The Influence of Titanium Dioxide Particle Size on the Photo-Protective Properties of Pharmaceutical Preparations and Their Effectiveness Assessment Using Hyperspectral Imaging Methods
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Analysis of the Full Wavelength Range
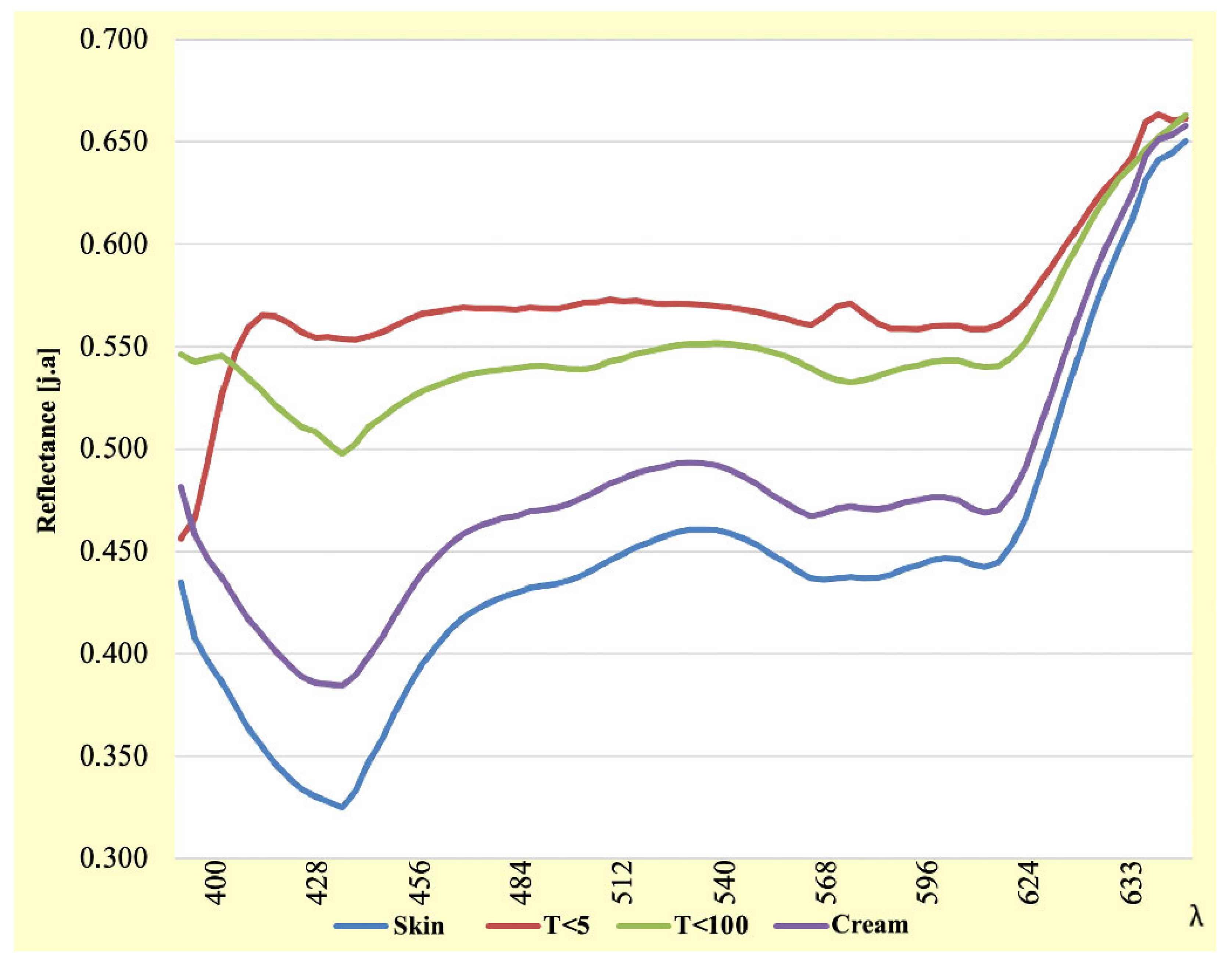
3.2. Analysis of the 400–633 nm Wavelength Range
3.3. Analysis of the 636–897 nm Wavelength Range
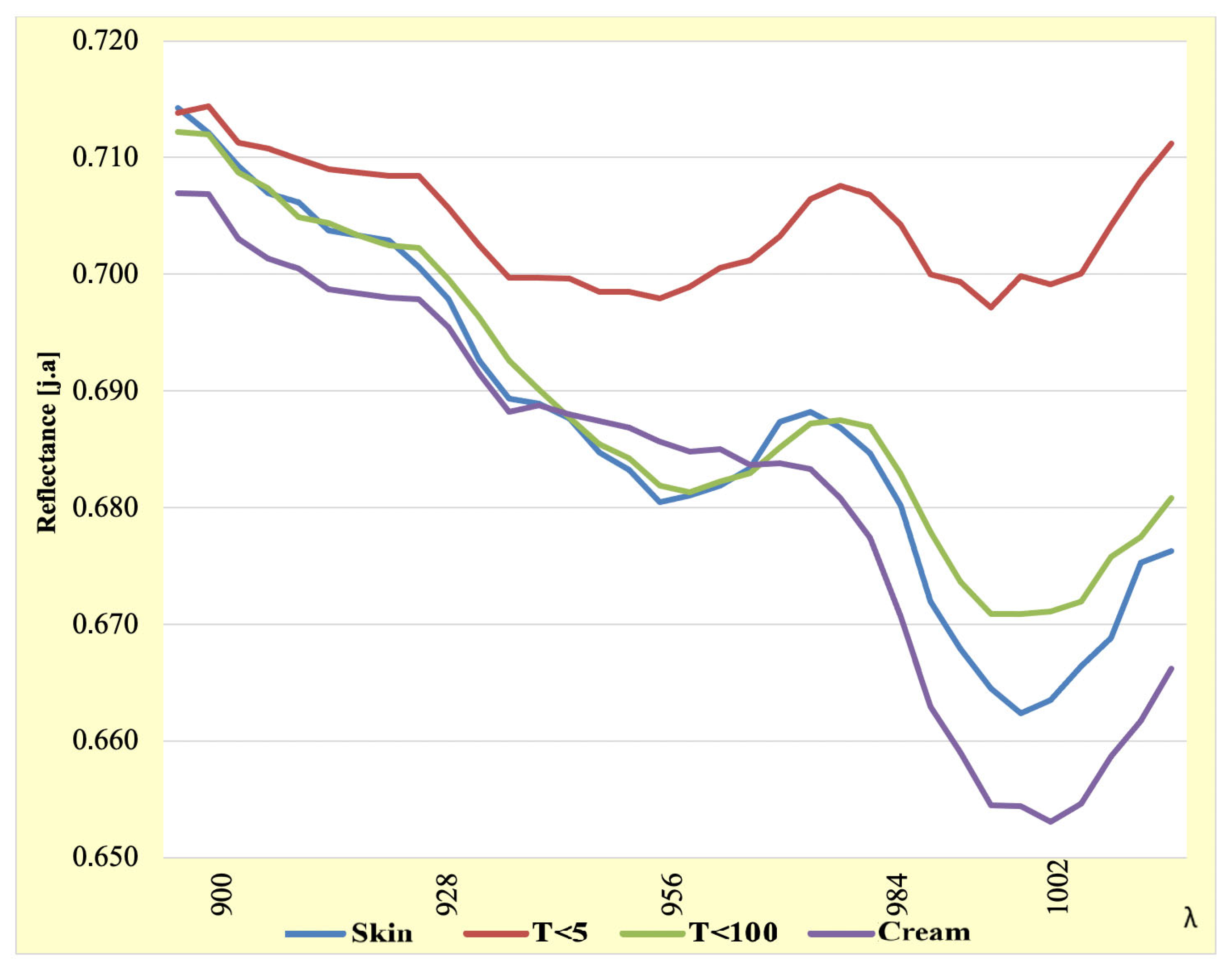
3.4. Analysis of the 900–1002 nm Wavelength Range
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Kamińska, M.; Hartman-Petrycka, M.; Bożek, M.; Krusiec-Świdergoł, B.; Nędza, M.; Wilczyński, S. Assessment of knowledge and selected attitudes among Silesians about effects of ultraviolet radiation on health. Przegl. Epidemiol. 2018, 72, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Zheng, M.; Fan, H.Y.; Liang, X.H.; Tang, Y.L. Ultraviolet (UV) radiation: A double-edged sword in cancer development and therapy. Mol. Biomed. 2024, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Duarte Romero, B.L.; Andersen, H.; Clarke, M.; Collins, L.G.; Dawson, T.; Hartel, G.; Lefevre, J.G.; Lucas, R.M.; McLeod, D.S.A.; et al. The effect of daily sunscreen application on vitamin D: Findings from the open-label, randomised, controlled Sun-D Trial. Br. J. Dermatol. 2025, ljaf310. [Google Scholar] [CrossRef]
- Abdel Azim, S.; Bainvoll, L.; Vecerek, N.; DeLeo, V.A.; Adler, B.L. Sunscreens part 1: Mechanisms and efficacy. J. Am. Acad. Dermatol. 2025, 92, 677–686. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef]
- Serpone, N. Sunscreens and their usefulness: Have we made any progress in the last two decades? Photochem. Photobiol. Sci. 2021, 20, 189–244. [Google Scholar] [CrossRef]
- Bens, G. Sunscreens. Adv. Exp. Med. Biol. 2014, 810, 429–463. [Google Scholar] [CrossRef]
- Buchalska, M.; Kras, G.; Oszajca, M.; Łasocha, W.; Macyk, W. Singlet oxygen generation in the presence of titanium dioxide materials used as sunscreens in suntan lotions. J. Photochem. Photobiol. A Chem. 2010, 213, 158–163. