Valorization of Date Seed Waste for Sustainable Dermocosmetic Sunscreens: Phytochemical Insights and Formulation Advances
Abstract
1. Introduction
2. Valorization of Date Seed Waste: A Sustainable Resource for Dermocosmetic Innovation
2.1. Phytochemical Composition: Unlocking Bioactivity from Agro-Residues
| Phytochemical Group | Identified Compounds | Extraction Protocol | Biological Function | References |
|---|---|---|---|---|
| Phenolic acids | 3,4-dihydroxybenzoic acid, ferulic acid, p-coumaric acid, caffeic acid, gallic acid | -MAE with acid hydrolysis pretreatment -UAE with NADES | Antioxidant, anti-inflammatory, inhibition of α-amylase, α-glucosidase | [33,47,48] |
| Flavonoids | Epicatechin, rutin, quercetin | -UAE, ultrasound –NADES | Anti-inflammatory, antioxidative defense antimicrobial activity | [47,49] |
| Tocopherols | α-, γ-, δ-Tocopherols | -Soxhlet (hexane, petroleum ether) -Supercritical CO2 Extraction | Antioxidant activity, improve oxidative stability | [50,51] |
| Fatty acids | Oleic, linoleic, palmitic acids, sterols | -Soxhlet (hexane, petroleum ether) -UAE with hexane, combined with hydrothermal pre-treatment, -UAAEE with thermal pretreatment | Cardio-protective role, hypocholesterolemic effect; oxidative and thermal stability | [50,51,52,53] |
| Polysaccharides | Heteropolysaccharides: galacturonic acid, glucose, mannose, fructose, galactose | -MA using DES systems -HWE -UAE -Ultrasonication-assisted green extraction | Antioxidant, antimicrobial, enzyme inhibition, anticancer, and prebiotic potential. | [23,54,55] |
2.2. Circular Bioeconomy and Green Valorization Pathways: From Waste to Wealth
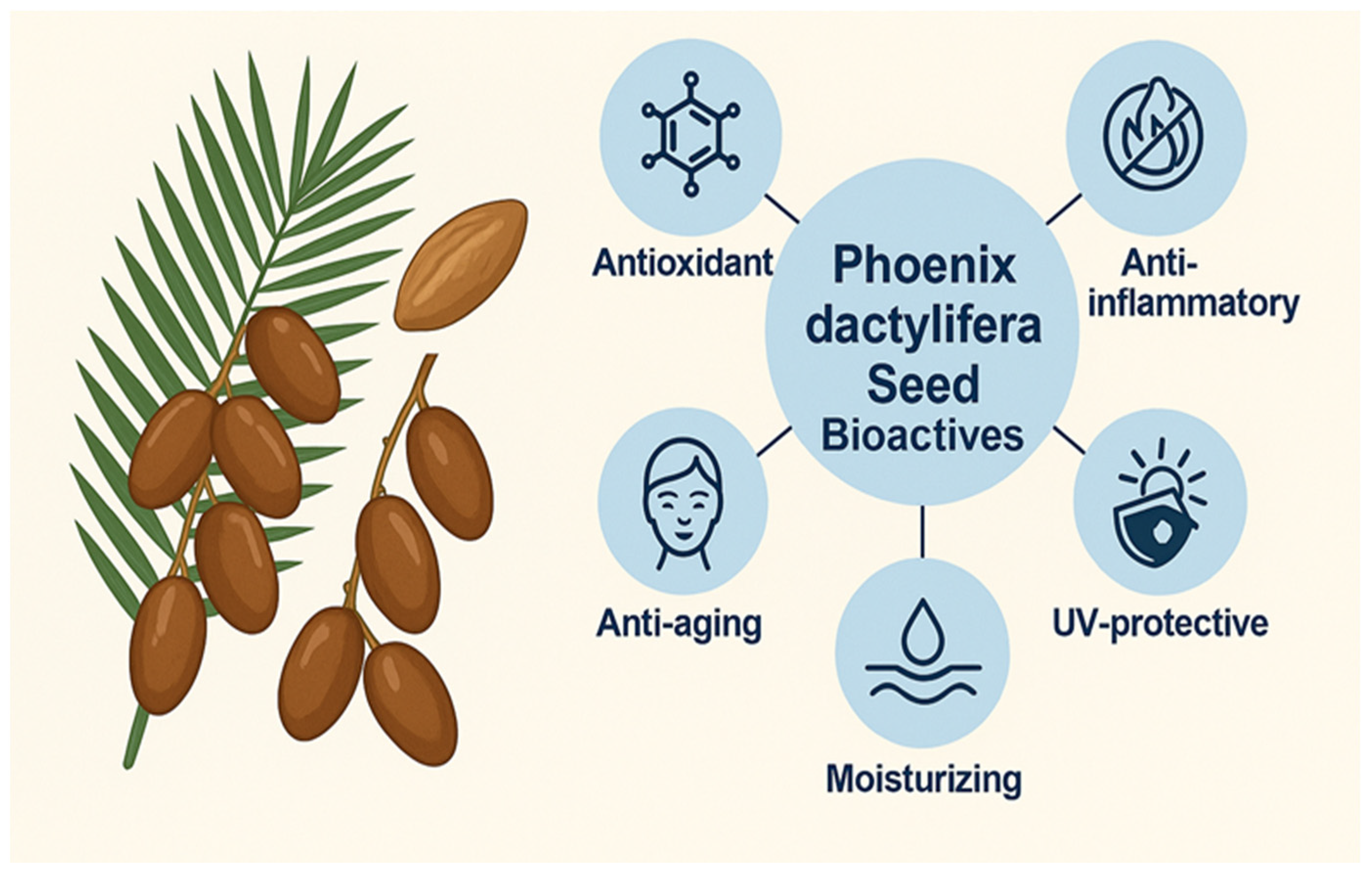
2.3. Pharmacological and Dermatological Potential: Bridging Systemic and Topical Benefits
| Activity | Mechanism | Test Model | Key Findings | References |
|---|---|---|---|---|
| Antioxidant | Free radical scavenging (DPPH, ABTS) | In vitro assays | Superior to Trolox in some aqueous extracts; solvent-dependent performance | [30,65] |
| Anti-inflammatory | Inhibition of protein denaturation, cytokine modulation | In vitro (protein denaturation) | Comparable to ibuprofen; relevant for reducing UV-induced inflammation | [61,62,65] |
| Photoprotective | UV absorption, SPF enhancement, ROS reduction | in vitro models | Enhanced SPF when combined with ZnO/TiO2; potential as natural UV booster | [66] |
| Antimicrobial | Bactericidal activity against skin pathogens | In vitro (E. coli, S. aureus) | Encapsulated extracts exhibited significant antimicrobial efficacy | [65,67,68] |
| Skin Moisturizing | Barrier enhancement, lipid replacement, water retention | Topical formulations with oils and emulsions | Improves hydration and reduces trans-epidermal water loss (TEWL) | [32,69,70] |
| Anti-aging | Collagen preservation, antioxidant protection, wrinkle reduction | In vivo and in vitro models | Comparable to active botanicals like green tea and resveratrol | [15,21] |
| Photostability Support | Protects UV filters and antioxidants from degradation | Nanocarrier formulations (SLNs, cyclodextrins) | Improves SPF longevity and antioxidant retention | [71] |
2.4. Regulatory Considerations, Traditional Usage, and Consumer Acceptance
3. Sunscreen Formulation and Photoprotection Mechanisms
3.1. Reassessing Conventional Sunscreens: Efficacy, Limitations, and Health Concerns
3.2. Botanical Actives and Bio-Based Delivery Platforms
| Delivery/ Formulation | Seed/Fruit Source | Key Bioactives | SPF Value | Bioactivity | References |
|---|---|---|---|---|---|
| Nanoemulgel (benchmark) | Carrot seed | Carotenoids, polyphenols | 20.28 | Anti-aging, wrinkle reduction, pore size control | [11] |
| Green lotion | Green tea & lemon | Polyphenols, flavonoids | 15.0 | Antioxidant, broad-spectrum UVR absorption | [18] |
| Ethyl acetate fraction sunscreen | Padina boergesenii (brown seaweed) | Polyphenols | 20.55 | Stable DPPH activity (54%), UVB-induced cytoprotection | [93] |
| Vegetable oils (commercial) | Carrot, raspberry, rosehip | Unsaturated fatty acids, carotenoids | <2.8 (in vitro) | Low UV absorption; SPF claims not substantiated | [94] |
| SLM sunscreen formulation | Rutin + UVA filter | Flavonoids | Not specified | Enhanced photostability and antioxidant synergy | [95] |
| Chitosan hydrogel sunscreen | Vanillin + DHBA | Polyphenols | Robust UV absorbance | Self-healing, antioxidant, skin-compatible delivery | [96] |
| Extracts, oil, nanocarriers | Grape seed | Catechins, epicatechins, gallic acid, polyphenols | 28.17 | SPF enhancement, photostability, water resistance, antioxidant, anti-aging, improved melanin, erythema, hydration, elasticity | [80,81,82,83,84,85] |
| Emulsions | Pomegranate peel | Ellagic acid, punicalagin, quercetin, rutin | 13.59–50.65 | SPF enhancement, antioxidant, antimicrobial | [86] |
| Extract | Papaya seed | Flavonoids, phenols, alkaloids, saponins, tannins | 12 | SPF, photoprotective | [87] |
| Carbopol emulgel | Nutmeg seed | Polyphenols, essential oil | >8 | SPF, stable physicochemical properties | [88] |
