A Polyherbal Formulation That Mitigates Cellular Damage in Narrowband UVB-Irradiated HaCaT Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
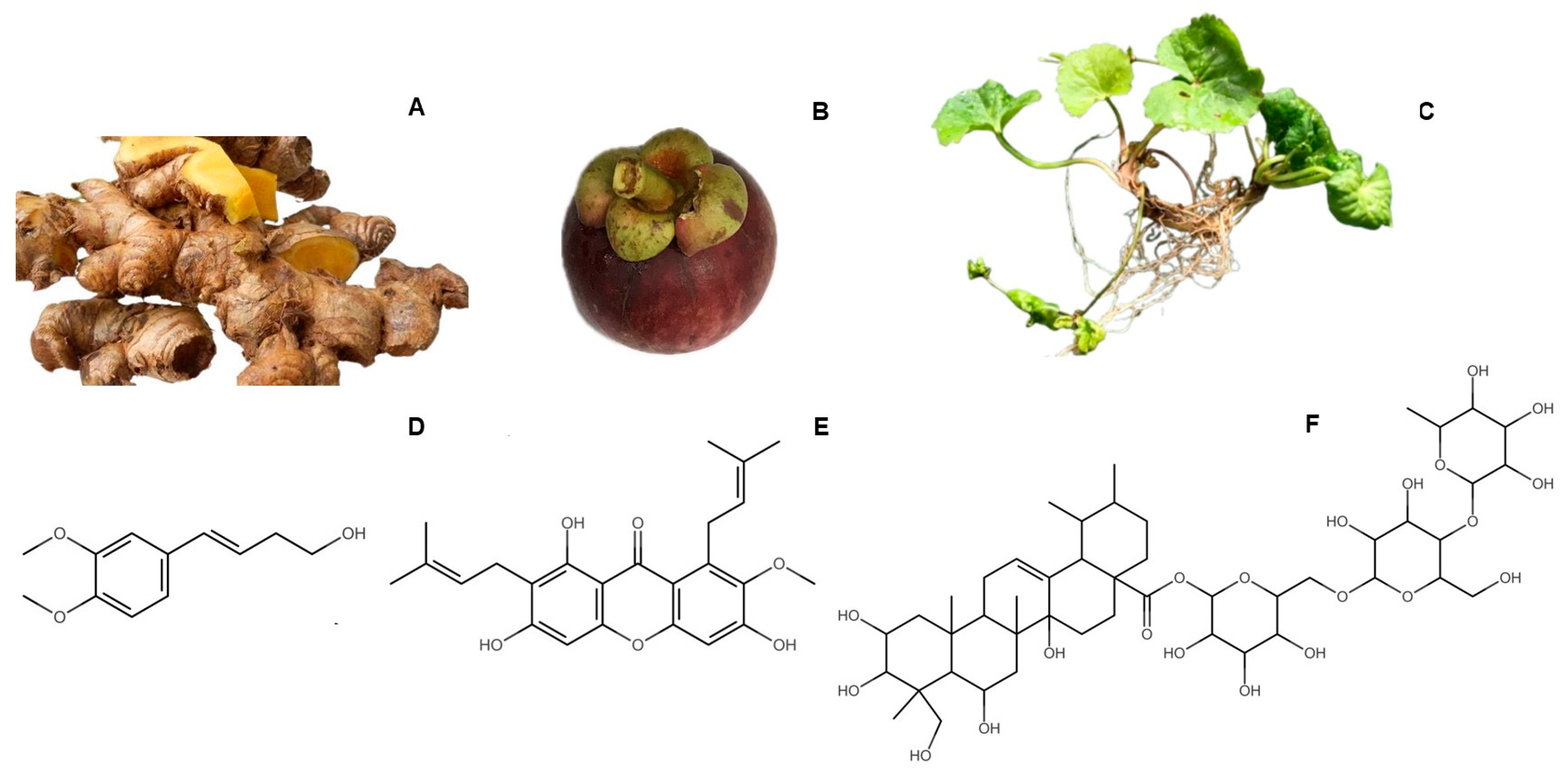
2.2. Plant Materials
2.3. Extract Preparation
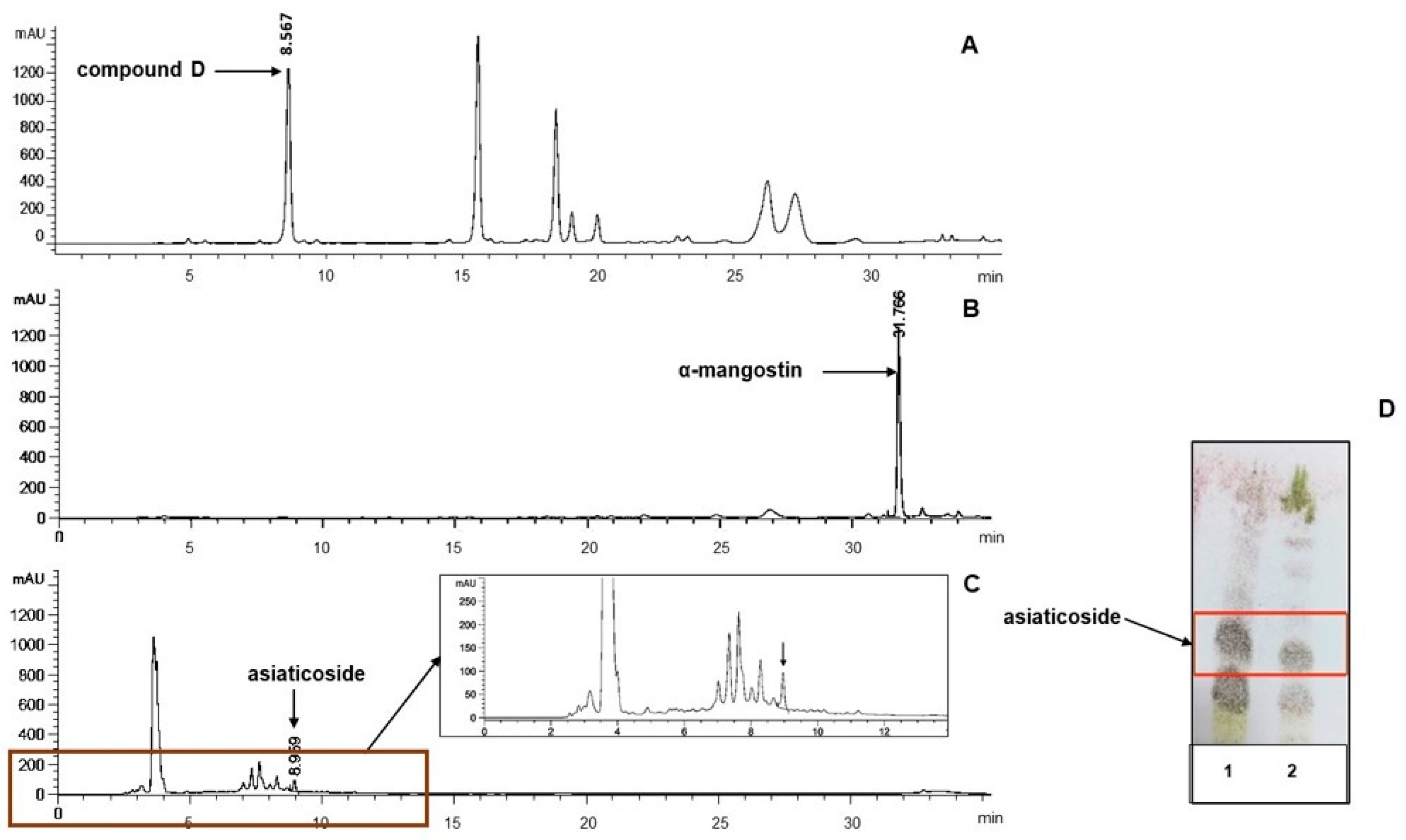
2.4. High-Performance Thin-Layer Chromatography (HPTLC)
2.5. High-Performance Liquid Chromatography (HPLC) Analysis
2.6. Chemical Characterization of the Herbal Extracts
2.6.1. Determination of Total Phenolic Contents
2.6.2. Determination of Total Flavonoid Contents
2.7. Antioxidant Assays
2.7.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radical Scavenging Assay
2.7.2. Superoxide Anion Scavenging Activity
2.8. Anti-Collagenase Activity Assay
2.9. NB-UVB-Exposed HACAT Assays
2.9.1. Cell Culture
2.9.2. Cell Viability Assay
2.9.3. NB-UVB Exposure
2.9.4. Cell Proliferation Assay on NB-UVB-Exposed HaCaT Cells
2.9.5. Apoptosis Assay
2.9.6. Intracellular ROS Assay
2.9.7. Quantitative Real-Time PCR (qPCR) of NB-UVB-Exposed HaCaT Cells
2.10. Statistical Analysis
3. Results
3.1. Extraction Yield and HPTLC Peak Area of Biomarkers
3.2. Chemical Characterization of the Extracts
3.2.1. HPLC Analysis of the Extracts
3.2.2. Total Phenolic Contents (TPCs) and Total Flavonoid Contents (TFCs)
3.3. Antioxidant and Anti-Collagenase Activities of the Extracts
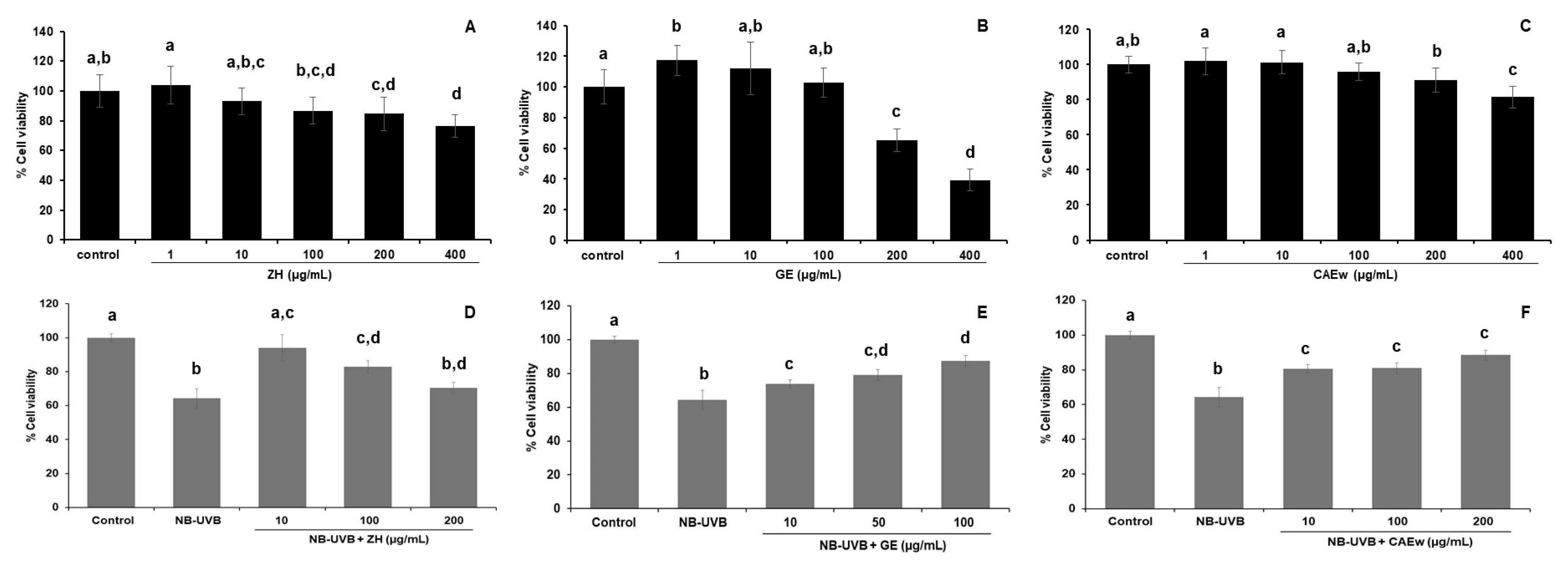
3.4. Effect of the Herbal Extracts on the Viability of NB-UVB-Exposed HaCaT
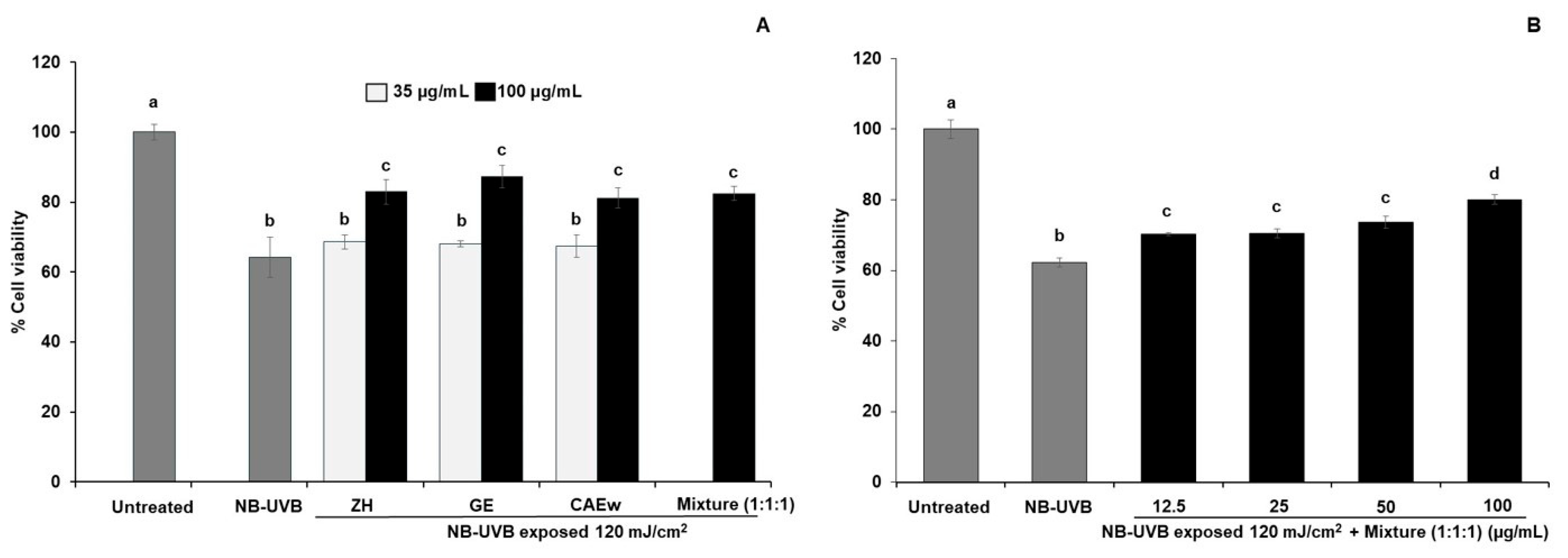
3.5. Effect of Polyherbal Mixture Ratios on the Viability of NB-UVB-Exposed HaCaT
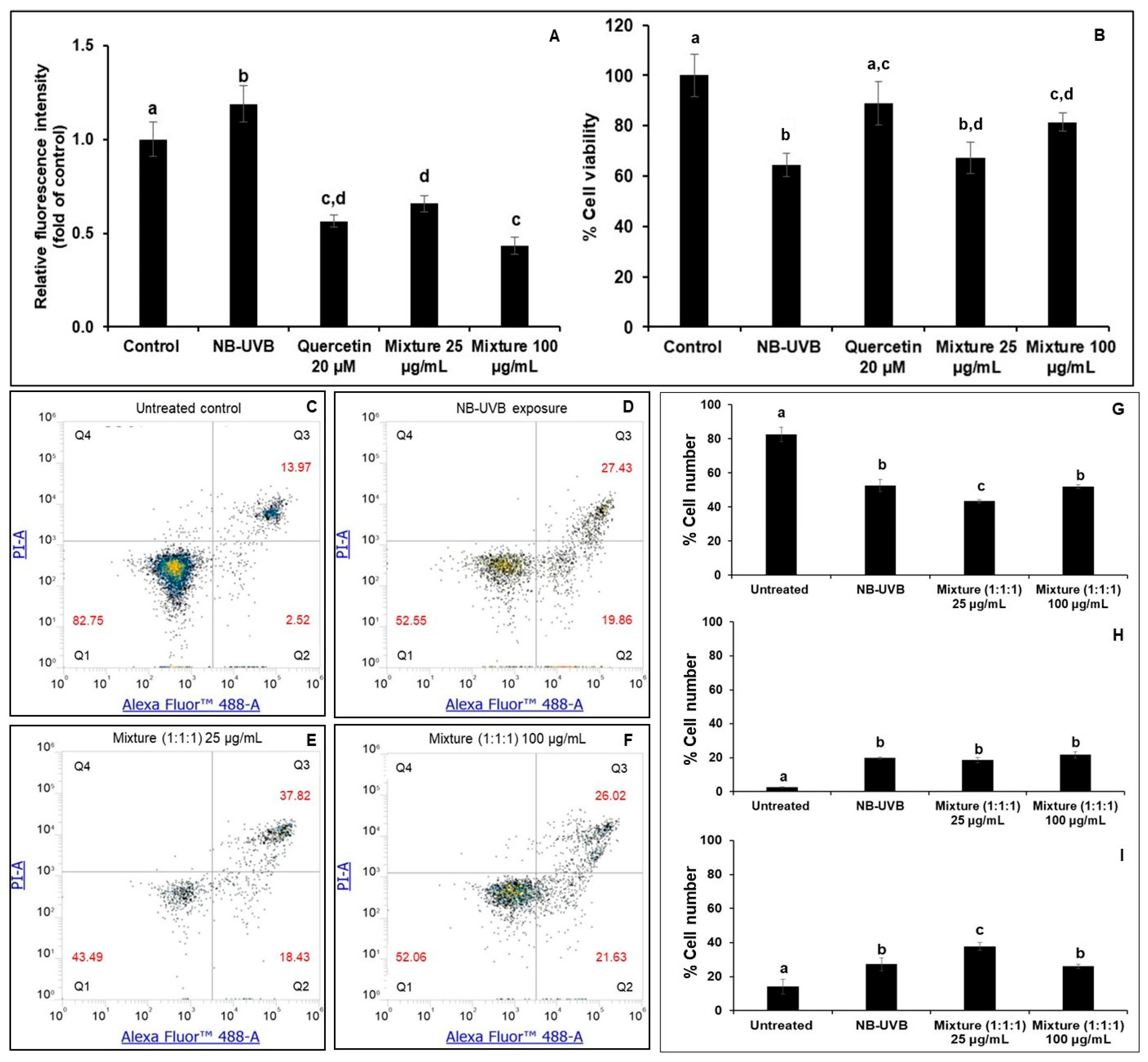
3.6. Effects of the Polyherbal Mixture on Intracellular Reactive Oxygen Species (ROS) and Cell Apoptosis on NB-UVB-Exposed HaCaT
3.7. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marrot, L.; Meunier, J.R. Skin DNA Photodamage and Its Biological Consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.A.; Jaenicke, K.F. Action Spectrum for Phototherapy of Psoriasis. J. Investig. Dermatol. 1981, 76, 359–362. [Google Scholar] [CrossRef]
- Yuehua, Y.; Khalaf, A.T.; Xiaoxiang, Z.; Xinggang, W. Narrow-Band Ultraviolet B and Conventional UVB Phototherapy in Psoriasis: A Randomised Controlled Trial. J. Appl. Sci. 2008, 5, 905–908. [Google Scholar] [CrossRef][Green Version]
