Abstract
This study aimed to investigate Malassezia furfur inhibitory activity of the fermented product from Ocimum sanctum and develop an antidandruff shampoo. The fermented product was obtained by the fermentation process of the aerial part of O. sanctum. Total soluble protein was detected in the fermented product with the amount of 65.32 ± 0.14 mg/100 mL, whereas there was no organic acid. The inhibitory activity against four strains of M. furfur (No. 133, 656, 6000, and 7966) of the fermented product and shampoos containing the fermented product were investigated by broth dilution and agar diffusion method, respectively. The fermented product possessed high antifungal activity with the minimum inhibitory concentrations for 50% (MIC50) of M. furfur 133, 656, 6000, and 7966 of 0.125, 0.25, 0.125, and 0.125 mg/mL, respectively. Interestingly, the antifungal activity against M. furfur 656 was comparable to that of ketoconazole. Shampoo formulation C, which was the best formulation in terms of characteristics and stability, obtained a high level of satisfaction scores in terms of hair smoothness, hair shine, ease in combing, frizz reduction, and triboelectric reduction while brushing. Additionally, the shampoo containing 2% (w/w) of the fermented product of O. sanctum also possessed inhibitory activity against M. furfur 133, 656, 6000, and 7966 with inhibition zones of 13.2 ± 1.6, 12.8 ± 1.1, 18.7 ± 0.3, and 17.0 ± 1.1 mm respectively. Therefore, this shampoo was suggested for use as an antidandruff shampoo.
1. Introduction
Nowadays, people pay more attention to cleansing themselves than in the past. For this reason, cleansing products are becoming popular, especially shampoo, which is very important in daily life in order to clean the scalp, remove excess oils or dandruff, and eliminate dead cells [1]. However, most of the commercial shampoos that are available in the market could cause scalp problems, such as dandruff, allergies, or irritation, and do the opposite to what consumers want and how shampoos are supposed to perform.
Dandruff, considered as a group of skin cells overproduced on the scalp, is the main scalp problem, and afflicts more than half of the people in the world [2]. Normally, each skin cell sheds individually, but sometimes, several cells accumulate and this leads to dandruff. The symptoms of dandruff can be an abnormal epidermis, scalp irritation, itching, and large scalp flakes [3]. Another important factor associated with dandruff is an abundance of Malassezia sp., which affects sensitive skin and causes scalp irritation [4,5]. Most commercial antidandruff shampoos employ chemicals as active ingredients. These chemicals may cause serious hair and scalp problems, such as irritation, itchy eyes, hair damage, and allergies. Therefore, natural compounds, which are friendly to the skin, could help to ease those problems. Consequently, the hair care products consisting of natural ingredients become more popular. People have recently become more focused on the benefits of natural products, particularly herbs. Therefore, natural compounds are widely used as remedial agents.
Ocimum sanctum Linn. has been well documented for its therapeutic potential, including usages for the treatment of wounds, catarrhal fever, otalgia, lumbago, hiccough, ophthalmia, gastric disorders, genitourinary disorders, skin diseases, various forms of poisoning, and psychosomatic stress disorders [6]. O. sanctum has also been investigated for various biological activities, including antimicrobial, antifungal, anti-septic, anti-stress, anti-cancer, and antioxidant activity [7,8,9,10]. Therefore, O. sanctum could have the potential to be used as a natural antidandruff agent.
There are various methods for extracting the bioactive compounds from O. sanctum [11,12,13]. However, fermentation by Lactobacillus plantarum was selected for the present study. The fermentation process is eco-friendly, and it improves the efficacy and lowers the side effect of medicinal plants. Moreover, the antidandruff activity of the fermented product from O. sanctum has not been reported before.
Therefore, the aim of this study was to investigate the inhibitory activity against Malassezia furfur of the fermented product from O. sanctum. Additionally, shampoos containing the fermented product from O. sanctum were developed and characterized.