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Ferrero, L.; Pissavini, M.; Marguerie, S.; Zastrow, L. Sunscreen in vitro spectroscopy: Application to UVA protection assessment and correlation with in vivo persistent pigment darkening. Int. J. Cosmet. Sci. 2002, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EU) No 1333/2008 of the European Parliament and of the Council as regards titanium dioxide (E 171) as a food additive. Off. J. Eur. Union 2022, L11, 1–3. [Google Scholar]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Catalano, R.; Bartolomei, V.; Labille, J. Release and fate of nanoparticulate TiO2 UV filters from sunscreen: Effects of particle coating and formulation type. Environ Pollut. 2021, 271, 116263. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. Investigating the use of titanium dioxide (TiO2) nanoparticles on the amount of protection against UV irradiation. Sci. Rep. 2023, 13, 9793. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sun-screens. J Am Acad Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef]
- Zvyagin, A.V.; Zhao, X.; Gierden, A.; Sanchez, W.; Ross, J.A.; Roberts, M.S. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J. Biomed. Opt. 2008, 13, 064031–064038. [Google Scholar] [CrossRef]
- Aggarwal, S.L.P.; Papay, F.A. Applications of multispectral and hyperspectral imaging in dermatology. Exp. Dermatol. 2022, 31, 1128–1135. [Google Scholar] [CrossRef]
- Zherebtsov, E.; Dremin, V.; Popov, A.; Doronin, A.; Kurakina, D.; Kirillin, M.; Meglinski, I.; Bykov, A. Hyperspectral imaging of human skin aided by artificial neural networks. Biomed. Opt. Express 2019, 10, 3545–3559. [Google Scholar] [CrossRef]
- Stolecka-Warzecha, A.; Wilczyński, S.; Pawlus, A.; Lebiedowska, A.; Chmielewski, Ł.; Niezgoda, Z. The Use of Hemispheric Directional Reflectance Method to Verify the Usefulness of Filters Protecting the Skin Against Infrared Radiation. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2663–2675. [Google Scholar] [CrossRef]
- Cole, C.; Shyr, T.; Ou-Yang, H. Metal oxide sunscreens protect skin by absorption, not by reflection or scattering. Photodermatol. Photoimmunol. Photomed. 2016, 32, 5–10. [Google Scholar] [CrossRef]
- Egerton, T.A.; Tooley, I.R. UV absorption and scattering properties of inorganic-based sunscreens. Int. J. Cosmet. Sci. 2012, 34, 117–122. [Google Scholar] [CrossRef]
- Vujovic, M.; Kostic, E. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: A Review of Toxicological Data. J. Cosmet. Sci. 70, 223–234. [PubMed]