- Coelho, M.M.V.; Apetato, M. The Dark Side of the Light: Phototherapy Adverse Effects. Clin. Dermatol. 2016, 34, 556–562. [Google Scholar] [CrossRef]
- Goulden, V.; Ling, T.C.; Babakinejad, P.; Dawe, R.; Eadie, E.; Fassihi, H.; Fityan, A.; Garibaldinos, T.; Ibbotson, S.H.; Novakovic, L.; et al. British Association of Dermatologists and British Photodermatology Group Guidelines for Narrowband Ultraviolet B Phototherapy 2022. Br. J. Dermatol. 2022, 187, 285–286. [Google Scholar] [CrossRef]
- Ade, I.; Wanandi, S.I.; Wuyung, P.E.; Hoemardani, A.S.D. Effect of Narrowband Ultraviolet B (311 nm) Exposure on Skin Carcinogenesis in Wistar Rats. J. Adv. Vet. Anim. Res. 2024, 11, 1105–1113. [Google Scholar] [CrossRef]
- Patel, V.R.; Saini, S.; Dwivedi, J.; Gupta, A.K.; Shrivastava, A.K.; Misra, A. Exploring the Concept and Scope of Polyherbal Formulations: A Comprehensive Review. Int. J. Herb. Med. 2025, 13, 9–16. [Google Scholar] [CrossRef]
- Karole, S.; Shrivastava, S.; Thomas, S.; Soni, B.; Khan, S.; Dubey, J.; Dubey, S.P.; Khan, N.; Jain, D.K. Polyherbal Formulation Concept for Synergic Action: A Review. J. Drug Deliv. Ther. 2019, 9, 453–466. [Google Scholar] [CrossRef]
- Singh, M.; Gautam, A.; Kumar, P. Chemical Stability of Polyherbal Formulations. Int. J. Environ. Health Sci. 2024, 6, 24–37. [Google Scholar] [CrossRef]
- Lohakul, J.; Chaiprasongsuk, A.; Jeayeng, S.; Saelim, M.; Muanjumpon, P.; Thanachaiphiwat, S.; Tripatara, P.; Soontrapa, K.; Lumlerdkij, N.; Akarasereenont, P.; et al. The Protective Effect of Polyherbal Formulation, Harak Formula, on UVA-Induced Photoaging of Human Dermal Fibroblasts and Mouse Skin via Promoting Nrf2-Regulated Antioxidant Defense. Front. Pharmacol. 2021, 12, 649820. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, N.; Arumugam, S.; Paramasivam, B.; Narasimhan, T.; Jayaraman, S.; Venkateasan, P.; Rajendran, S.; Elumalai, K. Formulation and Characterization of a Polyherbal Sunscreen Containing Camellia sinensis, Vitis vinifera, and Silybum marianum Extracts. Pharmacogn. Res. 2025, 17, 836–849. [Google Scholar] [CrossRef]
- Panthong, A.; Kanjanapothi, D.; Niwatananant, W.; Tuntiwachwuttikul, P.; Reutrakul, V. Anti-Inflammatory Activity of Compound D {(E)-4-(3′,4′-Dimethoxyphenyl)but-3-en-2-ol} Isolated from Zingiber cassumunar Roxb. Phytomedicine 1997, 4, 207–212. [Google Scholar] [CrossRef]