2. Materials and Methods
2.1. Plant Materials
The whole plant of O. sanctum was collected from Chiang Mai, Thailand. The plant was authenticated and voucher specimen number 023230 was deposited in the official Herbarium of the Faculty of Pharmacy, Chiang Mai University, Thailand. The plants were cleaned and dried in the oven at the temperature of 50 °C. The dried plants were then ground into powder using an electric blender (Panasonic-1061, Panasonic, Kadoma, Japan).
2.2. Microorganisms
Lactobacillus plantarum was obtained from the Health Innovation Institute, Chiang Mai, Thailand. Four strains of M. furfur, including 133, 656, 6000, and 7966, were isolated from clinical samples by the Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
2.3. Preparation of Extracts %
The dried plant powder of O. sanctum was extracted by the previously optimized fermentation process [14]. Before the fermentation, starter culture was prepared by activating the L. plantarum in De Man Rogosa and Sharpe (MRS) broth and incubated at 35 ± 2 °C for 24 h. The dried plant materials were mixed with water and cane sugar at the ratio of 0.5:10:1 in a sterile tank and inoculated by 10% w/w of L. plantarum culture (107 CFU/mL). The mixture was then incubated at 35 ± 2 °C for 30 days [15]. The fermented plant juice was collected and then filtered through sterile No.1 Whatman filter paper and dried using a freeze dryer (FreeZone 4.5 model 7750031, Labconco, Kansas, MO, USA). The fermented product was kept at 4 °C until further use.
2.4. Determination of Antifungal Susceptibility Testing of the Fermented Product of O. sanctum
The antifungal susceptibility test of M. furfur was determined by broth dilution method [16]. Four M. furfur strains, including No. 133, 656, 6000, and 7966, were isolated, sub-cultured onto modified Dixon agar (3.6% malt extract, 0.6% peptone, 2% ox bile, 1% Tween 40, 0.2% glycerol, 0.2% oleic acid, 1% olive oil, and 1.5% agar), and incubated at 32 ± 2 °C for 3–5 days before the antifungal susceptibility test. The fungal cells were suspended in 1% (w/w) Tween 80 in order to obtain an optical density of 2.4 McFarland, approximately 106 CFU/mL [16]. The sample was dissolved in dimethylsulfoxide (DMSO) using various concentrations, ranging from 0.004 to 1 mg/mL. Ketoconazole dissolved in DMSO at a concentration of 0.5 mg/mL was used as a positive control. Then, 100 µL of each filtrated sample was mixed with 100 µL of microbial suspension (106 CFU/mL) and incubated at 32 ± 2 °C for 48 h. The absorbance of each mixture was then measured at the wavelength of 565 nm by a microplate reader (Biochrom, Cambridge, UK). The results were reported as being minimum inhibitory concentrations for 50% (MIC50), which was the concentration that resulted in 50% growth reduction when compared with the extract-free SDB.
The minimal fungal concentrations (MFCs) assay was performed after the MICs method. The sample of the previous MICs assay was streaked on the modified Dixon agar. After the incubation at 32 ± 2 °C for 72 h, MFCs was determined as the lowest concentration, which showed no microbial growth. The experiments were performed in triplicate.
2.5. Determination of Organic Acids Content by High Performance Liquid Chromatography (HPLC)
The fermented product was analyzed for its organic acids content, including lactic acid and acetic acid, using HPLC analysis. The fermented product was dissolved in DMSO at the concentration of 1 mg/mL and filtered through a 0.45 μm HPLC filter (Millipore SLCR013NL, Millipore, Bedford, MA, USA). The filtered solution was further assessed by HPLC with an auto-sampler (Model HP 1100, Agilent Tecnologies, CA, USA). The injection volume was 20 μL. Separation was performed on a reversed phase column, which was the Phenomenex Luna® C18 (2) (150 mm × 4.6 mm, 5 µm) with a guard column (Phenomenex C18, 4 mm × 3 mm, 5 µm). The mobile phase, which was a 0.1% (v/v) phosphoric acid solution (pH 5.0), was allowed to flow at the rate of 0.5 mL min−1 at the temperature of 25 °C. The HPLC analyses were performed with a UV detector set at 210 nm.
