.; Okuwa, M.; Sanada, H.; Sugama, J. Comparison of ceramide retention in the stratum corneum between dry skin and normal skin using animal model with fluorescent imaging method. Skin Res. Technol. 1999, 25, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Bleck, O.; Abeck, D.; Ring, J.; Hoppe, U.; Vietzke, J.P.; Wolber, R.; Brandt, O.; Schreiner, V. Two Ceramide Subfractions Detectable in Cer(AS) Position by HPTLC in Skin Surface Lipids of Non-Lesional Skin of Atopic Eczema. J. Investig. Dermatol. 1999, 113, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Gooris, G.S. The Lipid Organisation in Human Stratum Corneum and Model Systems. Open Dermatol. J. 2010, 4, 10–13. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Gooris, G.S.; Dubbelaar, F.E.; Ponec, M. Phase behavior of skin barrier model membranes at pH 7.4. Cell Mol. Biol. 2002, 46, 979–992. [Google Scholar]
- Brandner, J.; Zorn-Kruppa, M.; Yoshida, T.; Moll, I.; Beck, L.; De Benedetto, A. Epidermal tight junctions in health and disease. Tissue Barriers 2015, 3, e974451. [Google Scholar] [CrossRef] [PubMed]
- Brandner, J.M. Importance of Tight Junctions in Relation to Skin Barrier Function. In Skin Barrier Function; Karger Publishers: Basel, Switzerland, 2016; Volume 49, pp. 27–37. [Google Scholar]
- Champagne, A.M.; Pigg, V.A.; Allen, H.C.; Williams, J.B. Presence and persistence of a highly ordered lipid phase state in the avian stratum corneum. J. Exp. Biol. 2018, 221, jeb176438. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; Chen, S.C.; Osterberg, L.; Brandt, S.; Von Grote, E.C.; Meckfessel, M.H. A daily skincare regimen with a unique ceramide and filaggrin formulation rapidly improves chronic xerosis, pruritus, and quality of life in older adults. Geriatr. Nurs. 2018, 39, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Chermprapai, S.; Broere, F.; Gooris, G.; Schlotter, Y.M.; Rutten, V.P.M.G.; Bouwstra, J.A. Altered lipid properties of the stratum corneum in Canine Atopic Dermatitis. Biochim. Biophys. Acta Biomembr. 2018, 1860, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Lademann, J.; Darvin, M.E. A depth-dependent profile of the lipid conformation and lateral packing order of the Raman microscopy. Analyst 2016, 141, 1981–1987. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. In vivo confocal Raman microscopic determination of depth pro fi les of the stratum corneum lipid organization in fl uenced by application of various oils. J. Dermatol. Sci. 2017, 87, 183–191. [Google Scholar] [CrossRef]
- Choi, M.J.; Maibach, H.I. Role of Ceramides in Barrier Function of Healthy and Diseased Skin. Am. J. Clin. Dermatol. 2005, 6, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Munoz-Garcia, A.; Jurkowitz, M.S.; Williams, J.B. beta-Glucocerebrosidase activity in the stratum corneum of house sparrows following acclimation to high and low humidity. Physiol. Biochem.Zool. 2008, 81, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Wertz, P.; Giannetti, A.; Seidenari, S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 1998, 78, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Raymond, I. The Efficacy of a Ceramide-based Cream in Mild-to-moderate Atopic Dermatitis. J. Clin. Aesthetic Dermatol. 2018, 11, 30–32. [Google Scholar]
- Eckl, K.M.; Tidhar, R.; Thiele, H.; Oji, V.; Hausser, I.; Brodesser, S.; Preil, M.L.; Önal-Akan, A.; Stock, F.; Müller, D.; et al. Impaired Epidermal Ceramide Synthesis Causes Autosomal Recessive Congenital Ichthyosis and Reveals the Importance of Ceramide Acyl Chain Length. J. Investig. Dermatol. 2013, 133, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Epidermal lipids, barrier function, and desquamation. J. Investig.Dermatol. 2003, 80, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Falluel-Morel, A.; Aubert, N.; Vaudry, D.; Desfeux, A.; Allais, A.; Burel, D.; Basille, M.; Vaudry, H.; Laudenbach, V.; Gonzalez, B.J. Interactions of PACAP and Ceramides in the Control of Granule Cell Apoptosis During Cerebellar Development. J. Mol. Neurosci. 2008, 36, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gaul, E.; Underwood, G.B. Relation of dew point & barometric pressure to chapping of normal skin. J. Investig. Dermatol. 2001, 19, 9–19. [Google Scholar]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef]