- Popov, A.P.; Lademann, J.; Priezzhev, A.V.; Myllylä, R. Effect of size of TiO2 nanoparticles embedded into stratum corneum on ultraviolet-A and ultraviolet-B sun-blocking properties of the skin. J. Biomed. Opt. 2005, 10, 064037. [Google Scholar] [CrossRef]
- Wokovich, A.; Tyner, K.; Doub, W.; Sadrieh, N.; Buhse, L.F. Particle size determination of sunscreens formulated with various forms of titanium dioxide. Drug Dev. Ind. Pharm. 2009, 35, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Lécureux, M.; Enoch, S.; Deumié, C.; Tayeb, G. Electromagnetic sunscreen model: Implementation and comparison between several methods: Step-film model, differential method, Mie scattering, and scattering by a set of parallel cylinders. Appl. Opt. 2014, 53, 6537–6545. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C. Solbeskyttelse med solcreme [Sun protection with sunscreens]. Ugeskr. Laeger 2025, 187, V05250383. [Google Scholar] [CrossRef]
- Ziglar, J.; Mohammad, T.F.; Gilaberte, Y.; Lim, H.W. Sunscreens: Updates on Sunscreen Filters and Formulations. Photodermatol. Photoimmunol. Photomed. 2025, 41, e70026. [Google Scholar] [CrossRef]
- Schalka, S.; dos Reis, V.M.; Cucé, L.C. The influence of the amount of sunscreen applied and its sun protection factor (SPF): Evaluation of two sunscreens including the same ingredients at different concentrations. Photodermatol. Photoimmunol. Photomed. 2009, 25, 175–180. [Google Scholar] [CrossRef]
- Kim, S.M.; Oh, B.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. The relation between the amount of sunscreen applied and the sun protection factor in Asian skin. J. Am. Acad. Dermatol. 2010, 62, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.; Yip, L.; McDonald, C.; Lin, F.; Liu, W.; Sullivan, J. Recommendations on Periprocedural Skincare for Energy-Based Dermatologic Procedures. Aesthet. Surg. J. Open Forum 2025, 7, ojaf039. [Google Scholar] [CrossRef]
- Le Digabel, J.; Questel, E.; Lauze, C.; Carballido, F.; Josse, G. In vivo evaluation of sunscreen application by multispectral imaging: A new tool for educating sunscreen users. Skin Res. Technol. 2023, 29, e13320. [Google Scholar] [CrossRef] [PubMed]
- Le Digabel, J.; Filiol, J.; Lauze, C.; Redoulès, D.; Josse, G. In vivo method for evaluating sunscreen protection against high-energy visible light. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 6–11. [Google Scholar] [CrossRef] [PubMed]