2.6. Determination of Total Soluble Protein Content
Total soluble protein content of the fermented product was determined by the Lowry’s method [17]. Briefly, 5 mL of the extract solution (1 mg/mL) was mixed with 50 mL of phosphate buffer (pH 7.0 ± 0.2). Then, 2 mL taken from the above solution was mixed with 2 mL of 20% trichloroacetic acid. After incubation at room temperature for 30 min, the mixture was centrifuged at 3000 rpm for 25 min, washed with acetone twice, and centrifuged again. The supernatant was then removed and the precipitate was dissolved in 5 mL of 0.1N NaOH solution. After that, 1 mL of the solution was mixed with 5 mL alkaline copper sulphate reagent and incubated at room temperature for 1 min. Then, 0.5 mL Folin’s reagent was added and allowed to stand for 30 min. The absorbance of the final solution was measured by using a UV spectrophotometer (Shimadzu UV-2450, Shimadzu, Kyoto, Japan) at 660 nm. Bovine serum albumin (BSA) was used as a standard compound.
2.7. Development of Shampoo Formulations
The shampoos were prepared using various ingredients. Different types of detergent, including anionic, sodium lauryl ether sulfate, and ammonium lauryl sulfate, were used as major ingredients of the shampoo formulations. Additional additives, such as preservative, foam builder, emollient, humectant, thickening agent, pearlescent, conditioning agent, antioxidant, pH modifier, solubilizing agent, and opacifying agent, were also added. The ingredients of each shampoo formulation are shown in Table 1.

Table 1.
Ingredients of shampoo formulations in this study.
2.8. Characterization of Shampoo Formulations
Shampoos formulated in the present study were characterized in a comparison with the commercial shampoos and Texapon N70. The codes of each shampoo investigated in the present study are shown in Table 2.

Table 2.
Codes of shampoo samples.
2.8.1. Physical Appearance
Approximately five grams of each sample was evaluated for the physical appearance by organoleptic inspections in terms of external appearance, homogeneity, color, and odor.
2.8.2. Determination of Foaming Ability and Foam Stability
Foaming ability was determined using the cylinder shake method [18]. Briefly, 1 g of sample or commercial shampoo was added into a cylinder and 50 mL of DI water was then added. The cylinders were covered with paraffin film and shaken ten times. The foam volume that was recorded immediately was reported as flash foam and the foam volume recorded four minutes after the shaking was reported as maximum foam. The experiment was performed in triplicate.
2.8.3. pH Measurement
The formulation was diluted in DI water in order to obtain the final concentration of 10% (v/v). The pH was measured by using a pH meter at room temperature. The experiment was performed in triplicate.
2.8.4. Determination of Wetting Time
The wetting time of the shampoo was investigated [19]. A paper was cut into a one-inch square and placed on the aqueous solution of 1% (w/w) shampoo. The time when the paper started becoming wet was recorded and reported as the wetting time. The experiment was performed in triplicate.
2.8.5. Determination of Solid Content
The solid content of the shampoo was investigated [18]. First, 4 g of the sample was placed in an evaporating dish and the total weight was recorded. The evaporating dish was placed in a water bath to allow evaporation to occur until it was completely evaporated. Then, the evaporating dish was weighed again. The percentage of solid content was then calculated using the follow equation:
where A was the total weight of the sample and evaporating dish after evaporation and B was the total weight of the sample and evaporating dish before evaporation. The experiment was performed in triplicate.
% solid content = [((A − B)/4) × 100]
2.8.6. Viscosity Measurement
The viscosity of each formulation was determined by using a Brookfield Rheometer (Model R/S-CPS, Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA) at room temperature. The experiment was performed in triplicate.
2.9. Stability Test of Shampoo Formulations
The stability tests of the shampoo formulations were determined by a heating–cooling cycle. The formulations were kept in a refrigerator at 4 °C for 24 h and in a hot air oven at 45 °C for 24 h. This heating–cooling cycle was repeated eight times. After that, the formulations were characterized in terms of physical appearance, pH, viscosity, solid content, foaming ability, and wetting time.