| Gel | Sunflower seed | Unsaturated fatty acids, polyphenols | 15.60 | SPF, good spreadability, viscosity | [89] |
| ZnO-based cream | Bitter melon seed | Alpha-oleo stearic acid, flavonoids, tannins, polyphenols, phytosterols | 24.27 | SPF, improved spreadability, reduced pigmentation/erythema transmission | [90,97] |
| Various formulations | Brazilian native seed oils (pomegranate, grape, prickly pear) | Unsaturated fatty acids, tocopherols, polyphenols | Up to 107.67 (with UV filters) | Synergistic SPF enhancement, UV absorption, antioxidant | [91] |
| Soxhlet extract | Ajwa date fruit | Phenolics, flavonoids | 17.09 | SPF, anti-tyrosinase, anti-collagenase, pigmentation control, elasticity improvement | [92] |
3.3. Advances in Delivery Technologies for Natural Sunscreen Agents
3.4. Beyond UV: Addressing Visible and Infrared Radiation
4. Toxicology, Safety, and Regulatory Aspects of Botanical Sunscreen Actives
4.1. Dermal Toxicity and Cellular Compatibility
4.2. Phototoxicity and Allergenicity of Botanical Ingredients
4.3. Standardization Challenges and Batch Variability
- Analytical validation using high-performance liquid chromatography (HPLC), mass spectrometry (MS), and UV-visible spectroscopy [20];
4.4. Global Regulatory Landscape and SPF Claims
4.5. Environmental Safety and Marine Impact of Sunscreen Ingredients
4.6. Ethical and Cultural Considerations in Botanical Sunscreen Development
5. Future Trends and Commercial Potential of Botanical-Based Sunscreens
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Passeron, T.; Gilaberte, Y.; Granger, C.; Leone, G.; Narda, M.; Schalka, S.; Trullas, C.; Masson, P.; Lim, H.w. Photoprotection of the Future: Challenges and Opportunities. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Moor, J.; Bowman, A.; Choudhary, H.; Brookes, J.; Brieva, P.; Birch-Machin, M.A. Sun Protection Products Protect Against UV-Induced Mitochondrial DNA Damage and Blue Light-Induced Cell Decline in Human Dermal Fibroblast Skin Cell Viability. Cosmetics 2025, 12, 128. [Google Scholar] [CrossRef]
- Abdel Azim, S.; Bainvoll, L.; Vecerek, N.; DeLeo, V.A.; Adler, B.L. Sunscreens Part 1: Mechanisms and Efficacy. J. Am. Acad. Dermatol. 2025, 92, 677–686. [Google Scholar] [CrossRef]
- Ziglar, J.; Mohammad, T.F.; Gilaberte, Y.; Lim, H.W. Sunscreens: Updates on Sunscreen Filters and Formulations. Photodermatol. Photoimmunol. Photomed. 2025, 41, e70026. [Google Scholar] [CrossRef]
- Saxe, J.K.; Dean, S.; Jones, R.L.; Mullins, L.A.; Reynertson, K.A. Development of a Novel Rinse-off Method for Improved Sunscreen Exposure Assessment. Integr. Environ. Assess. Manag. 2021, 17, 961–966. [Google Scholar] [CrossRef]
- Sun, A.; Wang, W.-X. Differentiation of Cellular Responses to Particulate and Soluble Constituents in Sunscreen Products. J. Hazard. Mater. 2024, 474, 134791. [Google Scholar] [CrossRef]
- Aguilera, J.; Vicente-Manzanares, M.; de Gálvez, M.V.; Herrera-Ceballos, E.; Rodríguez-Luna, A.; González, S. Booster Effect of a Natural Extract of Polypodium Leucotomos (Fernblock®) That Improves the UV Barrier Function and Immune Protection Capability of Sunscreen Formulations. Front. Med. 2021, 8, 684665. [Google Scholar] [CrossRef]
- Aswini, T.; Dhanusha, K.; Priya, K.; Shalini, R.; Sumithra, S.; Helen, W.; Aswini, T.; Dhanusha, K.; Priya, K.; Shalini, R.; et al. A Short Review on Natural Components in Sunscreen. World J. Biol. Pharm. Health Sci. 2024, 19, 218–224. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural Sun-Screening Compounds and DNA-Repair Enzymes: Photoprotection and Photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Arianto, A.; Bangun, H.; Lubis, S.; Siregar, C. The use of carrot seed oil (Daucus carota L.) to formulate nanoemulgels as an effective natural sunscreen and skin anti-aging. Int. J. Appl. Pharm. 2022, 14, 124–129. [Google Scholar] [CrossRef]
- Jin, C.; Kim, S.; Moon, S.; Jin, H.; Hahn, J.-S. Efficient Production of Shinorine, a Natural Sunscreen Material, from Glucose and Xylose by Deleting HXK2 Encoding Hexokinase in Saccharomyces Cerevisiae. FEMS Yeast Res. 2021, 21, foab053. [Google Scholar] [CrossRef]
- Luangpraditkun, K.; Kasemkiatsakul, P.; Sangnim, T.; Yammen, S.; Pajoubpong, J.; Vongsak, B. Anti-Senescence and Anti-Photoaging Activities of Mangosteen Pericarp Extract on UVA-Induced Fibroblasts. Cosmetics 2025, 12, 108. [Google Scholar] [CrossRef]
- Hegde, A.R.; Kunder, M.; Narayanaswamy, M.; Murugesan, S.; Furtado, S.; Veerabhadraiah, B.; Srinivasan, B. Advancements in Sunscreen Formulations: Integrating Polyphenolic Nanocarriers and Nanotechnology for Enhanced UV Protection. Environ. Sci. Pollut. Res. 2024, 31, 38061–38082. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.P.; Ribon, R.; Cascais, L.; Facchini, G.; Pinheiro, A.L.T.A.; Eberlin, S.; Maia Campos, P.M.B.G. Benefits of a Multifunctional Sunscreen Formulation Containing Nanoencapsulated Antioxidants in the Skin Protection Against UV Radiation and Blue Light: Clinical and Preclinical Studies. J. Cosmet. Dermatol. 2025, 24, e70282. [Google Scholar] [CrossRef] [PubMed]