- Haftek, M. Epidermal barrier disorders and corneodesmosome defects. Cell Tissue Res. 2015, 360, 483–490. [Google Scholar] [CrossRef]
- Hwang, K.; Kim, H.; Kim, D.J. Thickness of skin and subcutaneous tissue of the free flap donor sites: A histologic study. Microsurgery 2016, 36, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Janssens, M.; Van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Yoon, G.H.; Lee, H.C.; Jung, M.H.; Yu, S.I.; Yeon, S.J.; Min, S.K.; Kwon, Y.S.; Hwang, J.H.; Shin, H.S. Thermodynamic Insights and Conceptual Design of Skin-Sensitive Chitosan Coated Ceramide/PLGA Nanodrug for Regeneration of Stratum Corneum on Atopic Dermatitis. Sci. Rep. 2015, 5, 18089. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Khazanov, E.; Priev, A.; Shillemans, J.P.; Barenholz, Y.; Khazanov, E.; Priev, A.; Shillemans, J.P.; Barenholz, Y. Physicochemical and Biological Characterization of Ceramide-Containing Liposomes: Paving the Way to Ceramide Therapeutic Application Physicochemical and Biological Characterization of Ceramide-Containing Liposomes: Paving the Way to Ceramide Therapeutic. Langmuir 2008, 24, 6965–6980. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, W.R.; Kim, J.H.; Cho, E.C.; An, E.J.; Kim, J.W.; Oh, S.G. Fabrication of pseudo-ceramide-based lipid microparticles for recovery of skin barrier function. Colloids Surf. B Biointerfaces 2012, 94, 236–241. [Google Scholar] [CrossRef]
- Matsumoto, M.; Umemoto, N.; Sugiura, H.; Uehara, M. Difference in ceramide composition between “dry” and “normal” skin in patients with atopic dermatitis. Acta Derm-Venereol 1999, 79, 246–247. [Google Scholar] [CrossRef]
- Mauro, T.M. SC pH: Measurement, Origins, and Functions. In Skin Barrier; Elias, P.M., Feingold, K.R., Eds.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 223–229. [Google Scholar]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Gooris, G.S.; Barlow, D.J.; Lawrence, M.J.; Demé, B.; Bouwstra, J.A. Skin Lipids: Localization of Ceramide and Fatty Acid in the Unit Cell of the Long Periodicity Phase. Biophys. J. 2015, 108, 2670–2679. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Kariman, Z.; Kerckhove, L.; Van Gooris, G.S.; Bouwstra, J.A. Biochimica et Biophysica Acta The role of ceramide chain length distribution on the barrier properties of the skin lipid membranes. Biochim. Biophys. Acta BBA 2014, 1838, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Maibach, H.I. Nanotechnology in Cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 337–369. [Google Scholar]
- Nakaune-Iijima, A.; Sugishima, A.; Omura, G.; Kitaoka, H.; Tashiro, T.; Kageyama, S.; Hatta, I. Topical treatments with acylceramide dispersions restored stratum corneum lipid lamellar structures in a reconstructed human epidermis model. Chem. Phys. Lipids 2018, 215, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nemes, Z.; Steinert, P.M. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Neubert, R.H.; Sonnenberger, S.; Dobner, B.; Gray, C.W., Jr.; Barger, K.N.; Sevi-Maxwell, K.; Sommer, E.; Wohlrab, J. Controlled Penetration of a Novel Dimeric Ceramide into and across the Stratum Corneum Using Microemulsions and Various Types of Semisolid Formulations. Ski. Pharmacol. Physiol. 2016, 29, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Oltulu, P.; Ince, B.; Kökbudak, N.; Fındık, S.; Kilinc, F. Measurement of epidermis, dermis, and total skin thicknesses from six different body regions with a new ethical histometric technique. Turk. J. Plast. Surg. 2018, 26, 56–61. [Google Scholar] [CrossRef]