2.10. Evaluation of Conditioning Performance of Shampoo Formulations
The conditioning performance of the shampoo formulations was determined with slight modifications [20]. A tress of hair was washed by shaking it in a mixture of 10 g of the sample and 30 g DI water for 2 min in an Erlenmeyer flask. The tress of hair was then removed and rinsed with DI water until clean. Each tress was kept at room temperature until it was completely dry. The conditioning effects of the shampoo were evaluated by questionnaires in terms of smoothness, hair shine, ease in combing, frizz reduction, and triboelectric reduction. The questionnaires were answered by thirty volunteers after the examination of the tresses of hair. The conditioning effects were rated from 1 to 5 (1 = very poor; 2 = poor; 3= moderate; 4 = good; 5 = excellent).
2.11. Determination of Antifungal Susceptibility Testing of Shampoo Containing the Fermented Product of O. sanctum by Agar Well Diffusion Method
Four M. Furfur strains, including No. 133, 656, 6000, and 7966, were isolated, sub-cultured onto modified Dixon agar and incubated at 32 ± 2 °C for 3–5 days before the antifungal susceptibility test. The fungal cells were suspended in 1% Tween 80 in order to obtain an optical density of 2.4 McFarland, approximately 106 CFU/mL.
M. furfur was spread on the modified Dixon agar. The plates were holed with a sterile cork borer and 100 µL of each sample was added into them. The culture plates were incubated at 32 ± 2 °C for 48 h and the diameter (mm) of the zones of inhibition were measured. A formulated shampoo was used as a negative control. Commercial anti-dandruff shampoos (Nizoral and Head and Shoulder) were used as positive controls. The experiments were performed in triplicate.
2.12. Statistic Analysis
Statistical significance was assessed by the one-way analysis of variance (ANOVA) using the SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The level of significant difference was defined at p < 0.05.
3. Results and Discussion
3.1. Yield of the Fermented Product of O. sanctum
The physical appearance of the fermented product of O. sanctum was a brown semisolid mass with an agreeable odor. The yield was 11.93% (w/w). The fermented product of O. sanctum might contain phenolic compounds, sugars, organic acids, and pigments, which were released from the plant cell during the fermentation process [21].
3.2. Antifungal Susceptibility Testing of the Fermented Product of O. sanctum
Antifungal activities against four strains of M. furfur are presented in Table 3 and Table 4. The fermented product of O. sanctum was a potent extract that could inhibit all strains of M. furfur. Interestingly, the fermented product of O. sanctum possessed the comparable antifungal activity against M. furfur No. 656 to that of ketoconazole, a well-known broad-spectrum antifungal drug. The results were in agreement with the previous study, which reported that the fermented product could enhance the antibacterial activities of Rheum palmatum L. when compared with a non-fermented product [22]. Besides, several microbes, which were used as intermediate producers in the fermentation process, could break down the plant cell membrane and allow the bioactive compounds to release from the cells easier [23]. Moreover, some intermediate microbes could change the herbal ingredients to increase inhibitory activity [23]. Therefore, the fermented product could be used in various applications because of its inhibitory activity against M. furfur, which is known as the cause of several chronic skin diseases, such as dandruff, seborrhoeic dermatitis, pityriasis versicolor, atopic dermatitis, psoriasis, and both confluent and reticulate papillomatosis [24].

Table 3.
The minimum inhibitory concentrations required for 50% growth reduction when compared with the extract-free Sabouraud Dextrose Broth (MIC50) and minimal fungal concentrations (MFCs) of the fermented product of O. sanctum against four strains of M. furfur.

Table 4.
MFCs of ketoconazole and Fermented-bio of O. sanctum against M. furfur 656 at different concentrations.
3.3. Organic Acids and Total Soluble Protein Content of the Fermented Product
Because total soluble proteins and organic acids could be the promising compounds responsible for the antifungal activity of fermented products [25], therefore, both promising components were analyzed by means of Lowry’s and HPLC method, respectively. The total soluble protein content of the fermented product was 65.32 ± 0.14 mg/100 mL, while there was no organic acid detected. The soluble protein found in the extract could probably be derived from the release of the bacteria starter culture, L. plantarum, during the fermentation process. These proteins could be the key element that enhanced the antibacterial and antifungal activities of the fermented product, because bacteriocins, which were the major groups of ribosomal proteins synthesized during the fermentation process, can kill or inhibit the growth of other bacteria [25].
