1. Introduction
Wound healing is a complex, dynamic, and synchronized process involving a multitude of cells, cytokines, and growth factors. This complex biological cascade progresses through distinct yet overlapping phases: hemostasis, inflammation, proliferation, and remodeling. Throughout, cells cooperate and communicate through sophisticated signaling pathways to achieve rapid and effective repair of damaged tissue and restore skin integrity [
1]. The delicate balance of these interactions determines the quality of the healing outcome, influencing both functional recovery and aesthetic appearance [
2]. Optimal wound healing results in complete restoration of tissue structure and function without visible scarring. However, dysregulation of the healing process frequently leads to scar formation due to imbalances between cellular proliferation, extracellular matrix production, and tissue remodeling. These healing impairments manifest as various complications (aesthetic concerns, persistent itching, hyperpigmentation) often resulting in significant psychological distress and social challenges for affected individuals. Multiple factors influence healing outcomes, including wound depth, anatomical location, presence of infection, and genetic predisposition. Notably, ethnicity and skin pigmentation play crucial roles in determining scarring tendencies.
Clinically, cutaneous scars are classified into three primary categories: hypertrophic scars, keloids, and atrophic scars. Hypertrophic scars present as raised, thickened, and inflamed tissue confined to the original wound boundaries [
3]. Risk factors for their development include younger age, atopic predisposition, wound infection, and areas of high skin tension [
4]. Individuals with darker skin phototypes demonstrate significantly higher susceptibility to hypertrophic scarring. Epidemiological data reveals disparities: hypertrophic scarring affects approximately 16% of Black individuals, and the prevalence ratio between darker and lighter skin tones ranges from 5:1 to 15:1 [
4]. Keloids represent a more severe scarring variant, distinguished by their characteristic growth beyond the initial wound margins, invading surrounding healthy tissue [
3]. Conversely, atrophic scars appear as depressions below the surrounding skin surface, commonly resulting from conditions like acne or chicken pox. These concave scars develop when dermal tissue loss occurs, and insufficient collagen is produced during the healing process [
5].
Among all scar types, acne scars are among the most prevalent worldwide, with studies suggesting that moderate to severe acne scarring affects approximately 20% of those who experience acne [
6,
7]. Given acne’s high global prevalence, this represents a significant clinical challenge. Current therapeutic approaches for scar management can be classified into two primary categories: energy-based and non-energy-based [
8]. Energy-based technologies encompass ablative and non-ablative laser systems, fractional radiofrequency devices, intense pulsed light therapy, and plasma skin regeneration techniques. Those work by delivering controlled thermal energy to targeted tissue depths, stimulating collagen remodeling and epidermal renewal [
9]. Non-energy-based interventions include mechanical treatments such as subcision (to release tethered scars), microdermabrasion and dermabrasion (for surface irregularities), and microneedling (to induce controlled dermal injury). Chemical approaches like specialized peels and injectable treatments including dermal fillers further expand the therapeutic arsenal [
10]. Most treatments require multiple sessions, substantial financial investment from patients, and are often associated with considerable discomfort, downtime, and potential adverse effects such as post-inflammatory hyperpigmentation, particularly in darker skin types.
Several studies have shown that visible scarring is often associated with high levels of anxiety, depression, and associated social behaviors, with up to 87% reporting negative effects on their self-esteem [
11]. This psychological burden is more pronounced in individuals with darker skin phototypes who have both more severe scarring and greater pigmentary changes [
12]. Thus, in this study, we investigate a natural ingredient from Australia, Jellybush honey extract, for its potential to improve skin wound healing outcomes for diverse skin phototypes.
Jellybush honey is produced by bees collecting nectar from the
Leptospermum polygalifolium plant. This honey possesses numerous documented medicinal properties, including antibiotic, anti-inflammatory, and antiseptic activities. Its rich phytochemical profile, which compares favorably to New Zealand’s manuka honey [
13], positions it as a promising cosmetic active ingredient for reducing post-acne scar appearance in all skin types, including darker skin tones, as demonstrated in our clinical investigations, showing uniformized skin appearance within 8 weeks of use. To elucidate the mechanism of action of Jellybush honey extract, we assessed the expression of a broad spectrum of genes at 1, 3, and 6 days following manual injury in human skin explants. This controlled study compared gene expression profiles between injured untreated skin explants and explants treated topically with Jellybush honey extract, allowing us to identify the molecular pathways modulated by this natural ingredient during the wound healing process.
