1. Introduction
The efficacy of any cosmetic product containing an active ingredient is determined by two factors: The intrinsic activity of the molecule and the delivery of this molecule to its site of action. The intrinsic activity of an active ingredient, or its capability to express efficacy, dictates its functional profile but does not guarantee the efficacy of the final formulation containing the ingredient.
To express its function, the active ingredient must be delivered to the site of action at the right concentrations and the right time. This criterion applies to all types of active ingredients with any functionality and is of particular importance in dermo-cosmetic science [
1].
Because the skin is the most extended organ of the human body, these concepts are particularly relevant due to the large surface for application. However, to reach effective concentrations in the cutaneous tissues deeper layers, the uppermost barrier, the stratum corneum (SC), must be overcome. In addition to specific transport mechanisms, due to the lipophilic properties of the SC, hydrophilic molecules penetrate poorly. Therefore, achieving skin bioavailability of hydrophilic active substances, such as peptides or peptide-like substances, is challenging [
2].
The focus of the present study was to investigate the delivery of a simple dipeptide, L-carnosine, by topical application to human skin by means of a gel formulation containing L-carnosine in association with a magnesium ion, thus forming a carnosine complex. The aim of this complex is to improve the bioavailability by acting as a multifunctional ingredient with several roles, including antioxidant effects, buffering enzyme and sarcoplasmic reticulum calcium (Ca
2+) regulations [
3].
Carnosine (β-alanyl-L-histidine) is a dipeptide with a molecular weight of 226.23 Da and is very hydrophilic, with a partition coefficient (log P) of −2.972 ± 0.436 [
4]. As a component of several tissues, it has several biological roles, including pH buffering and excellent antioxidant properties [
5]. Carnosine is based on an indole nucleus (histidine-containing), particularly present in skeletal muscle [
6].
The degrading enzyme, carnosinase, is also present in most tissues in the body, excepting skeletal muscle. This may explain why carnosine concentrations are highest in this tissue.
In human skeletal muscle, the levels of carnosine ranges between 5–10 mM wet weight or 15–40 mmol/kg dry weight. Depending on the muscle mass these concentrations differ among animal species [
7].
Slow-twitch muscle fibers contain low carnosine level, animals that are characterized by prolonged hypoxic dives, frequent sprints, and explosive flight behaviors have higher initial concentrations [
7,
8,
9]. This fact is also confirmed in anaerobic sports (i.e., higher intramuscular concentrations of carnosine) [
8,
9,
10].
On the other side, carnosine supplementation does not increase plasma levels due to the high activity of carnosinase. Only 14% of the ingested carnosine was found in urine and because β-ALA and L-histidine are the precursors of carnosine, the research pointed towards their supplementation [
11].
Moreover, carnosine displays other physiological roles, including protection against oxidative stress. This latter is due to different mechanisms such as: Metal ion chelation, scavenging reactive oxygen species (ROS) and peroxyl radicals [
8]. ROS can arise from exercise in several proposed mechanisms, i.e., increased flow of electrons in the electron transport system, decrease in pH or increased respiration, all leading to oxygen release from hemoglobin with increase in pO2 in the tissues [
12]. Carnosine is involved in anti-aging processes, as it seems to influence the cellular lifespan and onset of age-related change with an apparent rejuvenation of senescent cells. Even if the mechanism of action of carnosine is not yet clear, in vitro and in vivo studies confirm the positive effects for skin and muscles in the presence of carnosine as a supplementation as well as in topical applications [
13]. A large body of literature has dealt with the intriguing properties of this small dipeptide, here following a few examples that support our interest toward this molecule for antiaging application.
The accumulation of advanced glycation end products (AGE) in the skin has been associated with skin aging, inhibition of glycation of extracellular matrix proteins can therefore help the structure and appearance of the skin. Carnosine has demonstrated anti-glycation activity when applied topically in ex vivo human skin explants. In this study, a facial cream showed a significant superior anti-glycation effect compared to aqueous carnosine solution (approximately double), thus indicating the importance of the vehicle [
14]. In another study [
15] the effect of carnosine in combination with urea and arginine, was evaluated against severe xerosis of the foot skin in patients with diabetes (a disease associated with accelerated ageing). The study continued for 8 months in patients with type 2 diabetes, with severe plantar xerosis, with an emollient glycerin-based cream (SEC). The use of a cream based on urea, arginine and carnosine increased skin hydration and improved skin dryness in type 2 diabetic patients compared to a commonly used emollient cream based on glycerol, with a greater effectiveness observed as early as 4 weeks after treatment.
