Laser-Assisted Exosome Delivery (LAED) with Fractional CO2 Laser: A Pilot Two-Case Report and Narrative Review
Abstract
1. Introduction
2. Laser-Assisted Drug Delivery (LADD)
3. Exosomes in Dermatology
4. Integrated Methods and Clinical Evidence
4.1. Case 1—Atrophic Acne Scars
4.2. Case 2—Diffuse Hyperpigmentation of the Face
5. Laser-Assisted Exosome Delivery (LAED)
6. Limitations
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCE | Adipose Stem Cell–derived Exosomes |
| ATP | Adenosine Triphosphate |
| CO2 | Carbon Dioxide |
| ECCA | Échelle d’Évaluation Clinique des Cicatrices d’Acné |
| Er:YAG | Erbium-doped Yttrium Aluminum Garnet |
| EU | Endotoxin Unit(s) |
| FACE-Q | Facial Aesthetic Clinical Evaluation Questionnaire |
| HIF-1 | Hypoxia-Inducible Factor-1 |
| ISO | International Organization for Standardization |
| LADD | Laser-Assisted Drug Delivery |
| LAED | Laser-Assisted Exosome Delivery |
| LAL | Limulus Amebocyte Lysate (assay) |
| LLLT | Low-Level Laser Therapy |
| MAZ(s) | Microablative Zone(s) |
| MSC(s) | Mesenchymal Stem Cell(s) |
| Nd:YAG | Neodymium-doped Yttrium Aluminum Garnet |
| NF-κB | Nuclear Factor kappa-B |
| OCT | Optical Coherence Tomography |
| PIH | Post-Inflammatory Hyperpigmentation |
| PRP | Platelet-Rich Plasma |
| PRO(s) | Patient-Reported Outcome(s) |
| PROM(s) | Patient-Reported Outcome Measure(s) |
| SPF | Sun Protection Factor |
| TNF-α | Tumor Necrosis Factor-alpha |
| UV | Ultraviolet |
| UVB | Ultraviolet B |
| VISIA® | VISIA® Complexion Analysis System |
References
- Goel, A.; Krupashankar, D.; Aurangabadkar, S.; Nischal, K.; Omprakash, H.; Mysore, V. Fractional lasers in dermatology—Current status and recommendations. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 369. [Google Scholar] [CrossRef]
- Cohen, S.R.; Goodacre, A.; Lim, S.; Johnston, J.; Henssler, C.; Jeffers, B.; Saad, A.; Leong, T. Clinical outcomes and complications associated with fractional lasers: A review of 730 patients. Aesthetic Plast. Surg. 2017, 41, 171–178. [Google Scholar] [CrossRef]
- Jih, M.H.; Kimyai-Asadi, A. Fractional photothermolysis: A review and update. Semin. Cutan. Med. Surg. 2008, 27, 63–71. [Google Scholar] [CrossRef]
- Bodendorf, M.O.; Grunewald, S.; Wetzig, T.; Simon, J.C.; Paasch, U. Fractional laser skin therapy. JDDG J. Ger. Soc. Dermatol. 2009, 7, 301–308. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lee, G.Y. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: A randomized controlled trial. Ann. Dermatol. 2011, 23, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Clementi, A.; Cannarozzo, G.; Guarino, L.; Zappia, E.; Cassalia, F.; Alma, A.; Sannino, M.; Longo, C.; Nisticò, S.P. Sequential fractional CO2 and 1540/1570 nm lasers: A narrative review of preclinical and clinical evidence. J. Clin. Med. 2025, 14, 3867. [Google Scholar] [CrossRef]
- Campolmi, P.; Cannarozzo, G.; Bennardo, L.; Clementi, A.; Sannino, M.; Nisticò, S.P. Fractional micro-ablative CO2 laser as therapy in penile lichen sclerosus. J. Lasers Med. Sci. 2021, 12, e61. [Google Scholar] [CrossRef]
