A Green and Rapid Analytical Method for the Determination of Hydroxyethoxyphenyl Butanone in Cosmetic Products by Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents
2.3. Samples
2.4. Proposed Method
2.4.1. Preparation of Standards and Sample Solutions
2.4.2. Chromatographic Analysis
3. Results and Discussion
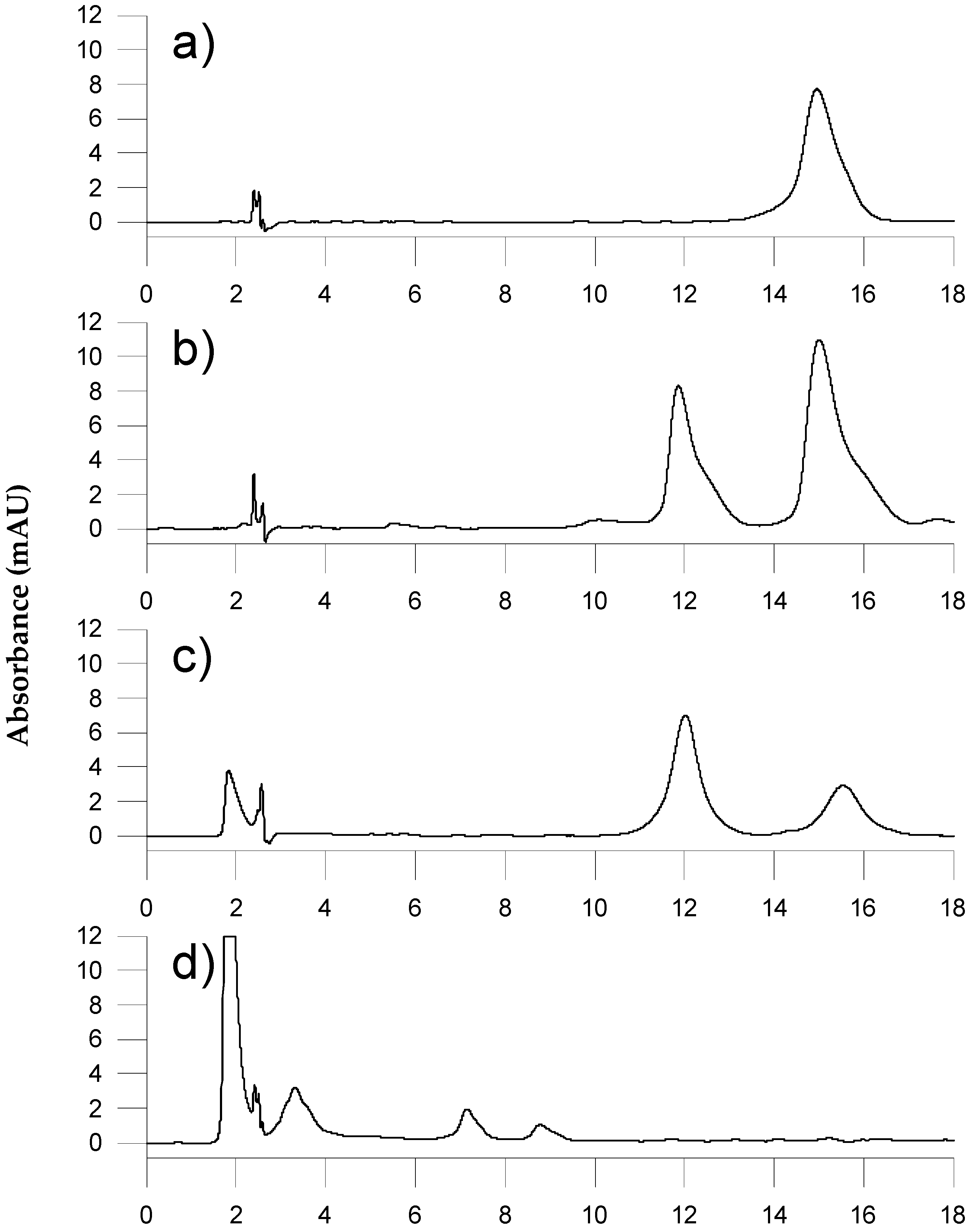
3.1. Study of the Chromatographic Variables
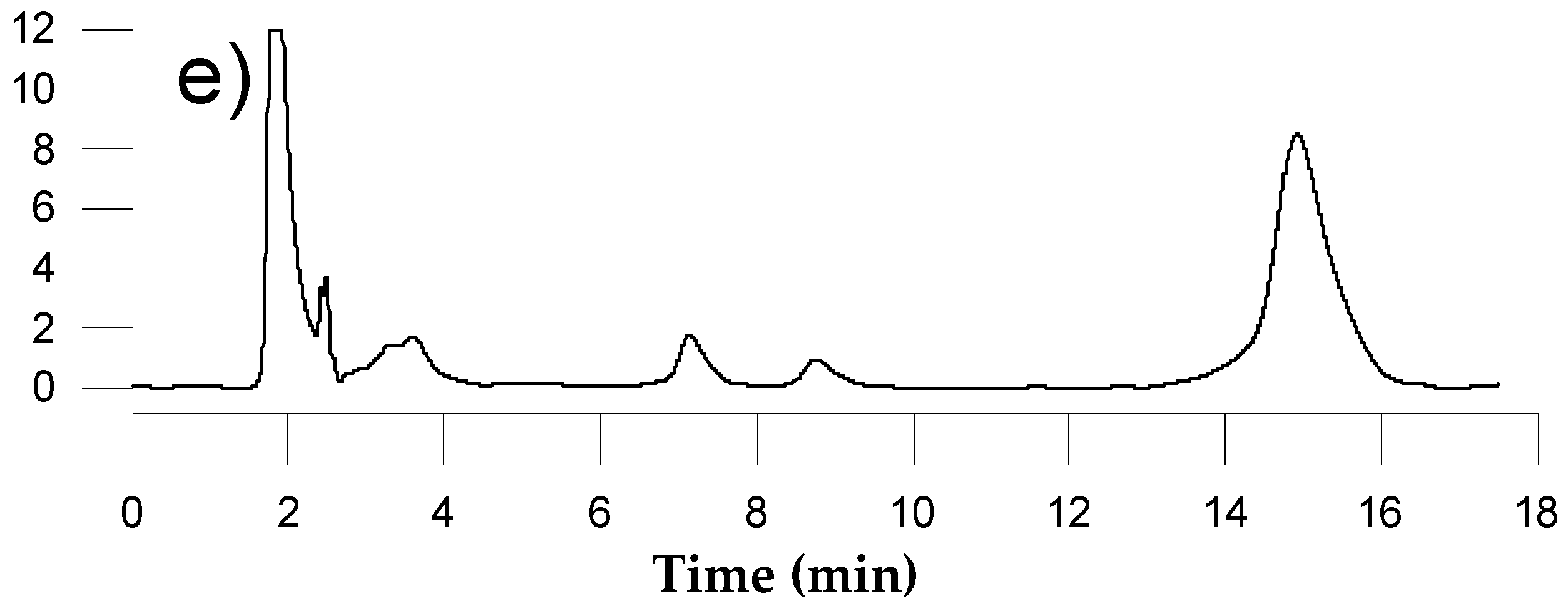
3.2. Study of the Sample Preparation
3.3. Analytical Figures of Merit of the Proposed Method
3.4. Analysis of Commercial Samples
3.5. Evaluation of the Greenness of the Proposed Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32009R1223 (accessed on 10 June 2018).
- Commission Regulation (EU) No. 358/2014 of 9 April 2014 Amending Annexes II and V to Regulation (EC) No. 1233/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32014R0358 (accessed on 10 June 2018).