In another study, the application of a low molecular weight peptides such as carnosine in geronto-cosmetology has been investigated in skin cell cultures of young and old rats, demonstrating, together with other small peptides, to stimulate the proliferation of skin fibroblasts by 29–45% [
16]. This effect was observed at lower concentrations and levels during skin aging in old cell cultures than in young cell cultures. These data open up prospects for a new approach to the creation of cosmetological substances at the base of small peptides such as carnosine.
Exposure to ultraviolet rays (UVR) is an important risk factor for skin aging and the development of non-melanoma skin cancers (NMSC). Although traditional sunscreens remain the pillar for preventing UVR-induced skin damage, they cannot provide complete protection against the full spectrum of molecular lesions associated with UVR exposure. The formation of pyrimidine cyclobutane dimers (CPD), as well as oxidative damage to the bases of DNA, including the formation of 8-oxo-7,8-dihydro-2′-deoxyguanosin (8OHdG) are among the key DNA lesions associated with photoaging and tumorigenesis. In addition to DNA lesions, UVR-induced free radical formation can lead to protein carbonylation (PC), an important form of irreversible protein damage that inactivates their biological function. In a recent study [
17] a new topical product composed of traditional sun filters, DNA repair enzymes and carnosine was compared with traditional commercial products based on sun filters, demonstrating, by comparative study, on irradiated skin biopsies, clear improvements in the oxidation markers used in the study, CPD, 8OHdG and PC. Thus reducing the risk of skin aging and NMSC.
It has been documented that cellular senescence associated with telomeres may contribute to certain age-related disorders, including increased incidence of cancer, wrinkles and decreased skin elasticity. It has been proposed that telomere length may not be a strong survival biomarker in older individuals, but it may be an informative biomarker of healthy aging. Natural compounds based on imidazole dipeptide carnosine may make it clinically possible to demonstrate that slowing down the rate of telomere shortening could slow down the human aging process in specific tissues where it is known that telomere shortening appears to be a distinctive sign of oxidative stress and disease. Preliminary longitudinal studies of elderly individuals, conducted in a recent study, suggest that longer telomeres associated with improved survival and oral nutritional support with non-hydrolysed carnosine are useful therapeutic tools for critical telomere length maintenance that can fundamentally be applied in prolonging life expectancy, increasing survival and the chronological age of an organism [
18].
Finally, since the 2000s, peptides are used in topical formulation for collagen stimulation, wound healing, “Botox-like” wrinkle smoothing, as well as for antioxidative and antimicrobial effects. Particularly, carnosine and N-acetylcarnosine alone in a water solution are able to reduce UVB erythema in human skin; both peptides showed antioxidant capacity, with a higher significance in conjunction with vehicles improving the substances’ skin penetration capabilities [
19].
Many future research opportunities are offered in view of these different physiological roles, in particular investigating its role to improve exercise performance and/or reduce muscular fatigue. It will also be important to investigate the role of different nutritional approaches in increasing carnosine levels in the muscle to optimize physiological activity and/or exercise capacity.
Goebel et al. [
2] investigated the penetration of carnosine through the SC in association with a penetration enhancer, such as pentylene glycol, in a hydrophilic vehicle, achieving a low concentration of the dipeptide in the SC. Taking this into account, the aim of our study, although preliminary, was to evaluate whether a strategy involving the complexation of carnosine can influence its bioavailability through the SC. As the complexing agent we selected the magnesium ion; its concentration is generally high in the SC but constantly low in all other layers [
20], suggesting that the magnesium ion plays a role in epidermal functions, such as barrier homeostasis and epidermal differentiation [
21].
2. Materials and Methods
2.1. Materials
All tested samples were from Pavia Farmaceutici s.r.l. (Copiano (PV), Italy).
The study considered three different samples as hydrogel formulations with the aim of evaluating the role of the complexing agent, magnesium alone, as a penetration enhancer of carnosine.
The different formulations tested are summarized in
Table 1.
The hydrogel termed as Sample A was designed to contain carnosine, magnesium sulfate, ethoxydiglycol, phenoxyethanol, ethylhexyl glycerin, sclerotium gum, glycerin and water. All the raw materials used for the preparation of the samples are compliant with Regulation 1223/2009 on cosmetic products.