- Murillo Vázquez, R.N.; Pacheco Moisés, F.P.; Nardello-Rataj, V.; Arratia-Quijada, J.; Carbajal Arízaga, G.G. Composite with Natural Ingredients and Layered Double Hydroxide Nanoparticles as Antioxidant and Sunscreen Powder Material. Mater. Sci. Eng. B 2023, 297, 116810. [Google Scholar] [CrossRef]
- Bozza, A.; Campi, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Current Regulatory and Market Frameworks in Green Cosmetics: The Role of Certification. Sustain. Chem. Pharm. 2022, 30, 100851. [Google Scholar] [CrossRef]
- Putri, F.R.; Sailah, I. Formulation Natural Ingredients Combination and Consumer Preference Product Sunscreen Lotion. IOP Conf. Ser. Earth Environ. Sci. 2022, 1063, 012008. [Google Scholar] [CrossRef]
- Lotos, E.-D.; Danila, A.; Vasiliu, A.-L.; Rosca, I.; Stroian, D.-V.; Simionescu, B.C.; Mihai, M. The Potential Emulsions of Xanthan Gum and Daucus Carota Macerated Oil in Functional Textiles for Skincare Applications: Formulation, Characterization, and Performance Evaluation. Colloids Surf. Physicochem. Eng. Asp. 2024, 682, 132960. [Google Scholar] [CrossRef]
- Subhash, A.J.; Bamigbade, G.B.; Tarique, M.; Al-Ramadi, B.; Abu-Jdayil, B.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. Bioactive Properties and Gut Microbiota Modulation by Date Seed Polysaccharides Extracted Using Ultrasound-Assisted Deep Eutectic Solvent. Food Chem. X 2024, 22, 101354. [Google Scholar] [CrossRef]
- Alharbi, K.L.; Raman, J.; Shin, H.-J. Date Fruit and Seed in Nutricosmetics. Cosmetics 2021, 8, 59. [Google Scholar] [CrossRef]
- Mohamadizadeh, M.; Dehghan, P.; Azizi-Soleiman, F.; Maleki, P. Effectiveness of Date Seed on Glycemia and Advanced Glycation End-Products in Type 2 Diabetes: A Randomized Placebo-Controlled Trial. Nutr. Diabetes 2024, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, S.C.; Edo, G.I.; Samuel, P.O.; Jikah, A.N.; Oloni, G.O.; Ezekiel, G.O.; Agbo, J.J. The Botanical Details, Pharmacological Activities and Industrial Applications of Date Seed (Phoenix dactylifera L.). Phytochem. Rev. 2025, 24, 927–951. [Google Scholar] [CrossRef]
- Mrabet, A.; Hamdi, A.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Date Seed By-Products as Source of Bioactive Ingredient for Healthy Cookies. Food Biosci. 2024, 61, 104543. [Google Scholar] [CrossRef]
- Jahan, E.; Nupur, A.H.; Majumder, S.; Chandra Das, P.; Aunsary, L.; Aziz, M.G.; Islam, M.A.; Rahman Mazumder, M.A. Physico-Chemical, Textural and Sensory Properties of Breads Enriched with Date Seed Powder. Food Humanity 2023, 1, 165–173. [Google Scholar] [CrossRef]
- Alsaiari, R.A.; Musa, E.M.; Rizk, M.A. Biodiesel Production from Date Seed Oil Using Hydroxyapatite-Derived Catalyst from Waste Camel Bone. Heliyon 2023, 9, e15606. [Google Scholar] [CrossRef]
- Cheng, F.; Feng, J.; Cao, Z.; Duan, Q.; Li, H. Efficacy and Safety of Topical Application of Plant-Based Products on Skin Aging in Healthy Individuals: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Cosmet. Dermatol. 2025, 24, e16710. [Google Scholar] [CrossRef]
- Kim, E.; Seo, H.H.; Shin, D.S.; Song, J.; Yun, S.K.; Lee, J.H.; Moh, S.H. Safety Validation of Plant-Derived Materials for Skin Application. Cosmetics 2025, 12, 153. [Google Scholar] [CrossRef]
- Swaidan, A.; Azakir, B.; Neugart, S.; Kattour, N.; Sokhn, E.S.; Osaili, T.M.; Darra, N.E. Evaluation of the Phenolic Composition and Biological Activities of Six Aqueous Date (Phoenix dactylifera L.) Seed Extracts Originating from Different Countries: A Comparative Analysis. Foods 2024, 13, 126. [Google Scholar] [CrossRef]
- Jayasree Subhash, A.; Babatunde Bamigbade, G.; Al-Ramadi, B.; Kamal-Eldin, A.; Gan, R.-Y.; Senaka Ranadheera, C.; Ayyash, M. Characterizing Date Seed Polysaccharides: A Comprehensive Study on Extraction, Biological Activities, Prebiotic Potential, Gut Microbiota Modulation, and Rheology Using Microwave-Assisted Deep Eutectic Solvent. Food Chem. 2024, 444, 138618. [Google Scholar] [CrossRef]
- Wu, Y.; Qian, Y.; Zhang, A.; Lou, H.; Yang, D.; Qiu, X. Light Color Dihydroxybenzophenone Grafted Lignin with High UVA/UVB Absorbance Ratio for Efficient and Safe Natural Sunscreen. Ind. Eng. Chem. Res. 2020, 59, 17057–17068. [Google Scholar] [CrossRef]
- Lestari, F.D.; Waspodo, N.N.; Nurdin, A.R.; Tabri, F.; Widita, W.; Zainuddin, A.A.; Lestari, F.D.; Waspodo, N.N.; Nurdin, A.R.; Tabri, F.; et al. Improvement in Skin Hydration Status Following 8% Ajwa Date (Phoenix dactylifera L.) Extract Lotion Application: A Clinical Trial on Xerosis Cutis Patients in an Elderly Population. Turk. J. Dermatol. 2024, 18. [Google Scholar] [CrossRef]
- Ranasinghe, M.; Mostafa, H.; Sivapragasam, N.; Stathopoulos, C.; Manikas, I.; Maqsood, S. Sustainable Approach for Defatted Date Seed Valorization through Ultrasonication-Based Green Extraction: A Prospective Approach for Nutraceutical Applications. Sustain. Chem. Pharm. 2023, 35, 101138. [Google Scholar] [CrossRef]