2. Materials and Methods
2.1. Jellybush Honey Extract
The studies described herein employed an aqueous extract of monofloral Australian Jellybush honey produced by Western honey bees (
Apis mellifera) foraging on coastal tea tree
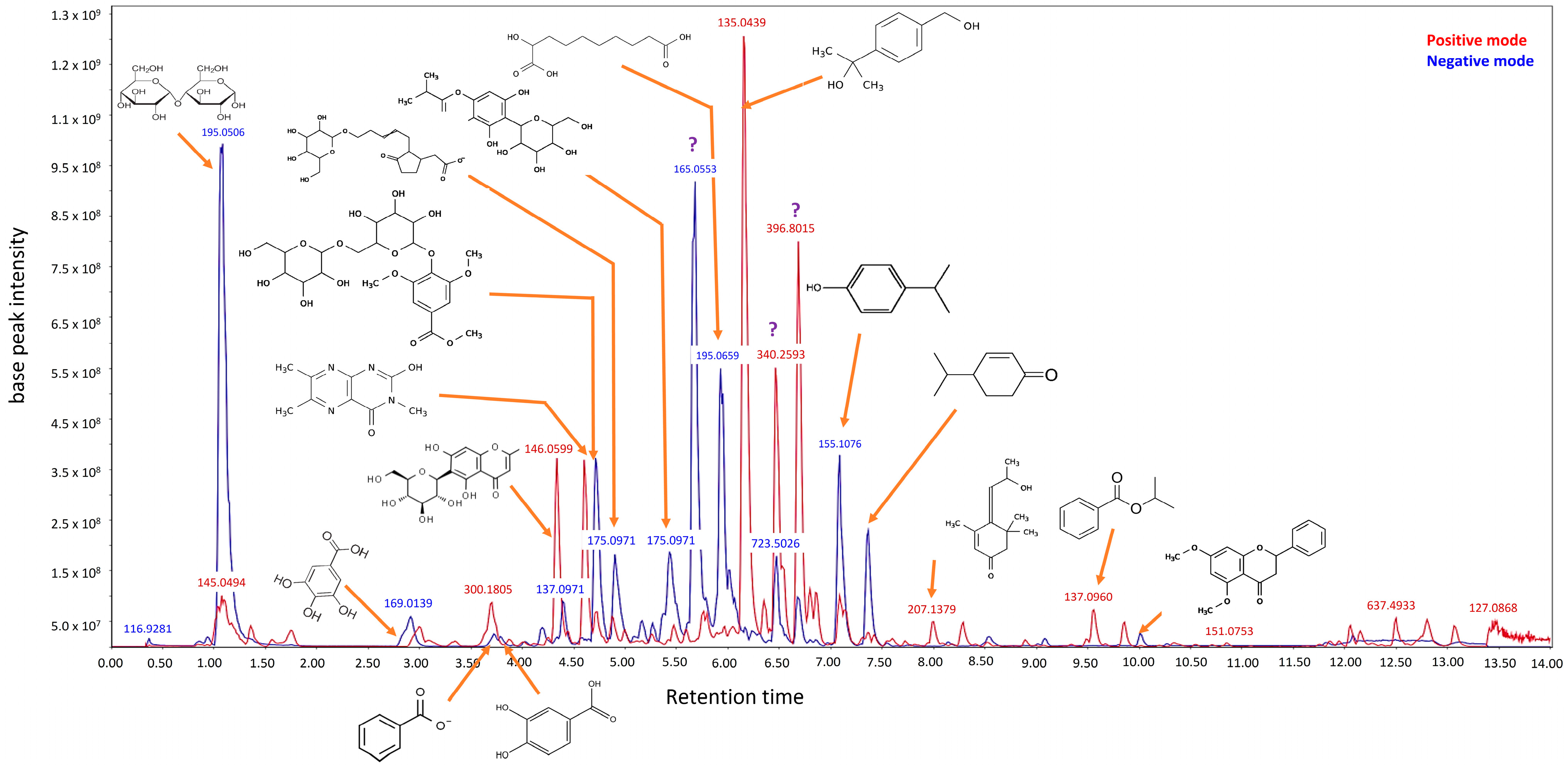
Leptospermum polygalifolium. The Jellybush honey was extracted in water at 95 °C for 10 min. We performed a critical separation step where the diluent was separated from solid components (including particulates and waxes) prior to incorporation into the glycerin excipient. These purification steps altered the composition of the original honey, justifying the designation of our ingredient as ‘Jellybush honey extract.’ Our ingredient was rich in several bioactive compounds, including leptosperin, which serves as the quality control marker, representing approximately 21 ppm as evaluated by HPLC (High Performance Liquid Chromatography) according to methods described by Brooks et al. [
14]. Furthermore, metabolomic studies using LC-HRMS/MS methods (Luna Omega Polar C18 column, Phenomenex, France) were performed on the ethanol fraction obtained through SPE (solid phase extraction) fractionation (
Scheme 1). This process eliminated major sugars and highly polar compounds, allowing us to concentrate on secondary metabolites. Our analysis revealed that the ingredient contains organic and phenolic acids and their derivatives, monoterpenoids, and carbohydrates.
2.2. Cell Cultures
Normal human dermal fibroblasts (NHDF) used in this study were isolated from healthy female donors aged 20 to 35 years undergoing plastic surgery, with the donors’ informed consent. NHDF were maintained in specific medium, named DMEM (Dulbecco Modified Eagle Medium, Sigma-Aldrich, Lyon, France, D5523) supplemented with 10% fetal bovin serum (FBS, Sigma-Aldrich, F7524) and 1% antibiotics (penicillin/streptomycin, Sigma-Aldrich, Lyon, France, P0781), at 37 °C under 5% CO2 and 95% humidity.
2.3. Inflammatory Activity
NHDF cells were seeded at 2 × 104 cells per well in 96-well plates in DMEM supplemented with 10% FBS (Sigma-Aldrich, Saint Quentin Fallavier, France, F7524). After 24 h, the medium was removed, and cells were treated with interleukin-1alpha (IL-1α) (0.002 ng/mL, Sigma Aldrich, Saint Quentin Fallavier, France, I2778) to induce interleukine-8 (IL-8) production in the presence of different concentrations of Jellybush honey extract or 10 µM dexamethasone (positive control, Sigma-Aldrich, Saint Quentin Fallavier, France, D1756) for an additional 24 h, alongside an untreated negative control. At the end of incubation period, the release of pro-inflammatory cytokine IL-8 was quantified by enzyme-linked immunosorbent assay (ELISA) following the supplier’s protocol (R&D System, Bio-Techne, Minneapolis, MN, USA, DY208).
2.4. Oxidative Activity
NHDF were seeded at 1 × 104 cells per well in 96-well plates. After 24 h, the medium was removed, and cells were washed with 100 μL of the ROS (reactive oxygen species) detector kit’s (ENZO, New York, NY, USA, ENZ-51011) wash buffer. Cells were then treated with ROS detector buffer containing either Jellybush honey or vitamin C (Sigma-Aldrich, Saint Quentin Fallavier, France, 95210-50G) at 50 μg/mL as a positive control for 30 min, alongside an untreated negative control. Subsequently, ROS production was induced by adding 200 µM pyocyanin (Sigma-Aldrich, Saint Quentin Fallavier, France, P00646). After 2 h of induction, ROS production was quantified by fluorescence staining following the manufacturer’s protocol.
2.5. Pro-Collagen I Production
NHDF were seeded at 2.5 × 104 cells per well in 96-well plates in DMEM supplemented with 10% FBS (Sigma-Aldrich, F7524) for 24 h, then serum-starved for 24 h to promote a quiescent cell state. Following this, cells were treated with different concentrations of Jellybush honey, or vitamin C (Sigma-Aldrich, 95210-50G) at 25 µg/mL as a positive control, alongside an untreated negative control, in serum-free DMEM for 24 h. Supernatants were collected at the end of the incubation period, and the production of pro-collagen I by fibroblasts was measured using an ELISA (R&D System, Abingdon, UK DY6220). Simultaneously, cell viability was assessed using the MTS method (Promega, Madison, USA G3582), following the provider’s instructions. Briefly, this colorimetric method allows detection of living cells by measuring mitochondrial metabolic activity, where enzymes from viable cells reduce the MTS compound into a soluble colored formazan product. The intensity of absorbance measured using a plate reader (Cytation 1, Agilent BioTek, Fisher Scientific SAS, Illkirch, France) at 450 nm/540 nm was proportional to the number of metabolically active cells. Pro-collagen I production was normalized to cell viability and expressed as a percentage relative to the untreated control.