3.4. Development of the Shampoo Formulation
Eight formulations of different shampoo types were developed, including creamy, clear, and pearlescent shampoo. The creamy shampoos include formulations A and H; the clear shampoo includes formulation B; and the pearlescent shampoos include formulations C, D, E, F, and G. Based on the physical appearance inspection, shampoo formulations A, B, C, G, and H had homogeneous textures. Meanwhile, formulations D, E, and F were separated into two layers. Therefore, these unstable formulations were excluded from the further studies. Only formulations A, B, C, G, and H were selected for the future characterizations. Foaming ability, which is a crucial property of shampoo to the consumer, is considered as an important parameter in the evaluation of shampoo. The result of foaming ability in Table 5 showed that the flash foam and maximum foam of shampoo formulations B, C, and H were similar to that of the commercial products, whereas formulation A could only produce a small amount of foam.

Table 5.
Characterization of formulated and marketed shampoo.
The pH of each shampoo is shown in Table 5. The pH of commercial shampoos (C1, C2, C3, and C4) were in the range of 6 to 7.5, which were neutral and good to use on the hair and scalp. Shampoo formulations A, C, and H showed comparable pH to that of the commercial products, whereas shampoo formulation B was a little bit acidic, which would be good for people with healthy hair and scalps because the normal pH of the scalp was 5.5. Moreover, the mild acidity of the shampoo could increase the hair’s quality, decrease eye irritation, and maintain the ecological balance of the scalp [26]. However, shampoo formulation G had higher pH than that of the commercial products, which would not be good for usage because the high pH would decrease the performance of the product concerning compatibility, irritation, friction, and frizz effect [27].
The solid content of each shampoo is shown in Table 5. Formulations A, B, C, and H had solid contents in the normal range of 20% to 30%, whereas formulation G had a higher solid content (35%). Commercial products, including C1, C2, and C3 had solid contents in the range of 20% to 30%, whereas C4 had a solid content lower than 20%. In general, the appropriate solid content for shampoo is between 20% and 30% [18]. Consequently, the shampoo with lower solid content could be rinsed out easily.
The wetting times of all the developed formulations were similar to that of the commercial products (Table 5). Since the wetting time relies on the wetting ability of the shampoo and normally depends on the concentration of the detergents in the formulation, the shampoos with high wetting times would be good for cleaning because they could diffuse and wet the hair shaft very well [19].
The viscosity of each shampoo is shown in Table 5. It was noted that the viscosity of the commercial shampoos varied. C1 had a very high viscosity. Most of the commercial shampoos had a viscosity in the range of 0.8 Pas to 1.4 Pas. Shampoo formulations B, C, and G had a similar viscosity to most of the commercial shampoos.
3.5. Evaluation of Conditioning Performance of Shampoo Formulation
The tresses of hair were evaluated for conditioning performance of the shampoo formulations by thirty volunteers, the majority of which were female (86.7%) aged between 21 and 30 years old. Shampoo formulations A, B, C, G, and H, which showed good characteristics and good stability, were evaluated for satisfaction compared to N70 and commercial antidandruff shampoos, including C1 and C2.
The conditioning performances of each shampoo are shown in Table 6. The results noted that volunteers were more satisfied by shampoo formulations A, B, C, G, and H than N70. The satisfaction scores of the developed shampoos were compared to that of the commercial antidandruff products in terms of smoothness, hair shine, ease in combing, frizz reduction, and triboelectric reduction. Interestingly, formulation C got the highest satisfaction score for smoothness (3.8 ± 0.8), hair shine (3.8 ± 0.8), ease in combing (3.6 ± 0.8), frizz reduction (3.4 ± 0.9), and triboelectric reduction (3.7 ± 1.0).

Table 6.
