1. Introduction
Cellulose is one of the most abundant biodegradable materials in nature. Currently, cellulose can be produced in different ways, all by natural synthesis procedures, plant photosynthesis, microbial synthesis, and synthetic methods [
1].
Microbial cellulose (or bacterial cellulose, BC) is produced by various species of bacteria, such as
Gluconacetobacter xylinus, in the presence of oxygen and glucose. Since 1886,
Gluconacetobacter xylinus has been used as the model microorganism for different bases and applied studies on cellulose. Microbial cellulose is an exopolysaccharide, a long chain polysaccharide consisting of sugars units, or sugar derivatives, joined by different links (e.g., 1–2 and 1–4). The molecular formula of bacterial cellulose (C
6H
10O
5) is the same as that of plant cellulose, but their physical and chemical features are different [
2].
Typically, bacterial cellulose exhibits a basic structure made of microfibrils composed of glucan chains interlocked by hydrogen bonds, so that a crystalline pattern (microfibrils) is obtained. BC possesses a higher purity, crystallinity index, and degree of polymerization in comparison to plant cellulose. Its fibrils are about 100 times thinner (diameter 20–100 nm), such that a very high surface area per unit mass is demonstrated. This feature, combined with its highly hydrophilic nature, results in a very high liquid-loading capacity. Its water-holding capacity (WHC) is over 100 times (by mass) higher than plant cellulose, and the WHC is 100–200 times its dry weight [
1,
2,
3].
The hydrogen bonds between its fibrillar units stabilize the whole structure, and define many of its mechanical properties. Tensile strength, maximum elongation, and elastic modulus, that characterize BC, depend on its own uniform ultrafine-fiber network structure, and the high planar orientation of the ribbon-like fibers, when compressed into sheets, results in good chemical stability [
3].
Bacterial cellulose shows great biocompatibility, not only because of its non-toxic effects on biological systems but, also, by eliciting an appropriate host response to ensure satisfactory performance during a specific application. Petersen and Gatenholm have pointed out that biocompatibility of BC for tissue engineering applications can be can be due to structure similarities with extracellular matrix components, such as collagen. In fact, collagen and BC nanofibers have similar diameters (around 100 nm), and are extracellularly assembled from precursor molecules into polymer chains [
4].
Due to its unique structural properties, BC has become of interest in different fields, such as in the textile industry, high quality paper production, food, pharmaceutical and medical devices, electronics, and acoustics [
1,
2]. In particular, the biomedical field exploits microbial cellulose as a natural, porous, nontoxic material in tissue-like products for both wound care and the regeneration of damaged or diseased organs. Due to its unique nanostructure and properties, BC is a natural candidate for numerous medical and tissue-engineered applications, such as wound dressings, bone tissue engineering and bone grafting, and cardiovascular applications [
3,
4,
5]. In wound healing systems, BC has great potential because it is biocompatible, adherent, elastic, and transparent, resulting in good permeability and water absorption capacity. Also, it maintains a moist environment in wounds, absorbing exudates, and shows low solubility and resistance to degradation [
1].
Biocompatibility allows for classification of BC among sustainable/renewable sources, thus suitable when developing value-added cosmetic products [
6].
It has been claimed that, due to its high water-holding capacity and good gas permeability, BC can be an appropriate carrier for delivering active ingredients to the skin, including moisturizers, whitening ingredients, and anti-wrinkling agents.
In recent years, studies have been developed for the use of BC as a substrate to deliver hydrophilic cosmetic compounds, in order to obtain masks with exfoliating and brightening effect, purifying hot clay masks, and anti-wrinkle patches [
7,
8,
9,
10,
11,
12].
A patented BC facial mask was fabricated with holes for the eyes, mouth, and nose. The author claimed that such a mask may be suitable for repeated or prolonged use for skin beautifying purposes, skin nutrition, and moisturizing and cosmetic effects [
11]. An American study describes a method for manufacturing masks from bacterial biocellulose by directly adding extracts of ginseng (known for its antioxidant and anti-inflammatory properties) to the culture broth; results show overall user satisfaction in terms of moist feel and skin elasticity in women aged 30+ years [
11].
However, available information about the use of BCs in the cosmetic industry and, also, clinical results, are rather scarce [
12].
The production of a safe and stable formulation is one of the more important issues to be addressed by cosmetic and pharmaceutical companies.
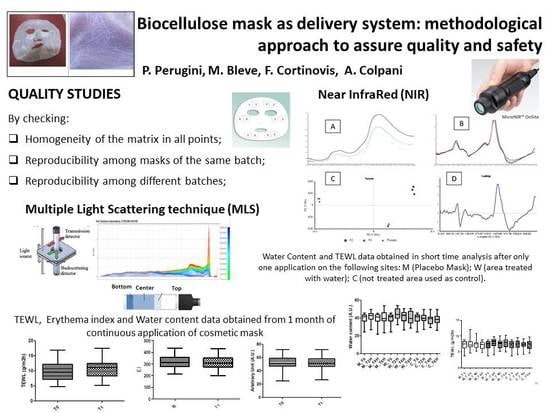
Stability evaluation is always crucial for colloidal systems, like emulsions and suspensions. They are intrinsically unstable, and particle migration (sedimentation or creaming) combined with particle aggregation might lead to partial or total separation, or the emulsion breaking. Characterizing these destructive processes and monitoring of stability are, therefore, essential. This is usually obtained through Aging tests, accelerating destabilization to detect potentially unstable formulations. Products are stored under specific conditions (temperature, humidity, light), then organoleptic properties, pH, and viscosity are analyzed. Besides ordinary visual inspections, non-destructive instrumental techniques (NIR and MLS) have been used to investigate the expected stability.
The aim of this paper has been to establish a protocol for the manufacture of BC face masks, and to identify appropriate methods for quality assessment of the finished product, establishing criteria to evaluate their shelf-life, as well.
Finally, specific “in vivo” tests have been designed in order to test such aspects as tolerability, safety, and efficacy of cosmetic formulations, and their effect on many different skin parameters upon application using a BC mask delivery system.
2. Materials and Methods
2.1. Materials
The bacterial cellulose sheets (BCS) used in this study were obtained from two manufacturers; sheets were supplied in stacks (20 sheets) soaked with a preserved water solution (phenoxyethanol 0.075%, iodopropynyl butylcarbamate 0.0015%). Both manufacturers stated that BC had been obtained by incubation of Gluconacetobacter xylinus in a sterile broth at 30 °C, for a period of up to 120 h, at a pH = 6.5–7.0, in static conditions. After partially de-watering the gel, the BC sheet had been die-cut to obtain a mask shape, with holes for eyes, nose, and mouth.