2.6. Human Skin Explant Cultures
Human skin tissues were obtained from surgical residues in full compliance with the Declaration of Helsinki and Article L.1243-4 of the French Public Health Code. The latter does not require prior authorization by an ethics committee for the collection and use of surgical wastes. Normal skin explants were prepared from abdominal tissue obtained during surgery from a 37-year-old Caucasian woman. Explants were stabilized in a proprietary medium at 37 °C, 5% CO2 for 24 h. On day 0 (D0), mechanical wounds were created in the center of each explant using a 3.5 mm diameter punch, while uninjured control explants were maintained in parallel. Immediately after wounding (D0) and on days 2, 4, 6, and 7, wounded explants were treated with Jellybush honey through topical application (2 µL/cm2). Half of the culture medium (1 mL) was replaced on days 2, 4, 6, and 7. On days 1, 2, 3, and 6 after treatment, two explants per test group, for subsequent gene expression analysis, were preserved in 700 μL of RNALater (Sigma-Aldrich, France, R0901) in order to preserve the integrity of ribonucleic acids in biological samples by rapid penetration into tissues to stabilize and protect cellular RNA in situ, inhibit RNases and prevent RNA degradation. Additionally, on day 0 and 10 post-treatment, three explants per test group were collected and fixed in buffered formalin solution to evaluate neo-epidermal tongue formation.
2.7. Gene Modulation
All RNA samples were extracted in triplicate using the ReliaPrep RNA Tissue Miniprep System (Promega, Madison, USA, Z6110). The extraction protocol included a DNase digestion step to eliminate genomic DNA contamination. Total RNA was eluted with 35 μL of RNase/DNase-free water and concentration was determined using a BioDrop spectrophotometer. RNA quality was assessed by microcapillary electrophoresis (Experion Automated Electrophoresis System, Bio-Rad, Marnes-la-Coquette, France) through measurement of the RNA Quality Indicator (RQI). For cDNA synthesis, 100 ng of high-quality RNA was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Marnes-la-Coquette, France, 1708890). RT reactions were validated using an exogenous RNA spike-in control. The expression of 29 genes associated with wound healing processes was evaluated by qPCR array. For qPCR reactions, cDNA was diluted 1:10 in RNAse/DNAse-free water and amplified with specific primer pairs using iTaq Universal SYBR Green Supermix (Bio-Rad, 1725120) for 40 cycles. Three housekeeping genes (B2M, GAPDH, and RPLP2) were included for normalization of target gene expression. Results are presented as fold expression relative to untreated unwounded conditions.
2.8. Wound Healing Ex Vivo
After 24 h of fixation in buffered formalin, the above tissue samples were dehydrated, embedded in paraffin, and sectioned at 5 µm thickness using a rotary microtome. Tissue viability and epidermal/dermal architecture were assessed using Masson’s trichrome staining (Goldner variant). Wound closure was evaluated by measuring the length of the neo-epidermal tongue using light microscopy (Leica DMLB, Olympus BX43 or BX63 microscope, Wetzlar, Germany) with calibrated image analysis software. Results are presented as percentage variation in neo-epidermal tongue length relative to the untreated control, quantitatively illustrating the progression of re-epithelialization.
2.9. Clinical Study
A double-blind, placebo-controlled clinical evaluation was conducted on 21 healthy volunteers (both male and female) aged 18 to 40 years, exhibiting bilateral visible acne scars. Inclusion criteria specified fresh scars or present 9–12 months, with a minimum of 2 scars per side of the face, with the presence of keloids. Skin phototypes represented were phototype II (10% of volunteers), III (38%), IV (10%), V (30%), and VI (10%). All subjects provided written informed consent prior to participation in accordance with ethical guidelines. Volunteers applied the test products on clean, dry skin under normal conditions of use twice a day (morning and evening), following a split-faced random design for 8 weeks (
Table 1). Clinical assessments were conducted at baseline (day 0), at 4 weeks, and at the end of the 8-week application period. Skin homogeneity, scar size, and overall appearance were evaluated using standardized VISIA-CR photography. Skin color intensity was assessed via the Individual Typological Angle (ITA°) parameter, where higher ITA° values indicate lighter skin coloration. The percentage of skin area affected by scars was quantified through segmentation of brown images, isolating pixel intensity values of dark spots from the other features to determine the percentage of skin surface occupied by a scar. Scar appearance was determined by measuring the evolution of the surface mean quadratic roughness (Spq) parameter over a defined region of interest (ROI) in VISIA-CR visible cross-polarized images. The distribution of gray levels in each image was measured, and the corresponding amplitude variations were analyzed. A decrease in the Spq parameter indicates a reduction in visual roughness, corresponding to improved scar appearance.
2.10. Statistics
All data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 10.5.0 (GraphPad Software Inc., version 10.6.1, La Jolla, CA, USA). Data were first assessed for normality using the Shapiro–Wilk test. Depending on the normality of distribution and homogeneity of variance, either ordinary one-way ANOVA or two-way ANOVA was performed, followed by Dunnett’s multiple comparison test for post hoc analysis. The statistical significance threshold was set at 5%, with significance levels indicated as follows: # p < 0.1 (trend), * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Bioactive Properties of Jellybush Honey Extract
Jellybush honey is known for its capacity to resolve inflammation and reduce oxidative stress. Based on this knowledge, we evaluated our extract’s ability to preserve these beneficial properties, essential for effective wound healing, using in vitro skin cell models. In parallel, we evaluated pro-collagen I production to determine the extract’s potential to modulate extracellular matrix synthesis, a key factor in preventing scarring and promoting optimal tissue regeneration.
