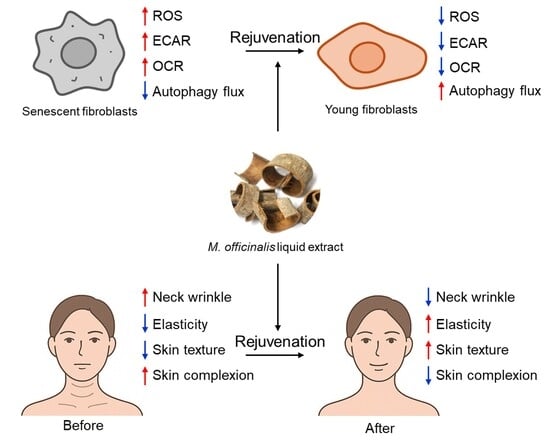
Liquid Extract from the Bark of Magnolia officinalis Rejuvenates Skin Aging Through Mitochondrial ROS Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of M. officinalis Liquid Extract
2.3. Preparation of a Cream Containing M. officinalis Liquid Extract
2.4. Flow Cytometric Analysis of ROS, Mitochondrial Membrane Potential (MMP), Autophagy Flux, Mitochondrial Mass and Autofluorescence
2.5. Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) Analysis
2.6. Fluorescence Analysis of Mitophagy
2.7. Quantitative PCR (qPCR) Analysis
2.8. Western Blot Analysis
2.9. High-Performance Liquid Chromatography (HPLC) Analysis
2.10. Clinical Trials
2.11. Measurement of Neck Wrinkles
2.12. Measurements of Skin Elasticity
2.13. Measurements of Skin Texture
2.14. Measurements of Skin Complexion
2.15. Statistical Analysis
3. Results
3.1. M. officinalis Liquid Extract Ameliorates Mitochondrial Function
3.2. M. officinalis Liquid Extract Restores Mitochondrial Metabolic Function
3.3. M. officinalis Liquid Extract Induces Restoration of Mitophagy and Autophagy Activity
3.4. M. officinalis Liquid Extract Rejuvenates Senescence-Associated Phenotypes
3.5. The Cream Containing M. officinalis Liquid Extract Is Effective in Reducing Neck Wrinkles
3.6. The Cream Containing M. officinalis Liquid Extract Is Effective in Enhancing Skin Elasticity
3.7. The Cream Containing M. officinalis Liquid Extract Is Effective in Improving Skin Texture
3.8. The Cream Containing M. officinalis Liquid Extract Is Effective in Improving Skin Complexion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, J.Y.; Lee, H.; Song, E.S.; Kuk, M.U.; Joo, J.; Oh, S.; Kwon, H.W.; Park, J.T.; Park, S.C. Targeting Mitochondrial Metabolism as a Strategy to Treat Senescence. Cells 2021, 10, 3003. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Elias, P.M. Skin barrier function. Curr. Allergy Asthma Rep. 2008, 8, 299–305. [Google Scholar] [CrossRef]
- Boismal, F.; Serror, K.; Dobos, G.; Zuelgaray, E.; Bensussan, A.; Michel, L. Skin aging: Pathophysiology and innovative therapies. Med. Sci. 2020, 36, 1163–1172. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef]
- Hussen, N.H.A.; Abdulla, S.K.; Ali, N.M.; Ahmed, V.A.; Hasan, A.H.; Qadir, E.E. Role of antioxidants in skin aging and the molecular mechanism of ROS: A comprehensive review. Asp. Mol. Med. 2025, 5, 100063. [Google Scholar] [CrossRef]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Poivre, M.; Duez, P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Univ.-Sci. B 2017, 18, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jeong, E.Y.; Kim, Y.H.; Park, J.H.; Yoon, J.H.; Lee, Y.J.; Lee, S.H.; Nam, Y.K.; Cha, S.Y.; Park, J.S.; et al. Identification of senescence rejuvenation mechanism of Magnolia officinalis extract including honokiol as a core ingredient. Aging 2025, 17, 497–523. [Google Scholar] [CrossRef]
- Chwil, M.; Dzida, K.; Terlecka, P.; Gruľová, D.; Matraszek-Gawron, R.; Terlecki, K.; Kasprzyk, A.; Kostryco, M. Antibacterial, Photoprotective, Anti-Inflammatory, and Selected Anticancer Properties of Honokiol Extracted from Plants of the Genus Magnolia and Used in the Treatment of Dermatological Problems—A Review. Int. J. Mol. Sci. 2025, 26, 8737. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the Multifunctional Activities of Magnolol. Biomed. Res. Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Du, Y.; Li, J.; Guo, T.; Luo, S. Mitophagy in Cell Death Regulation: Insights into Mechanisms and Disease Implications. Biomolecules 2024, 14, 1270. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of plant extracts cosmetics in the field of anti-aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- Henderson, L.M.; Chappell, J.B. Dihydrorhodamine 123: A fluorescent probe for superoxide generation? Eur. J. Biochem. 1993, 217, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-Based Flow Cytometry for Detecting Mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.E.; Kim, Y.; Kwon, S.; Kim, M.; Kim, Y.H.; Kim, J.H.; Park, T.J.; Kang, H.Y. Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics 2018, 8, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Song, E.S.; Lee, Y.H.; Lee, K.S.; So, B.; Park, J.H.; Yoon, J.H.; Kim, D.; Kim, M.; Kwon, H.W.; et al. Dehydroacteoside rejuvenates senescence via TVP23C-CDRT4 regulation. Exp. Gerontol. 2025, 207, 112800. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, X.; Huang, R.; Yan, K.; Wang, M.; Liu, W.; Zeng, X.; Ren, X.; Gong, H. Characterization of morphological and chemical changes using atomic force microscopy and metabolism assays: The relationship between surface wax and skin greasiness in apple fruit. Front. Plant Sci. 2024, 15, 1489005. [Google Scholar] [CrossRef]
- Piérard, G.E. EEMCO guidance for the assessment of skin colour. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 1–11. [Google Scholar] [CrossRef]
- Plitzko, B.; Loesgen, S. Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism. Bio-Protocol 2018, 8, e2850. [Google Scholar] [CrossRef]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013, 1, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Davan-Wetton, C.S.A.; Montero-Melendez, T. An optimised protocol for the detection of lipofuscin, a versatile and quantifiable marker of cellular senescence. PLoS ONE 2024, 19, e0306275. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, Y.K.; Park, S.S.; Park, S.H.; Eom, S.Y.; Lee, Y.S.; Lee, W.J.; Jang, J.; Seo, D.; Kang, H.Y.; et al. Mid-old cells are a potential target for anti-aging interventions in the elderly. Nat. Commun. 2023, 14, 7619. [Google Scholar] [CrossRef]
- Qi, C.; Lan, H.; Ye, J.; Li, W.; Wei, P.; Yang, Y.; Guo, S.; Lan, T.; Li, J.; Zhang, Q.; et al. Slit2 promotes tumor growth and invasion in chemically induced skin carcinogenesis. Lab. Investig. 2014, 94, 766–776. [Google Scholar] [CrossRef]
- Pieńkowska, N.; Bartosz, G.; Pichla, M.; Grzesik-Pietrasiewicz, M.; Gruchala, M.; Sadowska-Bartosz, I. Effect of antioxidants on the H2O2-induced premature senescence of human fibroblasts. Aging 2020, 12, 1910–1927. [Google Scholar] [CrossRef]
- Toussaint, O.; Medrano, E.E.; von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000, 35, 927–945. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.L.; Man, K.M.; Huang, P.H.; Chen, W.C.; Chen, D.C.; Cheng, Y.W.; Liu, P.L.; Chou, M.C.; Chen, Y.H. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules 2010, 15, 6452–6465. [Google Scholar] [CrossRef]
- Singh, D.; Nandni; Bedi, N. Honokiol as a next-generation phytotherapeutic: Anticancer, neuroprotective, and nanomedicine perspectives. Pharmacol. Res.-Mod. Chin. Med. 2025, 17, 100713. [Google Scholar] [CrossRef]
- Olas, B. The Cardioprotective Effect of Magnolia officinalis and Its Major Bioactive Chemical Constituents. Int. J. Mol. Sci. 2025, 26, 4380. [Google Scholar] [CrossRef] [PubMed]