Satisfaction scores of shampoo formulations in the terms of smoothness, hair shine, ease in combing, frizz reduction, and triboelectric reduction.
The best formulation that got the highest overall satisfaction score was shampoo formulation C, which was a creamy shampoo with the flash foam and maximum foam of 46.67 and 68.33 mL, respectively. The pH was 7.81, which is an appropriate value for use on the scalp. The solid content was 28.55%, which is perfect for rinsing out. The wetting time was 26.58 min and the viscosity was 1.18 Pas. Furthermore, this formulation got the highest satisfaction score in terms of smoothness, hair shine, ease in combing, frizz reduction, and triboelectric reduction. Therefore, shampoo formulation C was selected for the incorporation of the fermented product from O. sanctum.
3.6. Antifungal Susceptibility Testing of Shampoo Containing the Fermented Product of O. sanctum
The results of antifungal activity of the shampoos containing 2% (FO2), 5% (FO5), and 10% (w/w) (FO10) of the fermented product of O. sanctum against M. furfur 133, M. furfur 656, M. furfur 6000, and M. furfur 7966, respectively, compared with commercial antidandruff shampoo and formulation C are shown in Table 7 and Figure 1. The results noted that the FO10 had a larger inhibition zone than those of the shampoos containing FO2 and FO5 of the fermented product of O. sanctum. The inhibition zones against M. furfur 133, M. furfur 656, M. furfur 6000, and M. furfur 7966 were 16.1 ± 1.5, 14.6 ± 1.4, 17.2 ± 0.5, and 16.2 ± 1.3 mm, respectively. Meanwhile, formulation C also showed the inhibitory activity against M. furfur 133, M. furfur 6000, and M. furfur 7966 with the inhibition zones of 7.3 ± 0.6, 16.0 ± 1.0, and 14.6 ± 0.8 mm, respectively. Moreover, FO2, FO5, and FO10 had less antifungal activity against the four strains of M. furfur than the C1 and C2.

Table 7.
Antifungal activity of shampoo containing the fermented product of O. sanctum.
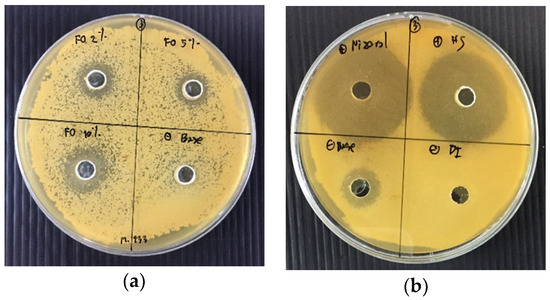
Figure 1.
(a) Antimicrobial susceptibility of shampoos containing the fermented product of O. sanctum. Shampoo containing the fermented product of O. sanctum. Top left: shampoo containing 2% (w/w) of the fermented product of O. sanctum (FO2); top right: shampoo containing 5% (w/w) of the fermented product of O. sanctum (FO5); bottom left: shampoo containing 10% (w/w) of the fermented product of O. sanctum (FO10); bottom right: shampoo base (Base). (b) Antimicrobial susceptibility of commercial shampoos; top left: C1; top right: C2; bottom left: formulation C; bottom right: DI water.
Shampoos containing different concentrations of the fermented product of O. sanctum did not show different antifungal activities (p > 0.05). However, FO10 was not stable as it separated into two layers after the heating–cooling stability test. Therefore, FO2 was suggested for further use because the higher amount of the extract could not enhance the M. furfur inhibitory activity. Additionally, the inhibitory effect of the shampoo formulations could be as a result of other ingredients that also possess antimicrobial activity, such as sodium laureth sulfate, cocamide diethanolamine, cocamidopropyl betaine, cetrimonium chloride, and propylene glycol [28,29,30,31], because these compounds, especially cationic surfactants, could interact with the cellular membranes of microorganisms [32]. The use of FO could be combine with the other ingredients of shampoo, possessing antimicrobial activity, in order to improve the efficacy of the product.
