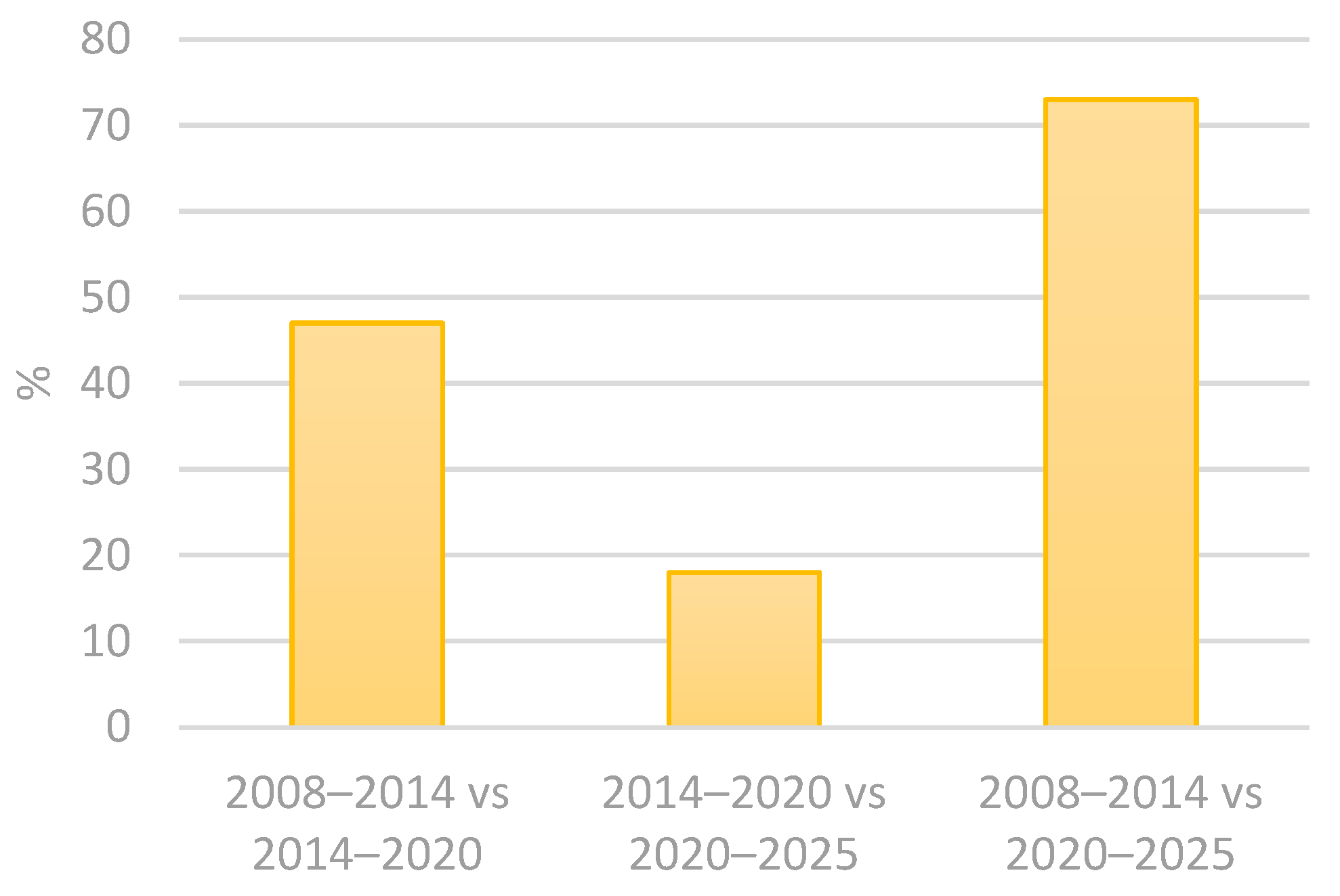Abstract
European legislation defines cosmetics as substances or mixtures designed to contact external body parts for cleaning, protection, fragrance, maintenance, or appearance modification. Cosmetic regulation has become increasingly important in recent years, as the number of consumers continues to grow. One of the major challenges of the cosmetic industry is effectively communicating to consumers the critical need to avoid using expired products for several safety reasons, with microbial contamination being among the most significant concerns. A key research priority involves understanding how bacterial and fungal populations commonly proliferate within cosmetic formulations. Regulatory standards strictly prohibit specific microorganisms in finished cosmetic products, as specified in EMA guidelines, making microbiological assessment an essential component of product evaluation. This review examines the prevalence, risks, and control measures associated with microbial contamination in cosmetic products. Special attention is given to the most isolated microorganisms, factors contributing to contamination, and current preservation strategies in the cosmetic industry.
1. Introduction
Contamination of cosmetic products may occur during production, storage and consumers’ daily handling. In cases when products show no visible defects, the cosmetic industry faces liability for product-related contamination only under two circumstances [1]. Consumer handling and storage practices for cosmetics typically do not adhere to the strict standards maintained during manufacturing and distribution phases. Upon first opening, products become subject to variable yet persistent contamination from household environmental conditions and dermal contact with users [2,3].
The application of cosmetics, particularly make-up, represents a widespread global practice intrinsically linked to cultural expression and personal confidence. Safe product manufacturing needs comprehensive knowledge of safety parameters for cosmetic formulations when applied to their intended bodily regions [3]. According to EU Regulation (EC) No. 1223/2009, cosmetics are substances or mixtures intended to come into contact with external parts of the body (for cleaning, protecting, perfuming, maintaining, or altering their appearance). This regulation also established that quality control plays a fundamental role in ensuring consumers’ safety [4]. The Good Manufacturing Practices (GMP) are the set of instructions ensuring risk minimization during product manufacturing, including microbiological analysis [5]. Therefore, microbiological control is a critical aspect in cosmetics production, ensuring that the product remains within safe parameters for a proper amount of time without a human health risk of microbial contamination [6]. In response to these challenges, the cosmetic industry has developed multiple methodologies to guarantee end-product safety. Despite extensive regulation, industry accountability terminates upon delivery of compliant products to consumers. Subsequently, contamination resulting from environmental exposure and user practices falls under consumer responsibility. Maintaining product quality during use requires strict adherence to manufacturer guidelines. Failure to observe these conditions generates public health risks, as cosmetic products may function as fomites and establish novel pathways for microbial dissemination [7]. Consequently, expiration dates must be prominently displayed, and consumers require education regarding packaging label conventions [8]. Notwithstanding their expanding global usage, cosmetic products may function as vectors for microbial proliferation [9].
For these reasons, regular cosmetic/make-up consumers and cosmetic/make-up service providers should maintain and establish safe use practices to avoid microbial cross-contamination [10]. The cosmetics sector grapples with a significant issue: ensuring consumers understand why expired beauty products pose serious health risks, particularly due to the threat of microbial growth. Research efforts are increasingly targeting the mechanisms by which bacteria and fungi develop and multiply within cosmetic formulations. The combination of water, organic compounds, and minerals in these products establishes favorable conditions for microbial proliferation [11]. Comprehension of these contamination dynamics is critical for maintaining consumer safety and product quality standards. European Medicines Agency guidelines explicitly prohibit the presence of specific microorganisms in finished cosmetic products (e.g., Staphylococcus aureus, Candida albicans), thereby establishing microbiological testing as an indispensable element of product evaluation procedures [12]. This review analyzes microbial contamination in cosmetic products, focusing on how common it is, what risks it poses, and how it can be controlled. Furthermore, it emphasizes the microorganisms most often found in contaminated products, the factors that lead to contamination, and the preservation methods currently used by cosmetic manufacturers.
2. Materials and Methods
A bibliographic search for this narrative review was conducted using PubMed, Science Direct, and Web of Science databases with keywords and MeSH terms related to “cosmetic”, “biological contamination”, and “facial makeup”. Studies were included if they focused on cosmetic regulation or cosmetic contamination; were original research, systematic reviews, or meta-analyses; were published within the last decade (2014–2024) unless considered foundational; were written in English. Additionally, ISO standards were also used to establish acceptance parameters for good practices in finished cosmetic products before their use by consumers.