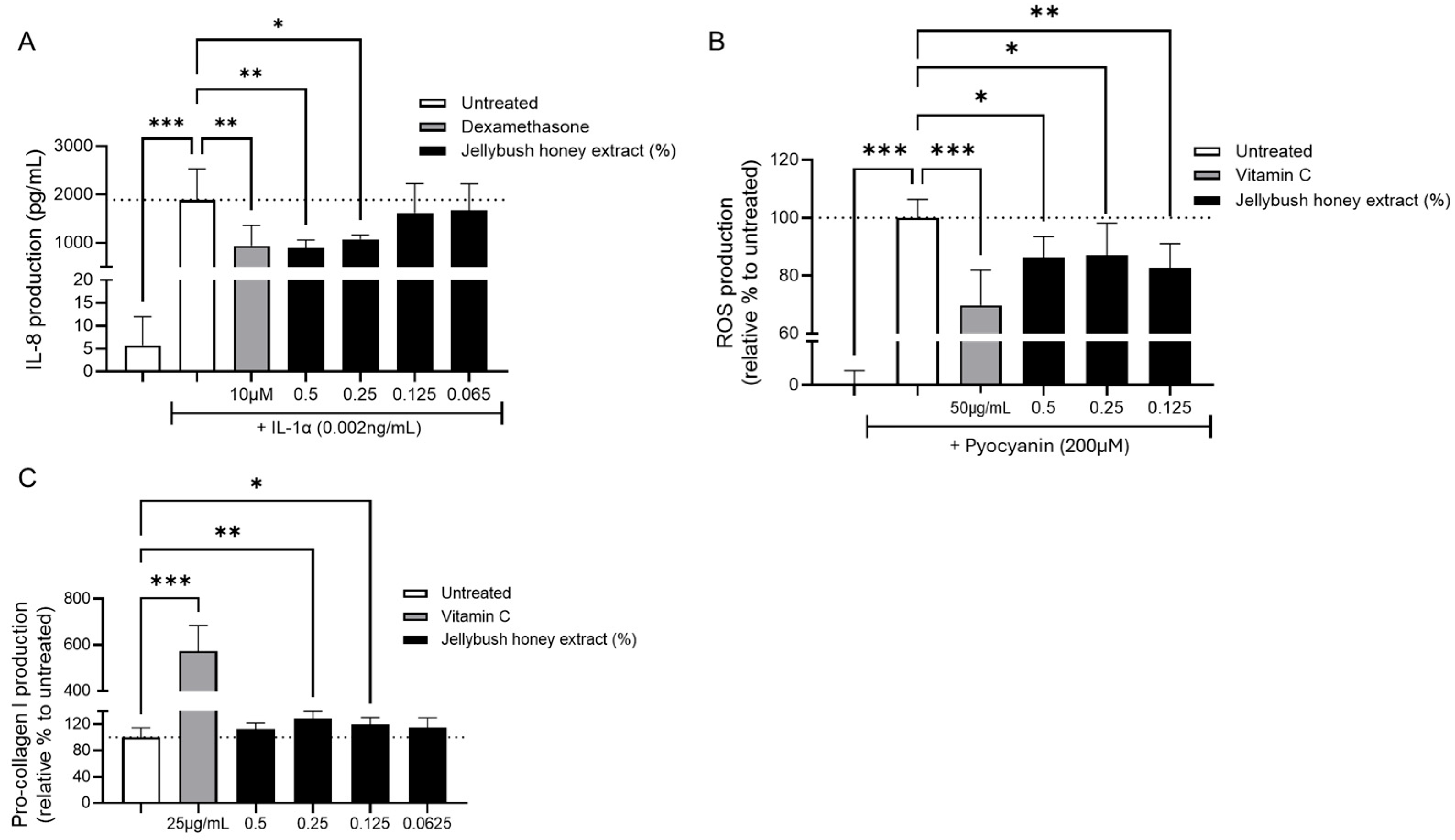
The anti-inflammatory potential of Jellybush honey extract was evaluated by measuring IL-8 production in IL-1α-stimulated NHDF cultures. As anticipated, IL-1α stimulation significantly increased IL-8 production. Notably, treatment with either 0.5% or 0.25% Jellybush honey extract significantly reduced IL-8 production, with efficacy comparable to dexamethasone (
Figure 1A).
The extract’s antioxidant activity was assessed in NHDF cells stimulated with pyocyanin, a toxin from
P. aeruginosa known to significantly increase ROS production. Treatment of cells with Jellybush honey extract, regardless of the concentration tested, significantly reduced ROS production by an average of 14.6%, compared to 30% for the benchmark antioxidant vitamin C (
Figure 1B).
Therefore, we evaluated Jellybush honey extract’s ability to enhance collagen synthesis. NHDF cells were treated with different concentrations of Jellybush honey extract, and we demonstrated a modest but significant increase in pro-collagen I synthesis: compared to the vitamin C control, which increased pro-collagen I by 472% relative to untreated cells, Jellybush honey extract at 0.25% significantly increased production by 28% (
Figure 1C).
These results demonstrate that Jellybush honey extract can promote healthy skin closure without excessive or insufficient collagen production.