- Papageorgiou, S.; Varvaresou, A.; Tsirivas, E.; Demetzos, C. New alternatives to cosmetics preservation. J. Cosmet. Sci. 2010, 61, 107–123. [Google Scholar] [PubMed]
- Narayanan, M.; Sekar, P.; Pasupathi, M.; Mukhopadhyay, T. Self-preserving personal care products. Int. J. Cosmet. Sci. 2017, 39, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Herman, A. Antimicrobial ingredients as preservative booster and components of self-preserving cosmetic products. Curr. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Commission Decision of 9 February 2006 Amending Decision 96/335/EC Establishing an Inventory and a Common Nomenclature of Ingredients Employed in Cosmetic Products (2006/257/EC). Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32006D0257 (accessed on 10 June 2018).
- Scientific Committee on Consumer Safety (SCCS), Opinion on Ethylzingerone—‘Hydroxyethoxyphenyl Butanone’ (HEPB) Cosmetics Europe No. P98. Adopted on 7 April 2017. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_203.pdf (accessed on 10 June 2018).
- González-Abellán, E.F.; Martínez-Perez, D. Quality control of cosmetic products: Specific legislation on ingredients. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–53. ISBN 978-0-444-63508-2. [Google Scholar]
- Alvarez-Rivera, G.; Llompart, M.; Lores, M.; Garcia-Jares, C. Preservatives in cosmetics: Regulatory aspects and analytical methods. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–224. ISBN 978-0-444-63508-2. [Google Scholar]
- Aoyama, A.; Doi, T.; Tagami, T.; Kajimura, K. Simultaneous determination of 11 preservatives in cosmetics by high-performance liquid-chromatography. J. Chromatogr. Sci. 2014, 52, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Bellver, R.; Chisvert, A.; Salvador, A. Vortex-assisted emulsification semimicroextraction for the analytical control of restricted ingredients in cosmetic products: determination of bronopol by liquid chromatography. Anal. Bioanal. Chem. 2016, 408, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Vrouvaki, I.; Chisvert, A.; Salvador, A. Determination of alternative preservatives in cosmetic products by vortex-assisted liquid-liquid semimicroextraction and liquid chromatography. Talanta 2016, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Chisvert, A.; Alonso, M.J.; Hernandorena, S.; Salvador, A. Determination of free formaldehyde in cosmetics containing formaldehyde-releasing preservatives by reversed-phase dispersive liquid-liquid microextraction and liquid chromatography with post-column derivatization. J. Chromatogr. A 2018, 1543, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Hsu, W.C.; Lu, Y.C.; Weng, J.R.; Feng, C.H. Determination of parabens using two microextraction methods coupled with capillary liquid chromatography-UV detection. Food Chem. 2018, 241, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, J.B.; Canas, B.J.; Zhou, W.L.; Wang, P.G.; Rua, D.; Krynitsky, A.J. Determination of methylisothiazolinone and methylchloroisothiazolinone in cosmetic products by ultra high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015, 38, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Khosrowshahi, E.M.; Khorram, P. Simultaneous derivatization and air-assisted liquid-liquid microextraction of some parabens in personal care products and their determination by GC with flame ionization detection. J. Sep. Sci. 2013, 36, 3571–3578. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.B.; LaFon, W.; Burford, M.D. Determination of iodopropynyl butylcarbamate in cosmetic formulations utilizing pulsed splitless injection, gas chromatography with electron capture detector. J. Chromatogr. A 2017, 1516, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Sarhadi, A.; Bazregar, M.; Asghari, A.; Mirparizi, E. Rapid derivatization and extraction of paraben preservatives by fast syringe-assisted liquid-liquid microextraction and their determination in cosmetic and aqueous sample solutions by gas chromatography. Anal. Methods 2017, 9, 5963–5969. [Google Scholar] [CrossRef]