- Rempel, V.; Fuchs, A.; Hinz, S.; Karcz, T.; Lehr, M.; Koetter, U.; Müller, C.E. Magnolia Extract, Magnolol, and Metabolites: Activation of Cannabinoid CB2 Receptors and Blockade of the Related GPR55. ACS Med. Chem. Lett. 2013, 4, 41–45. [Google Scholar] [CrossRef]
- Kim, E.; Cho, G.; Won, N.G.; Cho, J. Age-related changes in skin bio-mechanical properties: The neck skin compared with the cheek and forearm skin in Korean females. Ski. Res. Technol. 2013, 19, 236–241. [Google Scholar] [CrossRef]
- Fujimura, T.; Haketa, K.; Hotta, M.; Kitahara, T. Loss of skin elasticity precedes to rapid increase of wrinkle levels. J. Dermatol. Sci. 2007, 47, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Varela-Gómez, F.; Sandoval-García, A.; Valeria Cabrera-Rios, K. Signs of skin aging: A review. Int. J. Res. Med. Sci. 2024, 12, 2674–2679. [Google Scholar] [CrossRef]
- Kim, J.C.; Park, T.J.; Kang, H.Y. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef] [PubMed]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e11. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on Skin and Intrinsic Aging: Biological, Environmental, and Therapeutic Insights. J. Cosmet. Dermatol. 2025, 24, e16688. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Li, R.; Gao, T. Role of Mitochondrial Dynamics in Skin Homeostasis: An Update. Int. J. Mol. Sci. 2025, 26, 1803. [Google Scholar] [CrossRef]
- Yang, D.; Le, J.; Xiao, S.; Cui, Y.; Zhu, W.; Otsuki, K.; Li, W.; Xu, J.; Feng, F.; Zhang, J. Hyperoside Promotes Mitochondrial Autophagy Through the miR-361-5p/PI3K/Akt/mTOR Signaling Pathway, Thereby Improving UVB-Induced Photoaging. Antioxidants 2025, 14, 1401. [Google Scholar] [CrossRef]
- Wang, M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxidative Med. Cell. Longev. 2019, 2019, 8135985. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.; Manson Brown, S.; Cross, S.J.; Mehta, R. Defining Skin Quality: Clinical Relevance, Terminology, and Assessment. Dermatol. Surg. 2021, 47, 974–981. [Google Scholar] [CrossRef]
- Brito, S.; Baek, M.; Bin, B.H. Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1403. [Google Scholar] [CrossRef]
- Lagouvardos, P.; Spyropoulou, N.; Polyzois, G. Perceptibility and acceptability thresholds of simulated facial skin color differences. J. Prosthodont. Res. 2018, 62, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Lin, W.; Dong, Y.; Li, L.; Yi, F.; Meng, Q.; Li, Y.; He, Y. Statistical analysis of age-related skin parameters. Technol. Health Care 2021, 29, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Udén, P.; Robinson, P.H.; Mateos, G.G.; Blank, R. Use of replicates in statistical analyses in papers submitted for publication in Animal Feed Science and Technology. Anim. Feed Sci. Technol. 2012, 171, 1–5. [Google Scholar] [CrossRef]
- Zakharkin, S.O.; Kim, K.; Bartolucci, A.A.; Page, G.P.; Allison, D.B. Optimal allocation of replicates for measurement evaluation studies. Genom. Proteom. Bioinform. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef]








| Cell Line Name | Company Name | Catalogue Number | Medium Condition | Culture Condition |
|---|---|---|---|---|
| Human dermal fibroblasts | ATCC, Manassas, VA, USA | PCS–201–010 | Dulbecco’s modified Eagle’s medium (10–013–CV; Corning, Corning, NY, USA) 10% fetal bovine serum (30006; SPL Life Sciences, Pocheon, Republic of Korea) 100 U/mL penicillin & 100 μg/mL streptomycin (SV30079.01; Hyclone, Logan, UT, USA) | Cells were cultivated in 5% CO2 at 37 °C. |
| Analysis | Dye | Catalogue Number | Concentration | Staining Condition | Calculation Method |
|---|---|---|---|---|---|