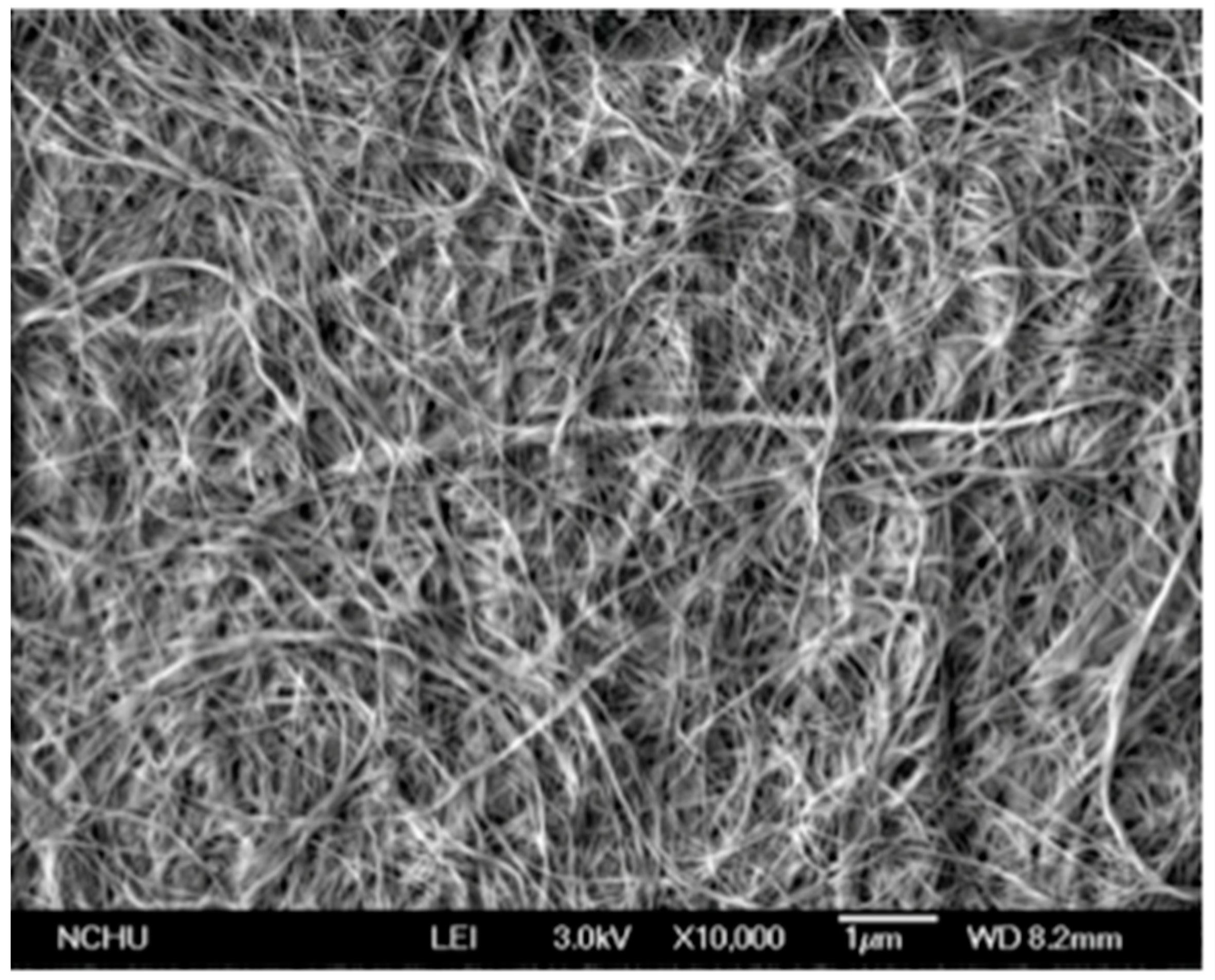
The diameter of the fibers is about 40 nm, so that the ultrafine net of BC has a very smooth network of microfibrils, perfect to fit skin (
Figure 1).
2.2. Preparation of Face Masks
In this work, placebo masks (PM) were prepared, starting from BCS in the following way: bacterial cellulose sheets (BCS, batch 1603, Supplier 2) were folded, embedded with 15 mL of solution placebo (P) containing water 95.7%, butylene glycol 3%, phenoxyethanol 0.9%, ethylhexylglycerin 0.1%, and xanthan gum 0.3%.
The folded masks were inserted into Polyethylene terephthalate (PET)/aluminum/ Polyethylene (PE) sachets, thermo-sealed, and stored for 1 week at room temperature before analysis.
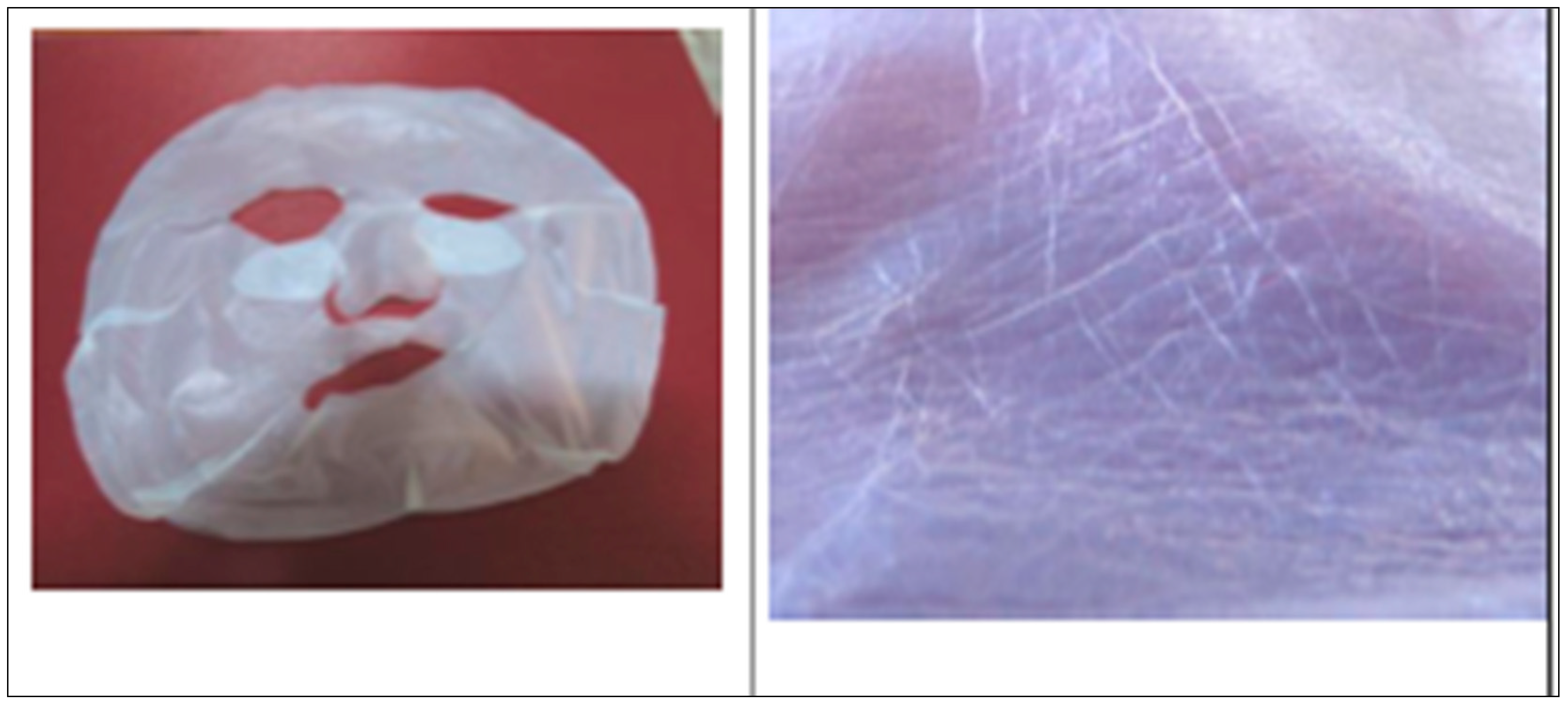
Figure 2 shows unfolded face placebo masks, as an example.
Table 1 shows all samples investigated in this study.
The “in vitro” analyses were performed on
- -
bacterial cellulose sheets (BCS),
- -
placebo masks (PM);
- -
solution P before and after squeezing the masks.
2.3. Stability Testing
For these purposes, two non-destructive techniques, near-infrared spectroscopy (MicroNIR™ Pro Spectrometer, JDSU, Milpitas, CA, USA) and multiple light scattering (MLS, Turbiscan Tower®, Formulaction, Toulouse, France), were used.
2.3.1. Near-Infrared (NIR) Analysis
In recent years, NIR spectroscopy has gained wide acceptance within the pharmaceutical industry for raw material testing, product quality control, and process monitoring. The growing pharmaceutical interest in NIR spectroscopy is probably a direct result of its major advantages over other analytical techniques, namely, an easy sample preparation without any pretreatments, the use of fiber optic probes for measurement of samples far from the spectrometer, and the capacity to predict chemical and physical sample parameters from one single spectrum [
13,
14].