- Roux, J.; Horton, L.; Babadjouni, A.; Kincaid, C.M.; Mesinkovska, N.A. Ferulic Acid Use for Skin Applications: A Systematic Review. J. Clin. Aesthetic Dermatol. 2025, 18, 38–42. [Google Scholar]
- Tabolacci, E.; Tringali, G.; Nobile, V.; Duca, S.; Pizzoferrato, M.; Bottoni, P.; Clementi, M.E. Rutin Protects Fibroblasts from UVA Radiation through Stimulation of Nrf2 Pathway. Antioxidants 2023, 12, 820. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Salabat, A. Microemulsion and Microemulsion Gel Formulation for Transdermal Delivery of Rutin: Optimization, in-Vitro/Ex-Vivo Evaluation and SPF Determination. J. Dispers. Sci. Technol. 2022, 43, 1848–1857. [Google Scholar] [CrossRef]
- Elias, M.L.; Israeli, A.F.; Madan, R. Caffeine in Skincare: Its Role in Skin Cancer, Sun Protection, and Cosmetics. Indian J. Dermatol. 2023, 68, 546–550. [Google Scholar] [CrossRef]
- Terto, M.V.C.; Gomes, J.M.; Araújo, D.I.A.F.; Silva, T.S.; Ferreira, J.M.; Souza, J.J.N.; Silva, M.S.; Tavares, J.F. Photoprotective Activity of Plectranthus Amboinicus Extracts and HPLC Quantification of Rosmarinic Acid. Rev. Bras. Farmacogn. 2020, 30, 183–188. [Google Scholar] [CrossRef]
- Huerta-Madroñal, M.; Caro-León, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vázquez-Lasa, B. Chitosan—Rosmarinic Acid Conjugates with Antioxidant, Anti-Inflammatory and Photoprotective Properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef]
- Touti, R.; Renoux, P.; Nocairi, H.; Douezan, S.; Passeron, T.; Josso, M. Standardized In Vivo Method Using High-Resolution Diffuse Reflectance Spectroscopy for Evaluating Sunscreen Effectiveness Against Ultraviolet A and High-Energy Visible Light. Photodermatol. Photoimmunol. Photomed. 2025, 41, e70044. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Tsim, K.W.K.; Iempridee, T.; Ruktanonchai, U.; Lourith, N. Camelia Seed and Its Functional Properties Promising for Dermatological Applications. Discov. Food 2025, 5, 204. [Google Scholar] [CrossRef]
- Zhang, W.-R.; Zhang, Q.-R.; Zhou, Z.-Y.; Zhang, Y.-F.; Li, X.-W.; Shen, H.-Y.; Tang, L.-F.; Xiang, Q. Oxidative-Inflammatory Modulation of Skin Lipid Metabolism by Squalane, Oleic Acid, and Linoleic Acid. Cosmetics 2025, 12, 130. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Hrdinová, I.; Opálka, L.; Boncheva Bettex, M.; Vávrová, K. Time-Dependent Differences in the Effects of Oleic Acid and Oleyl Alcohol on the Human Skin Barrier. Mol. Pharm. 2023, 20, 6237–6245. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Q.; Zheng, J.; Gu, H.; Chen, K.; Jin, H.; He, C.; Xu, A.-E.; Xu, J.; Zhang, J.; et al. Efficacy and Safety of a Topical Moisturizer Containing Linoleic Acid and Ceramide for Mild-to-Moderate Psoriasis Vulgaris: A Multicenter Randomized Controlled Trial. Dermatol. Ther. 2020, 33, e14263. [Google Scholar] [CrossRef]
- Simard, M.; Tremblay, A.; Morin, S.; Martin, C.; Julien, P.; Fradette, J.; Flamand, N.; Pouliot, R. α-Linolenic Acid and Linoleic Acid Modulate the Lipidome and the Skin Barrier of a Tissue-Engineered Skin Model. Acta Biomater. 2022, 140, 261–274. [Google Scholar] [CrossRef]
- Nip, J.; Ilarslan, H.; Villa, A.; Mihalov, D.; Misra, M.; Samaras, S.D.; Feng, L.; Arcella, S.; Bajor, J.; Mayes, A.E. Topically Applied, Fatty Acid-Containing Formulations Provide Superior Barrier Benefits in an Ex Vivo Tape-Stripped Skin Model. Int. J. Cosmet. Sci. 2024, 46, 506–515. [Google Scholar] [CrossRef]
- Airouyuwa, J.O.; Souka, U.; Maqsood, S. Utilization of Accelerated Solvent Extraction and Deep Eutectic Solvents as Synergistic Green Extraction Technique for the Recovery of Bioactive Compounds from Date Palm (Phoenix dactylifera L.) Seeds. J. Mol. Liq. 2025, 425, 127185. [Google Scholar] [CrossRef]
- Mostafa, H.; Hamdi, M.; Airouyuwa, J.O.; Maqsood, S. Efficient Valorization of Date Fruit Processing By-Product through Nano- and Green-Extraction Technology: A Response Surface Methodology-Based Optimization Study. Biomass Convers. Biorefinery 2024, 14, 12857–12875. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Screening Factors to Affect Ultrasound-Assisted Extraction of (Poly)Phenols from Date Palm Seeds. Front. Chem. 2024, 12, 1409393. [Google Scholar] [CrossRef]
- Halabi, Y.; Nasri, C.; Guezzane, C.E.; Harhar, H.; Gharby, S.; Bellaouchou, A.; Warad, I.; Abdelkader, Z.; Tabyaoui, M. date palm Phoenix dactilifera L. seed oil: Variety effects on physicochemical characteristics, fatty acid composition, sterol and tocol contents. J. Microbiol. Biotechnol. Food Sci. 2023, 12, e5725. [Google Scholar] [CrossRef]
- Al-Hussain, M.A.; Ali, D.; Asiri, S.; Khalid Alqahtani, N.; Makki, H.; Mohamed, H.A.; Ali, S.; Al-Senaien, W.A. Evaluation of the Fatty Acid Profiles, Antioxidant Activities, and Total Phenol and Vitamin E Contents of Three Types of Saudi Date Seed Oils. Food Res. 2025, 9, 87–92. [Google Scholar] [CrossRef]