Samples B and C were formulated with the same components with the exception of magnesium for the former and carnosine for the latter.
The concentration of magnesium salt in samples A and C was 1.64%, while the concentration of L-carnosine in samples A and B was 1.50%.
Sample A was prepared by adding all the ingredients to the water solution, at room temperature under stirring; the gelling agent sclerotium gum, at a concentration of 0.8%, was added as the last ingredient. The solution was maintained under stirrer mixing, until the complete hydration of the polymer and gelation process was complete.
The samples appear as transparent and colorless gels, pH 5.5 ± 1.0, density 0.90 ± 0.10 g/mL, viscosity (Brooksfield RV, Brooksfield-Urai Spa, Assago (Milan), Italy), spindle 6, rpm 10) 2500–4500 cps.
Sample A was stated as a cosmetic product. It was dermatologically tested (Occlusive Patch Test), applied under occlusive conditions on the healthy skin of 20 volunteers, and it resulted as a non-irritant if applied on human skin. The microbiological stability of Sample A was tested via a Challenge test, that confirmed the preservative system used in the formulation was compliant with a 24-month shelf-life granted by the manufacturer Pavia Farmaceutici srl.
Samples B and C were used as models in this study and they were prepared with the same procedures and raw materials reported for sample A.
2.2. EpiDermTM Reconstructed Human Epidermis (RHE) Model
Reconstructed human epidermis (RHE) was used as a human skin tissue model. The EpiDermTM RHE model consists of a three-dimensional epidermal tissue grown at an air-liquid interface from normal human keratinocytes. The model is histologically similar to the human epidermis, presenting all differentiated cellular layers, and features a functional permeability barrier. The EpiDermTM Tissue Model was purchased from MatTek (MatTek In Vitro Life Science Laboratories, Bratislava, Slovak Republic).
Upon reception, tissues were transferred to SkinEthicTM Maintenance Medium and kept at 37 °C in a humidified 5% CO2 atmosphere until hydrogel application.
2.3. Hydrogel Application on EpiDermTM RHE and Sample Collection
Before the application of the three hydrogels, the medium was removed, and fresh medium was added. Then, 10 μL of the three different hydrogels was topically applied on the surface of a set of RHEs in a single dose for 24 h or in a repeated application at 24 h for a total of 48 h in another set of RHEs. Control tissues were exposed to the vehicle medium alone. All exposed hydrogels and control samples were assayed in triplicate using three sets of RHE for each group.
At each time point, tissue medium aliquots were collected and centrifuged at 1000× g for 20 min at 4 °C to remove insoluble impurities and tissue debris. The clear supernatants were stored at −20 °C until analysis. After washing with PBS and snap-freezing in liquid nitrogen, the RHE tissues were homogenized in a glass beaker with 150 µL of PBS on ice. The homogenates were then centrifuged at 5000× g for 5 min to obtain the supernatants, which were stored at −20 °C until analysis.
2.4. ELISA for Carnosine
Carnosine levels in the RHE culture media and homogenates collected at different time points (24 or 48 h) were determined using a competitive ELISA kit (Elabscience Biotechnology Co., Ltd., Houston, TX, USA) according to the manufacturer’s instructions. All of the samples were assayed in duplicate. A calibration curve was performed using carnosine as a standard. The optical absorbance was measured with a microplate reader at 450 nm, and results are expressed as ng/mL. The lower limit of detection for carnosine was 8.438 ng/mL.
2.5. Statistical Analysis
Means ± standard deviations (SD) were calculated, and a Student’s t test was used for the evaluation of statistical significance. A p-value of <0.05 was considered significant.
2.6. Tissue Treatment and Sample Collection
Before the treatments, the medium was removed, and fresh medium was added. For the treatments, 10 μL of the three different samples (abbreviated as A: Carnosine complex; B: Free carnosine, C: Magnesium salt) was topically applied over RHE in a single dose for 24 h or in a repeated application at 24 h for a total of 48 h. Control tissue (CTRL) was exposed only to the medium alone. Because no differences between 24 and 48 h were observed in control tissues, the samples were pooled and presented as the control group. All mixtures and control samples were assayed in triplicate using three sets of RHEs for each group.