The combined use of near-infrared (NIR) spectroscopy and multivariate spectral processing chemometric techniques has enabled the development of effective methods for establishing the composition of complex samples with acceptable levels of analytical properties, such as accuracy, precision, and throughput [
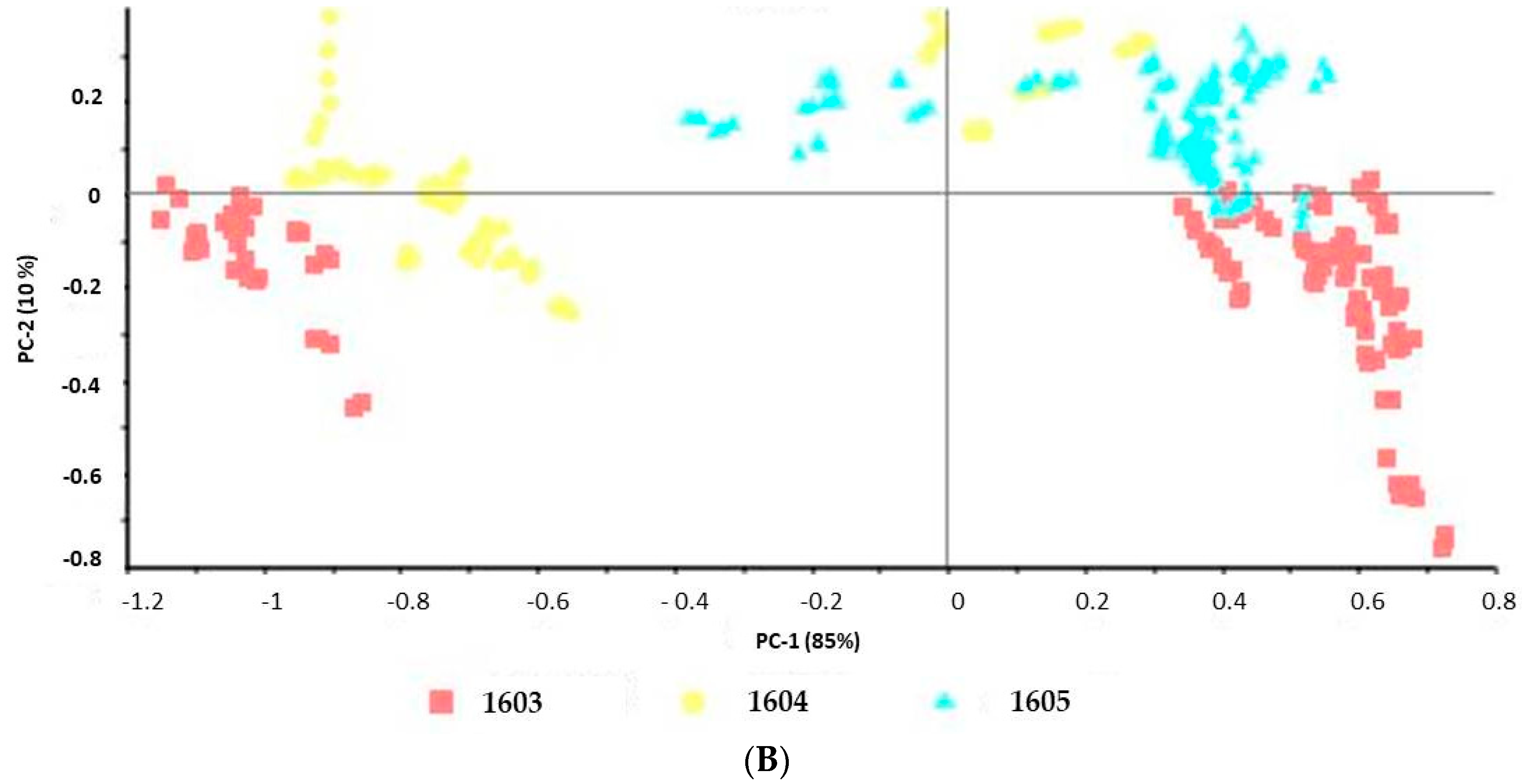
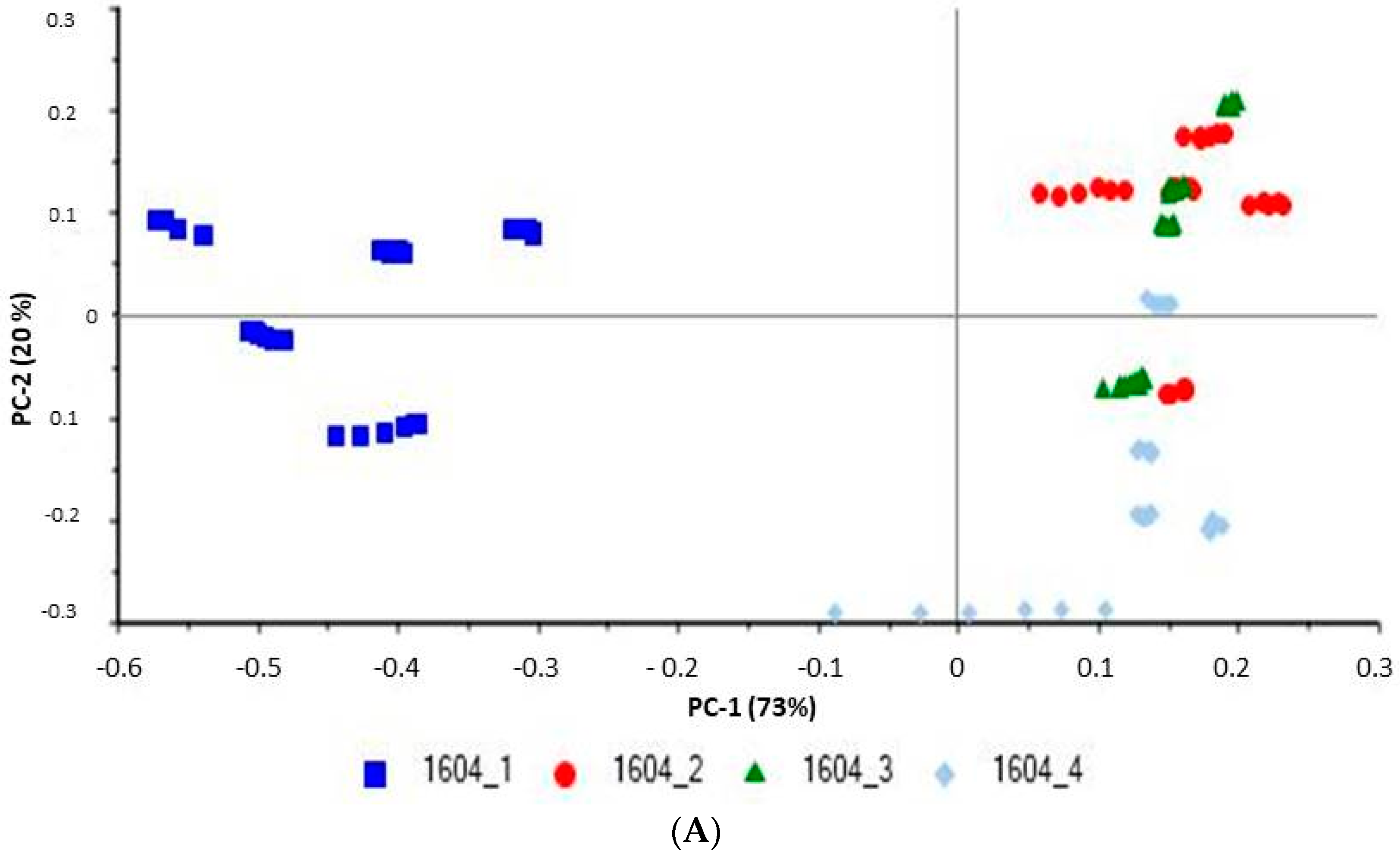
15]. In this work, we applied principal component analysis (PCA) and global indices (viz., the hydroxyl value), obtained from the NIR spectrum of the sample, to define the limit of acceptability. The models, thus obtained, are accurate enough for use in quality control analyses of cosmetic preparations, and provide an effective alternative to existing conventional global methods [
16].