- Pappas, A.; Kendall, A.C.; Brownbridge, L.C.; Batchvarova, N.; Nicolaou, A. Seasonal changes in epidermal ceramides are linked to impaired barrier function in acne patients. Exp. Dermatol. 2018, 27, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Pilgram, G.; Van Der Meulen, J.; Gooris, G.; Koerten, H.; Bouwstra, J. The influence of two azones and sebaceous lipids on the lateral organization of lipids isolated from human stratum corneum. Biochim. Biophys. Acta BBA Biomembr. 2001, 1511, 244–254. [Google Scholar] [CrossRef]
- Pilgram, G.S.; Vissers, D.C.; Van Der Meulen, H.; Koerten, H.K.; Pavel, S.; Lavrijsen, S.P.; Bouwstra, J.A. Aberrant Lipid Organization in Stratum Corneum of Patients with Atopic Dermatitis and Lamellar Ichthyosis. J. Investig. Dermatol. 2001, 117, 710–717. [Google Scholar] [CrossRef]
- Qurt, M.; Esentürk, İ.; Birteksöz Tan, S.; Erdal, M.S.; Araman, A.; Güngör, S. Voriconazole and sertaconazole loaded colloidal nano-carriers for enhanced skin deposition and improved topical fungal treatment. J. Drug Deliv. Sci. Technol. 2018, 48, 215–222. [Google Scholar] [CrossRef]
- Rabionet, M.; Gorgas, K.; Sandhoff, R. Ceramide synthesis in the epidermis. Biochim. Biophys. Acta BBA Mol. CellBiol. Lipids 2014, 1841, 422–434. [Google Scholar] [CrossRef]
- Rawlings, A.; Voegeli, R. Stratum corneum proteases and dry skin conditions. Cell Tissue Res. 2013, 351, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V. Skin Waxes: Their Composition, Properties, Structures and Biological Significance. In Waxes: Chemistry, MolecularBiology & Functions; Hamilton, R.J., Ed.; The Oily Press: Dundee, UK, 2004; pp. 221–256. [Google Scholar]
- Roger, M.; Fullard, N.; Costello, L.; Bradbury, S.; Markiewicz, E.; O’Reilly, S.; Darling, N.; Ritchie, P.; Määttä, A.; Karakesisoglou, I.; et al. Bioengineering the microanatomy of human skin. J. Anat. 2019, 234, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Wohlrab, J.; Neubert, R.H. Controlled penetration of ceramides into and across the stratum corneum using various types of microemulsions and formulation associated toxicity studies. Eur. J. Pharm. Biopharm. 2014, 86, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Schade, H. Zur physikalischen chemie der hautoberflache. Arch. Dermatol. Syph. 2008, 154, 690–716. [Google Scholar] [CrossRef]
- Schmitt, T.; Lange, S.; Sonnenberger, S.; Dobner, B.; Demé, B.; Neubert, R.H.; Gooris, G.; Bouwstra, J.A. Determination of the influence of C24 D/(2R)-and L/(2S)-isomers of the CER[AP] on the lamellar structure of stratum corneum model systems using neutron diffraction. Chem. Phys. Lipids 2017, 209, 29–36. [Google Scholar] [CrossRef]
- Školová, B.; Janůšová, B.; Zbytovská, J.; Gooris, G.; Bouwstra, J.; Berka, P.; Roh, J.; Palát, K.; Hrabálek, A.; Slepička, P.; et al. Ceramides in the Skin Lipid Membranes: Length Matters. Langmuir 2013, 29, 15624–15633. [Google Scholar]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Environ. Factors Ski. Dis. 2016, 49, 8–26. [Google Scholar]
- Sondari, D.; Haryono, A.; Harmami, S.B.; Randy, A. Influence of Palmitoyl Pentapeptide and Ceramide III B on the Droplet Size of Nanoemulsion. In Southeast Asian International Advances in Micro/Nano-Technology; SPIE Press: Bellingham, WA, USA, 2010; Volume 7743, p. 77430D. [Google Scholar]
- Spada, F.; Barnes, T.M.; Greive, K.A. Skin hydration is significantly increased by a cream formulated to mimic the skin’s own natural moisturizing systems. Clin. Cosmet. Investig. Dermatol. 2018, 11, 491–497. [Google Scholar] [CrossRef]