3. Results
3.1. Global Policies on Microbial Contamination of Products
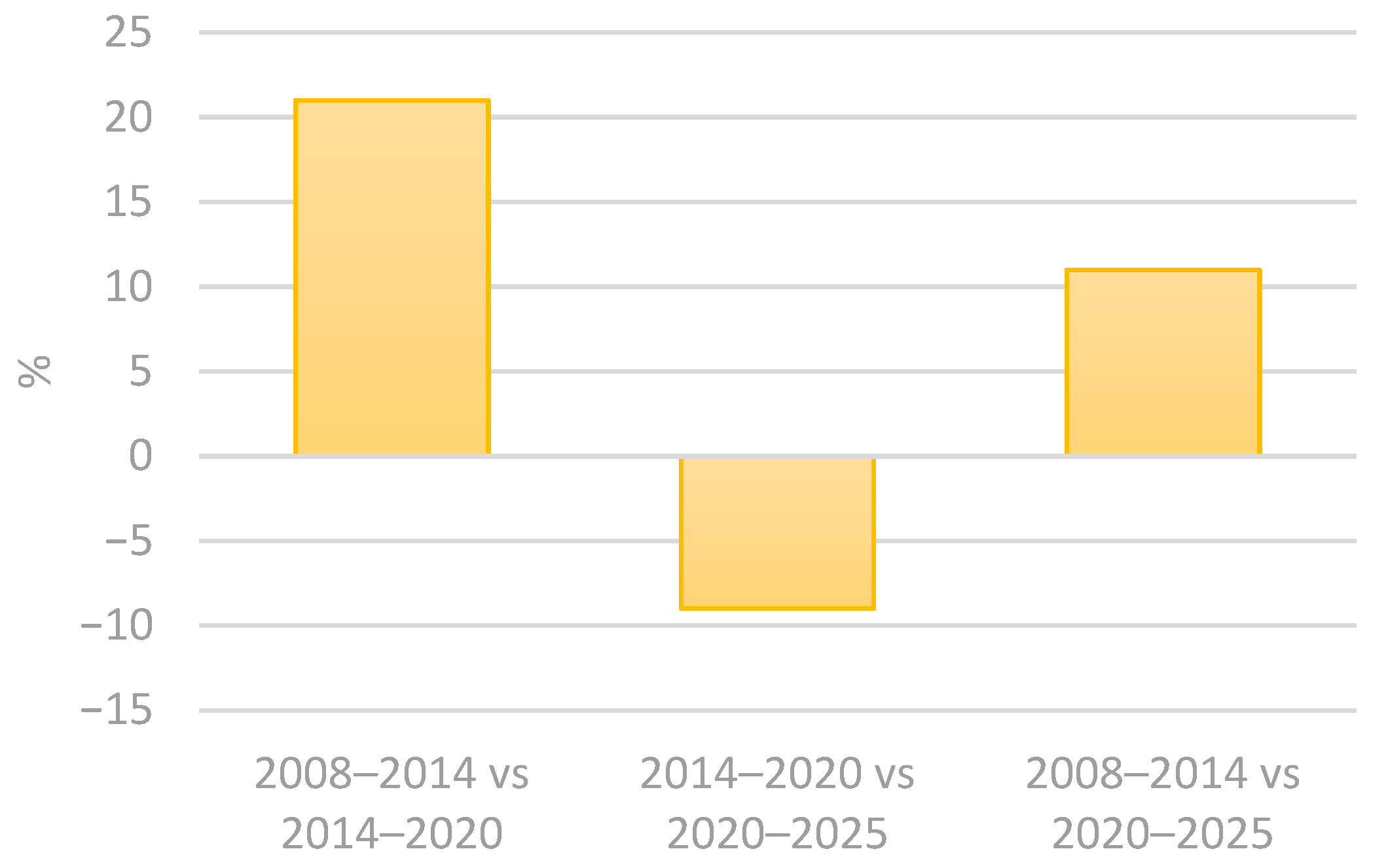
Contamination in cosmetic product studies usually takes into consideration the intended contact of the utensils and the skin surface. However, accidental contact with areas such as the oral cavity, nose and ocular epithelium should also be considered, as these tissues exhibit structural and immunological characteristics distinct from keratinized skin [6,13]. The European Commission considers microbiological contamination a risk to consumers’ health and has established an alert system for unsafe products. The Rapid Alert System for all dangerous consumer products (RAPEX) allows for almost immediate recalls of batches of cosmetic and health products that are deemed unsafe for the public [14]. Using the advanced filters in the RAPEX system, select all in the country of origin and all notifying countries, followed by selecting “microbiological risk”, in product categories select “cosmetics”. The results obtained from these filtering methods in the 2005–2025 period are that 215 cosmetic products from 37 different countries of origin had microorganism contamination. The results are better explored in the following tables (Table 1 and Table 2 and Figure 1, Figure 2 and Figure 3) [15]. The results indicate a 21% increase in the notified products from 2008 to 2020, followed by a 9% decline in the subsequent period, from 2014 and 2025. Overall, from 2008 to 2025, there is an accumulated growth of 11% (Table 1 and Figure 1) [15]. In addition, the number of notifying countries really increased by approximately 47% from 2008–2014 to 2014–2020 and by 17% from 2014–2020 to 2020–2025. Overall, there was a 72% cumulative increase from 2008 to 2025 (Table 2 and Figure 2 and Figure 3) [15]. The analysis also revealed that the number of countries of origin increased by 13% from 2008–2014 to 2014–2020, followed by a smaller rise of 4% from 2014–2020 to 2020–2025. Overall, there was a 13% cumulative increase from 2008–2014 to 2020–2025 (Table 1) [15].

Table 1.
Summary of notified products, notifying countries, and countries of origin from 2005 to 2025.

Table 2.
Data analysis from the RAPEX system from 2008 to 2025 regarding the number of notified products and countries.
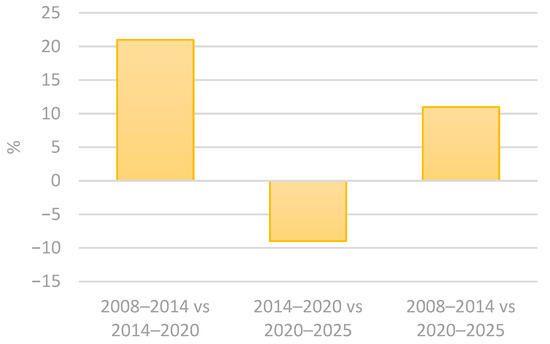
Figure 1.
Percentage variation in the number of notified products between consecutive and commutative property periods: 2008–2014 vs. 2014–2020; 2014–2020 vs. 2020–2025; and 2008–2014 vs. 2020–2025.
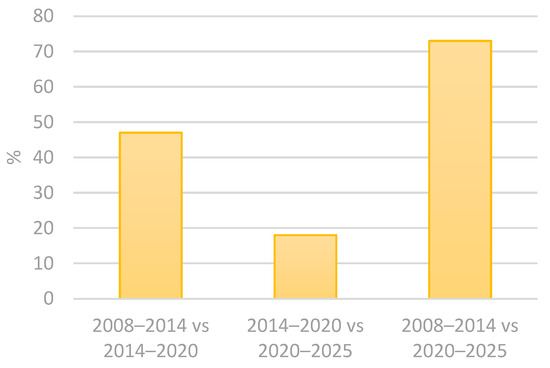
Figure 2.
Percentage variation between periods of notifying countries between consecutive and commutative property periods: 2008–2014 vs. 2014–2020; 2014–2020 vs. 2020–2025; and 2008–2014 vs. 2020–2025.
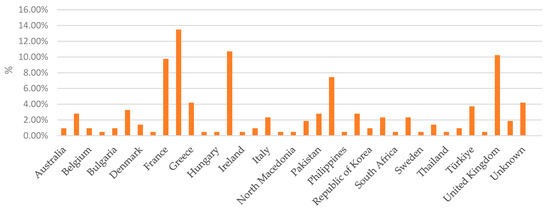
Figure 3.
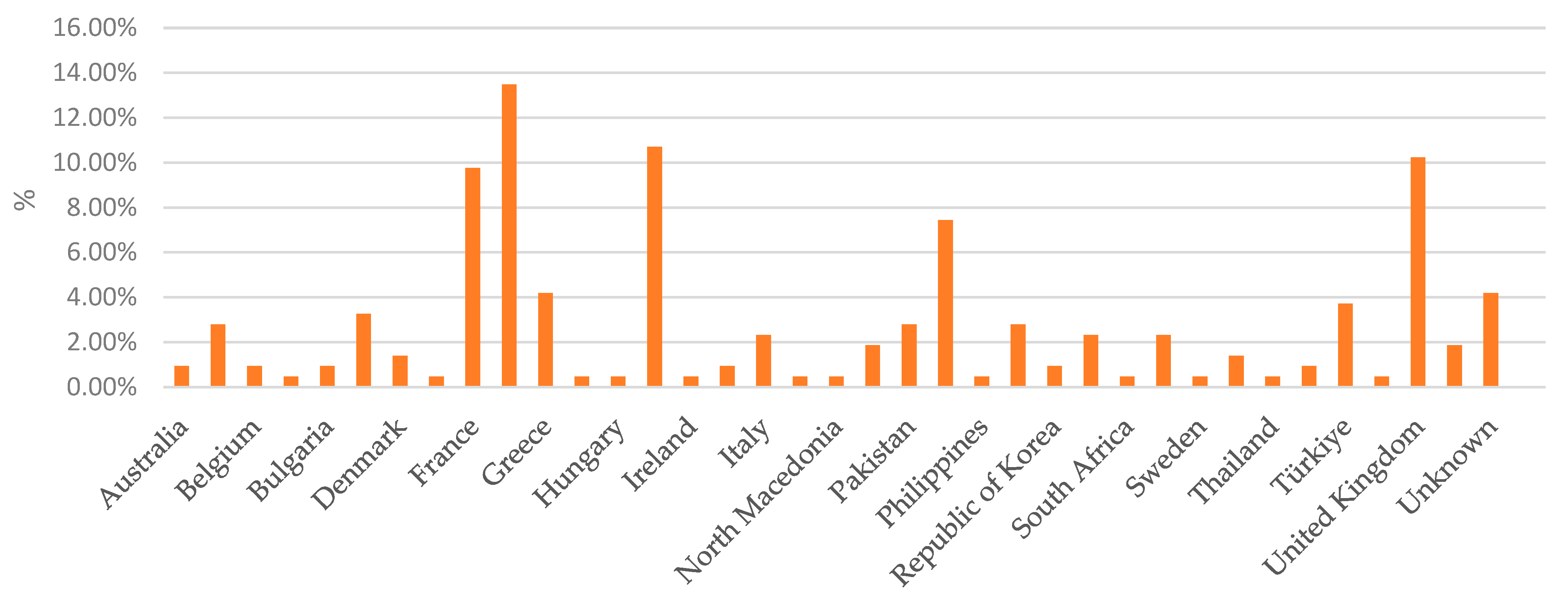
Percentage distribution of notifications by country of origin, illustrating the proportion of notifications received from each country.