4. Conclusions
The fermented product of O. sanctum possessed potent antifungal activity against four strains of M. furfur. Therefore, it could be used as an active ingredient in the development of antidandruff shampoo. Shampoo formulation C was the best formulation because of its good characteristics, good stability, and the fact that it obtained the highest satisfaction score when evaluated by 30 volunteers, especially in terms of smoothness, hair shine, ease in combing, frizz reduction, and triboelectric reduction. Additionally, 2% (w/w) of the fermented product of O. sanctum was suggested for use as an active component in the shampoo as it could inhibit the growth of M. furfur and provide the antidandruff shampoo with a good external appearance and good stability. Additionally, the fermentation process could lower the cost of production, and it is friendly to the environment, harmless, less toxic, and produces less hazardous solvent waste when compared with other extraction methods.
Author Contributions
Conceptualization, W.C.; Methodology, C.P., S.S., and P.C.; Investigation, C.P.; Writing—Original Draft Preparation, C.P. and W.C.; Supervision, W.C., S.S., and P.C.; Project Administration, W.C.; Funding Acquisition, W.C.
Funding
This research was funded by Graduated School of Chiang Mai University and Faculty of Pharmacy, Chiang Mai University.
Acknowledgments
We would like to thank Mr. Michael Mayo for improving the use of English in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandran, S.; Vipin, K.V.; Augusthy, A.R.; Lindumol, K.V.; Shirwaikar, A. Development and evaluation of antidandruff shampoo based on natural sources. J. Pharm. Phytother. 2013, 1, 2321–5895. [Google Scholar]
- Xu, Z.; Wang, Z.; Yuan, C.; Liu, X.; Yang, F.; Wang, T.; Wang, J.; Manabe, K.; Qin, O.; Wang, X.; et al. Dandruff is associated with the conjoined interactions between host and microorganisms. Sci. Rep. 2016, 6, 24877. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.; Altenburg, M.; Bailey, D. Milady’s Standard Cosmetology; Cengage Learning: New York, NY, USA, 2002; pp. 221–222. ISBN 1-56253-880-2. [Google Scholar]
- Gupta, A.K.; Batra, R.; Bluhm, R.; Boekhout, T.; Dawson, T.L. Skin diseases associated with Malassezia species. J. Am. Acad. Dermatol. 2004, 51, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, A.C.; Bulmer, G.S. The antifungal action of dandruff shampoos. Mycopathologia 1999, 147, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Tulsi: The Indian holy power plant. Nat. Prod. Rad. 2006, 5, 279–283. [Google Scholar]
- Rana, M.M.; Sayeed, M.A.; Nasrin, S.; Islam, M.; Rahman, M.M.; Alam, M.F. Free radical scavenging potential and phytochemical analysis of leaf extract from Ocimum sanctum Linn. J. Agric. Sci. Technol. 2015, 11, 1615–1623. [Google Scholar]
- Basak, P.; Mallick, P.; Mazumder, S.; Verma, A.S. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of tulsi (Ocimum sanctum) leaves. Adv. Pharmacol. Toxicol. 2014, 15, 19–29. [Google Scholar]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Zinnai, A. A Simplified Method to Estimate Sc-CO2Extraction of Bioactive Compounds from Different Matrices: Chili Pepper vs. Tomato By-Products. Appl. Sci. 2017, 7, 361. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of Phenol-Enriched Olive Oil with Phenolic Compounds Extracted from Wastewater Produced by Physical Refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, S.; Rajan, S.; Thirunalasundari, T.; Jeeva, S. Antifungal activity of Ocimum sanctum Linn. (Lamiaceae) on clinically isolated dermatophytic fungi. Asian Pac. J. Trop. Med. 2011, 4, 654–657. [Google Scholar] [CrossRef]