3.2. Jellybush Honey Extract Timely Coordinates Wound Healing Gene Expression
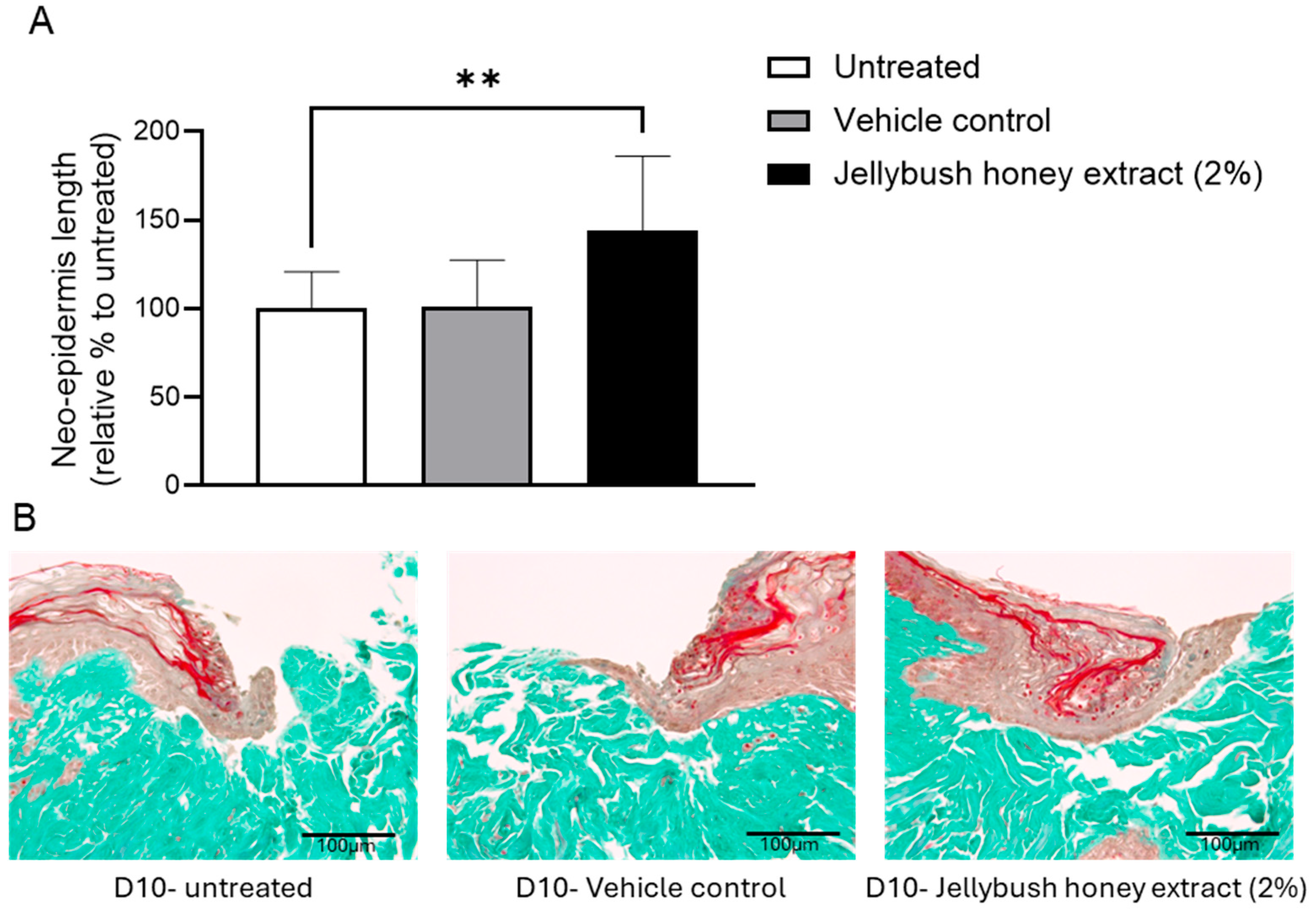
The potential ability of Jellybush honey extract to improve skin closure was evaluated using injured human skin explants. After 10 days of treatment, the length of the neo-epidermis formed to close the wound was 44% greater with Jellybush honey extract compared to vehicle control (
Figure 2).
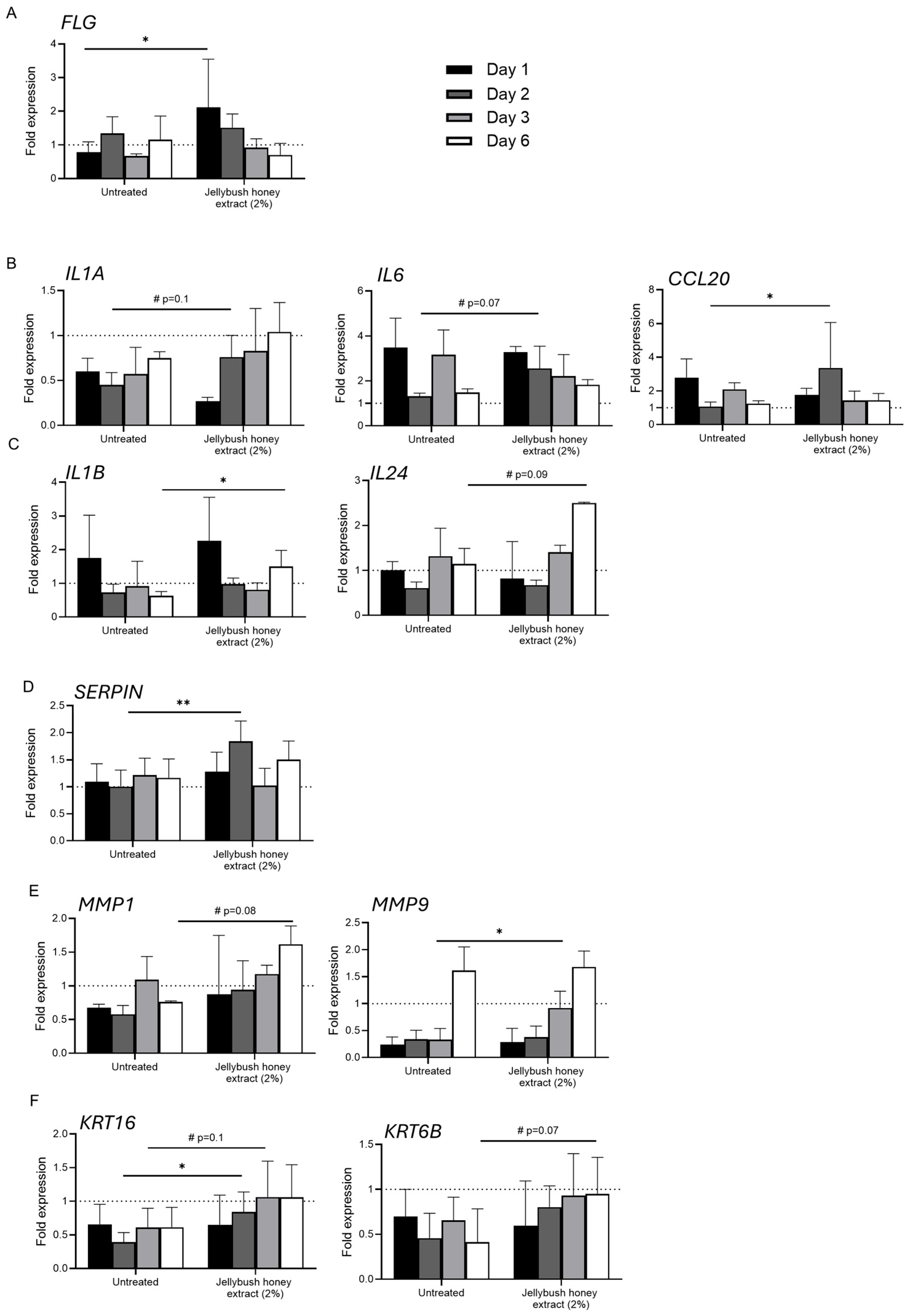
In addition to observing the formation of neo-epidermis, the wound healing process was monitored by gene expression analysis. Human skin explants were injured and treated with Jellybush honey extract. The cellular response linked to inflammation and matrix modulation was evaluated (
Figure 3). Firstly, we observed that after 24 h of treatment, Jellybush honey extract significantly increased the expression of
FLG (filaggrin) transcript compared to untreated conditions (
Figure 3A). This early expression underlined the immediate activation of an adaptive response to reinforce the skin barrier around the wound, in order to prevent transepidermal water loss and reduce microbial infection.