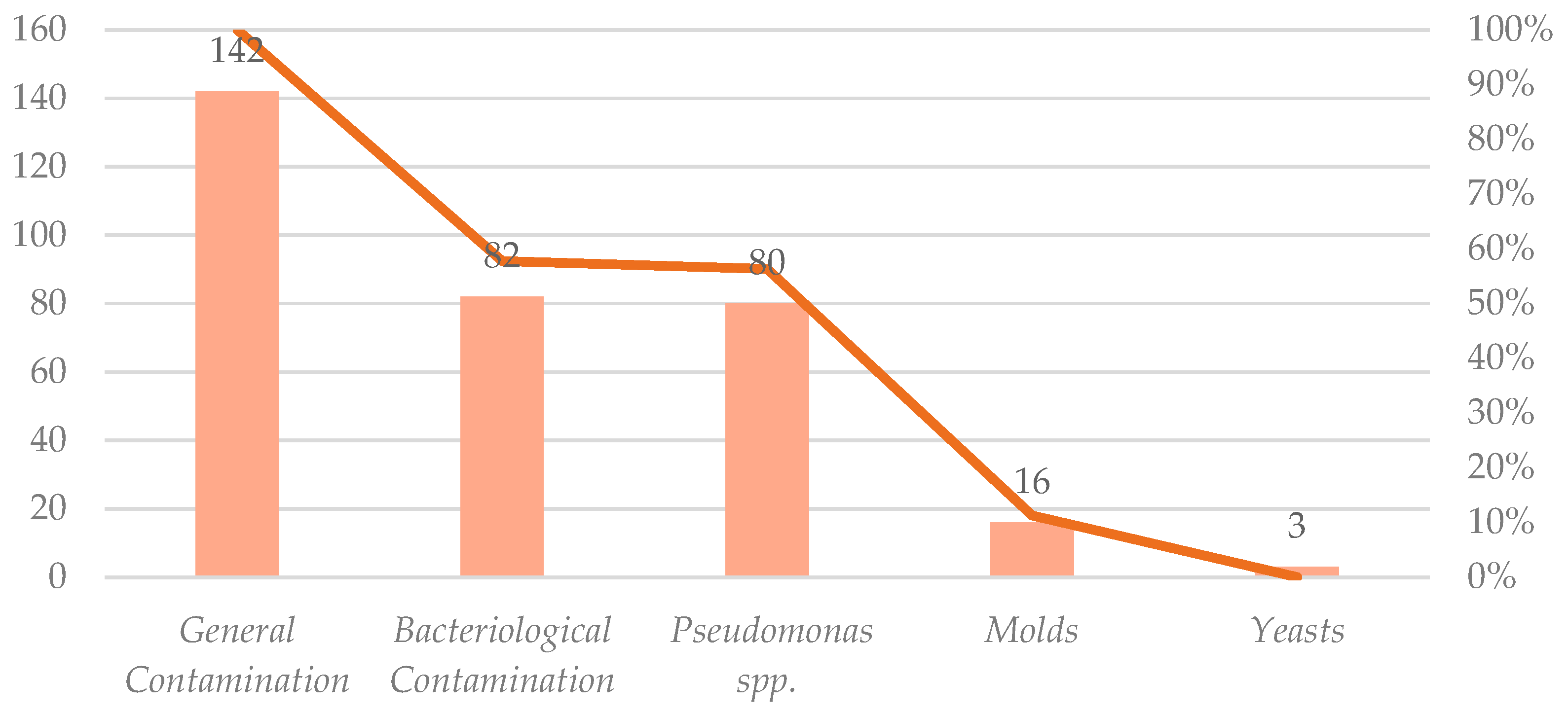
From the Food and Drug Administration (FDA) enforcement report, it was possible to collect a database from classified dates from 2014 to 2025 with the filter’s product type “cosmetics”, and reason for recall “contamination” (Table 3). There were 142 results for contaminated cosmetic products. For the second search in the filter, reasons for recall, the term “bacteria” had 82 results associating cosmetic products with bacterial contaminants. Among 142 cosmetic products recalled due to microbiological contamination between 2005 and 2025, Pseudomonas spp. was the most frequently identified microbial contaminant present in 80 cases, representing 56.34% of all microbiologically contaminated samples. Molds were reported in 16 cases, and yeasts were identified in three cases. These classifications were retrieved from the reason for recall field in the FDA notification data. Figure 4 provides a more detailed breakdown of these findings in a visual representation.

Table 3.
Enforcement report data analysis from the FDA data.
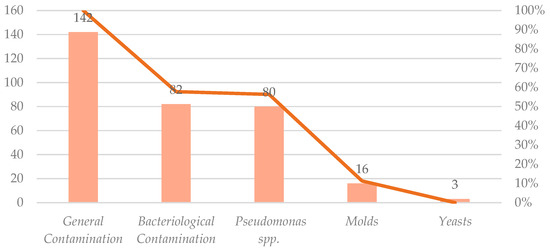
Figure 4.
Distribution of contaminant categories for a total of 142 recalls.
General contamination accounted for all 142 recalls, followed by bacteriological contamination, 82 recalls (58%), and Pseudomonas spp., 80 recalls (56%). Molds and yeasts are less frequent, with 16 (11%) and three recalls (2%), respectively [16].
Regarding the cosmetic products associated with microbiological contamination, and according to the FDA enforcement reports, RAPEX in notifications, and independent studies, the identified contamination includes bacteria, yeasts, and molds, highlighting microbiological risks in commonly used products such as mascara, cream, soaps, and lip products.
The most frequently identified microorganisms include Staphylococcus spp. and Bacillus cereus. Several products, particularly mascara, lip products, foaming soaps, and body or facial creams, were associated with multiple contaminants, including opportunistic pathogens and environmental bacteria. In addition to bacteria, yeasts and modes such as Candida albicans, Candida parapsilosis, and Aspergillus spp. were also reported, especially in body lotions and creams. These findings highlight the potential public health risks. The risks caused by microbiological contamination in cosmetic products highlight the need for strict daily quality control measures in manufacturing and post-market surveillance (Table 4).

Table 4.
Common contaminants: FDA vs RAPEX vs independent studies.
While RAPEX and FDA data pertain to finished products that fall within manufacturers’ liability regarding contamination, independent research primarily examines products following consumer usage.