NIR spectroscopy is used as non-destructive mask characterization for two different purposes:
- ✓
To test the homogeneity of the cellulose sheets, investigating the overall quality of the biocellulose item, and assess/validate the BCS supplier;
- ✓
To check the manufacturing process of face masks, and use spectroscopy as a quality control tool over time.
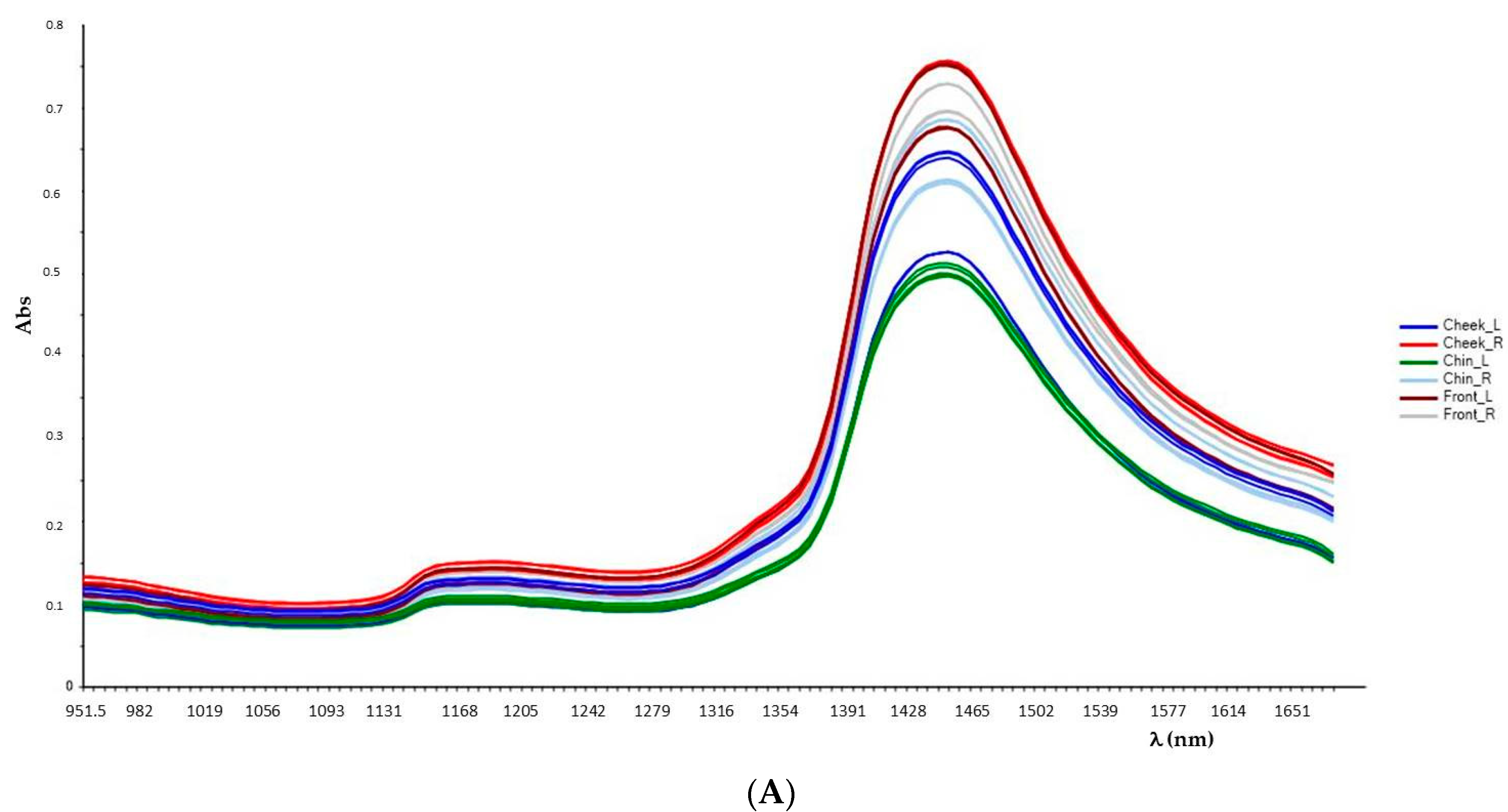
Both sheets and masks to be analyzed were extracted from the sachet and completely unfolded, stretched, and laid on the work surface, looking for visible stains or folds. Then, three specific areas to be scanned were selected (the same for all performed analyses) with reference to actual face areas: upper section (corresponding to forehead), median section (cheek), and lower section (chin). For each area, the points analyzed are split between the right and the left, as shown in the
Figure 3. The right/left name is referred to the viewer’s point of view, with the sample lying on the work surface.
At least 3 samples from each batch and 3 different batches of sheets and placebo masks were analyzed.
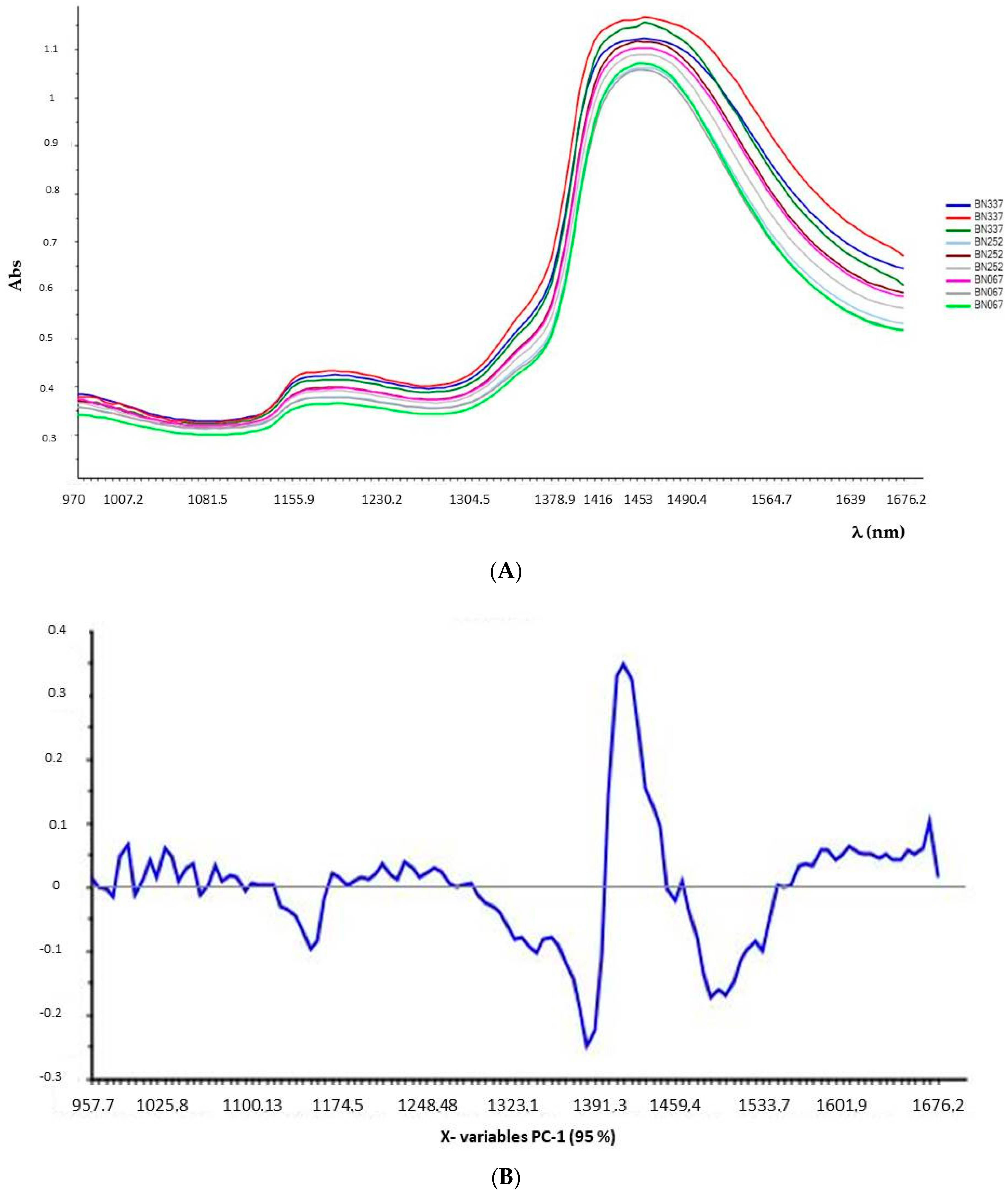
For the NIR spectroscopy, the scanning wavelength was in the range of 950–1650 nm. In this region, each constituent of a complex organic mixture has unique absorption properties, due to the stretching and bending vibrations in molecular bonds [
13].