- Ong, M.W.S.; Bashir, S.J. Fractional laser resurfacing for acne scars: A review. Br. J. Dermatol. 2012, 166, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; Manela-Azulay, M.; Cuzzi, T. Photoaging and the clinical utility of fractional laser. Clin. Cosmet. Investig. Dermatol. 2016, 9, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Amato, S.; Nisticò, S.P.; Clementi, A.; Stabile, G.; Cassalia, F.; Dattola, A.; Rizzuto, G.; Cannarozzo, G. Multispectral imaging and OCT-guided precision treatment of rhinophyma with CO2 and dye lasers: A comprehensive diagnostic and therapeutic approach. Cosmetics 2024, 11, 221. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Hop, I.; Branewska, J.; Ostrowska, B.; Matysek, M.; Olszanicka, A.; Imioło, J.; Maciąg, A.; Niemiec, R.; Galas, A. CO2 ablative fractional laser—Mechanism of action and assessment of safety, effectiveness and possible side effects: Literature review. J. Educ. Health Sport 2023, 21, 72–77. [Google Scholar] [CrossRef]
- Nisticò, S.P.; Bennardo, L.; Zingoni, T.; Pieri, L.; Fusco, I.; Rossi, F.; Magni, G.; Cannarozzo, G. Synergistic sequential emission of fractional 10,600 and 1540 nm lasers for skin resurfacing: An ex vivo histological evaluation. Medicina 2022, 58, 1308. [Google Scholar] [CrossRef]
- Berlin, A.L.; Hussain, M.; Phelps, R.; Goldberg, D.J. A prospective study of fractional scanned nonsequential carbon dioxide laser resurfacing: Clinical and histopathologic evaluation. Dermatol. Surg. 2009, 35, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Fife, D.J.; Fitzpatrick, R.E.; Zachary, C.B. Complications of fractional CO2 laser resurfacing: Four cases. Lasers Surg. Med. 2009, 41, 179–184. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, S.; Kaur, T.; Bassi, R. Complications of fractional ablative carbon dioxide laser in various aesthetic procedures: A retrospective study. Int. J. Res. Dermatol. 2019, 5, 664–667. [Google Scholar] [CrossRef]
- Ramsdell, W.M. Fractional CO2 laser resurfacing complications. Semin. Plast. Surg. 2012, 26, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Lodi, G.; Cassalia, F.; Sannino, M.; Cannarozzo, G.; Baroni, A.; Amato, S.; Zappia, E.; Pellacani, G.; Nisticò, S.P. Blue light therapy in dermatological practice: A review. Cosmetics 2025, 12, 30. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef]
- Yousefian, F.; Espinoza, L.; Yadlapati, S.; Lorenc, Z.P.; Gold, M. A comprehensive review of the medical and cosmetic applications of exosomes in dermatology. J. Cosmet. Dermatol. 2024, 23, 1224–1228. [Google Scholar] [CrossRef]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Basu, J.; Ludlow, J.W. Exosomes for repair, regeneration and rejuvenation. Expert. Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoo, S.-M.; Park, H.H.; Lim, H.J.; Kim, Y.L.; Lee, S.; Seo, K.W.; Kang, K.S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, S.; Wu, K.; Zhao, R.; Cao, L.; Wang, H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J. Cosmet. Dermatol. 2020, 19, 574–581. [Google Scholar] [CrossRef]
- Novis, T.; Gomes Menezes, A.H.; Vilaça Lima, L.C.; Lötvall, J.; Borges do Nascimento, I.J.; Maeda Takiya, C. The therapeutic, diagnostic, and prognostic values of extracellular vesicles (exosomes) in dermatology: A systematic review. JAAD Rev. 2024, 1, 135–174. [Google Scholar] [CrossRef]
- Semsarzadeh, N.; Andrasik, W.; Khetarpal, S. Stem cells and exosomes in aesthetic medicine. Adv. Cosmet. Surg. 2021, 4, 59–70. [Google Scholar] [CrossRef]