3.2. Major Bacterial and Fungal Cosmetic Contamination
Evidence consistently demonstrates that cosmetic products become contaminated with microorganisms through user handling and sharing practices [52]. While populations in similar environments typically share comparable bacterial/fungal strain profiles, age groups and genders within these populations show distinct variations due to differences in skin composition characteristics. Each person maintains a unique microbial ecosystem on their skin, with stability maintained through mutually beneficial relationships between microorganisms and their human host. When cosmetics are applied, they can either support this natural microbial balance or disrupt it, potentially leading to skin dysbiosis where harmful bacteria outcompete beneficial ones [53]. In addition, this unique skin microbiome with distinct antibiotic resistance patterns varies between populations; shared cosmetics may serve as an unmonitored source of antibiotic-resistant bacteria transmission [54]. When individuals with less resistant microbial communities use contaminated cosmetic products, they face increased health risks, particularly if they have underlying biological vulnerabilities that make them susceptible to opportunistic infections [5]. An interesting study evaluated 467 samples from five categories (lipstick, eyeliner, mascara, lip gloss and beauty blenders). The results show that 13 genera of both bacteria and fungi species were present, such as Staphylococcus, Pseudomonas, Escherichia, Candida, and Cryptococcus [8]. Another study showed that from a total of 320 samples, 56.6% were contaminated with microbial agents, 63.88% were identified in cream body products, and 20% were found in eye-dedicated products. The highest S. aureus count was >4000 CFU/g, which was found on toner and facewash. The highest C. albicans contamination count was >20,000 CFU/g of product on a lipstick [55]. In the Dhaka metropolis, it was reported that of the 27 samples tested, 21 were contaminated beyond the accepted limits. The highest proportion of non-compliance was shown by powders with 88.9% contamination, followed by lipsticks with 87.5%, and finally creams (60%) [56]. A study on microbial contamination of commercial cosmetic products showed that Staphylococcus spp. is present as a contaminant in cosmetic products [49]. Another study, with the aim of isolating and characterizing microorganisms in cosmetics, tested samples with approximately 12 months of use in six distinct categories. From the products, microorganisms belonging to P. aeruginosa, Serratia liquifaciens and C. parapsilosis were recovered They were referred to in the study as opportunistic pathogens with the ability to cause skin irritation and infections in cases of wounded epithelium [36]. P. aeruginosa are easily adapted to different kinds of environments, making pertinent the topic of sharing products and crossing microbes among the sharing individuals and prophylactic cleaning strategies to prevent sources of skin diseases [54]. Folliculitis associated with P. aeruginosa has been described in epilation material and bath sponges, both of which are part of the cosmetic industry. Sponge materials are also used in make-up applications due to their practicality, but the sponge matrix becomes moist with increasing use, becoming a perfect site for P. aeruginosa proliferation. There is a thin line between saprophyte colonization and opportunistic infections. The integrity of the skin and the immune system are the barriers that determine this equilibrium. Therefore, it is very important to prevent adding another multi-resistance source to our day-to-day life, avoiding contributing to the growing lack of new antibiotics being discovered and increased hospitalizations associated with microbe resistance [57]. In one study, microbial contamination was detected in 56% of the assessed samples: face washes and toners were shown to have elevated levels of S. aureus with counts higher than 4000 CFU/gram of product. One S. aureus isolate had the ability to produce enterotoxin [55]. In a study of used cosmetics and beauty blenders used as application tools for cosmetic products, it was found that 79–90% of all used products were contaminated with bacteria. The bacterial loads ranged between 10 × 102 to 10 × 103 CFU/mL, and beauty blenders contained an average load of >10 × 106 CFU/mL. Samples with over 4000 CFU/mL of S. aureus were found in toner and face wash samples [55]. Cosmetic products used during the tattooing process showed microbiological contamination in 4.7% of the samples that were identified during testing, and the results were contaminated with various opportunistic pathogens, including S. aureus [58]. In 50 cosmetic brands, S. aureus was the most common contaminant detected and isolated. Results showed 27% contamination in products considered low quality, whereas in products considered high quality, 41% contamination was detected [59]. E. coli is another important contaminant. It can sustain its reproductive abilities in environments containing titanium dioxide and zinc oxide, which are chemical compounds used in the pharmaceutical and cosmetic industry. These metals are commonly used as colorants for eyeshadows, blush and face powders. There is a correlation between the presence of dipeptides and the enrichment of E. coli, suggesting the production of proteases by this species [60]. Dipeptides are used in cosmetics as an antiaging agent, meaning that products that use this class of active ingredients may be one of the sources of susceptibility to E. coli contamination, at least in antiaging cosmetic products [60,61]. With regards to Bacillus spp., they are particularly concerning due to their ability to form biofilms and resist preservatives [62,63]. In the presence of oxygen, they can sporulate, giving them the ability to persist until the right environment to proliferate. B. cereus has been described as a strain able to survive the third challenge test above the permitted CFU count, whilst in other strains (e.g., C. albicans and P. aeruginosa), growth was unaffected by the number of challenges. [64].
Other reports also show relevant results. A total of 85 tattoo and permanent makeup inks were tested. Among them, 42 inks were found to be contaminated with microorganisms, representing 49% of the samples. Specifically, 33 inks were contaminated with bacteria, two showed fungal contamination, and seven exhibited both bacterial and fungal growth. Using 16S rDNA sequencing, 83 bacterial isolates were identified, encompassing 20 genera and 49 species. Bacillus spp. were the most prevalent, accounting for 53 isolates, followed by Lysinibacillus fusiformis with 7% and P. aeuruginosa with a representation of 5% in samples. In 34 isolates, 41% were classified as potentially clinically relevant, including Pseudomonas aeruginosa and Dermococcus baratrhy, and Rosiomonas mucosa species prevailed. This species was previously implicated in human skin infections. These findings highlight that commercially available tattoo and permanent makeup inks in the US market may harbor diverse microbiota, including opportunistic pathogenic bacteria [65]. A study conducted in Egypt on samples of hair care items detected that out of 140 cosmetic samples, 31 (22.14%) were contaminated with bacteria, fungi, or both. Out of ten samples, three were contaminated with E. coli (30%). Out of nine samples, four were contaminated with S. aureus (44.4%). Out of nine samples, two were contaminated with P. aeruginosa (22.2%) [66].
Individuals with immunodeficiencies are more susceptible to fungal infections. Candida spp. are fungal species, known to be rapidly developing resistance to antifungals, in particular to triazoles. They are also known to be able to form strong biofilms in both biotic (e.g., human mucosae) and abiotic surfaces (e.g., cosmetics), which increase their virulence, tolerance to drugs, and resilience. A notably high contamination has been found in lipstick samples, exceeding 20,000 CFU/g of product. Moreover, three out of the 12 C. albicans isolates could produce protease, as well as other enzymes able to degrade skin tissue and increase the risk of infection REF. Other studies have shown that mascara and liquid foundations are particularly susceptible to fungal contamination due to their water content and repeated consumer contact [67]. Furthermore, shared cosmetic kits have been reported (IC = 95%) to be contaminated with fungi species (19.2%) [68]. These findings reveal the potential health hazards linked to the fungal contamination of cosmetic products used after their intended shelf life, showing how crucial it is to educate consumers about respecting the expiration dates indicated in the information labels [55].
Aspergillus species are on the World Health Organization (WHO) list of highest priority [69] for their virulence and known pathology associated with the genera. Aspergillosis is a recognized common fungal infection with a great impact on immunocompromised patients [21]. Like other fungi, Aspergillus spp. has already been found as a mascara contaminant and, in an occupational pathology-associated study, was found to lead to respiratory issues such as allergic rhinitis [49,70]. Penicillium is an environmental, opportunistic, fast-growing fungus, a strong spore and enzyme producer, and economically important in the food and drug industry, but is also found in cosmetic products, in shared kits in beauty salons [68].
As in other cases, the FDA, EU, and the International Organization for Standardization (ISO) guidelines provide frameworks for best practices in various industries, including cosmetics. These guidelines ensure security, consistency and quality in cosmetics, and consumers’ safety. Table 5 describes the ISO standards for cosmetics that ensure compliance with the FDA and EU contamination limits regulations in Table 6.

Table 5.
ISO standards for cosmetics (microbiology). List of ISO standards applicable to microbiological testing and risk assessment in cosmetic products, including detection methods, microbiological limits, and quality control procedures.

Table 6.
FDA vs. EU standards for microbiological limits for cosmetic products. Microbiological quality. Limits for cosmetic products established by the FDA [83] and the European Union [84,85].
There are several essential risk reduction practices in the industry to ensure safety and quality in production. The strategies implemented in the cosmetic industry are based on good manufacturing practices, including environmental, procedural, and personal-related controls (Table 7).

Table 7.
Risk reduction in the industry according to GMP.
Good manufacturing practices play a crucial role in minimizing microbiological and chemical risks in the cosmetic industry. Effective cleaning and sanitization procedures are essential at all stages of production. Segregation of areas in materials prevents cross-contamination, while ongoing staff training and the use of personal protective equipment help maintain hygiene. Standards control storage conditions, ensuring the stability of raw products and finished products. Continuous microbiological monitoring detects bacteriological and fungal contamination, and chemical testing verifies ingredient and production quality. Additionally, equipment must undergo proper qualification, calibration and maintenance to ensure process reliability and compliance with safety standards [86]. It outlines the key control areas involved in maintaining microbial and chemical quality during pharmaceutical/cosmetic manufacturing processes. It summarizes the best practices involved in the process, such as cleaning and sanitization, personnel hygiene, environmental monitoring, chemical and microbiological testing, and technological optimization to ensure compliance with GMP and standards, and to minimize contamination risks throughout the production cycle [87].