| Md | Q1 | Q3 | Min | Max | Mean | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | 0.683 | 0.446 | 0.727 | 0.325 | 0.758 | 0.609 | 0.141 | |||
| T < 5 μm | 0.699 | 0.569 | 0.718 | 0.456 | 0.739 | 0.656 | 0.073 | Skin vs. T < 5 μm * | ||
| T < 100 nm | 0.685 | 0.545 | 0.724 | 0.497 | 0.742 | 0.648 | 0.084 | Skin vs. T < 100 nm * | T < 5 μm vs. T < 100 nm ns | |
| Cream | 0.684 | 0.478 | 0.723 | 0.384 | 0.754 | 0.621 | 0.121 | Skin vs. Cream ns | T < 5 μm vs. Cream * | T < 100 nm vs. Cream * |
| Md | Q1 | Q3 | Min | Max | Mean | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | 0.440 | 0.409 | 0.457 | 0.325 | 0.651 | 0.445 | 0.074 | |||
| T < 5 | 0.567 | 0.560 | 0.571 | 0.456 | 0.664 | 0.570 | 0.034 | Skin vs. T < 5 μm * | ||
| T < 100 | 0.541 | 0.535 | 0.550 | 0.497 | 0.663 | 0.550 | 0.036 | Skin vs. T < 100 nm * | T < 5 μm vs. T < 100 nm * | |
| Cream | 0.472 | 0.456 | 0.490 | 0.384 | 0.658 | 0.480 | 0.062 | Skin vs. Cream * | T < 5 μm vs. Cream * | T < 100 nm vs. Cream * |
| λ [nm] | Skin | T < 5 | T < 100 | Skin | λ [nm] | Skin | T < 5 | T < 100 | Cream |
|---|---|---|---|---|---|---|---|---|---|
| 400 | 0.435 | 0.456 | 0.546 | 0.481 | 518 | 0.461 | 0.571 | 0.551 | 0.493 |
| 403 | 0.407 | 0.466 | 0.543 | 0.458 | 521 | 0.461 | 0.570 | 0.552 | 0.493 |
| 406 | 0.396 | 0.494 | 0.544 | 0.446 | 524 | 0.460 | 0.570 | 0.552 | 0.492 |
| 409 | 0.386 | 0.526 | 0.546 | 0.437 | 527 | 0.459 | 0.569 | 0.551 | 0.490 |
| 413 | 0.375 | 0.547 | 0.541 | 0.427 | 530 | 0.456 | 0.568 | 0.550 | 0.487 |
| 416 | 0.364 | 0.559 | 0.535 | 0.417 | 534 | 0.453 | 0.567 | 0.549 | 0.483 |
| 419 | 0.355 | 0.565 | 0.528 | 0.409 | 537 | 0.449 | 0.565 | 0.548 | 0.478 |
| 422 | 0.346 | 0.565 | 0.522 | 0.402 | 540 | 0.445 | 0.564 | 0.545 | 0.474 |
| 425 | 0.339 | 0.562 | 0.516 | 0.395 | 543 | 0.441 | 0.562 | 0.543 | 0.470 |
| 428 | 0.334 | 0.557 | 0.511 | 0.389 | 546 | 0.437 | 0.561 | 0.539 | 0.467 |
| 431 | 0.330 | 0.554 | 0.508 | 0.386 | 549 | 0.436 | 0.565 | 0.536 | 0.468 |
| 434 | 0.328 | 0.555 | 0.503 | 0.385 | 552 | 0.437 | 0.570 | 0.534 | 0.471 |
| 437 | 0.325 | 0.554 | 0.497 | 0.384 | 555 | 0.438 | 0.571 | 0.533 | 0.472 |
| 440 | 0.333 | 0.553 | 0.503 | 0.390 | 558 | 0.437 | 0.566 | 0.534 | 0.471 |