Tissue medium aliquots were collected after 24 and 48 h of treatment and centrifuged for 20 min to remove insoluble impurities and tissue debris at 1000× g at 4 °C. The clear supernatants were stored at −20 °C until analysis. After washing with PBS and snap-freezing in liquid nitrogen, the RHE tissues were homogenized in a glass beaker with 150 µL of PBS on ice. The homogenates were then centrifuged for 5 min at 5000× g to obtain the supernatants, which were stored at −20 °C until analysis.
4. Discussion
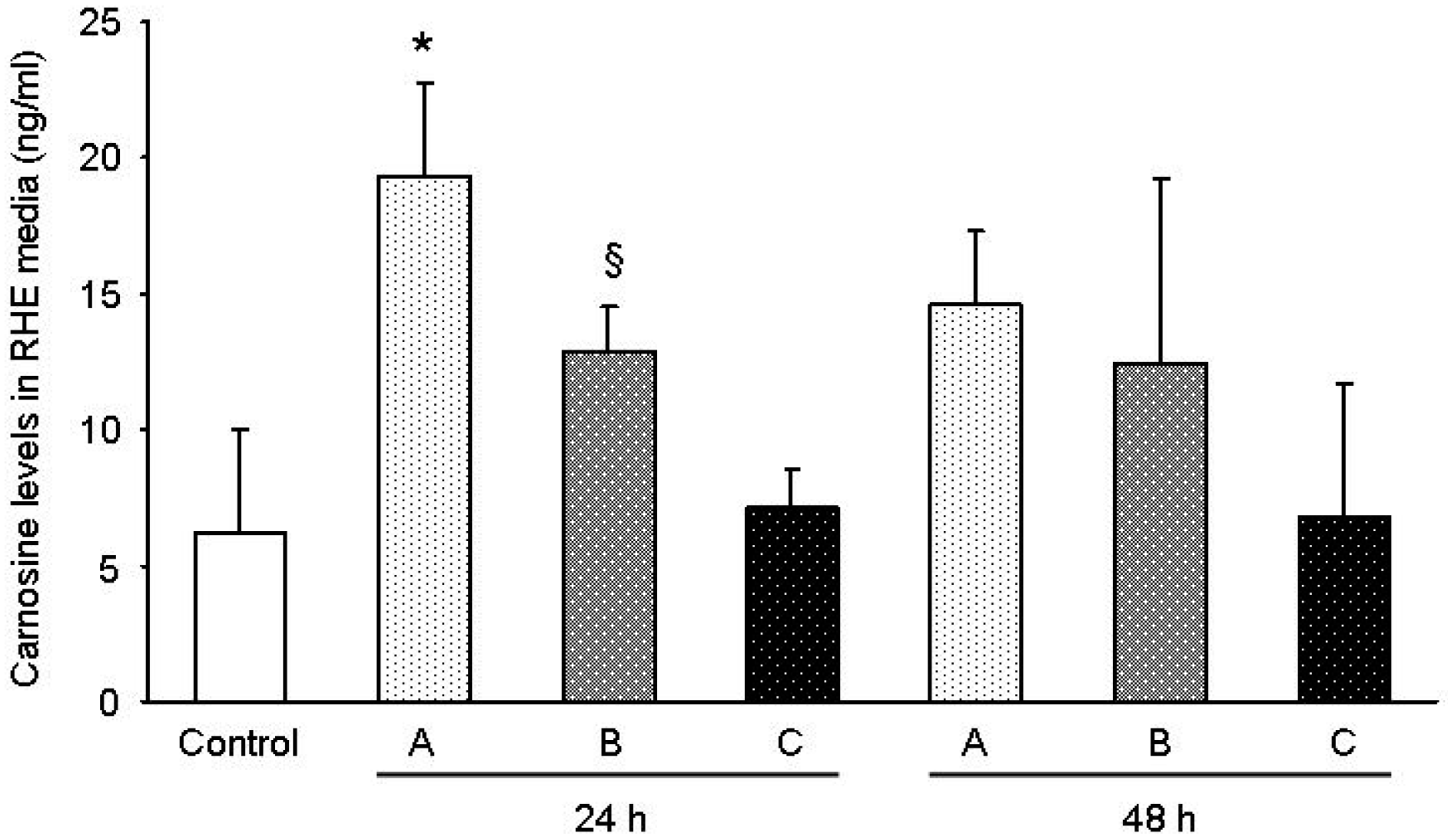
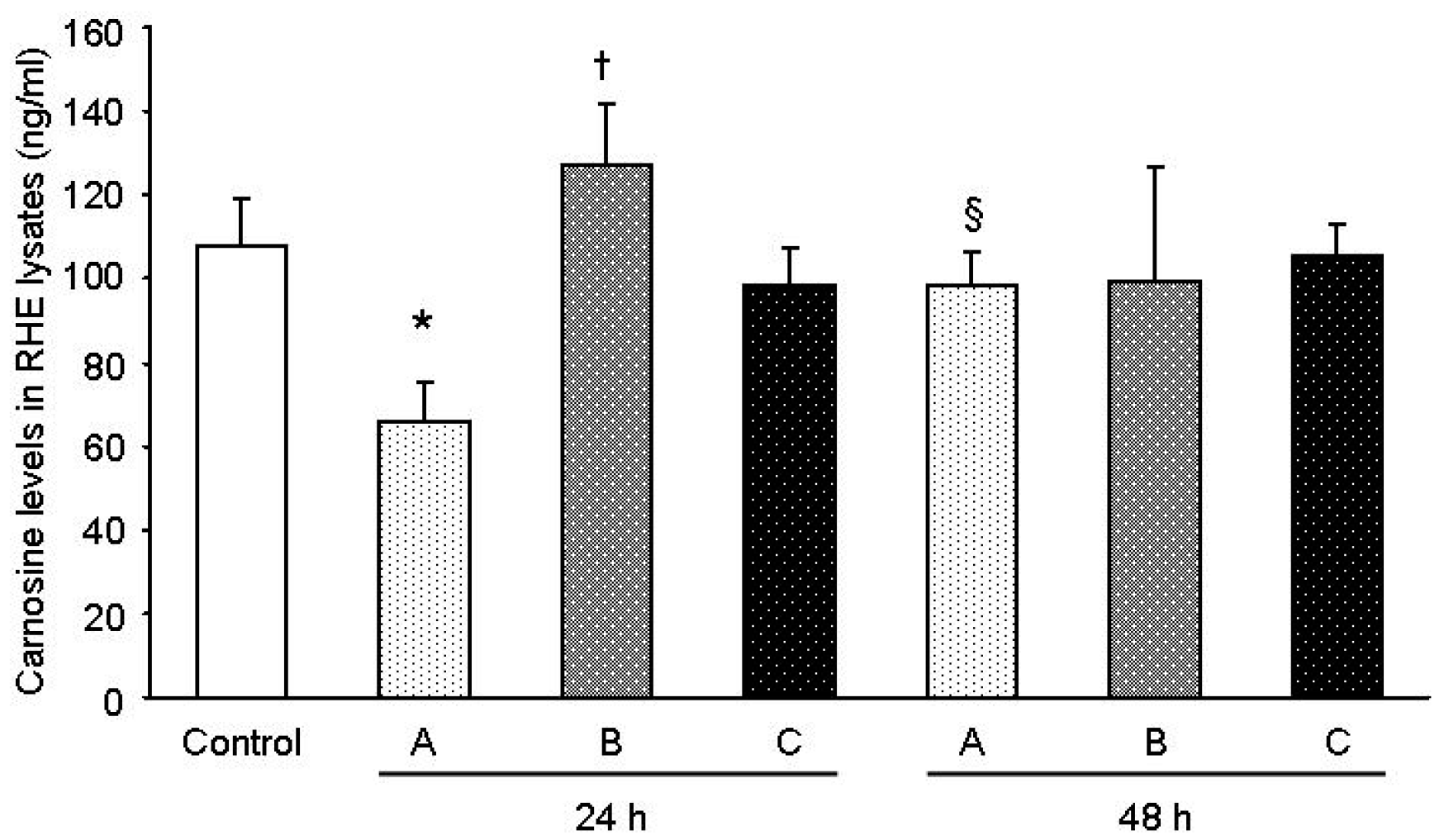
The results obtained in this study support the view that the gel formulation based on the carnosine-magnesium complex allows for superior delivery of carnosine in the lower skin layer. According to the data, there was a 1.5× improvement in the carnosine concentration detected in the medium underneath the RHE layer following the application of sample A with respect to B, which only contained carnosine. This fact is consistent with the observation of a 1.9× residual quantity of carnosine on the surface of the RHE tissues for sample B with respect to A. As expected in sample C, the carnosine level was comparable to the concentration of carnosine recorded for the control tissue. Furthermore, recent studies [
19] have already dealt with the issue related to the difficulties in delivering carnosine through diet and the necessity to develop an increased delivery on a tissue exposed to oxidation as skin, along with the possibility also to reach deeper layers. Schagen et al. [
19] found an improvement using emulsions instead of solutions. In our case, the use of a chelating metal, greatly improved delivery in a simple formulation as a gel achieving better results. These preliminary results indicate that there are still a great possibility to ameliorate carnosine skin delivery by combination of metal chelation and better performing vehicles.