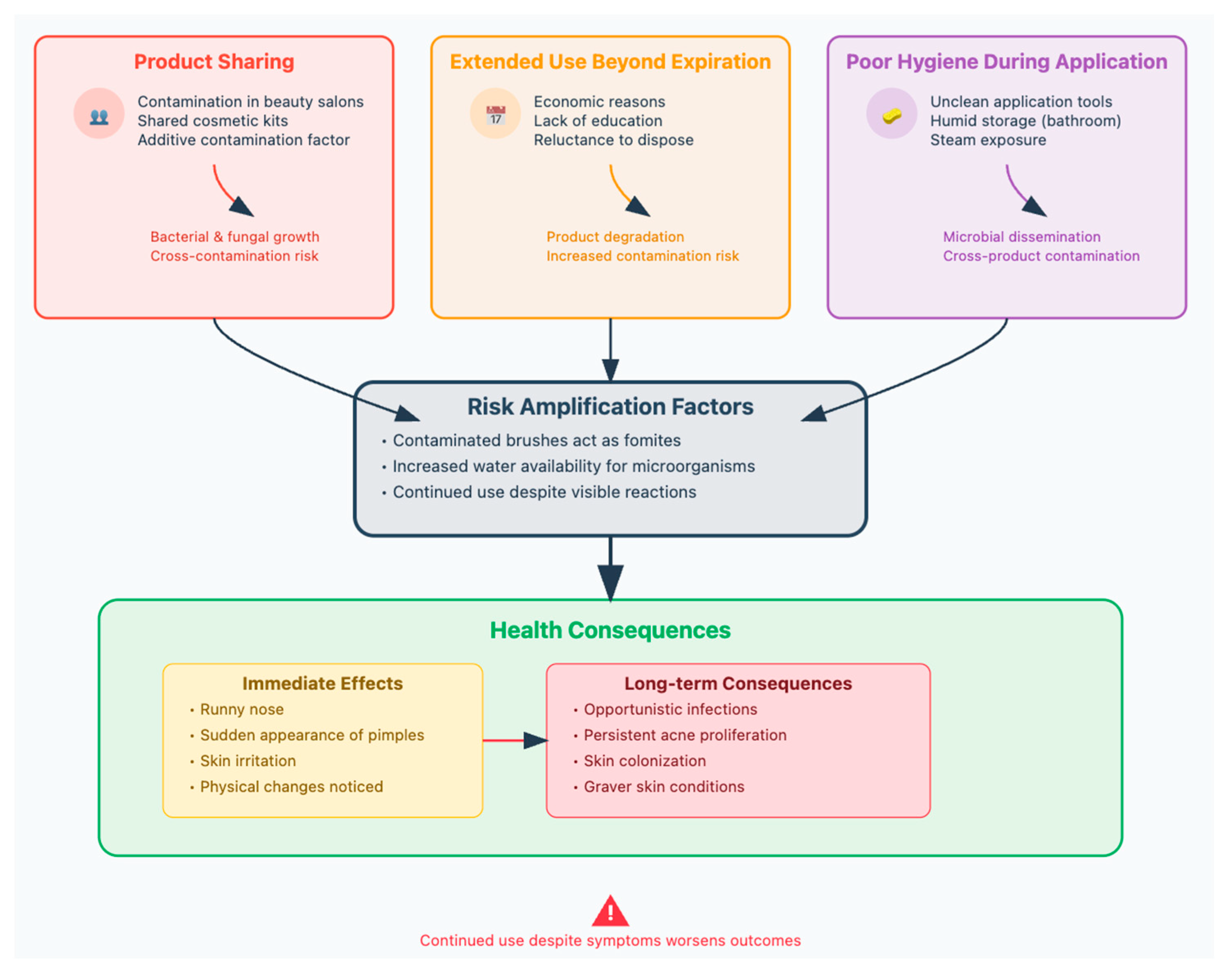
4. Beyond Manufacturing: Consumer Environment Risks
Consumers’ practices lead to risk amplification, which then results in health consequences that can progress from immediate to long-term if not addressed. Continued use despite any symptoms significantly worsens outcomes (Table 8) (Figure 5) [88,89].

Table 8.
Consumers’ common practices that allow microbial growth in cosmetic products.
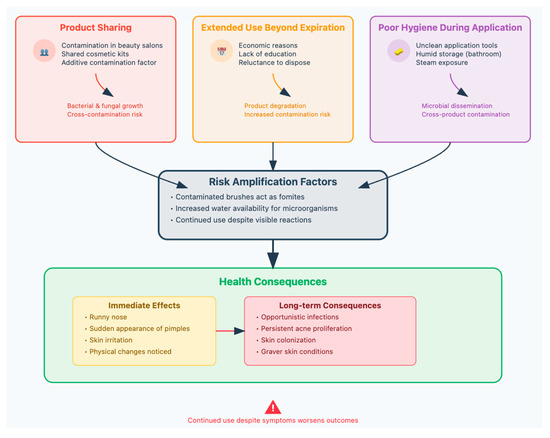
Figure 5.
Common practices among consumers, associated with cosmetics, that can decrease products’ security.
5. Preventing and Controlling Microbial Growth in Cosmetic Products
Microbiological contamination poses significant risks to cosmetic manufacturers, leading to production batch rejection and costly product recalls that damage brand reputation and consumer trust [3]. Market Introduction Authorization (MIA) holders must maintain strict hygiene and quality control to protect consumer health, particularly vulnerable populations, including children, the elderly, and immunosuppressed patients [87,93].
From the user’s perspective, contamination prevention is challenging due to inadequate protocols outside factory settings. Poor environmental control, lack of health risk awareness, and problematic consumer practices (product sharing, improper storage) contribute to microbial growth [84]. Products are considered low microbiological risk when they meet specific criteria, such as pH below 3.0 or above 10.0 (hostile to microbial growth); alcohol content above 20% (antimicrobial properties) [82,94]; filling temperature above 65 °C (reduces manufacturing contamination); water activity of 0.75 or lower [95]; high organic solvent content; and hydrogen peroxide above 3.0% (inhibits bacterial growth) [84].
In order to prevent and control microbial growth, preservatives are selected based on toxicological safety, antimicrobial efficacy, formulation compatibility, chemical stability, regulatory compliance, and environmental impact. Challenge tests verify preservative effectiveness by deliberately introducing microbial strains under controlled conditions and monitoring their reduction over time [82,87,93]. Several advanced preservation strategies are being researched: in microencapsulation, natural preservatives are enclosed within protective matrices to shield them from environmental factors and enable controlled release while enhancing stability; and multiple natural preservatives with complementary mechanisms are combined to achieve a broader antimicrobial spectrum while reducing individual preservative concentrations, minimizing toxicity and skin irritation [96]. These strategies represent essential developments as manufacturers seek secure products that address both microbiological and toxicological concerns while maintaining regulatory compliance [97,98].
Chemical Preservatives and Physical Methods
Naturally occurring phenolic compounds serve as commonly used bio-preservatives for extending finished product shelf life (Table 9). However, safety considerations have emerged regarding their performance compared to synthetic chemical preservatives.

Table 9.
Main chemical preservatives used in cosmetic products [6,84].
Poor hygiene during application and persistent use promotes loss of product stability and ongoing microbial proliferation both within the products and on the skin surface, creating opportunities for pathogenic infections. Without proper intervention, these minor issues can escalate into serious dermatological conditions, including acne, chronic hyperpigmentation, facial eczema, and extensive bacterial colonization of the skin by S. aureus [102,103,104].
Pump dispensers, pressurized delivery, or unit dose are described to provide appropriate protection from contamination during use of the cosmetic products [105]. Airless pumps are a good method for preventing aerobic microorganism development, since the formulations are not allowed to be in contact with oxygen [106]. Nevertheless, it is important to account for their limitations, such as particle diameters, making the prototype design and validation before its use in cosmetics indispensible [107]. Also, they do not prevent anaerobic development, therefore it is very important to continue to develop studies on preservative efficacy in such conditions [106]. Single-use applicators are an ideal choice for secondary packaging for their ability to provide physical protection against general contamination provided by consumer use [105].
6. Conclusions and Future Perspectives
Cosmetic products are a common source of microbial contamination, primarily due to consumer handling practices. While the pharmaceutical industry is bound by strict regulations and guidelines, end users often lack education on proper usage and hygiene. This gap undermines the industry’s efforts, as expectations for product safety are held to pharmaceutical standards but consumer behaviors fall far below that scope. Improper hygiene, disregard for expiration or usage timelines, and inadequate handling habits accelerate product degradation—either through additive microbial contamination or the loss of preservative efficacy. As a result, consumers may unknowingly expose themselves to dermatological and ocular pathologies, not because of formulation failure, but due to preservative breakdown or cumulative misuse, or pre-existing microbiological contamination.
Emerging trends in cosmetic preservation include natural compounds such as Rubus rosaefolius extract [108], essential oil from plants [109], and henna [110], which are some of the natural substances being studied for their preservative, antimicrobial, and antifungal activities, respectively. Natural antimicrobial compounds are alternatives for consumers who present with allergies to the traditional preservatives used most often in the cosmetic industry, but they do not offer the industry the same levels of confidence when compared with chemical preservatives and cosmetic stability. Also, natural compounds are more susceptible to degradation, posing a less effective form of preservative when compared with chemical preservatives [111]. Smart packaging technologies are a trend in the current cosmetic scenario for enhancing functionality and product performance, enabling traceability and sustainability through intelligent design, and facilitating personalized consumer experiences [112]. Between 2012 and 2016, an active packaging technology was developed by the EU, which allows us to produce new packages that are able to release natural preservatives at an adequate rate and amount into cosmetic products, lowering the need for preservative quantities in cosmetic formulations [113]. This is very useful both for allergy reasons and for product formulation stability [114], as the tables mentioned in that “Supplementary Materials” section.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics12050198/s1, Table S1: Current applications and future directions of smart packaging technologies in the cosmetic industry; Table S2: Suggestions for a multi-faced approach to avoid cosmetic microbial contamination during cosmetic use [112,113,114,115,116,117,118].