| 444 | 0.347 | 0.555 | 0.511 | 0.399 | 561 | 0.437 | 0.561 | 0.536 | 0.471 |
| 447 | 0.359 | 0.557 | 0.516 | 0.408 | 565 | 0.439 | 0.559 | 0.538 | 0.472 |
| 450 | 0.372 | 0.560 | 0.520 | 0.419 | 568 | 0.442 | 0.559 | 0.540 | 0.474 |
| 453 | 0.384 | 0.564 | 0.525 | 0.430 | 571 | 0.443 | 0.559 | 0.541 | 0.475 |
| 456 | 0.395 | 0.566 | 0.528 | 0.439 | 574 | 0.446 | 0.560 | 0.543 | 0.477 |
| 459 | 0.404 | 0.567 | 0.531 | 0.447 | 577 | 0.447 | 0.560 | 0.543 | 0.477 |
| 462 | 0.411 | 0.568 | 0.533 | 0.453 | 580 | 0.446 | 0.560 | 0.543 | 0.475 |
| 465 | 0.417 | 0.569 | 0.536 | 0.458 | 583 | 0.444 | 0.559 | 0.541 | 0.471 |
| 468 | 0.422 | 0.569 | 0.537 | 0.462 | 586 | 0.442 | 0.559 | 0.540 | 0.469 |
| 471 | 0.425 | 0.569 | 0.538 | 0.464 | 589 | 0.445 | 0.560 | 0.540 | 0.470 |
| 475 | 0.428 | 0.568 | 0.539 | 0.466 | 592 | 0.453 | 0.565 | 0.545 | 0.478 |
| 478 | 0.430 | 0.568 | 0.539 | 0.467 | 596 | 0.466 | 0.571 | 0.552 | 0.491 |
| 481 | 0.432 | 0.569 | 0.540 | 0.469 | 599 | 0.484 | 0.580 | 0.563 | 0.508 |
| 484 | 0.433 | 0.569 | 0.541 | 0.470 | 602 | 0.505 | 0.589 | 0.575 | 0.527 |
| 487 | 0.434 | 0.568 | 0.540 | 0.471 | 605 | 0.525 | 0.599 | 0.588 | 0.546 |
| 490 | 0.436 | 0.570 | 0.539 | 0.473 | 608 | 0.545 | 0.609 | 0.600 | 0.565 |
| 493 | 0.439 | 0.571 | 0.539 | 0.476 | 611 | 0.565 | 0.619 | 0.612 | 0.583 |
| 496 | 0.442 | 0.572 | 0.540 | 0.479 | 614 | 0.582 | 0.627 | 0.622 | 0.598 |
| 499 | 0.446 | 0.573 | 0.543 | 0.483 | 617 | 0.598 | 0.634 | 0.632 | 0.612 |
| 503 | 0.449 | 0.572 | 0.544 | 0.486 | 620 | 0.612 | 0.642 | 0.638 | 0.624 |
| 506 | 0.452 | 0.573 | 0.546 | 0.488 | 624 | 0.632 | 0.660 | 0.646 | 0.643 |
| 509 | 0.455 | 0.571 | 0.548 | 0.490 | 627 | 0.641 | 0.664 | 0.652 | 0.651 |
| 512 | 0.457 | 0.571 | 0.549 | 0.491 | 630 | 0.645 | 0.661 | 0.658 | 0.654 |
| 515 | 0.460 | 0.571 | 0.551 | 0.493 | 633 | 0.651 | 0.662 | 0.663 | 0.658 |
| Md | Q1 | Q3 | Min | Max | Mean | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | 0.729 | 0.718 | 0.740 | 0.658 | 0.758 | 0.724 | 0.023 | |||
| T < 5 | 0.718 | 0.711 | 0.723 | 0.665 | 0.739 | 0.713 | 0.017 | Skin vs. T < 5 μm * | ||
| T < 100 | 0.725 | 0.716 | 0.733 | 0.668 | 0.742 | 0.720 | 0.017 | Skin vs. T < 100 nm * | T < 5 μm vs. T < 100 nm * | |
| Skin | 0.726 | 0.714 | 0.739 | 0.664 | 0.754 | 0.722 | 0.021 | Skin vs. Cream * | T < 5 μm vs. Cream * | T < 100 nm vs. Cream ns |