- Amigh, S.; Taghian Dinani, S. Combination of Ultrasound-Assisted Aqueous Enzymatic Extraction and Cooking Pretreatment for Date Seed Oil Recovery. Heat Mass Transf. 2020, 56, 2345–2354. [Google Scholar] [CrossRef]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Sindic, M.; Jiménez-Araujo, A. Optimization of Date Seed Oil Extraction Using the Assistance of Hydrothermal and Ultrasound Technologies. Grasas Aceites 2022, 73, e457. [Google Scholar] [CrossRef]
- Dhahri, M.; Sioud, S.; Alsuhaymi, S.; Almulhim, F.; Haneef, A.; Saoudi, A.; Jaremko, M.; Emwas, A.-H.M. Extraction, Characterization, and Antioxidant Activity of Polysaccharides from Ajwa Seed and Flesh. Separations 2023, 10, 103. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Subhash, A.J.; Tarique, M.; al-Ramadi, B.; Abu-Jdayil, B.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. Date Pomace Polysaccharide: Ultrasonic-Assisted Deep Eutectic Solvent Extraction, Physicochemical Properties, Biological Activities, Gut Microbiota Modulation, and Rheological Properties. Chem. Biol. Technol. Agric. 2024, 11, 79. [Google Scholar] [CrossRef]
- Castagna, A.; Aboudia, A.; Guendouz, A.; Scieuzo, C.; Falabella, P.; Matthes, J.; Schmid, M.; Drissner, D.; Allais, F.; Chadni, M.; et al. Transforming Agricultural Waste from Mediterranean Fruits into Renewable Materials and Products with a Circular and Digital Approach. Materials 2025, 18, 1464. [Google Scholar] [CrossRef]
- Kiesler, R.; Franke, H.; Lachenmeier, D.W. A Comprehensive Review of the Nutritional Composition and Toxicological Profile of Date Seed Coffee (Phoenix dactylifera). Appl. Sci. 2024, 14, 2346. [Google Scholar] [CrossRef]
- Mihajlović, I.; Hgeig, A.; Novaković, M.; Gvoić, V.; Ubavin, D.; Petrović, M.; Kurniawan, T.A. Valorizing Date Seeds into Biochar for Pesticide Removal: A Sustainable Approach to Agro-Waste-Based Wastewater Treatment. Sustainability 2025, 17, 5129. [Google Scholar] [CrossRef]
- Faiad, A.; Alsmari, M.; Ahmed, M.M.Z.; Bouazizi, M.L.; Alzahrani, B.; Alrobei, H. Date Palm Tree Waste Recycling: Treatment and Processing for Potential Engineering Applications. Sustainability 2022, 14, 1134. [Google Scholar] [CrossRef]
- Dbeibia, A.; Khiari, R.; Mihoubi, D.; Zeghonda, N.; Boudhrioua, N. Drying Kinetics of Deglet Enour Date Seeds, Antioxidant, Antibacterial, Antidiabetic and Bio-Preservative Potencies. Waste Biomass Valorization 2025, 16, 4381–4397. [Google Scholar] [CrossRef]
- Saryono; Warsinah; Isworo, A.; Sarmoko. Anti-Inflammatory Activity of Date Palm Seed by Downregulating Interleukin-1β, TGF-β, Cyclooxygenase-1 and -2: A Study among Middle Age Women. Saudi Pharm. J. 2020, 28, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, Z.A.; Abdelazeem, S.; Abdel Jaleel, G.A.; Ali, M.E.; Mohamed, A.; Salah, A.; Raslan, M.A. Anti-Inflammatory and Anti-Rheumatic Effects of Phoenix dactylifera L. (Date Palm) Seed by Controlling Cytokines and Inhibiting JAK1/STAT3 Pathway on CFA-Induced Arthritis Rat and Its Phytochemical Profiling. J. Ethnopharmacol. 2024, 329, 118138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-R.; Aldosari, S.A.; Vidyasagar, P.S.P.V.; Nair, K.M.; Nair, M.G. Antioxidant and Anti-Inflammatory Assays Confirm Bioactive Compounds in Ajwa Date Fruit. J. Agric. Food Chem. 2013, 61, 5834–5840. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Wang, S.-S.; Tsai, T.-C.; Ko, W.-P.; Chang, T.-M. Phoenix dactylifera L. Seed Extract Exhibits Antioxidant Effects and Attenuates Melanogenesis in B16F10 Murine Melanoma Cells by Downregulating PKA Signaling. Antioxidants 2020, 9, 1270. [Google Scholar] [CrossRef]
- Cherfi, I.; Benaissa, A.; Mahboub, N.; Benmoussa, M.T.; Ahmadi, I.; Dif, B.; Djaber, I. Phytochemical Analysis and Bioactivity Evaluation of Phoenix dactylifera L. Ethanolic Extract and Cosmetic Cream: Antibacterial, Antioxidant, and Anti-Inflammatory Properties. Biomed. Chromatogr. BMC 2025, 39, e70199. [Google Scholar] [CrossRef]
- Warsinah; Ekowati, H.; Baroroh, H.N. The Phytochemical Screening, Total Phenolic and Photoprotective Potential of Date Palm Seeds (Phoenix dactylifera L.). Int. J. Sci. Res. Updat. 2022, 4, 143–149. [Google Scholar] [CrossRef]
- Farousha, K.; Rangaraj, V.M.; K, R.; Abu Haija, M.; Banat, F. Development of Date Seed Extract Encapsulated MCM-41: Characterization, Release Kinetics, Antioxidant and Antibacterial Studies. Food Biosci. 2023, 53, 102563. [Google Scholar] [CrossRef]
- Bhaskaracharya, R.K.; Bhaskaracharya, A.; Stathopoulos, C. A Systematic Review of Antibacterial Activity of Polyphenolic Extract from Date Palm (Phoenix dactylifera L.) Kernel. Front. Pharmacol. 2023, 13, 1043548. [Google Scholar] [CrossRef]
- Lestari, U.; Farid, F.; Yuliawati, Y.; Pratama, S. Irritation Test and Effectiveness of Facial Humidity Skin from Peel Off Gel Mask Based of Date Palm Seeds Powder (Phoenix dactylifera). J. Bio-Geo Mater. Dan Energi 2021, 1, 1–5. [Google Scholar] [CrossRef]
- Tijare, R.B.; Wargantiwar, S.S.; George, S.; Shastri, D.R.; Watkar, M.R.; Bodhankar, M.M.; Bobad, D.K. Formulation of Tinted Lip Gloss from Lotus Flower and Date Seed Oil. Magna Sci. Adv. Biol. Pharm. 2022, 6, 001–010. [Google Scholar] [CrossRef]