- Gundom, T.; Sukketsiri, W.; Panichayupakaranant, P. Phytochemical Analysis and Biological Effects of Zingiber cassumunar Extract and Three Phenylbutenoids: Targeting NF-κB, Akt/MAPK, and Caspase-3 Pathways. BMC Complement. Med. Ther. 2025, 25, 180. [Google Scholar] [CrossRef]
- Kaewchoothong, A.; Tewtrakul, S.; Panichayupakaranant, P. Inhibitory Effect of Phenylbutanoid-Rich Zingiber cassumunar Extracts on Nitric Oxide Production by Murine Macrophage-Like RAW264.7 Cells. Phytother. Res. 2012, 26, 1789–1792. [Google Scholar] [CrossRef]
- Maquart, F.X.; Chastang, F.; Simeon, A.; Birembaut, P.; Gillery, P.; Wegrowski, Y. Triterpenes from Centella asiatica Stimulate Extracellular Matrix Accumulation in Rat Experimental Wounds. Eur. J. Dermatol. 1999, 9, 289–296. [Google Scholar]
- Nema, N.K.; Maity, N.; Sarkar, B.K.; Mukherjee, P.K. Matrix Metalloproteinase, Hyaluronidase and Elastase Inhibitory Potential of Standardized Extract of Centella asiatica. Pharm. Biol. 2013, 51, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Liu, T.; Liu, Y.; Li, J.; Jing, Y.; Yang, M.; Zhang, H.; Jiang, M.; Wu, H.; Yang, W.; et al. Anti-Photoaging Activity of Triterpenoids Isolated from Centella asiatica. Phytochemistry 2024, 228, 114246. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, X.; Chen, L. Asiaticoside Delays Senescence and Attenuates Generation of ROS in UV Exposure Cells through Regulation of TGFβ1/Smad Pathway. Exp. Ther. Med. 2022, 24, 667. [Google Scholar] [CrossRef]
- Kalick, L.S.; Khan, H.A.; Maung, E.; Baez, Y.; Atkinson, A.N.; Wallace, C.E.; Day, F.; Delgadillo, B.E.; Mondal, A.; Watanapokasin, R.; et al. Mangosteen for Malignancy Prevention and Intervention: Current Evidence, Molecular Mechanisms, and Future Perspectives. Pharmacol. Res. 2023, 188, 106630. [Google Scholar] [CrossRef]
- Wang, F.; Ma, H.; Liu, Z.; Huang, W.; Xu, X.; Zhang, X. α-Mangostin Inhibits DMBA/TPA-Induced Skin Cancer through Inhibiting Inflammation and Promoting Autophagy and Apoptosis by Regulating PI3K/Akt/mTOR Signaling Pathway in Mice. Biomed. Pharmacother. 