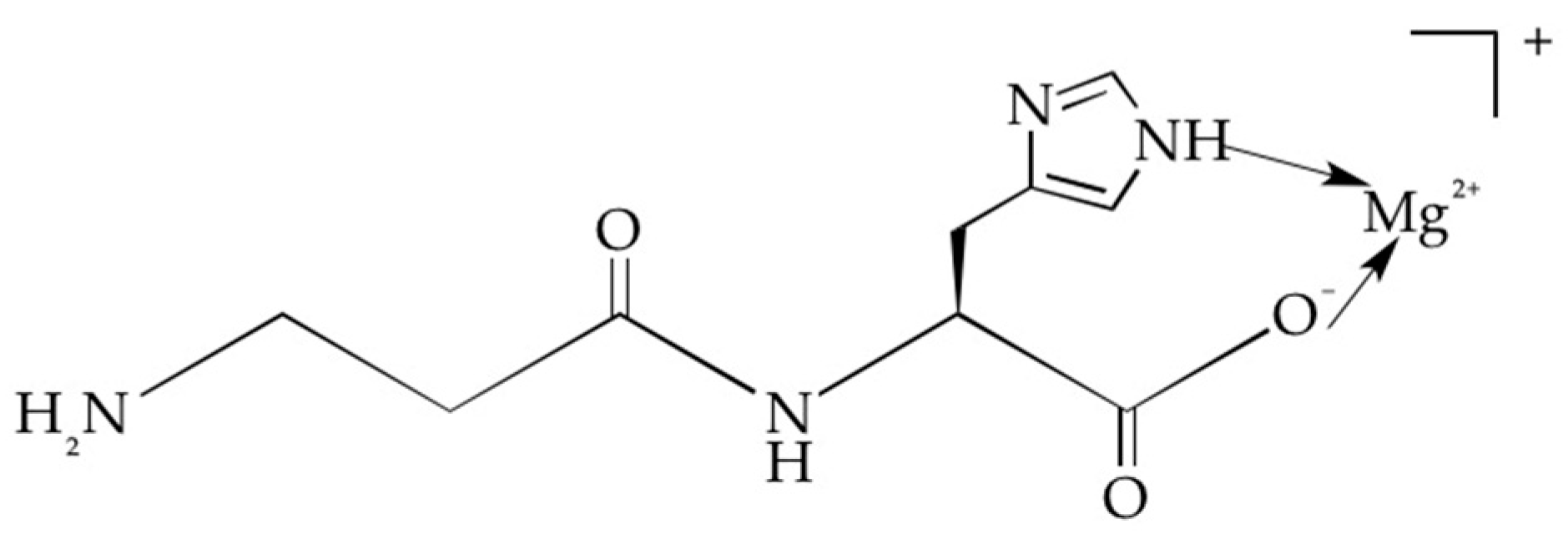
It is clear that an explanation of the higher topical availability of carnosine obtained in the presence of magnesium requires further investigation. However, we can advance the hypothesis that because carnosine is a polydentate ligand, it offers several potential binding sites: the two imidazole nitrogens, one carboxylic acid group and the amino groups. In aqueous solution, carnosine deprotonates the carboxylic function (Pk = 2.6) [
22] to form a negatively charged anionic species. Therefore, coordination with magnesium occurs through the interaction of the carboxylic function and the N1 nitrogen of the imidazole ring to form a 1:1 cationic carnosine-magnesium complex (
Figure 3) with a stability constant on the order of 103. [
23].
The positively charged complex in which carnosine is coordinated with the Mg2+ ion, which may be due to its small dimensions (86 pm), should experience an intense electric field and could therefore better interact with the electronic charges of the nitrogen atoms present on the membrane proteins and on the long chain glycosaminoglycans, thus favoring transmembrane transport of the amino acid.