Author Contributions
Conceptualization, C.F.R. and F.A.M.S.; methodology, all authors; validation, C.F.R. and F.A.M.S.; formal analysis, all authors; investigation, all authors; data curation, C.F.R. and J.D.d.S.; writing—original draft preparation, all authors.; writing—review and editing, C.F.R. and F.A.M.S.; supervision, C.F.R. and F.A.M.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received internal funding from 1H-TOXRUN—CESPU: PhytoCandd_GI2-CESPU-2023—Preventive and therapeutic potential of novel plants extracts in oral candidiasis, and ToxiBug4Ca-GI2-CESPU-2025 Polymorphic toxins and Insect-Derived Peptides: potential anti-Candida and anti-biofilm effects.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roslan, M.A.; Aziz, N.A.; Rani, A.R.A.; Roslan, R.; Mohamad, C.A.C. Product Liability Law: Producer’s Liability for Harmful Cosmetic Products. Malays. J. Soc. Sci. Humanit. (MJSSH) 2023, 8, e002323. Available online: https://www.researchgate.net/publication/371180163_Product_Liability_Law_Producer’s_Liability_for_Harmful_Cosmetic_Products (accessed on 28 June 2025). [CrossRef]
- Hye, W.; Yae, S.S.; Min, S.R. Risk Factors Influencing Contamination of Customized Cosmetics Made On-the-Spot: Evidence from the National Pilot Project for Public Health. Sci. Rep. 2020, 10, 1561. Available online: https://www.researchgate.net/publication/338945011_Risk_factors_influencing_contamination_of_customized_cosmetics_made_on-the-spot_Evidence_from_the_national_pilot_project_for_public_health (accessed on 28 June 2025). [PubMed]
- European Commission. SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation—12th Revision—European Commission. Available online: https://health.ec.europa.eu/publications/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision_en (accessed on 30 May 2025).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://data.europa.eu/eli/reg/2009/1223/oj/eng (accessed on 28 June 2025).
- European Commission. EudraLex—Volume 4: Good Manufacturing Practice (GMP) Guidelines. Available online: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 28 June 2025).
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akhand, S. Potential Contamination in Cosmetics: A Review. Syst. Rev. Pharm. 2023, 14, 641. [Google Scholar]
- Bashir, A.; Lambert, P. Microbiological study of used cosmetic products: Highlighting possible impact on consumer health. J. Appl. Microbiol. 2020, 128, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Bano, S.; Tunio, S.A.; Brohi, N.A.; Siddiqui, A. Evaluating the Bacterial Contamination in Used Cosmetic Products: A Potential Threat to Consumer’s Health. Proc. Pak. Acad. Sci. B. Life Environ. Sci. 2024, 61, 363–370. Available online: https://www.researchgate.net/publication/388798918_Evaluating_the_Bacterial_Contamination_in_Used_Cosmetic_Products_A_Potential_Threat_to_Consumer’s_Health (accessed on 10 June 2025). [CrossRef]
- FDA Authority Over Cosmetics: How Cosmetics Are Not FDA-Approved, but Are FDA-Regulated. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated#Who_is_responsible (accessed on 14 June 2025).
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: The evolution of culture media in clinical microbiology. New Microbes New Infect. 2020, 34, 100622. Available online: https://www.sciencedirect.com/science/article/pii/S2052297519301192 (accessed on 14 June 2025). [CrossRef]
- Detmer, A.; Jørgensen, C.; Nylén, D. A Guidance Document on Microbiological Control of Cosmetic Products; Danish Environmental Protection Agency: Odense, Denmark, 2010.
- Yazdani, M.; Elgstøen, K.B.P.; Utheim, T.P. Eye make-up products and dry eye disease: A mini review. Curr. Eye Res. 2022, 47, 1–11. [Google Scholar] [CrossRef]
- European Rapid Alert system for dangerous products (RAPEX)|Interoperable Europe Portal. 2020. Available online: https://interoperable-europe.ec.europa.eu/collection/rapex (accessed on 10 June 2025).
- European Commission. Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products 2005–2025. Available online: https://ec.europa.eu/safety-gate-alerts/screen/search (accessed on 14 June 2025).
- Enforcement Report. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm#tabNav_advancedSearch (accessed on 14 June 2025).
- Enforcement Report Juice Beauty Illuminating Eye Color 1.5 Grams (0.5 oz.); Color: Champagne Fard a Paupieres Positive for the Presence of Bacillus Cereus. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=129093 (accessed on 14 June 2025).
- Enforcement Report Juice Beauty Illuminating Eye Color 1.5 Grams (0.5 oz.); Color: Chocolate; Fard a Paupieres Presence of Bacillus Cereus. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=129094 (accessed on 14 June 2025).
- Enforcement Report Juice Beauty Illuminating Eye Color 1.5 Grams (0.5 oz.); Color: Cappuccino; Positive for the Presence of Bacillus Cereus. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=129095 (accessed on 14 June 2025).
- Pitt, T.L.; McClure, J.; Parker, M.D.; Amézquita, A.; McClure, P.J. Bacillus cereus in personal care products: Risk to consumers. Int. J. Cosmet. Sci. 2015, 37, 165–174. [Google Scholar] [CrossRef]
- Babalola, M.O.; Eze, M. Microbiological Quality and Characterization of Potential Pathogens Associated with Selected Brands of Commercial Cosmetic Products in Nigeria. Br. Microbiol. Res. J. 2015, 9, 1–17. Available online: https://www.researchgate.net/publication/281892369_Microbiological_Quality_and_Characterization_of_Potential_Pathogens_Associated_with_Selected_Brands_of_Commercial_Cosmetic_Products_in_Nigeria (accessed on 15 June 2025). [CrossRef]
- Almukainzi, M.; Alotaibi, L.; Abdulwahab, A.; Albukhary, N.; El Mahdy, A.M. Quality and safety investigation of commonly used topical cosmetic preparations. Sci. Rep. 2022, 12, 18299. [Google Scholar] [CrossRef]
- Consumer Product Information Database—Benefit Cosmetics recalled Golden Gate Glam Holiday 2017 Full Face Kit Since Samples of Eyebrow Products Have Tested Positive for Pseudomonas Aeruginosa. Available online: https://www.whatsinproducts.com/product_recalls/view/1/156 (accessed on 14 June 2025).
- Enforcement Report SANTEE Diamond Color Mascara, Bacillus lentus, Pseudomonas putida, and Achromobacter xylosoxidans. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=142213 (accessed on 14 June 2025).
- Enforcement Report Kleancolor Bow-Tux Volumizing Mascara Bacillus Cereus, Staphylococcus Epidermidis, and Staphylococcus Warneri Contamination. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=142856 (accessed on 14 June 2025).
- Enforcement Report Foaming Soap Contamination 4. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=126295 (accessed on 14 June 2025).
- Enforcement Report Foaming Soap Contamination. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=126292 (accessed on 14 June 2025).
- Enforcement Report Foaming Soap Contamination 2. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=126293 (accessed on 14 June 2025).
- Enforcement Report Foaming Soap 3. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=126294 (accessed on 14 June 2025).
- Enforcement Report Natural Wellness Splish Splash is Recalled Due to the Potential to be Contaminated with Pseudomonas Aeruginosa. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=165562 (accessed on 14 June 2025).
- Enforcement Report Pore Patrol Clay-to Foam Cleanser Recalled Due to the Presence of Pseudomonas Aeruginosa Bacteria. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=170852 (accessed on 14 June 2025).
- Enforcement Report Liberty Hair & Body Shampoo, Contains Generic E. coli and P. aeruginosa. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=179238 (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products of Pseudomonas Aeruginosa Shampoo. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/114453?lang=en (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products Basisches Shampoo Pseudomonas aeruginosa. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/110804?lang=en (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products “Shampoo—Hair Treatment Shampoo Pseudomonas aeruginosa. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/-16967 (accessed on 14 June 2025).
- Budecka, A.; Kunicka-Styczyńska, A. Microbiological contaminants in cosmetics—Isolation and characterization. Biotechnol. Food Sci. 2014, 78, 15–23. [Google Scholar]
- Enforcement Report KLM Hand & Body Moisturizing Lotion with Oatmeal P. auriginosa E Putida. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=127359 (accessed on 14 June 2025).
- Enforcement Report Medline Skintegrity Moisturizing Lotion. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=126935 (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products Hand Cream Pseudomonas aeruginosa. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/117426?lang=en (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products Facial Milk Product Poses a Microbiological Risk Due to the Presence of Pseudomonas aeruginosa. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/-13285 (accessed on 14 June 2025).
- Enforcement Report Glow Body Butter Pseudomonas. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=129372 (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products “Massage Cream—Massage und Pflege Balsam, 100% Naturrein—Sheabutter (Massage and Skin-Care Cream, 100% Pure Shea Butter) S. aureus. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/-15028 (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products Enterobacter Gergoviae. Eye Cream. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/-18256 (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products Eye Contour Cream—PEPTILYS Contour des Yeux Rhizobium Radiobacter, Soil Saprophyte. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/-13167 (accessed on 14 June 2025).
- Enforcement Report Eyebrow Products Have Tested Positive for Pseudomonas aeruginosa. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=159434 (accessed on 14 June 2025).
- Enforcement Report Eyebrow Products Have Tested Positive for Pseudomonas aeruginosa. 2. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=159435 (accessed on 14 June 2025).
- Enforcement Report Eyebrow Products Have Tested Positive for Pseudomonas aeruginosa 3. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=159504 (accessed on 14 June 2025).