2017, 92, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Monton, C.; Luprasong, C.; Suksaeree, J.; Songsak, T. Validated High Performance Liquid Chromatography for Simultaneous Determination of Stability of Madecassoside and Asiaticoside in Film Forming Polymeric Dispersions. Rev. Bras. Farmacogn. 2018, 28, 289–293. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, X.; He, W.H.; Xu, H.G.; Yuan, F.; Gao, Y.X. Investigation into the Antioxidant Activity and Chemical Composition of Alcoholic Extracts from Defatted Marigold (Tagetes erecta L.) Residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Sithisarn, P.; Rojsanga, P.; Sithisarn, P.; Kongkiatpaiboon, S. Antioxidant Activity and Antibacterial Effects on Clinical Isolated Streptococcus suis and Staphylococcus intermedius of Extracts from Several Parts of Cladogynos orientalis and Their Phytochemical Screenings. Evid.-Based Complement. Altern. Med. 2015, 2015, 908242. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tangyuenyongwatana, P. HPTLC Analysis of (E)-4-(3′,4′-Dimethoxyphenyl)but-3-en-1-ol in Zingiber cassumunar Roxb Rhizome Extract. Thai J. Pharm. Sci. 2017, 41, 1–6. [Google Scholar]
- Suksaeree, J.; Charoenchai, L.; Madaka, F.; Monton, C.; Sakunpak, A.; Charoonratana, T.; Pichayakorn, W. Zingiber cassumunar Blended Patches for Skin Application: Formulation, Physicochemical Properties, and In Vitro Studies. Asian J. Pharm. Sci. 2015, 10, 341–349. [Google Scholar] [CrossRef]
- Priprem, A.; Janpim, K.; Nualkaew, S.; Mahakunakorn, P. Topical Niosome Gel of Zingiber cassumunar Roxb. Extract for Anti-Inflammatory Activity Enhanced Skin Permeation and Stability of Compound D. AAPS PharmSciTech 2015, 17, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Pothitirat, W.; Gritsanapan, W. HPLC Quantitative Analysis Method for the Determination of α-Mangostin in Mangosteen Fruit Rind Extract. Thai J. Agric. Sci. 2009, 42, 7–12. [Google Scholar]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef]
- Hashim, P.; Sidek, H.; Helan, M.H.M.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene Composition and Bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef]
- Chaisawadi, A.; De-Eknamkul, W. Development of a New Analytical Method for Determination of Asiaticoside Content in Centella asiatica. Thai J. Pharm. Sci. 2012, 36, 205–208. [Google Scholar] [CrossRef]