| ROS | Dihydrorhodamine (DHR123) | 10056–1; Biotium, Fremont, CA, USA | 30 µM | 30 min at 37 °C | [DHR123 stained FITC MFI] − [DHR123 non–stained FITC MFI] |
| MitoSOX | M36008; Life Technologies, Carlsbad, CA, USA | 5 µM | 30 min at 37 °C | [MitoSOX stained phycoerythrin (PE) MFI] − [MitoSOX non–stained PE MFI] | |
| MMP | JC–10 | ENZ–52305; Enzo Life Sciences, Farmingdale, NY, USA | 0.6 µg/mL | 30 min at 37 °C | [JC–10 stained FITC MFI − JC–10 non–stained FITC MFI]/[JC–10 stained PE MFI − JC–10 non–stained PE MFI] |
| Mitochondrial mass | MitoTracker™ Deep Red FM Dye (MTDR) | M46753; Invitrogen, Waltham, MA, USA | 50 nM | 30 min at 37 °C | [MTDR stained APC MFI] − [MTDR non–stained APC MFI]. |
| Autofluorescence | No dye | Not available | Not available | 30 min at 37 °C | [FITC MFI] |
| Analysis | Antibody | Catalogue Number | Dilution in PBS | Staining Condition | Microscope |
|---|---|---|---|---|---|
| LC3B staining | anti-LC3B | A19665; Abclonal, Boston, MA, USA | 1:200 | overnight at 4 °C | A Carl Zeiss LSM 700 (Carl Zeiss, Oberkochen, Germany) |
| Alexa Fluor® 488 goat anti-rabbit IgG | A–11008; Invitrogen | 1:200 | 60 min at room temperature | ||
| OXPHOS staining | anti-OXPHOS cocktail | ab110411; Abcam, Cambridge, Cambridgeshire, UK | 1:200 | overnight at 4 °C | A Carl Zeiss LSM 700 (Carl Zeiss, Oberkochen, Germany) |
| Alexa Fluor® 647 goat anti-mouse IgG | A–28181; Invitrogen | 1:200 | 60 min at room temperature |
| Target | Orientation | Sequence (5′–3′) | Size (bp) |
|---|---|---|---|
| 36B4 (Accession number: NM_053275) | forward | CAGCAAGTGGGAAGGTGTAATCC | 23 |
| reverse | CCCATTCTATCATCAACGGGTACAA | 25 | |
| SLIT2 (Accession number: NM_004787.4) | forward | CAGAGCTTCAGCAACATGACCC | 22 |
| reverse | GAAAGCACCTTCAGGCACAACAG | 23 |
| Analysis | Antibody | Catalogue Number | Dilution in PBS | Staining Condition |
|---|---|---|---|---|
| p16 staining | anti-p16 | A11337; Abclonal | 1:500 | overnight at 4 °C |
| Horseradish peroxidase–conjugated antibody | sc–2357; Santa Cruz biotechnology; Dallas, TX, USA | 1:2000 | 60 min at room temperature | |
| Β-actin staining | anti-β-actin | sc–47778 HRP; Santa Cruz biotechnology | 1:5000 | overnight at 4 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lee, Y.H.; Jeong, E.Y.; Kim, Y.H.; Oh, S.; Yoon, J.H.; Park, J.H.; Lee, Y.J.; Kim, D.; So, B.; Kim, M.; et al. Liquid Extract from the Bark of Magnolia officinalis Rejuvenates Skin Aging Through Mitochondrial ROS Reduction. Cosmetics 2026, 13, 22. https://doi.org/10.3390/cosmetics13010022
Lee YH, Jeong EY, Kim YH, Oh S, Yoon JH, Park JH, Lee YJ, Kim D, So B, Kim M, et al. Liquid Extract from the Bark of Magnolia officinalis Rejuvenates Skin Aging Through Mitochondrial ROS Reduction. Cosmetics. 2026; 13(1):22. https://doi.org/10.3390/cosmetics13010022
Chicago/Turabian StyleLee, Yun Haeng, Eun Young Jeong, Ye Hyang Kim, Sekyung Oh, Jee Hee Yoon, Ji Ho Park, Yoo Jin Lee, Duyeol Kim, Byeonghyeon So, Minseon Kim, and et al. 2026. "Liquid Extract from the Bark of Magnolia officinalis Rejuvenates Skin Aging Through Mitochondrial ROS Reduction" Cosmetics 13, no. 1: 22. https://doi.org/10.3390/cosmetics13010022
APA StyleLee, Y. H., Jeong, E. Y., Kim, Y. H., Oh, S., Yoon, J. H., Park, J. H., Lee, Y. J., Kim, D., So, B., Kim, M., Kim, S. Y., Kwon, H. W., Byun, Y., Shin, S. S., & Park, J. T. (2026). Liquid Extract from the Bark of Magnolia officinalis Rejuvenates Skin Aging Through Mitochondrial ROS Reduction. Cosmetics, 13(1), 22. https://doi.org/10.3390/cosmetics13010022