For this analysis, the MicroNIR™ Pro Spectrometer, (JDSU, Milpitas, CA, USA) was used, with parameters reported in
Table 2. This technique presents some advantages with respect to other analytical techniques, for example, its ability to record the spectra of solid and liquid samples without prior manipulation. Furthermore, it is simple, rapid, and cost-effective [
14,
15].
Samples were placed on a non-reflective support, with a fixed and constant distance (3 mm) from the acquisition window. Three replicates for each sample were always used, and analyses performed at 21 °C ± 2 °C and 70% RH.
All the analyzed samples were white, and of uniform appearance (visual inspection).
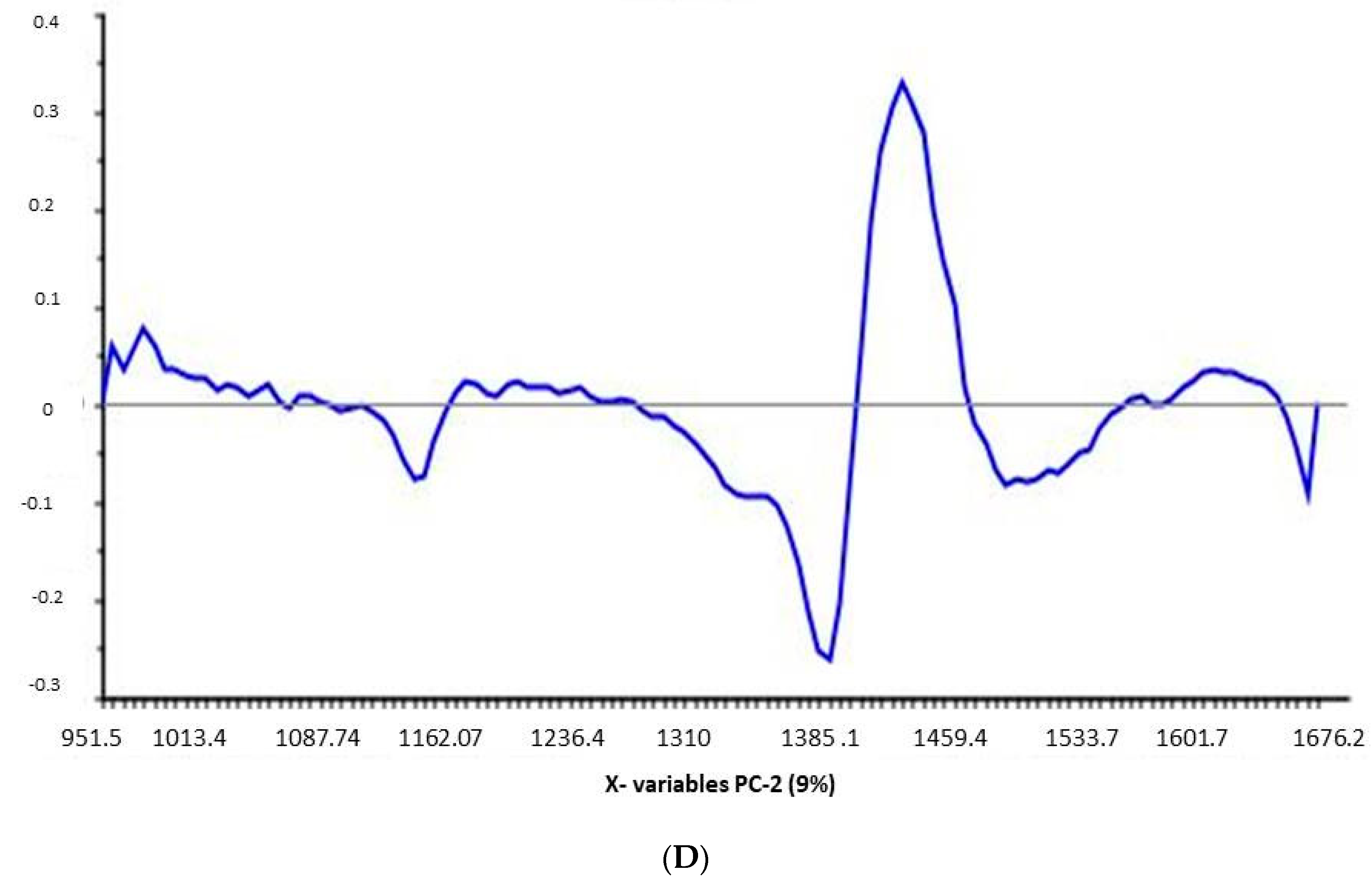
Data were evaluated using the Unscrambler® X software, version 10.4 (Camo software AS, Oslo, Norway). The spectra were pretreated by using standard normal variate (SNV) followed by the first derivative with Savitzky–Golay smoothing. Principal component analysis (PCA), on pretreated spectra, were performed on the obtained spectra.
2.3.2. Multiple Light Scattering (MLS) Analysis
MLS technique allows evaluation of optical characteristics for several formulations, from suspensions or emulsions to nanosystem or microsystem gels [
17,
18,
19]. With the multiple light scattering technique, a macroscopic fingerprint of the sample at a given time is obtained, providing useful information about changes in droplet size, distribution, and appearance of a creaming layer. On a smaller scale, MLS also offers a new approach to predict migration phenomena, like sedimentation and creaming (for emulsion and suspension), and size variation phenomena (like flocculation and aggregation). MLS provides a major advantage over current ageing tests. It is possible (i) to measure the stability of both opaque and concentrated colloidal dispersions with a single instrument; (ii) to detect instability phenomena much earlier and easier than is usually possible through a visual inspection [
20].
Other advantages represented by this technique are (i) a short evaluation time when compared to those of usual stability protocols (hours/days instead of weeks/months), (ii) a small sample required (20–30 g); (iii) no dilution nor sample preparation; the instrument works on the product as such.