- Dahabra, L.; Broadberry, G.; Le Gresley, A.; Najlah, M.; Khoder, M. Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention. Molecules 2021, 26, 1698. [Google Scholar] [CrossRef]
- Young, A.R. The Adverse Consequences of Not Using Sunscreens. Int. J. Cosmet. Sci. 2023, 45, 11–19. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Douki, T.; Sarkany, R.P.E.; Acker, S.; Herzog, B.; Young, A.R. Improved Photoprotection in the UVA/Visible Radiation Boundary Region Is Essential to Prevent DNA Damage, Oxidative Stress and Gene Expression Changes in Human Skin. JEADV Clin. Pract. 2025, 4, 440–450. [Google Scholar] [CrossRef]
- Passeron, T.; Lim, H.W.; Goh, C.-L.; Kang, H.Y.; Ly, F.; Morita, A.; Ocampo Candiani, J.; Puig, S.; Schalka, S.; Wei, L.; et al. Photoprotection According to Skin Phototype and Dermatoses: Practical Recommendations from an Expert Panel. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Mickoś, E.; Michalak, M.; Hartman-Petrycka, M.; Banyś, A.; Babczyńska, P.; Koprowski, R.; Wilczyński, S. Assessing Skin Photoprotection in the Infrared Range: The Reflectance Profiles of Cold-Pressed Plant Oils. Cosmetics 2025, 12, 80. [Google Scholar] [CrossRef]
- Breakell, T.; Kowalski, I.; Foerster, Y.; Kramer, R.; Erdmann, M.; Berking, C.; Heppt, M.V. Ultraviolet Filters: Dissecting Current Facts and Myths. J. Clin. Med. 2024, 13, 2986. [Google Scholar] [CrossRef]
- Stolecka, A.; Mielczarek, P.; Koziarska, M.; Gruszecka-Kosowska, A. Organic Ultraviolet Filters (OUVF) in Freshwater Bathing Areas: Necessary Sunscreen Protection versus Environmental Threat. Water Res. 2025, 279, 123423. [Google Scholar] [CrossRef]
- Saxe, J.K.; Mullins, L.; Jones, R.; Lewis, A., Jr.; Sun, F.; Reynertson, K.A. Influence of Sunscreen Formulation on the Transfer of Mineral and Organic Ultraviolet Filters from Skin to Seawater in Simulated Ocean Bathing Tests. Int. J. Cosmet. Sci. 2023, 45, 84–92. [Google Scholar] [CrossRef]
- Carrao, A.M.; Schmitt, C.N.; Dyer, S.D. Repurposing Consumer Sunscreen Habits and Practices Survey Data to Guide the Development of UV Filter Environmental Exposure Models and Risk Assessments. Int. J. Cosmet. Sci. 2023, 45, 93–100. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Effect of Grape Seed Extract on Skin Fibroblasts Exposed to UVA Light and Its Photostability in Sunscreen Formulation. J. Cosmet. Dermatol. 2021, 20, 1271–1282. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Chemical and Mechanical Accelerated and Long-Term Stability Evaluation of Sunscreen Formulation Containing Grape Seed Extract. J. Cosmet. Dermatol. 2022, 21, 6400–6413. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Clinical Study of Asian Skin Changes after Application of a Sunscreen Formulation Containing Grape Seed Extract. J. Cosmet. Dermatol. 2022, 21, 4523–4535. [Google Scholar] [CrossRef]
- Salem, Y.; Sunoqrot, S.; Rajha, H.N.; Abusulieh, S.; Afif, C.; Francis, H.; Touma, J.A.; Louka, N.; Maroun, R.G. Grape Seed Phenolic Extracts Encapsulation in Polymeric Nanoparticles: Characterization and in Vitro Evaluation against Skin Melanoma. J. Drug Deliv. Sci. Technol. 2024, 100, 106094. [Google Scholar] [CrossRef]
- de Souza Sanches, P.; de Oliveira Peixoto, L.M.; dos Santos, T.C.; da Silva, C.Q.; Brito, G.B.; da Silva Flores, Y.; Santos, N.F.; de Araújo, E.M.; Mourão, S.C.; Falcão, D.Q. Influence of Grape Seed Oil on Sun Protection Factor in Sunscreen Formulations: A Study Using Central Composite Design Approach. Drug Anal. Res. 2022, 6, 40–45. [Google Scholar] [CrossRef]
- Francisco, K.M.; Allam, S.T.; Caldona, A.K.; De Jesus, M.D.; Lubaton, N.; Sandoval, S.; Baylon, I.; Tugade, C. Effectiveness of Using Hydrophobic Silica Aerogel and Grape Seed Extract in Creating a Sunscreen Formula. IOP Conf. Ser. Earth Environ. Sci. 2024, 1372, 012077. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Ivanov, I.; Vrancheva, R.; Mazova, N.; Nikolova, K. Pomegranate Peels: A Promising Source of Biologically Active Compounds with Potential Application in Cosmetic Products. Cosmetics 2025, 12, 169. [Google Scholar] [CrossRef]
- Nursanto, E.B.; Wijaya, R.; Sari, A.P. Flavonoid Extraction From Papaya (Carica Papaya L.) Seed Using Ultrasound—Assisted Extraction Method and Determination of Its SPF Value. J. Emerg. Supply Chain Clean Energy Process Eng. 2023, 2, 75–84. [Google Scholar] [CrossRef]
- Khafifa, I.N.; Shabrina, A.; Rochman, M.F. Stability and sunscreen activity of nutmeg seed oil emulgel with carbopol 940 variation as gel base. J. Farm. Sains Dan Prakt. 2022, 8, 145–154. [Google Scholar] [CrossRef]
- Zulkarnain, A.K.; Faridhotu, F.; Pr, I.N. Optimization of Gelling Agent of Sunflower (Helianthus Annuus) Seed Oil Gel and Its Stability and Activity Test In Vitro as Sunscreen. Maj. Obat Tradis. 2022, 27, 247–256. [Google Scholar] [CrossRef]
- Winarti, L.; Dewinta, A.F.; Rosyidi, V.A. The Effect of Additional Bitter Gourd Seed Oil on the Effectiveness of Zinc Oxide Sunscreen Cream. Pharm. Educ. 2024, 24, 77–82. [Google Scholar] [CrossRef]