The expression of
IL1A (interleukin 1 alpha) under Jellybush honey extract treatment was decreased by approximately two-fold compared to untreated control. However, a significant progressive peak began after 2 days of treatment, while
IL1A transcript levels in untreated conditions remained unchanged. The transcription of
CCL20 (chemokine ligand 20) and
IL6 was also significantly increased after 2 days of Jellybush honey extract treatment (
Figure 3B). Other significant modulations were observed in the inflammatory response included the downregulation of
IL1B (interleukin 1 beta) transcripts and conversely, the upregulation of
IL24 after 6 days of treatment. These findings suggest that Jellybush honey extract exerts a complex and timely regulated modulation of inflammation, with a dynamic rebalancing of the inflammatory profile rather than simple suppression. Indeed,
SERPIN transcripts were also increased after 2 days of treatment, modulating the pro- and anti- inflammatory response (
Figure 3D).
In parallel, we observed that the transcription of
MMP1 (matrix metalloprotease 1) followed by
MMP9 was progressively increased earlier compared to untreated conditions, with
MMP1 showing a 3-fold higher increase compared to
MMP9 after day 1 of Jellybush honey extract treatment, versus a 1.6-fold difference for untreated conditions. Moreover, compared to untreated conditions, we observed a significant difference between Day 4 and Day 6 for
MMP9 and
MMP1, respectively. This suggests that Jellybush honey extract treatment improves matrix remodeling to facilitate cell migration (
Figure 3E). These changes are concomitant with increased expression of
KRT16 (keratin 16) and
KRT6B transcripts which are involved in proliferation and re-epithelization (
Figure 3F).
Overall, these results demonstrate that Jellybush honey extract treatment in our conditions exhibits a sophisticated timed regulation of key processes essential for effective wound healing. Indeed, the extract promotes skin repair by reinforcing barrier function, orchestrating a balanced inflammatory response, facilitating matrix remodeling, and stimulating re-epithelialization, all in a timely coordinated manner. These findings support the potential use of Jellybush honey extract as an effective natural therapeutic agent for wound management and skin regeneration.
3.3. Clinical Efficacity of Jellybush Honey Extract in Scar Repair Across Diverse Skin Phototypes
Wound healing is a fine-tuned mechanism, and dysregulation in any phase of this process can lead to visible skin damage. Excessive matrix remodeling through overproduction of collagen leads to hypertrophic scars, whereas slowed remodeling creates atrophic scars. These skin irregularities are associated with decreased self-esteem. Moreover, individuals with higher phototypes are more affected than those with lighter skin due to their greater number of melanocytes, which increase melanin production in parallel with collagen synthesis. Thus, hypertrophic scars are often associated with hyperpigmentation, while the opposite occurs with atrophic scars.
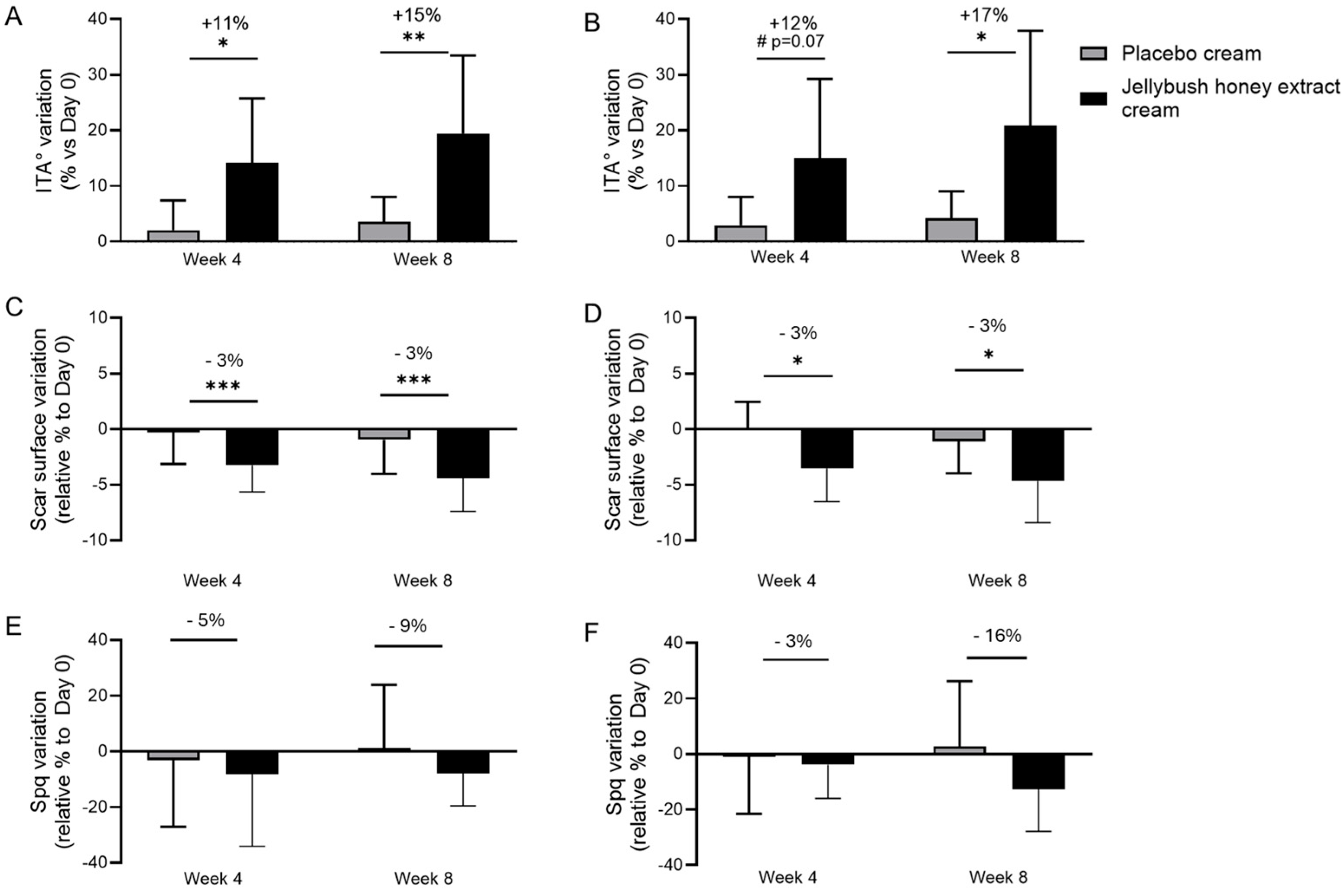
In a double-blinded clinical study, the efficacy of Jellybush honey extract in repairing skin damage related to wound healing was evaluated on volunteers of different phototypes who exhibited bilateral fresh or 9–12 months-old scars. Over an 8-week period, volunteers applied a gel-cream containing 2% Jellybush honey extract on one side of the face versus a placebo cream on the other side. After 4 and 8 weeks, skin homogeneity was evaluated using the ITA° parameter on the cheeks of volunteers. We observed a significant increase in the ITA° parameter by 15% with Jellybush honey extract cream application compared to placebo cream (
Figure 4A). Similar results were observed with volunteers having phototypes V and VI, where the ITA° parameter significantly increased by 17% after 8 weeks of application (
Figure 4B).