- Ye, N.S.; Shi, P.Z.; Li, J.; Wang, Q. Application of graphene as solid phase extraction absorbent for the determination of parabens in cosmetic products by capillary electrophoresis. Anal. Lett. 2013, 46, 1991–2000. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, N.; Luo, C.Y.; Wang, X.X.; Sun, C.J. Simultaneous determination of seven preservatives in cosmetics by dispersive liquid-liquid microextraction coupled with high performance capillary electrophoresis. Anal. Methods 2013, 5, 2391–2397. [Google Scholar] [CrossRef]
- Huang, J.Q.; Hu, C.C.; Chiu, T.C. Determination of seven preservatives in cosmetic products by micellar electrokinetic chromatography. Int. J. Cosmet. Sci. 2013, 35, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Lee, J.Y.; Hu, C.C.; Chiu, T.C. On-line concentration and separation of parabens by micellar electrokinetic chromatography using polymer solutions. J. Chin. Chem. Soc. 2014, 61, 453–460. [Google Scholar] [CrossRef]
- Lopez-Gazpio, J.; Garcia-Arrona, R.; Millan, E. Application of response function methodology for the simultaneous determination of potential fragrance allergens and preservatives in personal care products using micellar electrokinetic chromatography. Anal. Bioanal. Chem. 2014, 406, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gazpio, J.; Garcia-Arrona, R.; Millan, E. Simultaneous determination of multiclass preservatives including isothiazolinones and benzophenone-type UV filters in household and personal care products by micellar electrokinetic chromatography. Electrophoresis 2015, 36, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Zotou, A.; Sakla, I.; Tzanavaras, P.D. LC-determination of five paraben preservatives in saliva and toothpaste samples using UV detection and short monolithic column. J. Pharm. Biomed. 2010, 53, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Carreira, F.C.; Canaes, L.S.; Campos, F.A.D.; Cruz, L.M.D.; Rath, S. Determination of parabens in shampoo using high performance liquid chromatography with amperometric detection on a boron-doped diamond electrode. Talanta 2011, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abbasghorbani, M.; Attaran, A.; Payehghadr, M. Solvent-assisted dispersive micro-SPE by using aminopropyl-functionalized magnetite nanoparticle followed by GC-PID for quantification in aqueous matrices. J. Sep. Sci. 2013, 36, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Simultaneous in-cell derivatization pressurized liquid extraction for the determination of multiclass preservatives in leave-on cosmetics. Anal. Chem. 2010, 82, 9384–9392. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Lamas, J.P.; Garcia-Jares, C.; Llompart, M. Pressurized liquid extraction-gas chromatography-mass spectrometry analysis of fragrance allergens, musks, phthalates and preservatives in baby wipes. J. Chromatogr. A 2015, 1384, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Alvarez-Rivera, G.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Analysis of multi-class preservatives in leave-on and rinse-off cosmetics by matrix solid-phase dispersion. Anal. Bioanal. Chem. 2011, 401, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Guerra, E.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Development of multianalyte method based on micro-matrix-solid-phase dispersion for the analysis of fragrance allergens and preservatives in personal care products. J. Chromatogr. A 2014, 1344, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. In-vial micro-matrix-solid phase dispersion for the analysis of fragrance allergens, preservatives, plasticizers, and musks in cosmetics. Cosmetics 2014, 1, 171–201. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, M.; Lamas, J.P.; Sanchez-Prado, L.; Llompart, M.; Garcia-Jares, C.; Lores, M. Development of a solid-phase microextraction gas chromatography with microelectron-capture detection method for the determination of 5-bromo-5-nitro-1,3-dioxane in rinse-off cosmetics. J. Chromatogr. A 2010, 1217, 6634–6639. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Vila, M.; Lores, M.; Garcia-Jares, C.; Llompart, M. Development of a multi-preservative method based on solid-phase microextraction-gas chromatography-tandem mass spectrometry for cosmetic analysis. J. Chromatogr. A 2014, 1339, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Konieczka, P.; Migaszewski, Z.M.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAc-Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
| IUPAC Name | CAS Number | Chemical Structure | Molecular Weight | pKa | Log Ko/w |
|---|---|---|---|---|---|
| 4-(3-Ethoxy-4-hydroxyphenyl)-2-butanone | 569646-79-3 | 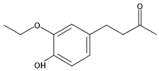 | 208.25 g·mol−1 | 10.19 | 1.68 |
| Slope (mAU min mL·µg−1) a | 19.0 ± 0.1 | ||
| Intercept (mAU min) a | −7 ± 6 | ||
| R2 b | 0.9997 | ||
| Instrumental LOD (µg·mL−1) c | 0.3 | ||
| Instrumental LOQ (µg·mL−1) d | 0.9 | ||
| Method LOD (µg·g−1) c | 30 | ||
| Method LOQ (µg·g−1) d | 90 | ||
| Intra-day Repeatability (RSD, %) e | 2.5 (5 µg·mL−1) | 0.2 (25 µg·mL−1) | 0.9 (50 µg·mL−1) |
| Inter-day Repeatability (RSD, %) e | 1.2 (5 µg·mL−1) | 1.2 (25 µg·mL−1) | 4.7 (50 µg·mL−1) |
| Sample | Concentration (%, w/w) a | Recovery Values (%) a | |||
|---|---|---|---|---|---|
| 0.05%, w/w | 0.25%, w/w | 0.50%, w/w | |||
| 1 | Laboratory-made cream (HEPB at 0.36%, w/w) | 0.34 ± 0.01 | 86 ± 2 | 102 ± 5 | 90 ± 5 |
| 2 | Liquid hand soap 1 | n.d. | 98 ± 4 | 102 ± 2 | 101 ± 1 |
| 3 | Liquid hand soap 2 | n.d. | 100 ± 4 | 100 ± 1 | 99 ± 1 |
| 4 | Make-up | n.d. | 93 ± 2 | 90 ± 1 | 89 ± 2 |
| 5 | Moisturizing cream | 0.083 ± 0.002 | 103 ± 8 | 92 ± 5 | 91 ± 1 |
| 6 | Shampoo | n.d. | 99 ± 1 | 100 ± 1 | 99 ± 1 |
| 7 | Sunscreen 1 | n.d. | 97 ± 1 | 98 ± 1 | 100 ± 1 |
| 8 | Sunscreen 2 | n.d. | 89 ± 2 | 90 ± 2 | 87 ± 2 |
| Proposed Method | Penalty Points |
|---|---|
| Sample preparation | |
| Dilution with water (10 mL/sample) | 0 |
| Ultrasound-assisted lixiviation | 0 |
| Instrument | |
| LC-UV/Vis | 1 |
| Mobile phase reagents | |
| Ethanol (≈3.3 mL/sample) | 2 |
| Acetic acid (≈0.1 mL/sample) | 4 |
| Wastes | 8 |
| Total penalty points: 15 | |
| Analytical Eco-Scale total score: 85 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles, P.; Benedé, J.L.; Mata-Martín, A.; Chisvert, A.; Salvador, A. A Green and Rapid Analytical Method for the Determination of Hydroxyethoxyphenyl Butanone in Cosmetic Products by Liquid Chromatography. Cosmetics 2018, 5, 44. https://doi.org/10.3390/cosmetics5030044
Miralles P, Benedé JL, Mata-Martín A, Chisvert A, Salvador A. A Green and Rapid Analytical Method for the Determination of Hydroxyethoxyphenyl Butanone in Cosmetic Products by Liquid Chromatography. Cosmetics. 2018; 5(3):44. https://doi.org/10.3390/cosmetics5030044
Chicago/Turabian StyleMiralles, Pablo, Juan L. Benedé, Aylén Mata-Martín, Alberto Chisvert, and Amparo Salvador. 2018. "A Green and Rapid Analytical Method for the Determination of Hydroxyethoxyphenyl Butanone in Cosmetic Products by Liquid Chromatography" Cosmetics 5, no. 3: 44. https://doi.org/10.3390/cosmetics5030044
APA StyleMiralles, P., Benedé, J. L., Mata-Martín, A., Chisvert, A., & Salvador, A. (2018). A Green and Rapid Analytical Method for the Determination of Hydroxyethoxyphenyl Butanone in Cosmetic Products by Liquid Chromatography. Cosmetics, 5(3), 44. https://doi.org/10.3390/cosmetics5030044