- Department of Medical Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia 2016; Department of Medical Sciences: Nonthaburi, Thailand, 2016; ISBN 9786161131913. [Google Scholar]
- Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Girsang, E.; Amalia, A.; Wibowo, S.H.B.; Kusuma, H.S.W.; Rizal. Anti-Aging Effects of Mangosteen Peel Extract and Its Phytochemical Compounds: Antioxidant Activity, Enzyme Inhibition and Molecular Docking Simulation. Trop. Life Sci. Res. 2020, 31, 127–144. [Google Scholar] [CrossRef]
- Fitri, E.W. The Effectiveness of Topical Mangosteen Pericarp Extract on the Collagen of Mice Skin Exposed to Ultraviolet B. Am. J. Clin. Exp. Med. 2016, 4, 88–93. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Jung, E.; Kim, Y.; Park, J.; Park, J.; Hong, S.; Kim, J.; Hyun, C.; Kim, Y.S.; Park, D. Asiaticoside Induces Human Collagen I Synthesis through TGFβ Receptor I Kinase (TβRI Kinase)-Independent Smad Signaling. Planta Med. 2006, 72, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Chopra, D.; Patel, S.K.; Negi, S.; Srivastav, A.K.; Ratnasekhar, C.; Bala, L.; Dwivedi, A.; Ray, R.S. Superoxide Anion Radical Induced Phototoxicity of 2,4,5,6-Tetraminopyrimidine Sulfate via Mitochondrial-Mediated Apoptosis in Human Skin Keratinocytes at Ambient UVR Exposure. Food Chem. Toxicol. 2022, 164, 112990. [Google Scholar] [CrossRef]
- Kulms, D.; Schwarz, T. Molecular Mechanisms of UV-Induced Apoptosis. Photodermatol. Photoimmunol. Photomed. 2000, 16, 195–201. [Google Scholar] [CrossRef]
- Aufiero, B.M.; Talwar, H.; Young, C.; Krishnan, M.; Hatfield, J.S.; Lee, H.K.; Wong, H.K.; Hamzavi, I.; Murakawa, G.J. Narrow-Band UVB Induces Apoptosis in Human Keratinocytes. J. Photochem. Photobiol. B 2006, 82, 132–139. [Google Scholar] [CrossRef]
- Lin, W.T.; Chen, Y.J.; Kuo, H.N.; Kumar, S.; Abomughaid, M.M.; Kumar, S. Ultraviolet B-Induced Oxidative Damage in Human Skin Keratinocytes Is Alleviated by Pinus morrisonicola Leaf Essential Oil through Activation of the Nrf2-Dependent Antioxidant Defense System. Redox Rep. 2025, 30, 2527427. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Dańczak-Pazdrowska, A.; Brzezińska, M. Centella asiatica in Dermatology: An Overview. Phytother. Res. 2014, 28, 1117–1124. [Google Scholar] [CrossRef]