This improvement in skin homogeneity was associated with scar size reduction. Indeed, after 8 weeks of application, volunteers who applied Jellybush honey extract showed a significant decrease in skin area occupied by scars, with a 3% reduction across all volunteers and for volunteers with higher phototypes (
Figure 4C,D).
To further demonstrate the efficacy of Jellybush honey extract, scars were categorized based on their profile: atrophic or hypertrophic. Through evaluation of skin roughness, we observed that atrophic scars decreased in depth by 9% compared to placebo (
Figure 4E), while hypertrophic scars showed a 16% reduction in volume (
Figure 4F).
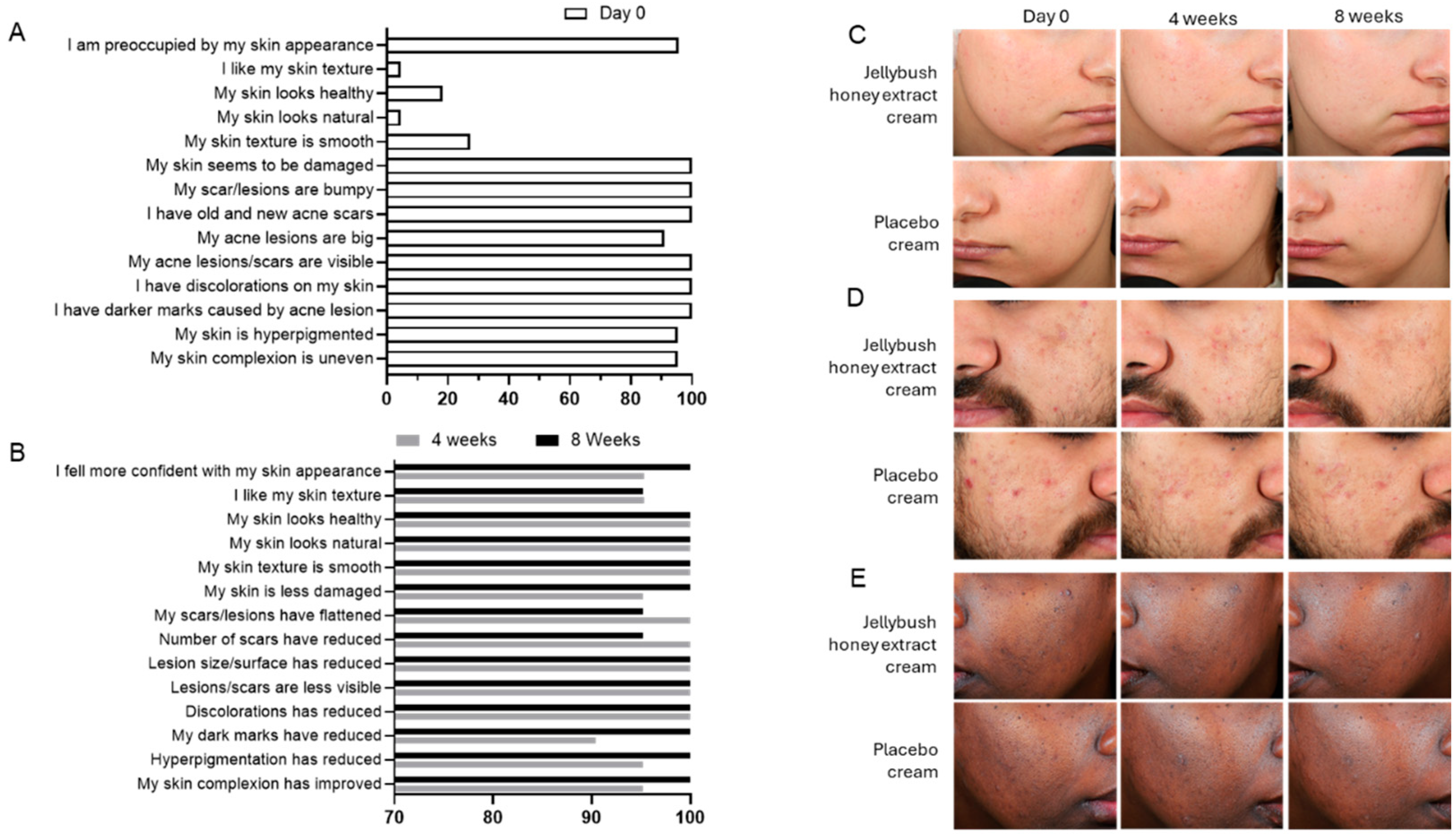
Collectively, these results explain the improved skin perception reported by volunteers, which was associated with visual skin improvements evaluated through self-assessment questionnaires. As illustrated in
Figure 5, self-esteem was significantly enhanced in correlation with improved skin perception and increased skin repair after 4 weeks of cream application (
Figure 5B) compared to the baseline evaluation conducted on day 0 (
Figure 5A).
4. Discussion
Skin appearance plays a crucial role in self-confidence and social interactions [
11,
12,
13,
14,
15]. When natural biological repair mechanisms become dysregulated, visible damage can result. Wound healing, a fundamental skin repair process occurring throughout an individual’s life, may become compromised, potentially leading to visible hypertrophic or atrophic scar formation. Here, we propose a natural ingredient from Australia able to modulate wound healing signaling and reduce post-acne scarring across skin phototypes.