| λ [nm] | Skin | T < 5 | T < 100 | Cream | λ [nm] | Skin | T < 5 | T < 100 | Cream |
|---|---|---|---|---|---|---|---|---|---|
| 636 | 0.658 | 0.665 | 0.668 | 0.664 | 766 | 0.732 | 0.719 | 0.728 | 0.727 |
| 639 | 0.662 | 0.667 | 0.671 | 0.668 | 769 | 0.729 | 0.718 | 0.726 | 0.725 |
| 642 | 0.667 | 0.670 | 0.675 | 0.672 | 772 | 0.728 | 0.718 | 0.725 | 0.723 |
| 645 | 0.671 | 0.672 | 0.678 | 0.675 | 776 | 0.727 | 0.717 | 0.724 | 0.721 |
| 648 | 0.674 | 0.673 | 0.681 | 0.678 | 779 | 0.726 | 0.718 | 0.724 | 0.721 |
| 651 | 0.677 | 0.675 | 0.684 | 0.680 | 782 | 0.727 | 0.719 | 0.725 | 0.723 |
| 655 | 0.680 | 0.678 | 0.687 | 0.683 | 785 | 0.727 | 0.719 | 0.724 | 0.722 |
| 658 | 0.682 | 0.680 | 0.689 | 0.685 | 788 | 0.726 | 0.719 | 0.724 | 0.722 |
| 661 | 0.686 | 0.682 | 0.692 | 0.688 | 791 | 0.729 | 0.721 | 0.727 | 0.724 |
| 664 | 0.688 | 0.684 | 0.694 | 0.690 | 794 | 0.733 | 0.724 | 0.729 | 0.728 |
| 667 | 0.690 | 0.687 | 0.697 | 0.692 | 797 | 0.739 | 0.728 | 0.732 | 0.734 |
| 670 | 0.693 | 0.688 | 0.699 | 0.694 | 800 | 0.739 | 0.727 | 0.733 | 0.735 |
| 673 | 0.694 | 0.690 | 0.700 | 0.695 | 803 | 0.742 | 0.729 | 0.733 | 0.737 |
| 676 | 0.697 | 0.692 | 0.702 | 0.698 | 807 | 0.743 | 0.730 | 0.734 | 0.739 |
| 679 | 0.700 | 0.695 | 0.705 | 0.700 | 810 | 0.745 | 0.731 | 0.735 | 0.741 |
| 682 | 0.702 | 0.697 | 0.706 | 0.703 | 813 | 0.744 | 0.730 | 0.734 | 0.741 |
| 686 | 0.706 | 0.700 | 0.710 | 0.707 | 816 | 0.748 | 0.732 | 0.737 | 0.744 |
| 689 | 0.711 | 0.704 | 0.714 | 0.712 | 819 | 0.754 | 0.735 | 0.739 | 0.748 |
| 692 | 0.714 | 0.705 | 0.715 | 0.716 | 822 | 0.758 | 0.739 | 0.742 | 0.754 |
| 695 | 0.718 | 0.708 | 0.718 | 0.720 | 825 | 0.751 | 0.734 | 0.738 | 0.750 |
| 698 | 0.721 | 0.709 | 0.720 | 0.723 | 828 | 0.744 | 0.727 | 0.734 | 0.741 |
| 701 | 0.724 | 0.711 | 0.722 | 0.726 | 831 | 0.745 | 0.728 | 0.733 | 0.741 |
| 704 | 0.729 | 0.714 | 0.725 | 0.731 | 835 | 0.748 | 0.729 | 0.734 | 0.743 |
| 707 | 0.732 | 0.715 | 0.727 | 0.735 | 838 | 0.746 | 0.728 | 0.733 | 0.742 |
| 710 | 0.735 | 0.716 | 0.730 | 0.738 | 841 | 0.745 | 0.727 | 0.731 | 0.740 |
| 714 | 0.738 | 0.718 | 0.732 | 0.741 | 844 | 0.743 | 0.726 | 0.731 | 0.738 |
| 717 | 0.739 | 0.717 | 0.732 | 0.742 | 847 | 0.740 | 0.724 | 0.728 | 0.735 |
| 720 | 0.736 | 0.715 | 0.730 | 0.740 | 850 | 0.737 | 0.723 | 0.727 | 0.732 |