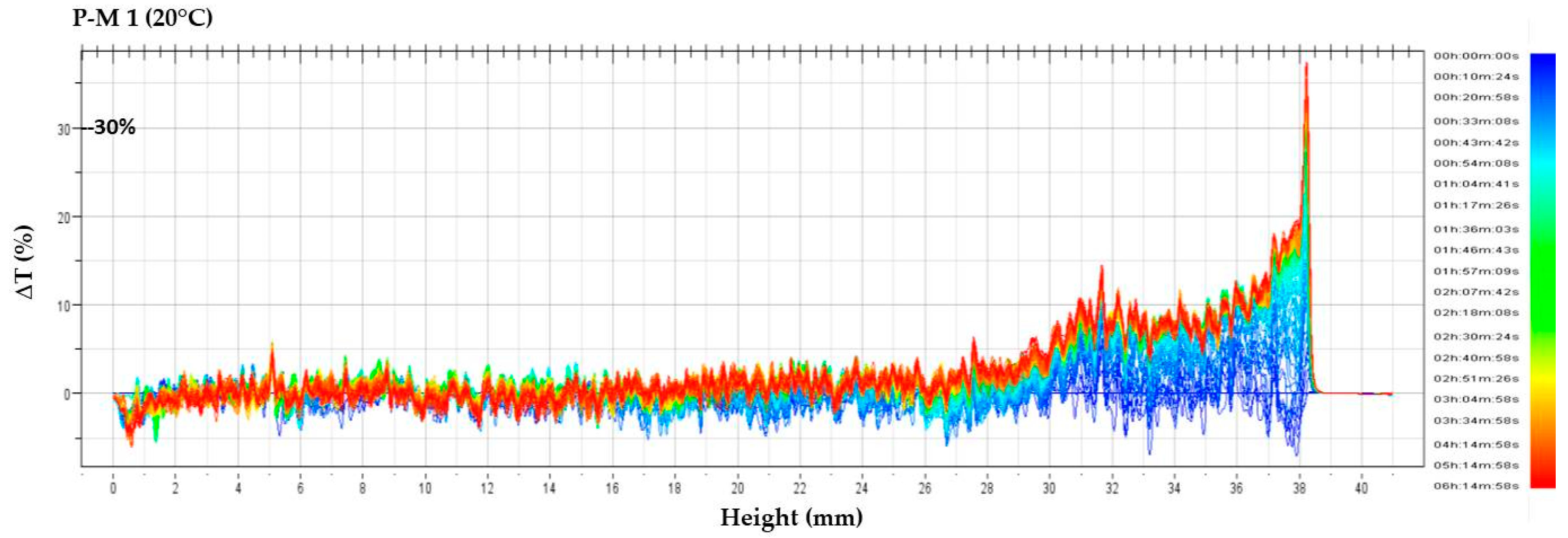
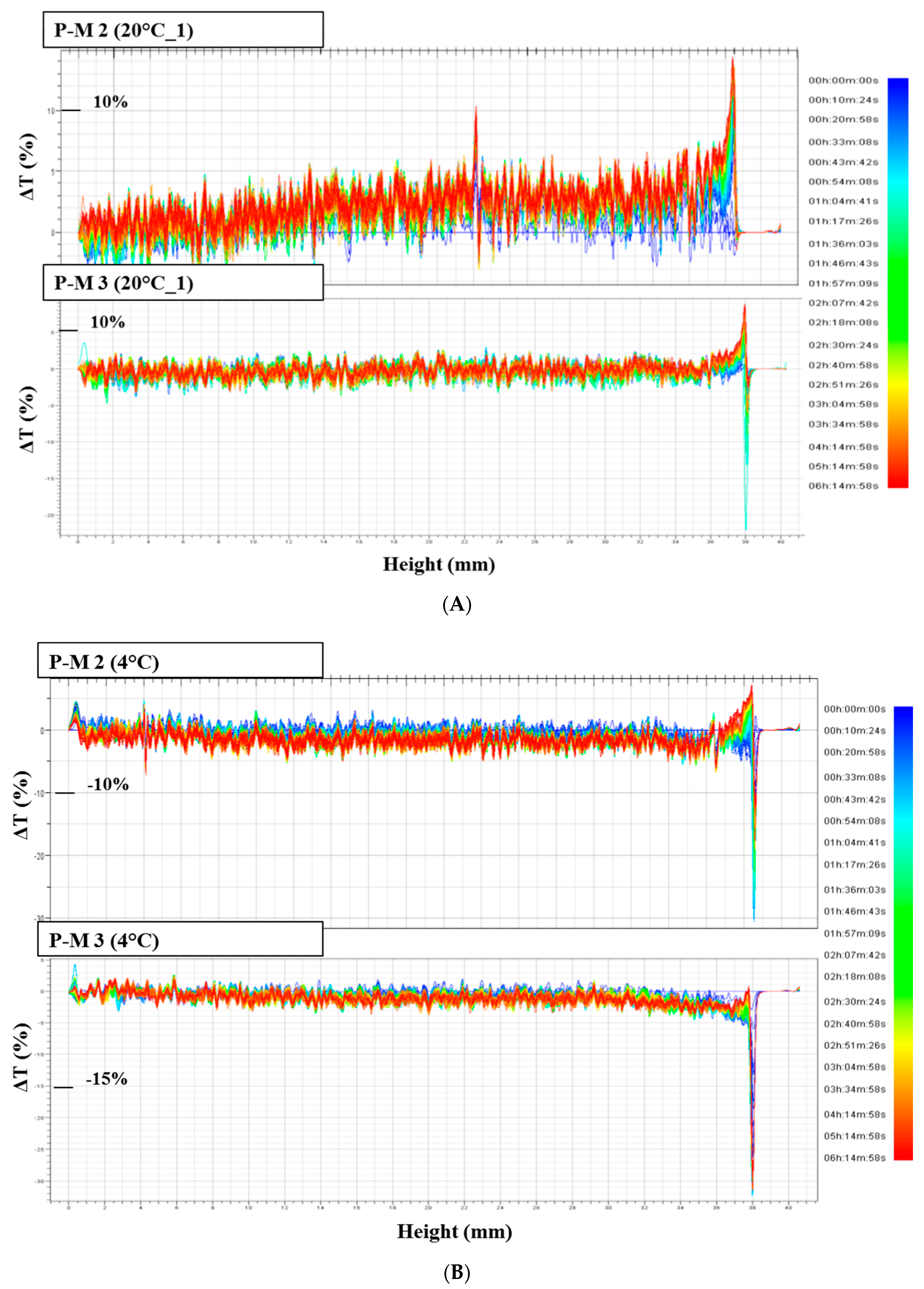
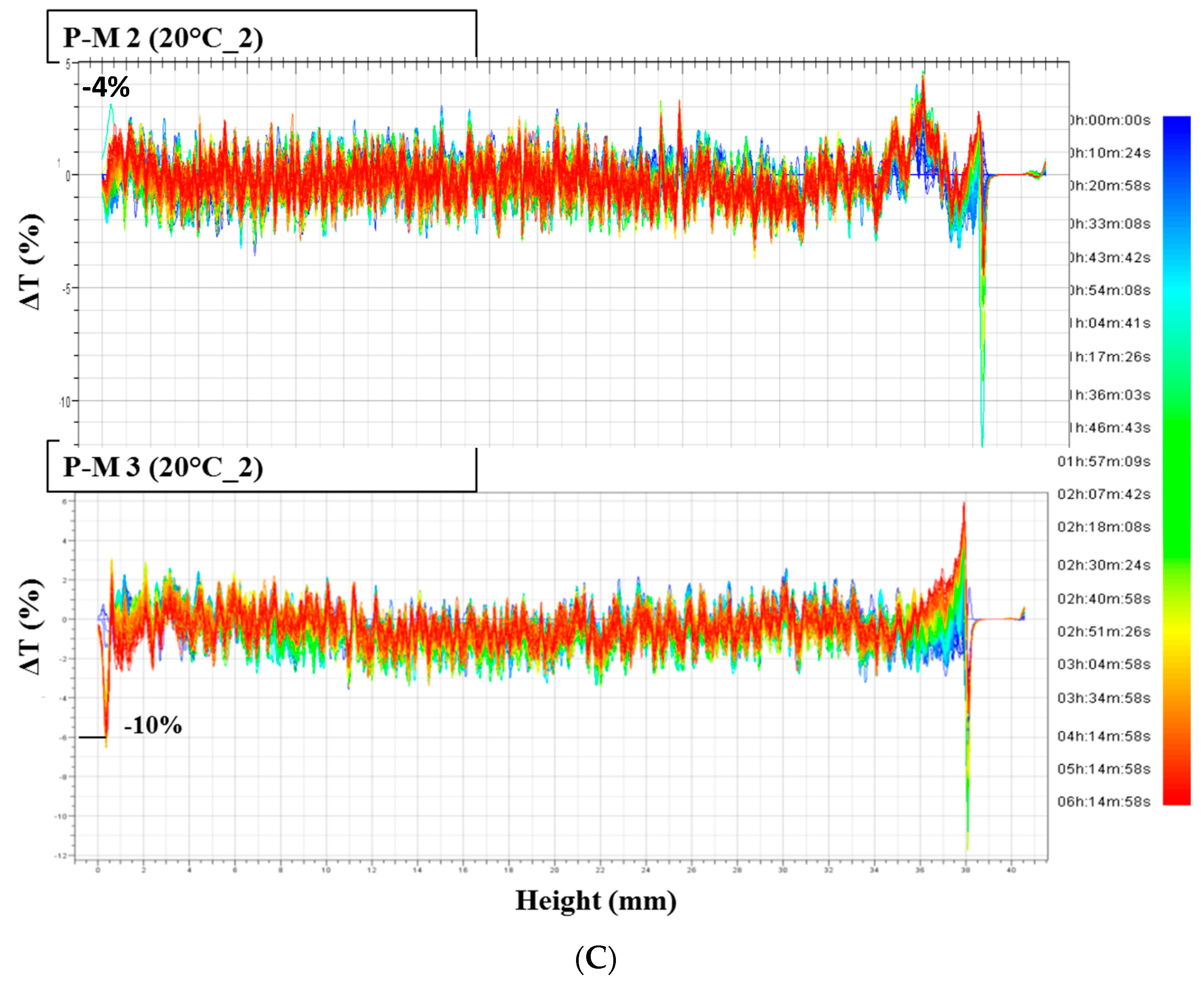
Destabilization phenomena by multiple light scattering were investigated by Turbiscan Tower® (Formulaction—France). Solutions were transferred in the specific glass vials and they were scanned, each 40 µm, by a pulsed near-infrared light source (λ = 880 nm). The instrument is equipped with two synchronous detectors: the transmission detector receives light through the sample at an angle of 0 degrees from the incident beam; the backscattering detector receives the light scattered by the sample at an angle of 135 degrees from the incident beam. Three different sequences of analyses were established: six hours at 20 °C, six hours at 4 °C and, again, six hours at 20 °C, in order to simulate a thermal shock.
For each cycle of analysis, 181 scans were acquired every 25 s. All samples, before analysis, were kept at room temperature (at 25 °C).
Solutions obtained by squeezing placebo masks, from three different batches manufactured over a period of 12 months, were analyzed using the MLS technique, in order to evaluate the possible interaction between the cellulose matrix and the solution used to soak the biopolymer.
The solutions obtained by mask squeezing and, then, analyzed as reported below:
- -
P-M 1 (from PM batch BN337, 1 year old),
- -
P-M 2 (from PM batch BN067, six months old),
- -
P-M 3 (from PM batch BN252, just produced)
Two parameters were evaluated and compared: transmission variations (ΔT), and Turbiscan stability index (TSI). Only transmission variations over ±10% were considered significant. TSI sums all the variations detected in the sample in terms of size and/or distribution. The higher is the TSI, the worse the stability. Visual evaluation (by image capture) and pH measurements (by pH-meter 827 pH Lab/Metrohm, Italy) were performed before and after Turbiscan cycles.
2.4. In Vivo Testing
2.4.1. Study Design
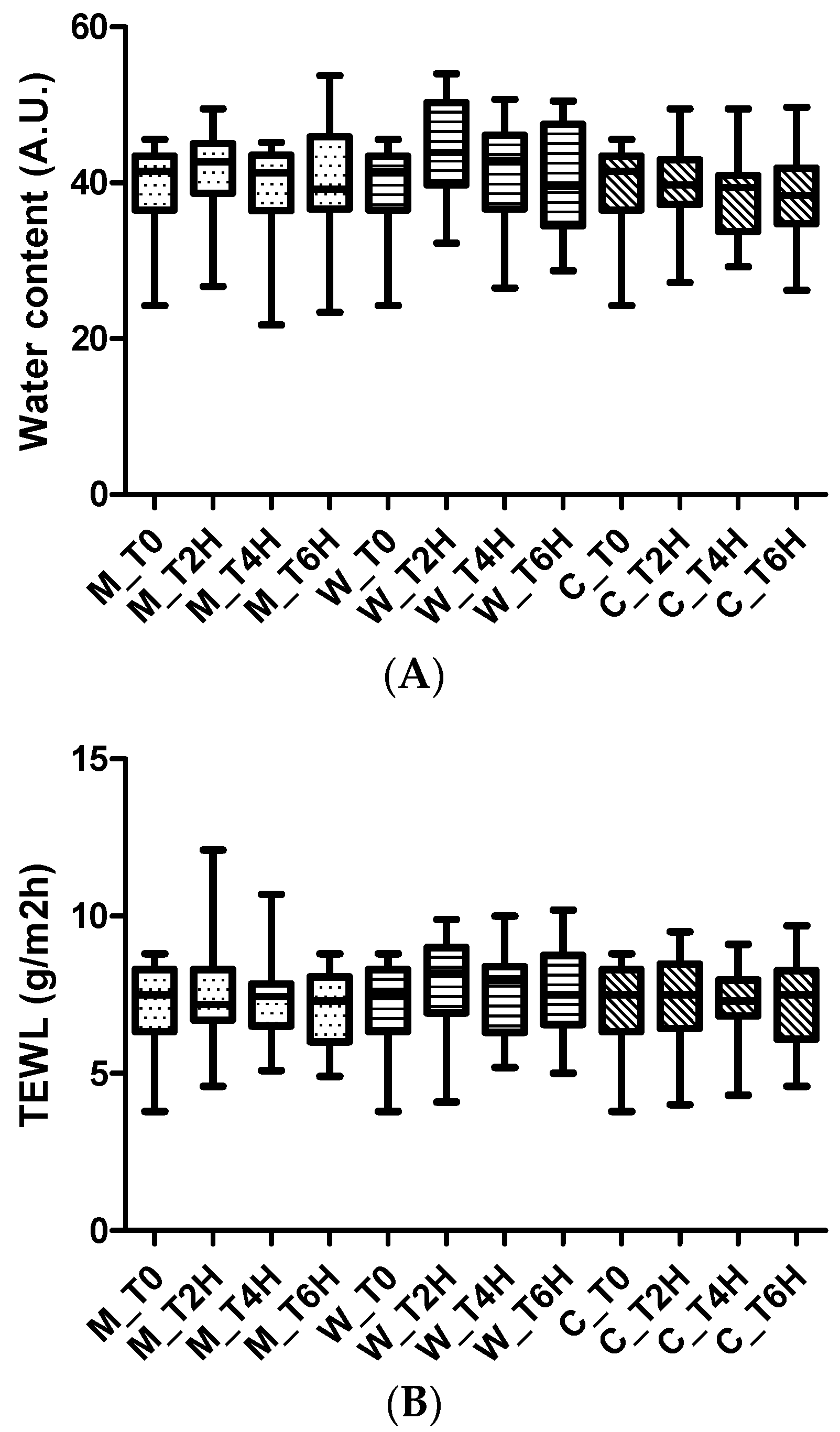
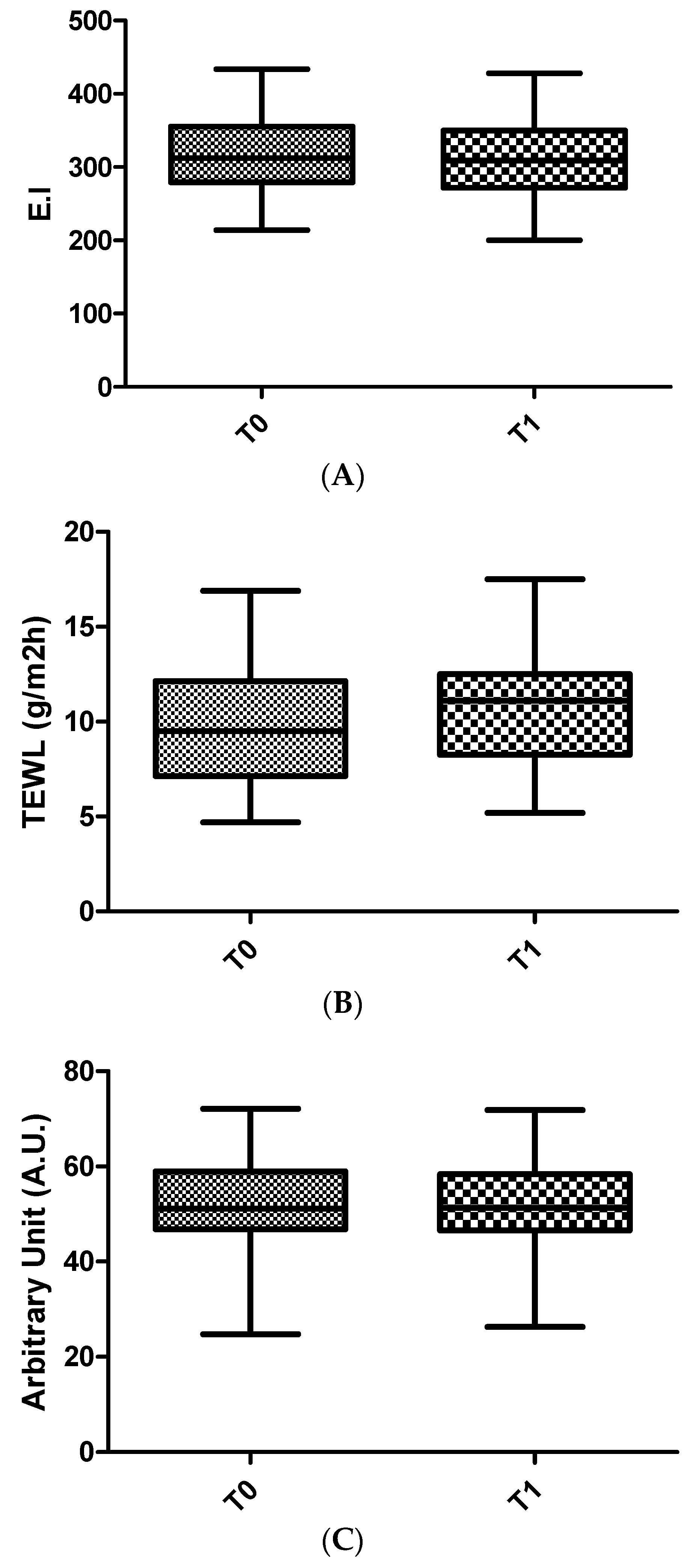
In order to investigate the safety of the biocellulose masks applied to skin, different in vivo studies have been performed on placebo masks. In particular, short-term effects after single application, and long-term effects after one month of use, were investigated.
In the short-term study, the effect of biocellulose masks was evaluated after one application by measuring the water content before the application (t0), and 2 h (t2), 4 h (t4), and 6 h (t6) after application. A site treated with water was used as reference.
In the long-term study, the safety and effect of biocellulose masks was evaluated after 1 month of application 3 times a week.