- Im, A.-R.; Kim, Y.-M.; Chin, Y.-W.; Chae, S. Protective Effects of compounds from Garcinia mangostana L. (Mangosteen) Against UVB Damage in HaCaT Cells and Hairless Mice. Int. J. Mol. Med. 2017, 40, 1941–1949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, J.; Bao, Y.; Wang, Y.; Zheng, H.; Guo, H.; Zhang, L.; Guo, R.; Yang, L. Protective Activity of Alpha-Mangostin Against UVB-Induced Injury in HaCaT Cells by Modulating the Ceramide and MAPK and NF-ΚB Signaling Pathways. J. Food Biochem. 2023, 2023, 4702866. [Google Scholar] [CrossRef]
- Khalil, C.; Shebaby, W. UVB Damage Onset and Progression 24 h Post Exposure in Human-Derived Skin Cells. Toxicol. Rep. 2017, 4, 441–449. [Google Scholar] [CrossRef]
- Kim, S.; You, D.H.; Han, T.; Choi, E.M. Modulation of Viability and Apoptosis of UVB-Exposed Human Keratinocyte HaCaT Cells by Aqueous Methanol Extract of Laver (Porphyra yezoensis). J. Photochem. Photobiol. B 2014, 141, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, Through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Piao, M.J.; Susara Ruwan Kumara, M.H.; Kim, K.C.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Diphlorethohydroxycarmalol Suppresses Ultraviolet B-Induced Matrix Metalloproteinases via Inhibition of JNK and ERK Signaling in Human Keratinocytes. Biomol. Ther. 2015, 23, 557–563. [Google Scholar] [CrossRef]
- Mittraphab, Y.; Nagata, M.; Matsumoto, M.; Shimizu, K. Antioxidant and Protective Effect of Acetone Extract of Entada Phaseoloides Leaves on UVB-Irradiated Human Epidermal Keratinocytes (HaCaT cells) by Inhibiting COX-2, iNOS, and Caspase-3 Activation. Nat. Prod. Commun. 2022, 17, 1934578X221078627. [Google Scholar] [CrossRef]
- Tyas, D.A.; Wijayanti, N.; Nuringtyas, T.R.; Wahyuono, S. Protective Effects of Zingiber cassumunar Roxb. Extract Against UVB-Induced Oxidative Stress in Wistar Albino Rats (Rattus Novergicus Berkenhout, 1769). Indones. J. Biotechnol. 2024, 29, 25–32. [Google Scholar] [CrossRef]
- Sukhonthasilakun, S.; Mahakunakorn, P.; Naladta, A.; Nuankaew, K.; Nualkaew, S.; Yenjai, C.; Nualkaew, N. Anti-Inflammatory Effects of Derris Scandens Extract on Narrowband-Ultraviolet B Exposed HaCaT Human Keratinocytes. J. Ayurveda Integr. Med. 2023, 14, 100693. [Google Scholar] [CrossRef] [PubMed]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Ha, R.; Cho, W.K.; Kim, E.; Jang, S.J.; Kim, J.