| 723 | 0.737 | 0.715 | 0.729 | 0.739 | 853 | 0.734 | 0.722 | 0.725 | 0.730 |
| 726 | 0.738 | 0.717 | 0.729 | 0.738 | 856 | 0.731 | 0.720 | 0.723 | 0.727 |
| 729 | 0.743 | 0.721 | 0.732 | 0.742 | 859 | 0.732 | 0.720 | 0.723 | 0.725 |
| 732 | 0.745 | 0.723 | 0.734 | 0.744 | 862 | 0.729 | 0.719 | 0.722 | 0.723 |
| 735 | 0.746 | 0.723 | 0.735 | 0.744 | 866 | 0.727 | 0.718 | 0.721 | 0.722 |
| 738 | 0.746 | 0.723 | 0.735 | 0.744 | 869 | 0.728 | 0.718 | 0.720 | 0.720 |
| 741 | 0.745 | 0.723 | 0.735 | 0.743 | 872 | 0.725 | 0.717 | 0.720 | 0.718 |
| 745 | 0.744 | 0.723 | 0.735 | 0.741 | 875 | 0.724 | 0.718 | 0.720 | 0.718 |
| 748 | 0.743 | 0.723 | 0.735 | 0.740 | 878 | 0.725 | 0.718 | 0.720 | 0.718 |
| 751 | 0.740 | 0.721 | 0.733 | 0.737 | 881 | 0.724 | 0.718 | 0.719 | 0.716 |
| 754 | 0.740 | 0.722 | 0.733 | 0.736 | 884 | 0.724 | 0.718 | 0.719 | 0.716 |
| 757 | 0.739 | 0.722 | 0.733 | 0.734 | 887 | 0.720 | 0.717 | 0.717 | 0.714 |
| 760 | 0.736 | 0.720 | 0.731 | 0.732 | 890 | 0.719 | 0.715 | 0.716 | 0.711 |
| 763 | 0.734 | 0.720 | 0.729 | 0.730 | 893 | 0.717 | 0.715 | 0.715 | 0.710 |
| 897 | 0.717 | 0.715 | 0.714 | 0.708 |
| Md | Q1 | Q3 | Min | Max | Mean | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | 0.686 | 0.676 | 0.701 | 0.662 | 0.714 | 0.687 | 0.015 | |||
| T < 5 | 0.704 | 0.700 | 0.708 | 0.697 | 0.714 | 0.704 | 0.005 | Skin vs. T < 5 μm * | ||
| T < 100 | 0.686 | 0.681 | 0.702 | 0.671 | 0.712 | 0.689 | 0.013 | Skin vs. T < 100 nm ns | T < 5 μm vs. T < 100 nm * | |
| Cream | 0.685 | 0.666 | 0.698 | 0.653 | 0.707 | 0.682 | 0.017 | Skin vs. Cream ns | T < 5 μm vs. Cream * | T < 100 nm vs. Cream * |
| λ [nm] | Skin | T < 5 | T < 100 | Cream | λ [nm] | Skin | T < 5 | T < 100 | Cream |
|---|---|---|---|---|---|---|---|---|---|
| 900 | 0.714 | 0.714 | 0.712 | 0.707 | 952 | 0.681 | 0.699 | 0.681 | 0.685 |
| 903 | 0.712 | 0.714 | 0.712 | 0.707 | 956 | 0.682 | 0.701 | 0.682 | 0.685 |
| 906 | 0.709 | 0.711 | 0.709 | 0.703 | 959 | 0.683 | 0.701 | 0.683 | 0.684 |
| 909 | 0.707 | 0.711 | 0.707 | 0.701 | 962 | 0.687 | 0.703 | 0.685 | 0.684 |
| 912 | 0.706 | 0.710 | 0.705 | 0.700 | 965 | 0.688 | 0.706 | 0.687 | 0.683 |
| 915 | 0.704 | 0.709 | 0.704 | 0.699 | 968 | 0.687 | 0.708 | 0.687 | 0.681 |
| 918 | 0.703 | 0.709 | 0.703 | 0.698 | 971 | 0.685 | 0.707 | 0.687 | 0.677 |