Skin parameters investigated in these studies were
- ➢
Erythema index
- ➢
Water content of the stratum corneum
- ➢
Skin barrier integrity
All “in vivo” protocol studies have been carried out according to the Helsinki declaration (Ethical Principles for Medical Research Involving Human Subjects). For both studies, 89 healthy female volunteers were recruited according to the following general inclusion criteria:
- -
good general health;
- -
absence of cutaneous diseases;
- -
people that do not show cutaneous lesions or other lesions in the interested area that could interfere with the study evaluation;
- -
people that do not present a history of hypersensitivity to the common components of the cosmetic formulations;
- -
women that are not pregnant nor breastfeeding;
- -
people that agreed not to undergo other treatments for the entire duration of the test in the treated area;
- -
subjects that signed the informed consent form and following the common exclusion criteria:
- -
subjects that did not meet the inclusion criteria above;
- -
subjects under pharmacological therapy;
- -
participation in similar study by at least 60 days;
- -
subjects with no known allergies to any components of the product.
2.4.2. Instrumental Evaluation
The instrumental evaluation was carried out at the beginning of each study, and at predetermined times, depending on the aim of the study. In all cases, the mask was applied 3 times a week, on the cleaned face, and 20 min after it was removed.
All measurements were made in an air-conditioned room with controlled temperature and humidity (T 22 °C, RH 70 ± 5%); subjects were preconditioned in such room for at least 15 min before the measurements.
The instruments used in the evaluation of the skin properties, such as water content, skin barrier integrity, and the erythema index, involve contact between the skin and a series of probes that do not cause discomfort, pain, or damage the skin.
The water content of the stratum corneum, reflecting the hydrating effect of the product, was evaluated by Corneometer CM 825 (Cutometer MPA580, Courage & Khazaka, Cologne, Germany). Corneometry is a technology used to assess the hydration of the outer layer of the epidermis: the stratum corneum [
21]. Since skin is a dielectric medium, all variations in hydration result in corresponding changes in the skin capacity [
22]. The device used in our trial was equipped with a 49 mm
2 surface probe that allows precise measurements in 1 s within a 10–20 μm depth range in the stratum corneum. The parameters were expressed as an arbitrary score scale (0–100 A.U.).