- Enforcement Report Eyebrow Products Have Tested Positive for Pseudomonas aeruginosa. 4. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=159505 (accessed on 14 June 2025).
- Suchitra, G.; Sanchita, C.; ResearchGate. Microbial Contamination of Commercial Cosmetic Products—A Review on Methods for Evaluation of Microbiological Safety and Guidelines Governing the Quality. Available online: https://www.researchgate.net/publication/364111619_MICROBIAL_CONTAMINATION_OF_COMMERCIAL_COSMETIC_PRODUCTS_-_A_REVIEW_ON_METHODS_FOR_EVALUATION_OF_MICROBIOLOGICAL_SAFETY_AND_GUIDELINES_GOVERNING_THE_QUALITY (accessed on 15 June 2025).
- Enforcement Report Gentle Steps Baby Lotion positive for, E. coli. Available online: https://www.accessdata.fda.gov/scripts/ires/index.cfm?Product=190431 (accessed on 14 June 2025).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products Skin Lotion—Ziegenbutter-Crème/Goat Butter Cream P. aeruginosa. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReport/alertDetail/-15102 (accessed on 14 June 2025).
- Vieira Hde, M.; Reis PCde, S.; Rocha, K.C.G.; Cardoso, A.M. Contaminação microbiológica em maquiagens de uso compartilhado. Rev. Bras. Mil. Ciênc. 2025, 11. Available online: https://rbmc.org.br/rbmc/article/view/184 (accessed on 30 May 2025). [CrossRef]
- Conwill, A.; Kuan, A.C.; Damerla, R.; Poret, A.J.; Baker, J.S.; Tripp, A.D.; Alm, E.J.; Lieberman, T.D. Anatomy promotes neutral coexistence of strains in the human skin microbiome. Cell Host Microbe. 2022, 30, 171–182.e7. [Google Scholar] [CrossRef] [PubMed]
- Rybtke, M.; Hultqvist, L.D.; Givskov, M.; Tolker-Nielsen, T. Pseudomonas aeruginosa Biofilm Infections: Community Structure, Antimicrobial Tolerance and Immune Response. J. Mol. Biol. 2015, 427, 3628–3645. [Google Scholar] [CrossRef] [PubMed]
- Ghias, M.; Fozouni, L. Assessment of Microbial Contamination and Metabolite Exposure in Cosmetic Products Used in Women’s Beauty Salons. Iran. J. Public Health 2024, 53, 1175–1183. [Google Scholar]
- Nusrat, N.; Ahmad Zahra, M.; Ahmed, A.; Haque, F. Assessment of potential pathogenic bacterial load and multidrug resistance in locally manufactured cosmetics commonly used in Dhaka metropolis. Sci. Rep. 2023, 13, 7787. [Google Scholar] [CrossRef]
- Morand, A.; Morand, J.J. Pseudomonas aeruginosa en dermatologie [Pseudomonas aeruginosa in dermatology]. Ann. Dermatol. Vénéréologie 2017, 144, 666–675. [Google Scholar] [CrossRef]
- Neuhaus, S.; Brockmann, S.; Al Dahouk, S.; Dieckmann, R. Survey on microbial contamination of opened skin care products used for tattooing. J. Appl. Microbiol. 2023, 134, lxad243. Available online: https://academic.oup.com/jambio/article/doi/10.1093/jambio/lxad243/7330802 (accessed on 10 July 2025). [CrossRef]
- Alshehrei, F.M. Isolation and Identification of Microorganisms associated with high-quality and low-quality cosmetics from different brands in Mecca region -Saudi Arabia. Saudi J. Biol. Sci. 2023, 30, 103852. [Google Scholar] [CrossRef]
- Vaccari, F.; Zhang, L.; Giuberti, G.; Grasso, A.; Bandini, F.; García-Pérez, P.; Copat, C.; Lucini, L.; Dall’aSta, M.; Ferrante, M.; et al. The impact of metallic nanoparticles on gut fermentation processes: An integrated metabolomics and metagenomics approach following an in vitro digestion and fecal fermentation model. J. Hazard. Mater. 2023, 453, 131331. [Google Scholar] [CrossRef]
- Edison, B.L.; Parsa, R.; Dufort, M.; Tierney, N.K.; Green, B.A.; Farris, P.K. Acetyl Dipeptide-31 Amide: A Novel Cosmetic Anti-Inflammatory Peptide That Demonstrates Anti-Aging, Firming, and Lifting Benefits. JDDonline—J. Drugs Dermatol. 2025, 24, 23–33. Available online: https://jddonline.com/articles/acetyl-dipeptide-31-amide-novel-cosmetic-anti-inflammatory-peptide-that-demonstrates-anti-aging-firming-lifting-benefits-S1545961625P8786X/ (accessed on 30 May 2025). [CrossRef]
- Yossa, N.; Bell, R.; Tallent, S.; Brown, E.; Binet, R.; Hammack, T. Genomic characterization of Bacillus cereus sensu stricto 3A ES isolated from eye shadow cosmetic products. BMC Microbiol. 2022, 22, 240. [Google Scholar] [CrossRef]
- Fessia, A.; Sartori, M.; García, D.; Fernández, L.; Ponzio, R.; Barros, G.; Nesci, A. In vitro studies of biofilm-forming Bacillus strains, biocontrol agents isolated from the maize phyllosphere. Biofilm 2022, 4, 100097. Available online: https://www.sciencedirect.com/science/article/pii/S2590207522000314 (accessed on 3 September 2025). [CrossRef] [PubMed]
- Yossa, N.; Arce, G.; Smiley, J.; Huang, M.J.; Yin, L.; Bell, R.; Tallent, S.; Brown, E.; Hammack, T. Survival and detection of Bacillus cereus in the presence of Escherichia coli, Salmonella enteritidis, Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans after rechallenge in make-up removers. Int. J. Cosmet. Sci. 2018, 40, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Nho, S.; Kim, S.; Kweon, O.; Howard, P.; Moon, M.; Sadrieh, N.; Cerniglia, C. Microbiological survey of commercial tattoo and permanent makeup inks available in the United States. J. Appl. Microbiol. 2018, 124, 1294–1302. [Google Scholar] [CrossRef]
- Zaghloul, R.; Abou-Aly, H.; Hanafy, E.; Emam, M. Microbial contamination of some cosmetics and personal care items in egypt. Egypt. J. Appl. Sci. 2015, 30, 424–441. [Google Scholar]
- Muhammed, H.J. Bacterial and Fungal Contamination in Three Brands of Cosmetic Marketed in Iraq. Iraqi J. Pharm. Sci. 2011, 20, 38–42. Available online: https://www.researchgate.net/publication/359562947_Bacterial_and_Fungal_Contamination_in_Three_Brands_of_Cosmetic_Marketed_in_Iraq (accessed on 31 May 2025). [CrossRef]
- Dadashi, L.; Dehghanzadeh, R. Investigating incidence of bacterial and fungal contamination in shared cosmetic kits available in the women beauty salons. Health Promot. Perspect 2016, 6, 159–163. [Google Scholar] [CrossRef]
- WHO. Fungal Priority Pathogens List to Guide Research, Development and Public Health Action, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; p. 1.
- Ahmed, M.A.E.-G.E.-S.; Abbas, H.S.; Kotakonda, M. Fungal Diseases Caused by Serious Contamination of Pharmaceuticals and Medical Devices, and Rapid Fungal Detection Using Nano-Diagnostic Tools: A Critical Review. Curr. Microbiol. 2023, 81, 1–10. Available online: https://link.springer.com/article/10.1007/s00284-023-03506-7 (accessed on 8 July 2025). [CrossRef] [PubMed]
- ISO 4973:2023 (En). Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:4973:ed-1:v1:en (accessed on 14 June 2025).
- ISO 11930:2019 (En). Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:11930:ed-2:v1:en (accessed on 14 June 2025).
- ISO 16212:2017 (En). Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:16212:ed-2:v1:en (accessed on 14 June 2025).
- ISO 17516:2014 (En). Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:17516:ed-1:v1:en (accessed on 14 June 2025).
- ISO 18415:2017 (En); Cosmetics—Microbiology—Detection of Specified and Non-Specified Microorganisms. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:18415:ed-2:v1:en (accessed on 14 June 2025).
- ISO 18416:2015/Amd 1:2022 (En); Cosmetics—Microbiology—Detection of Candida albicans—AMENDMENT 1. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:18416:ed-2:v2:amd:1:v1:en (accessed on 14 June 2025).
- ISO/TR 19838:2016 (En); Microbiology—Cosmetics—Guidelines for the Application of ISO Standards on Cosmetic Microbiology. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:tr:19838:ed-1:v1:en (accessed on 14 June 2025).
- ISO 21148:2017 (En); Cosmetics—Microbiology—General Instructions for Microbiological Examination. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:21148:ed-2:v1:en (accessed on 14 June 2025).
- ISO 21149:2017 (En); Cosmetics—Microbiology—Enumeration and Detection of Aerobic Mesophilic Bacteria. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:21149:ed-2:v1:en (accessed on 14 June 2025).
- ISO 21322:2020 (En); Cosmetics—Microbiology—Testing of Impregnated or Coated Wipes and Masks. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:21322:ed-1:v1:en (accessed on 14 June 2025).