- Pereira, M.D.; Mota, M.D.; de Paula Pereira, N. Photoprotective Efficacy of Seed Oils from Fruits of Brazilian Flora: A Scoping Review. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2025, 24, 1195–1215. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.; Abd Gani, S.S.; Effendi Halmi, M.I.; Zaidan, U.H. Evaluation of Anti-Tyrosinase, Anti-Collagenase, and In Vitro Sun Protection Factor (SPF) of Ajwa Date Fruit (Phoenix dactylifera L.). Pertanika J. Trop. Agric. Sci. 2024, 47, 707–719. [Google Scholar] [CrossRef]
- Soleimani, S.; Yousefzadi, M.; Babaei Mahani Nezhad, S.; Pozharitskaya, O.N.; Shikov, A.N. Potential of the Ethyl Acetate Fraction of Padina Boergesenii as a Natural UV Filter in Sunscreen Cream Formulation. Life 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Ácsová, A.; Hojerová, J.; Janotková, L.; Bendová, H.; Jedličková, L.; Hamranová, V.; Martiniaková, S. The Real UVB Photoprotective Efficacy of Vegetable Oils: In Vitro and in Vivo Studies. Photochem. Photobiol. Sci. 2021, 20, 139–151. [Google Scholar] [CrossRef]
- Martins, R.M.; de Siqueira Martins, S.; Barbosa, G.L.F.; das Neves e Silva, E.G.; Fonseca, M.J.V.; de Freitas, L.A.P. Natural Component and Solid Lipid Microparticles of Solar Filter in Sunscreen: Photoprotective and Photostability Effect Enhancement. J. Drug Deliv. Sci. Technol. 2023, 88, 104860. [Google Scholar] [CrossRef]
- Heydari, N.; Karimi, A.R.; Momeni, H.R.; Azadikhah, F.; Etemadi, T. Chitosan Schiff-Base Hydrogel Sunscreen: A Multifunctional Hybrid Network with Antioxidant, Ultraviolet-Shielding, and Self-Healing Properties. ACS Omega 2025, 10, 8250–8261. [Google Scholar] [CrossRef]
- Winarti, L.; Refayani, E.P. Use of Bitter Melon Seed Oil (Momordica Charantia) to Improve the Photoprotective Effect of Sunscreen Formulations. Pharmaciana 2024, 14, 346–355. [Google Scholar] [CrossRef]
- Bordes, C.; Bolzinger, M.-A.; El Achak, M.; Pirot, F.; Arquier, D.; Agusti, G.; Chevalier, Y. Formulation of Pickering Emulsions for the Development of Surfactant-Free Sunscreen Creams. Int. J. Cosmet. Sci. 2021, 43, 432–445. [Google Scholar] [CrossRef]
- Ezekwe, N.; Maghfour, J.; Kohli, I. Visible Light and the Skin. Photochem. Photobiol. 2022, 98, 1264–1269. [Google Scholar] [CrossRef]
- Deravi, L.F.; Cui, I.; Martin, C.A. Using Cephalopod-Inspired Chemistry to Extend Long-Wavelength Ultraviolet and Visible Light Protection of Mineral Sunscreens. Int. J. Cosmet. Sci. 2024, 46, 941–948. [Google Scholar] [CrossRef]
- Nunes, K.C.; Alves, B.L.; dos Santos, R.S.; de Araújo, L.A.; Bergamasco, R.; Bruschi, M.L.; Ueda-Nakamura, T.; Lautenschlager, S.d.O.S.; Nakamura, C.V. Exploring Skin Biometrics, Sensory Profiles, and Rheology of Two Photoprotective Formulations with Natural Extracts: A Commercial Product Versus a Vegan Test Formulation. Cosmetics 2025, 12, 112. [Google Scholar] [CrossRef]
- Mansuri, R.; Diwan, A.; Kumar, H.; Dangwal, K.; Yadav, D. Potential of Natural Compounds as Sunscreen Agents. Pharmacogn. Rev. 2021, 15, 47–56. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/homepage (accessed on 26 July 2025).
- Regulation—1223/2009—EN—Cosmetic Products Regulation—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2009/1223/oj/eng (accessed on 26 July 2025).
- WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants. Available online: https://www.who.int/publications/i/item/9241546271 (accessed on 30 August 2025).
- ISO 16128-1:2016; Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients and Products—Part 1: Definitions for Ingredients. ISO: Geneva, Switzerland, 2016. Available online: https://standards.iteh.ai/catalog/standards/iso/d618bccf-c844-441e-8b89-21cb0a7b3f34/iso-16128-1-2016 (accessed on 30 August 2025).
- International Organization for Standardization. Determination of Sunscreen UVA Photoprotection in Vitro; International Organization for Standardization: Geneva, Switzerland, 2021. [Google Scholar]
- International Organization for Standardization. Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF); International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- International Organization for Standardization, I. Cosmetics—Sun Protection Test Methods—Hybrid Diffuse Reflectance Method; International Organization for Standardization: Geneva, Switzerland, 2024. [Google Scholar]
- International Organization for Standardization. Cosmetics—Sun Protection Test Methods—In Vitro Spectral Absorbance-Based Method for Sun Protection Factor (SPF) Determination; International Organization for Standardization: Geneva, Switzerland, 2024. [Google Scholar]
- Pissavini, M.; Pouradier, F.; Lapalud, P.; Batzer, J.; Contier, M.; Matts, P. The Double Plate In Vitro SPF Test Method (ISO23675:2024) Is a Reliable Means of Measuring the Performance of Sunscreen Products with High Concentrations of Inorganic-Only UV Filters. Photochem. Photobiol. Sci. 2025. under review. [Google Scholar] [CrossRef]