- El-Soud, N.H.A.; Deabes, M.; El-Kassem, L.A.; Khalil, M. Chemical composition and antifungal activity of Ocimum basilicum L. essential oil. Open Access Maced. J. Med. Sci. 2015, 3, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Gunendren, M.; Nordin, S.S.; Ramachandran, M.R.; Samad, N.A. Effect of Ocimum sanctum (Tulsi) aqueous leaf extract on prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT) of human plasma. J. Biomed. Clin. Sci. 2017, 2, 62–68. [Google Scholar]
- Sirilun, S. Fermentation Kinetics of Morinda citrifolia linn and Antimicrobial Activity of Its Products. Ph.D. Thesis, Chiang Mai Univercity, Chiang Mai, Thailand, 23 September 2005. [Google Scholar]
- Chaiyasut, C.; Kruatama, C.; Sirilun, S. Breaking the spores of Ganoderma lucidum by fermentation with Lactobacillus plantarum. Afr. J. Biotechnol. 2010, 9, 7379–7382. [Google Scholar]
- Iatta, R.; Immediato, D.; Montagna, M.T.; Otranto, D.; Cafarchia, C. In vitro activity of two amphotericin B formulations against Malassezia furfur strains recovered from patients with bloodstream infections. Med. Mycol. 2015, 53, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Al Badi, K.; Khan, S.A. Formulation, evaluation and comparison of the herbal shampoo with the commercial shampoos. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 301–305. [Google Scholar] [CrossRef]
- Manikar, A.R.; Jolly, C.I. Evaluation of commercial herbal shampoos. Int. J. Cosmet. Sci. 2000, 22, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Boonme, P.; Pakpayat, N.; Yotmanee, K.; Kunlawijitrungsee, S.; Maneenuan, D. Evaluation of Shampoos Containing Silicone Quaternary Microemulsion. J. Appl. Pharm. Sci. 2011, 1, 59. [Google Scholar]
- Dey, T.B.; Kuhad, R.C. Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 2014, 4, 120–127. [Google Scholar]
- Zhang, Y.; Zhou, L.; Ma, W.; Shi, X.; Zhang, H.; Shi, X. Bidirectional solid fermentation using Trametes robiniophila Murr. for enhancing efficacy and reducing toxicity of rhubarb (Rheum palmatum L.). J. Tradit. Chin. Med. Sci. 2017, 4, 306–313. [Google Scholar] [CrossRef]
- Wu, T.; Wang, N.; Zhang, Y.; Xu, X. Advances in the study on microbial fermentation and transformation of traditional Chinese medicine. Afr. J. Microbiol. Res. 2013, 7, 1644–1650. [Google Scholar]
- Sreelatha, G.L.; Babu, U.V.; Sharath Kumar, L.M.; Soumya, K.; Sharmila, T. Investigation on biochemical characterisation and in vitro antifungal efficacy of plant extracts on Malassezia furfur. Int. J. Pharma Bio Sci. 2015, 6, 1027–1041. [Google Scholar]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Baran, R.; Maibah, H.I. Cosmetic dermatology in children. In Text Book of Cosmetic Dermatology, 2nd ed.; CRC Press: London, UK, 1998; pp. 507–508. ISBN 1853174785. [Google Scholar]
- Dias, M.F.R.G.; Almeida, D.A.M.; Cecato, P.M.R.; Adriano, A.R.; Pichler, J. The shampoo pH can affect the hair: Myth or reality. Int. J. Trichol. 2014, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, T.; Koskela, M. Antibacterial and antifungal properties of propylene glycol, hexylene glycol, and 1,3-butylene glycol in vitro. Acta Derm. Venereol. 1991, 71, 148–150. [Google Scholar] [PubMed]
- Crider, K.; Luciow, C.; Fuls, J.L.; Rodgers, N.D. Antibacterial Composition with Low Amounts of Surfactant and Antibacterial Actives. U.S. Patent No. 9,131,682, 15 September 2015. [Google Scholar]
- Curtis, M.A. Antimicrobial Composition. U.S. Patent No. 7,754,770, 13 July 2010. [Google Scholar]
- Moen, H.K.; Cunningham, C.T.; Hoffman, D.R.; Wenzel, S.W. Antimicrobial Cleansing Compositions. U.S. Patent No. 9,232,790, 12 January 2016. [Google Scholar]
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic surfactants derived from lysine: Effects of their structure and charge type on antimicrobial and hemolytic activities. J. Med. Chem. 2011, 54, 989–1002. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).