- ISO 22717:2015 (En); Cosmetics—Microbiology—Detection of Pseudomonas aeruginosa. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:22717:ed-2:v1:en (accessed on 14 June 2025).
- ISO 29621:2017 (En); Cosmetics—Microbiology—Guidelines for the Risk Assessment and Identification of Microbiologically Low-Risk Products. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:29621:ed-2:v1:en (accessed on 14 June 2025).
- FDA 177960. Available online: https://www.fda.gov/media/177960/download (accessed on 14 June 2025).
- Uzdrowska, K.; Górska-Ponikowska, M. Preservatives in cosmetics technology. Aesthetic Cosmetol. Med. 2023, 12, 73–78. Available online: https://www.researchgate.net/publication/370551193_Preservatives_in_cosmetics_technology (accessed on 1 June 2025). [CrossRef]
- Scientific Committee on Consumer Safety (SCCS). Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation—12th Revision. 2023 May. Report No.: SCCS/1647/22. Available online: https://www.cirs-ck.com/files/bjaxyplqmqyo/content/2023/06/co905jgkgkjk.pdf (accessed on 14 June 2025).
- FDA Guidance for Industry. Available online: https://www.fda.gov/media/86366/download (accessed on 15 June 2025).
- Regulation—1223/2009—EN—Cosmetic Products Regulation—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2009/1223/oj/eng (accessed on 15 June 2025).
- Giacomel, C.B.; Dartora, G.; Dienfethaeler, H.S.; Haas, S.E. Investigation on the use of expired make-up and microbiological contamination of mascaras. Int. J. Cosmet. Sci. 2013, 35, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.G.; Hiremath, P.; Divya, K.; Abhiram, P. A survey study on adverse effects of synthetic cosmetics. Int. J. Community Med. Public Health 2023, 10, 4147–4152. Available online: https://www.researchgate.net/publication/375156388_A_survey_study_on_adverse_effects_of_synthetic_cosmetics (accessed on 15 June 2025).
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H. An Investigation into Incidences of Microbial Contamination in Cosmeceuticals in the UAE: Imbalances between Preservation and Microbial Contamination. Cosmetics 2020, 7, 92. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, Y.; Chang, Y.; Liang, X.; Zhang, H.; Lin, X.; Qing, K.; Zhou, X.; Luo, Z. The Effects of Ventilation, Humidity, and Temperature on Bacterial Growth and Bacterial Genera Distribution. Int. J. Environ. Res. Public Health 2022, 19, 15345. [Google Scholar] [CrossRef]
- Lee, S.; Patel, K.; Howard, M.; Tate, B. Allergic contact dermatitis associated with rubber-based cosmetic sponge. Contact Dermat. 2023, 89, 382–383. [Google Scholar] [CrossRef]
- ISO 22716:2007(en); Cosmetics—Good Manufacturing Practices (GMP)—Guidelines on Good Manufacturing Practices. ISO: Geneva, Switzerland, 2007. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:22716:ed-1:v2:en (accessed on 15 June 2025).
- Stroppel, L.; Schultz-Fademrecht, T.; Cebulla, M.; Blech, M.; Marhöfer, R.J.; Selzer, P.M.; Garidel, P. Antimicrobial Preservatives for Protein and Peptide Formulations: An Overview. Pharmaceutics. 2023, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Cundell, T. The role of water activity in the microbial stability of non-sterile pharmaceutical drug products. Eur Pharm Rev. 2015, 20, 58–63. [Google Scholar]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2016, 33, 1–17. Available online: https://www.researchgate.net/publication/284754922_Encapsulation_of_cosmetic_active_ingredients_for_topical_application_-_a_review (accessed on 8 July 2025). [CrossRef]
- Joujou, F.M.; Darra, N.E.; Rajha, H.N.; Sokhn, E.S.; Alwan, N. Evaluation of synergistic/antagonistic antibacterial activities of fatty oils from apricot, date, grape, and black seeds. Sci. Rep. 2024, 14, 6532. [Google Scholar] [CrossRef] [PubMed]
- Chandrayan, A.; Hood, M.M. Natural Preservatives in Cosmetics: Efficacy, Stability, and Regulatory Considerations. Available online: https://www.iosrphr.org/papers/vol14-issue4/E1404013740.pdf (accessed on 1 June 2025).
- Matwiejczuk, N.; Galicka, A.; Brzóska, M.M. Review of the safety of application of cosmetic products containing parabens. J Appl Toxicol. 2020, 40, 176–210. [Google Scholar] [CrossRef]
- Freeman, H.L.R.; Lexology. 2013 EU Scientific Committee on Consumer Safety Confirms That Parabens in Cosmetic Products Do Not Pose Health Risks When Used at Authorised Concentrations. Available online: https://www.lexology.com/library/detail.aspx?g=120c16d3-3cae-4d3a-9326-8bee2965482e (accessed on 28 June 2025).
- European Commission. Directorate General for Health and Food Safety. Opinion on methylparaben (CAS No. 99-76-3, EC No. 202-785-7). LU: Publications Office; 2024. Available online: https://data.europa.eu/doi/10.2875/86920 (accessed on 28 June 2025).
- Cherian, P.; Zhu, J.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.J.G.; Shank, R.C.; Slaga, T.J.; et al. Amended Safety Assessment of Parabens as Used in Cosmetics. Int. J. Toxicol. 2020, 39 (Suppl. 1), 5S–97S. [Google Scholar] [CrossRef]
- Phenoxyethanol. Available online: https://go.drugbank.com/drugs/DB11304 (accessed on 28 June 2025).
- Tang, Z.; Du, Q. Mechanism of action of preservatives in cosmetics. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100054. [Google Scholar] [CrossRef]
- Which Cosmetic Products Don’t Require Challenge Test?—Ceway Blog. Available online: https://news.ceway.eu/cosmetic-challenge-test/ (accessed on 18 June 2025).
- André, A.; Debande, L.; Marteyn, B. The selective advantage of facultative anaerobes relies on their unique ability to cope with changing oxygen levels during infection. Cell Microbiol. 2021, 23, e13338. [Google Scholar] [CrossRef]
- Cocchietto, M.; Blasi, P.; Lapasin, R.; Moro, C.; Gallo, D.; Sava, G. Microencapsulation of Bioactive Principles with an Airless Spray-Gun Suitable for Processing High Viscous Solutions. J. Funct. Biomater. 2013, 4, 312–328. [Google Scholar] [CrossRef]
- Ostrosky, E.A.; Marcondes, E.M.C.; Nishikawa, S.d.O.; Lopes, P.S.; Varca, G.H.C.; Pinto, T.d.J.A.; Consiglieri, T.V.O.; Baby, A.R.; Velasco, M.V.R.; Kaneko, T.M. Rubus rosaefolius extract as a natural preservative candidate in topical formulations. AAPS PharmSciTech 2011, 12, 732–737. [Google Scholar] [CrossRef]
- Barbieri, N.; Costamagna, M.; Gilabert, M.; Perotti, M.; Schuff, C.; Isla, M.I.; Benavente, A. Antioxidant activity and chemical composition of essential oils of three aromatic plants from La Rioja province. Pharm. Biol. 2016, 54, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Rahmoun, N.; Boucherit-Otmani, Z.; Boucherit, K.; Benabdallah, M.; Choukchou-Braham, N. Antifungal activity of the Algerian Lawsonia inermis (henna). Pharm. Biol. 2013, 51, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Shkreli, R.; Terziu, R.; Memushaj, L.; Dhamo, K.; Malaj, L. Selected Essential Oils as Natural Ingredients in Cosmetic Emulsions: Development, Stability Testing and Antimicrobial Activity. Indian. J. Pharm. Educ. Res. 2023, 57, 125–133. [Google Scholar] [CrossRef]
- Ajanta Bottle Blog. Smart Packaging in Cosmetics: 7 Powerful Technologies Revolutionizing the Industry. Available online: https://www.ajantabottle.com/blog/smart-packaging-in-cosmetics-ajanta/ (accessed on 28 June 2025).
- ACTICOSPACK: Reducing the Levels of Preservatives in Cosmetic Products. 2022. Available online: https://itene.com/en/success-stories/acticospack-preservatives-cosmetics/ (accessed on 28 June 2025).
- Lidén, C.; Andersson, N.; White, I.R. Preservatives in non-cosmetic products: Increasing human exposure requires action for protection of health. Contact Dermat. 2022, 87, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Fofana, O.; Packaging Gateway. Smart Packaging Revolution: QR Codes and AR Lead the Way. 2024. Available online: https://www.packaging-gateway.com/features/smart-packaging-revolution/ (accessed on 28 June 2025).
- Makeup Artist Sanitation Standard—The Makeup Standard. Available online: https://themakeupstandard.org/service/makeup-artist-sanitation-standard/ (accessed on 18 June 2025).
- Tran, T.T.; Hitchins, A.D. Microbial survey of shared-use cosmetic test kits available to the public. J. Ind. Microbiol. 1994, 13, 389–391. [Google Scholar] [CrossRef]
- Cosmetic Facts by the FDA. Available online: https://www.fda.gov/media/93074/download (accessed on 14 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).