This consideration of skin color diversity is particularly important as individuals with highly pigmented skin are more susceptible to developing complex scarring associated with excessive melanin production resulting in increased hyperpigmentation. During the wound healing process, melanocytes released into the wound site stimulate fibroblast proliferation and promote excessive fibrosis, directly contributing to scar formation [
16]. Our Australian natural ingredient addresses these complex healing challenges, offering a solution that respects and effectively treats unique scarring concerns across diverse skin tones.
The In Vitro results demonstrated that our Jellybush honey extraction process preserves the known anti-inflammatory and antioxidant properties of Jellybush honey [
17]. Controlling inflammation is crucial to prevent chronic inflammatory responses that can lead to skin damage. Moreover, the modest increase in pro-collagen production observed in NHDF cells treated with our extract highlights the capacity of Jellybush honey extract to mediate skin repair while simultaneously preventing the formation of both atrophic and hypertrophic scars [
18].
Through Ex Vivo studies, we demonstrated that Jellybush honey extract coordinates multiple mechanisms involved in wound healing. We first observed that Jellybush honey acts immediately to reinforce the skin barrier through early expression of filaggrin transcript, helping to prevent water loss and reduce infection [
19]. Moreover, we observed that Jellybush honey extract exerts a complex and timely regulated modulation of inflammation, which is crucial for removing damaged cells and pathogens. Indeed, the initial decrease in
IL1A expression, followed by a progressive peak after 2 days of treatment, indicates a two-stage mechanism of action. This is combined with an increase in
CCL20 and
IL6 expression after 2 days of treatment, coupled with a decrease in
IL1B over time and an increase in IL24 after 6 days (
Figure 3). The progressive modulation of gene expression observed supports a gradual transition toward skin repair. Recent research demonstrates that
IL24 is upregulated in epithelial stem cells, and enhances wound repair by promoting re-epithelization, stimulating vascular regeneration and activating fibroblasts [
20]. This healing progression is further evidenced by the increased expression of re-epithelization markers
KRT6B and
KRT16, suggesting the extract’s capacity to restore epidermal integrity [
21]. In addition, the timed and regulated modulation promoted by the extract treatment was underlined by sequential up-regulation of
MMP1 from day 1 followed by
MMP9, indicating earlier matrix restructuring to facilitate cell migration [
22]. Gene expression analysis demonstrates that Jellybush honey extract facilitates wound healing through balanced biological modulation rather than through extreme suppression or stimulation. This
equilibrium is further confirmed by its dual capacity to moderately enhance collagen production (
Figure 1C) while simultaneously reducing ROS generation (
Figure 1B). This calibrated activity represents a key therapeutic advantage in wound management. Clinical evidence indicates that wound healing complications often arise from imbalances: excessive collagen synthesis leads to hypertrophic scarring, while insufficient production results in atrophic scars [
23]. Similarly, the ROS
equilibrium is critical; both oxidative excess and deficiency disrupt the intricate cellular signaling required for effective tissue repair [
24]. By maintaining these parameters within optimal therapeutic ranges, Jellybush honey extract supports physiological wound resolution while minimizing pathological scarring outcomes, offering a promising natural approach to skin regeneration (
Figure 2).
The double-blinded clinical study conducted validated the ability of Jellybush honey to reduce post-acne scarring on volunteers with diverse phototypes. The increase in ITA° (
Figure 4) recorded after 4 and 8 weeks of Jellybush honey cream application is also associated with an improvement of skin luminosity, and redness reduction (
Figure 5B). These results underline the visual perception of scar improvement and confirm the biological benefits of Jellybush honey extract in regulating the inflammation response, optimizing collagen production and controlling ROS levels. The extract’s precisely calibrated activity effectively maintains the delicate
equilibrium necessary for healthy wound resolution and scar reduction, as shown in
Figure 4, demonstrating a significant decrease in both the percentage of skin surface occupied by scars and the hypertrophic and atrophic roughness measured on volunteers’ skin (
Figure 4). Indeed, the timed coordination of
MMP1 and
MMP9 upregulation observed in our Ex Vivo model can directly explain the clinical reduction in scar roughness through the modulation of matrix remodeling [
22,
25]. Similarly, the early
FLG upregulation and subsequent
IL24 expression pattern likely contributes to the improved skin homogeneity and increased ITA° values observed in the clinical study, particularly in higher phototypes where barrier dysfunction often exacerbates pigmentation irregularities [
19,
20,
26]. These fine modulations are dependent on multiple factors including scar age, depth, and individual healing capacity, which can explain the modest average obtained for scar area reduction. However, scar remodeling is a dynamic process that typically continues for 12–24 months, with the most significant changes occurring within the first year [
4,
27]. The mechanism of action of Jellybush honey extract suggests potential for continuous, non-linear scar improvement over extended periods of application, beyond the 8-week timeframe of our study.
5. Conclusions
In conclusion, our Jellybush honey extract preserves the immunomodulatory properties characteristic of honey, offering an innovative natural ingredient capable of enhancing wound healing through timed, controlled modulation of signaling pathways, promoting healthy tissue repair without visible scarring [
12]. Unlike conventional treatments such as silicones sheets or corticosteroids injections, which are associated with side effects, Jellybush honey extract manages scarring by targeting multiple mechanisms of dysfunctional wound healing [
8,
28]. Compared to other natural ingredients studied for scar management, such as
centella or
cumin, which demonstrate some efficacity in reducing scar appearance and inflammation but show limited effects on matrix remodeling [
29,
30], Jellybush honey extract demonstrates broader activity across the entire wound healing cascade, explaining strong clinical outcomes, particularly in volunteers with higher phototype, from whom treatment options are often limited by concerns of post-inflammatory hyperpigmentation [
31].
The study also shows that a modest improvement in scar appearance, delivered through application of a cream containing Jellybush honey extract, can yield disproportionately positive psychological outcomes [
11]. The significant improvement in self-assessment scores observed after just 4 weeks of Jellybush honey application (
Figure 5) corroborates previous findings that effective scar treatments provide benefits extending beyond physical appearance to encompass emotional well-being and social functioning [
32,
33].
By offering a natural solution that effectively addresses both the visible manifestations of scarring and their psychological consequences, Jellybush honey extract represents a holistic approach to wound healing that recognizes the intricate relationship between skin outcomes and quality of life.