- Ince, F.A.; Nayar, J.C.; Wong, A.L.; McClelland, J.A.; Holland, A.J.A.; Stern, H.; Abboud, M.; Mason, R.S.; Dixon, K.M. Uncovering the Efficacy of a Natural Homemade Sunscreen in Protection from Ultraviolet Radiation. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2025, 24, 1017–1029. [Google Scholar] [CrossRef]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant Polyphenols as Green Sunscreen Ingredients: A Systematic Review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef]
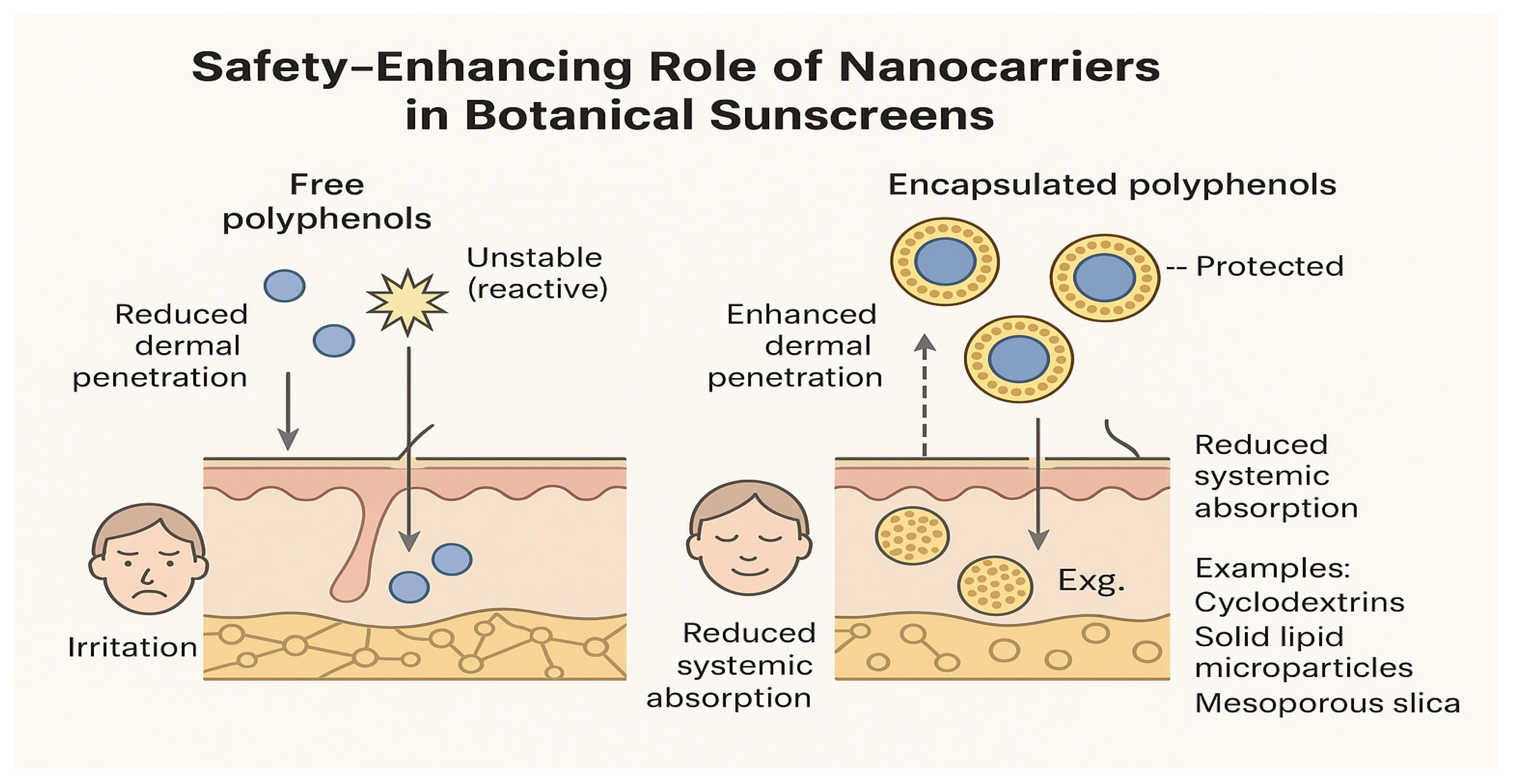

| Formulation Type | Carrier or Approach | Functional Benefit | Application Model | Ref. |
|---|---|---|---|---|
| Nanoencapsulation | MCM-41 mesoporous silica | Enhances antioxidant release; bactericidal effects; pH-responsive delivery | Biomedical and cosmetic | [67] |
| Solid Lipid Microparticles (SLMs) | Rutin co-loaded with UVA filter | Improves photostability and photoprotection of actives | Sunscreen formulations | [95] |
| Cyclodextrin Complexation | Inclusion with polyphenols or UV filters | Stabilizes actives, improves solubility, and enhances dermal delivery | Antioxidant sunscreens | [71] |
| Pickering Emulsions | ZnO and silica-coated TiO2 nanoparticles | surfactant-free stability; UV filtering and structuring agent | Oil-in-water natural sunscreen prototype | [98] |
| Nanoemulgel Systems | Natural oils (e.g., carrot, hibiscus, date seed) | Enhances SPF, reduces wrinkles, boosts absorption and spreadability | Topical cosmetic sunscreens | [11] |
| Hydrogel Networks | Chitosan + Vanillin + DHBA | UVA/UVB absorption; antioxidant synergy; self-healing and biocompatible delivery | Advanced photoprotective formulations | [96] |
| Textile Carriers | Emulsion-soaked fabrics (e.g., Daucus carota oil) | Occlusive barrier effect; prolonged skin hydration and antioxidant delivery | Transdermal textile delivery in skincare | [19] |
| Extraction Method | Key Solvent | Major Phytochemicals Identified | Antioxidant Activity | Estimated SPF Potential | Ref. |
|---|---|---|---|---|---|
| Aqueous Extract | Water | Caffeic acid, ferulic acid, gallic acid, epicatechin | High DPPH inhibition (70–85%), moderate FRAP | Low (SPF < 5) | [20,22] |
| Ethanolic Extract | 70% Ethanol | Epicatechin, rutin, quercetin, vanillic acid | Very high DPPH (90%+), strong FRAP and ABTS | Moderate (SPF 6–10) | [30,67,92] |
| Oil Extract | Cold-pressed | Tocopherols, unsaturated fatty acids, phytosterols | Moderate antioxidant (low polyphenol) | Low (SPF < 3) but enhances lipid barrier | [20,67] |
| Methanolic Extract | Methanol | Syringic acid, catechin, chlorogenic acid | High DPPH and FRAP, better phenolic yield | Moderate (SPF ~6) | [22] |
| Parameter | Synthetic UV Filters (e.g., Oxybenzone, Octinoxate) | Botanical Actives (e.g., Phoenix dactylifera L. Seed Oil/Extract) |
|---|---|---|
| Phototoxicity/Allergenicity | High potential for photoreactivity and allergic reactions | Low; generally well-tolerated with antioxidant and anti-inflammatory properties |
| Systemic Absorption | Documented systemic absorption and endocrine disruption risk | Minimal transdermal absorption; negligible systemic toxicity |
| Marine Impact | Contributes to coral bleaching, bioaccumulation | Biodegradable; low to no known aquatic toxicity |
| Regulatory Status | FDA-approved (monograph) ingredients; increasingly restricted | Not monograph-listed; requires safety and efficacy substantiation |
| Biocompatibility | May impair mitochondrial and lysosomal function in skin cells | Supports skin barrier function; reduces oxidative stress |
| Ethical Concerns | Some derived from petrochemicals, animal testing common | Vegan, halal, and cruelty-free compliant |
| Cultural/Religious Acceptability | May conflict with halal or vegan standards | Acceptable across major cultural and religious contexts |
| Sustainability | Non-renewable, synthetic origin | Derived from agri-food byproducts; promotes circular bioeconomy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siroukane, N.; Kheniche, A.; Souiki, L. Valorization of Date Seed Waste for Sustainable Dermocosmetic Sunscreens: Phytochemical Insights and Formulation Advances. Cosmetics 2025, 12, 225. https://doi.org/10.3390/cosmetics12050225
Siroukane N, Kheniche A, Souiki L. Valorization of Date Seed Waste for Sustainable Dermocosmetic Sunscreens: Phytochemical Insights and Formulation Advances. Cosmetics. 2025; 12(5):225. https://doi.org/10.3390/cosmetics12050225
Chicago/Turabian StyleSiroukane, Nassima, Abdelhakim Kheniche, and Lynda Souiki. 2025. "Valorization of Date Seed Waste for Sustainable Dermocosmetic Sunscreens: Phytochemical Insights and Formulation Advances" Cosmetics 12, no. 5: 225. https://doi.org/10.3390/cosmetics12050225
APA StyleSiroukane, N., Kheniche, A., & Souiki, L. (2025). Valorization of Date Seed Waste for Sustainable Dermocosmetic Sunscreens: Phytochemical Insights and Formulation Advances. Cosmetics, 12(5), 225. https://doi.org/10.3390/cosmetics12050225