-D.; Yi, C.-G.; Moh, S.H. Exploring the Benefits of Herbal Medicine Composite 5 (HRMC5) for Skin Health Enhancement. Curr. Issues Mol. Biol. 2024, 46, 12133–12151. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Lee, E.J.; Min, H.Y.; Kim, H.R.; Lee, S.; Seo, E.K. A Potential Cytotoxic Principle of Zingiber cassumunar. Nat. Prod. Sci. 2003, 9, 109–111. [Google Scholar]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic Effects of Curcumin: What is the Evidence? J. Cell. Physiol. 2018, 234, 10060–10071. [Google Scholar] [CrossRef]


| Sample | Biomarker | Mobile Phase | Spraying Reagent | Wavelength (nm) |
|---|---|---|---|---|
| Z. cassumunar extract | compound D | CHCl3:EtOAc (8.5:1.5) | - | 254 |
| G. mangostana extract | α-mangostin | Hexane–EtOAc (7:3) | - | 317 |
| C. asiatica extract | asiaticoside | CHCl3:MeOH:H2O (30:13:1) | anisaldehyde -H2SO4 | 530 |
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Accession No. |
|---|---|---|---|
| GAPDH | GAGAAGGCTGGGGCTCATTT | AGTGATGGCATGGACTGTGG | NM_002046.6 |
| COX-2 | TTGCATTCTTTGCCCAGCAC | ACCGTAGATGCTCAGGGACT | NM_000963.4 |
| iNOS | CCTGGAGGTGCTAGAGGAGT | ATCTCCGGTGTGGTAGGTGA | NM_000625.4 |
| MMP-1 | TGTGGTGTCTCACAGCTTCC | ATCTGGGCTGCTTCATCACC | NM_002421.4 |
| MMP-9 | ACGATGACGAGTTGTGGTCC | GGTTTCCCATCAGCATTGCC | NM_004994.3 |
| Plant/ (Biomarker) | Extract | % Yield of Dry Weight (w/w) | Peak Area of Biomarker (mAU) |
|---|---|---|---|
| Z. cassumunar/(Compound D) | 95% EtOH extract | 20.22 | 9856.9 |
| Hexane layer | 1.94 | 16,765.5 | |
| EtOAc layer | 3.19 | 5314.7 | |
| Hexane extract (ZH) | 14.76 | 16,687.4 | |
| Compound D (5 µg) | - | 4589.6 | |
| G. mangostana/ (α-Mangostin) | 95% EtOH extract (GE) | 17.08 | 48,775.2 |
| CH2Cl2 extract | 15.20 | 54,572 | |
| EtOAc extract | 16.50 | 16,550.4 | |
| n-BuOH extract | 8.65 | 1026.4 | |
| α-Mangostin (7.5 µg) | - | 65,191.85 | |
| C. asiatica/ (Asiaticoside) | 75% Ethanol extract | 21.26 | 3475.7 |
| EtOAc layer | 1.74 | 2317.2 | |
| n-BuOH layer | 2.10 | 17,028.8 | |
| Acetone–EtOH (2.6:7.4) extract | 24.12 | 12,567.2 | |
| Hexane layer | 9.19 | N.D. | |
| Aqueous layer (CAEw) | 10.14 | 8650.5 | |
| Asiaticoside (5 µg) | - | 10,345 |
| Extract | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) |
|---|---|---|