| 921 | 0.703 | 0.708 | 0.702 | 0.698 | 974 | 0.680 | 0.704 | 0.683 | 0.671 |
| 925 | 0.701 | 0.708 | 0.702 | 0.698 | 977 | 0.672 | 0.700 | 0.678 | 0.663 |
| 928 | 0.698 | 0.706 | 0.700 | 0.695 | 980 | 0.668 | 0.699 | 0.674 | 0.659 |
| 931 | 0.693 | 0.702 | 0.696 | 0.691 | 983 | 0.664 | 0.697 | 0.671 | 0.654 |
| 934 | 0.689 | 0.700 | 0.693 | 0.688 | 987 | 0.662 | 0.700 | 0.671 | 0.654 |
| 937 | 0.689 | 0.700 | 0.690 | 0.689 | 990 | 0.663 | 0.699 | 0.671 | 0.653 |
| 940 | 0.688 | 0.700 | 0.688 | 0.688 | 993 | 0.666 | 0.700 | 0.672 | 0.655 |
| 943 | 0.685 | 0.699 | 0.685 | 0.687 | 996 | 0.669 | 0.704 | 0.676 | 0.659 |
| 946 | 0.683 | 0.698 | 0.684 | 0.687 | 999 | 0.675 | 0.708 | 0.678 | 0.662 |
| 949 | 0.680 | 0.698 | 0.682 | 0.686 | 1002 | 0.676 | 0.711 | 0.681 | 0.666 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolecka-Warzecha, A.; Mickoś, E.; Śniecińska, D.; Malewicz-Skrabania, D.; Wilczyński, A.; Wilczyński, S. The Influence of Titanium Dioxide Particle Size on the Photo-Protective Properties of Pharmaceutical Preparations and Their Effectiveness Assessment Using Hyperspectral Imaging Methods. Cosmetics 2025, 12, 242. https://doi.org/10.3390/cosmetics12060242
Stolecka-Warzecha A, Mickoś E, Śniecińska D, Malewicz-Skrabania D, Wilczyński A, Wilczyński S. The Influence of Titanium Dioxide Particle Size on the Photo-Protective Properties of Pharmaceutical Preparations and Their Effectiveness Assessment Using Hyperspectral Imaging Methods. Cosmetics. 2025; 12(6):242. https://doi.org/10.3390/cosmetics12060242
Chicago/Turabian StyleStolecka-Warzecha, Anna, Elżbieta Mickoś, Daria Śniecińska, Dominika Malewicz-Skrabania, Adam Wilczyński, and Sławomir Wilczyński. 2025. "The Influence of Titanium Dioxide Particle Size on the Photo-Protective Properties of Pharmaceutical Preparations and Their Effectiveness Assessment Using Hyperspectral Imaging Methods" Cosmetics 12, no. 6: 242. https://doi.org/10.3390/cosmetics12060242
APA StyleStolecka-Warzecha, A., Mickoś, E., Śniecińska, D., Malewicz-Skrabania, D., Wilczyński, A., & Wilczyński, S. (2025). The Influence of Titanium Dioxide Particle Size on the Photo-Protective Properties of Pharmaceutical Preparations and Their Effectiveness Assessment Using Hyperspectral Imaging Methods. Cosmetics, 12(6), 242. https://doi.org/10.3390/cosmetics12060242