The assessment of skin barrier integrity, and the possible occlusive effect caused by the use of the product object of this study, have been performed by a Tewameter TM 300 (Cutometer MPA580, Courage & Khazaka, Cologne, Germany) [
22,
23]. Transepidermal water loss (TEWL) is assessed in terms of g/m
2h by a skin evaporimeter made of a small cylindrical open chamber (1 cm in diameter, 2 cm in height) with a couple of hygrometric sensors connected to a microprocessor plugged into a computer workstation. The device allows recording of TEWL values (ranging from 0 to 90 g/m
2h), as well as the relative humidity (ranging from 0 to 100%) and the probe temperature.
The erythema index (EI) was assessed using a reflectance spectrophotometer (Mexameter MX 18, Courage & Khazaka, Cologne, Germany).
2.4.3. Statistical Analysis
All data obtained were processed as both a descriptive statistical analysis, and as a paired t-test. A significance level of 5% was chosen, so changes were considered statistically significant for p < 0.05.
4. Conclusions
Bacterial cellulose is used, among several applications, for the manufacture of cosmetic masks, with beautifying purposes, to promote skin nutrition and moisture, plus other desirable cosmetics effects.
In order to develop stable, safe, and effective commercial products, it is essential to establish a protocol to check their quality and stability. So far, no methods, nor complete protocols for quality assurance of BC mask, have been published.
In this study, NIR and MLS techniques were applied to bacterial cellulose sheets and masks. These fast and reproducible techniques proved to be suitable methods for quality and stability testing. NIR proved to be able to detect changes in water and ingredients distribution in different areas of the mask, and to ascertain the influence of folding as well. Multiple light scattering was successful to evaluate stability, as well as possible interactions between the formulation and the cellulose matrix, leading to a more precise definition of the expiry date (shelf-life) attributable to these new delivery system.
If one refers to the tested products, the most likely shelf-life period for stable and safe commercially available BC masks would be 6 months.
Erythema index values and TEWL values, obtained during in vivo tests, highlighted the great tolerability of BC masks: skin parameters were not altered upon continued use, no occlusive effect was reported, nor had the skin barrier function been affected.
To summarize, bacterial cellulose may be a promising substrate to develop a new class of cosmetics, able to deliver a large number of hydrophilic/water-soluble substances onto the skin.