- Strugar, T.L.; Kuo, A.; Seite, S.; Lin, M.; Lio, P. Connecting the Dots: From Skin Barrier Dysfunction to Allergic Sensitization, and the Role of Moisturizers in Repairing the Skin Barrier. J. Drugs Dermatol. 2019, 18, 581. [Google Scholar]
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203–5221. [Google Scholar] [CrossRef]
- Tashiro, T.; Nakaune, A.; Kosugi, T.; Arakawa, J.; Mori, H.; Serizawa, S.; Suzuki, K.; Mori, F.; Orikasa, A.; Nakamura, Y. Development of functional cosmetics “ASTALIFT JELLY AQUARYSTA”. Fugifilm Res. Dev. 2011, 56, 1–4. [Google Scholar]
- Tessema, E.N.; Gebre-Mariam, T.; Paulos, G.; Wohlrab, J.; Neubert, R.H. Delivery of oat-derived phytoceramides into the stratum corneum of the skin using nanocarriers: Formulation, characterization and in vitro and ex-vivo penetration studies. Eur. J. Pharm. Biopharm. 2018, 127, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Gebre-Mariam, T.; Wohlrab, J.; Tessema, E.N.; Neubert, R.H. Potential Applications of Phyto-Derived Ceramides in Improving Epidermal Barrier Function. Ski. Pharmacol. Physiol. 2017, 30, 115–138. [Google Scholar]
- Tokudome, Y.; Saito, Y.; Sato, F.; Kikuchi, M.; Hinokitani, T.; Goto, K. Preparation and characterization of ceramide-based liposomes with high fusion activity and high membrane fluidity. Colloids Surf. B Biointerfaces 2009, 73, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Cho, Y.; Moradian, S.; Kim, J.; Nakajima, K.; Crumrine, D.; Park, K.; Ujihara, M.; Akiyama, M.; Shimizu, H.; et al. Neutral Lipid Storage Leads to Acylceramide Deficiency, Likely Contributing to the Pathogenesis of Dorfman–Chanarin Syndrome. J. Investig. Dermatol. 2010, 130, 2497–2499. [Google Scholar] [CrossRef] [PubMed]
- Vavrova, K.; Kovacık, A.; Opalka, L. Ceramides in the skin barrier. Eur. Pharm. J. 2017, 64, 1–8. [Google Scholar] [CrossRef]
- Wertz, P.W. Biochemistry of Human Stratum Corneum Lipids. In Skin Barrier; Elias, P.M., Feingold, K.R., Eds.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 33–42. [Google Scholar]
- Wertz, P.W.; Swartzendruber, D.C.; Madison, K.C.; Downing, D.T. The composition and morphology of epidermal cyst lipids. J. Investig. Dermatol. 2007, 89, 419–425. [Google Scholar] [CrossRef]
- Williams, A. Transdermal and Topical Drug Delivery: From Theory to Clinical Practice; Pharmaceutical Press: London, UK, 2003. [Google Scholar]
- Yu, H.; Valerio, M.; Bielawski, J. Fenretinide Inhibited de novo Ceramide Synthesis and Pro-inflammatory Cytokines Induced by A. actinomycetemcomitans. J. Lipid Res. 2012, 54, 189–201. [Google Scholar] [CrossRef]
- Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Mao, G.; Pappas, A.; Mack, M.C.; Walters, R.M.; Southall, M.D. Topically applied ceramide accumulates in skin glyphs. Clin. Cosmet. Investig. Dermatol. 2015, 8, 329–337. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahraman, E.; Kaykın, M.; Şahin Bektay, H.; Güngör, S. Recent Advances on Topical Application of Ceramides to Restore Barrier Function of Skin. Cosmetics 2019, 6, 52. https://doi.org/10.3390/cosmetics6030052
Kahraman E, Kaykın M, Şahin Bektay H, Güngör S. Recent Advances on Topical Application of Ceramides to Restore Barrier Function of Skin. Cosmetics. 2019; 6(3):52. https://doi.org/10.3390/cosmetics6030052
Chicago/Turabian StyleKahraman, Emine, Melis Kaykın, Hümeyra Şahin Bektay, and Sevgi Güngör. 2019. "Recent Advances on Topical Application of Ceramides to Restore Barrier Function of Skin" Cosmetics 6, no. 3: 52. https://doi.org/10.3390/cosmetics6030052
APA StyleKahraman, E., Kaykın, M., Şahin Bektay, H., & Güngör, S. (2019). Recent Advances on Topical Application of Ceramides to Restore Barrier Function of Skin. Cosmetics, 6(3), 52. https://doi.org/10.3390/cosmetics6030052