| ZH | 249.25 ± 5.19 | 148.64 ± 15.17 |
| GE | 398.29 ± 8.49 | 233.27 ± 9.90 |
| CAEw | 185.56 ± 4.78 | 54.12 ± 6.32 |
| Samples | IC50 (µg/mL) | ||
|---|---|---|---|
| Superoxide Anion Scavenging | DPPH Free Radical Scavenging | Collagenase Inhibition | |
| Z. cassumunar extract (ZH) | >250 # | 218.18 ± 8.46 | N.A. |
| G. mangostana extract (GE) | 52.67 ± 0.26 | 17.68 ± 0.38 | 77.21 ± 0.54 |
| C. asiatica extract (CAEw) | 188.83 ± 1.94 | 22.16 ± 0.25 | 1500.29 ± 23.48 |
| Compound D | 69.86 ± 0.75 | 115.32 ± 3.88 | 1368.35 ± 19.65 |
| α-Mangostin | 105.04 ± 1.63 | 11.01 ± 0.31 | 63.61 ± 1.51 |
| Asiaticoside | 289.70 ± 10.93 | 91.64 ± 1.47 | 209.65 ± 2.77 |
| Rutin | 27.13 ± 0.30 | N.D. | N.D. |
| Gallic acid | N.D. | 1.73 ± 0.95 | N.D. |
| 1,10-Phenanthroline monohydrate | N.D. | N.D. | 0.06 ± 0.00 µM |
| Treatments | Amount (µg/mL) | % Cell Viability * | % Increasing of Cell Viability * | ||
|---|---|---|---|---|---|
| ZH | GE | CAEw | |||
| Non-NB-UVB | - | - | - | 100.0 ± 2.7 a | - |
| NB-UVB | - | - | - | 62.3 ± 1.3 b | |
| ZH: GE: CAEw (1:1:1) | 33.3 | 33.3 | 33.3 | 80.1 ± 1.3 c | 28.4 |
| ZH: GE: CAEw (1:2:1) | 25.0 | 50.0 | 25.0 | 75.2 ± 1.9 d | 20.6 |
| ZH: GE: CAEw (10:1:10) | 47.6 | 4.8 | 47.6 | 73.2 ± 0.6 d | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teerapatpaisan, S.; Naladta, A.; Thapphasaraphong, S.; Nualkaew, N. A Polyherbal Formulation That Mitigates Cellular Damage in Narrowband UVB-Irradiated HaCaT Cells. Cosmetics 2025, 12, 241. https://doi.org/10.3390/cosmetics12060241
Teerapatpaisan S, Naladta A, Thapphasaraphong S, Nualkaew N. A Polyherbal Formulation That Mitigates Cellular Damage in Narrowband UVB-Irradiated HaCaT Cells. Cosmetics. 2025; 12(6):241. https://doi.org/10.3390/cosmetics12060241
Chicago/Turabian StyleTeerapatpaisan, Sineenad, Alisa Naladta, Suthasinee Thapphasaraphong, and Natsajee Nualkaew. 2025. "A Polyherbal Formulation That Mitigates Cellular Damage in Narrowband UVB-Irradiated HaCaT Cells" Cosmetics 12, no. 6: 241. https://doi.org/10.3390/cosmetics12060241
APA StyleTeerapatpaisan, S., Naladta, A., Thapphasaraphong, S., & Nualkaew, N. (2025). A Polyherbal Formulation That Mitigates Cellular Damage in Narrowband UVB-Irradiated HaCaT Cells. Cosmetics, 12(6), 241. https://doi.org/10.3390/cosmetics12060241