- Pinto, H.; Sánchez-Vizcaíno Mengual, E. Exosomes in the real world of medical aesthetics: A review. Aesthetic Plast. Surg. 2024, 48, 2513–2527. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic values of exosomes in cosmetics, skin care, tissue regeneration, and dermatological diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Park, G.H.; Kwon, H.H.; Seok, J.; Yang, S.H.; Lee, J.; Park, B.C.; Shin, E.; Park, K.Y. Combined treatment with human adipose tissue stem cell-derived exosome solution and microneedling for facial skin aging: 12-week prospective, randomized, split-face study. J. Cosmet. Dermatol. 2023, 22, 3418–3426. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, J.; He, L.; Khan, A.; Xiong, T.; Shen, H.; Li, Z. Exosomes-based advancements for application in medical aesthetics. Front. Bioeng. Biotechnol. 2022, 10, 1083640. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.C.; Omer Sulaiman, H.; Anderson, S.R.; Abtahi, A.R. The potential role of exosomes in aesthetic plastic surgery: A review of current literature. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5051. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the barriers of the skin: Exosome therapy. Biomater. Res. 2021, 25, 22. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-derived exosomes accelerate wound healing via anti-inflammation in diabetic rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Wu, Y.; Song, G.; Azizi, R.; Zamani, A. Mesenchymal stromal cells and their exosomes in skin wound healing: A comprehensive review. Stem Cell Res. Ther. 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ritter, T. The exosome—A naturally secreted nanoparticle—And its application to wound healing. Adv. Mater. 2016, 28, 5542–5552. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhou, S.; Zeng, L.; Gu, Q.; Xue, H.; Wang, F.; Feng, J.; Cui, S.; Shi, L. Exosome-based therapeutics in dermatology. Biomater. Res. 2025, 29, 0148. [Google Scholar] [CrossRef]
- Javeed, N.; Mukhopadhyay, D. Exosomes and their role in the micro/macro-environment: A comprehensive review. J. Biomed. Res. 2017, 31, 386–394. [Google Scholar] [CrossRef]
- Ersan, M.; Ozer, E.; Akin, O.; Tasli, P.N.; Sahin, F. Effectiveness of exosome treatment in androgenetic alopecia: Outcomes of a prospective study. Aesthetic Plast. Surg. 2024, 48, 4262–4271. [Google Scholar] [CrossRef]
- Lueangarun, S.; Cho, B.S.; Tempark, T. Hair repigmentation of poliosis circumscripta in androgenetic alopecia treated with exosomes and fractional picosecond laser. J. Cosmet. Dermatol. 2024, 23, 2307–2311. [Google Scholar] [CrossRef]
- Proietti, I.; Battilotti, C.; Svara, F.; Innocenzi, C.; Spagnoli, A.; Potenza, C. Microneedling plus exosomes for melasma: Efficacy and tolerability. Appl. Sci. 2024, 14, 7252. [Google Scholar] [CrossRef]
- Wang, T.; Gao, H.; Wang, D.; Zhang, C.; Hu, K.; Zhang, H.; Lin, J.; Chen, X. Stem cell-derived exosomes in melasma treatment and percutaneous penetration. Lasers Surg. Med. 2023, 55, 178–189. [Google Scholar] [CrossRef]
- Wenande, E.; Anderson, R.R.; Haedersdal, M. Fundamentals of fractional laser-assisted drug delivery: Methodology and data interpretation. Adv. Drug Deliv. Rev. 2020, 153, 169–184. [Google Scholar] [CrossRef]
- Sklar, L.R.; Burnett, C.T.; Waibel, J.S.; Moy, R.L.; Ozog, D.M. Laser-assisted drug delivery: A review of an evolving technology. Lasers Surg. Med. 2014, 46, 249–262. [Google Scholar] [CrossRef]
- Omi, T.; Numano, K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014, 23, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hædersdal, M.; Sakamoto, F.H.; Farinelli, W.A.; Doukas, A.G.; Tam, J.; Anderson, R.R. Fractional CO2 laser-assisted drug delivery. Lasers Surg. Med. 2010, 42, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.H.B.; Zhang, J.; Frame, J.; Najlah, M. Laser-assisted delivery of growth factors and vitamin C for scars: Randomised double-blind early clinical trial. Aesthetic Plast. Surg. 2021, 45, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.S.; Wulkan, A.J.; Rudnick, A.; Daoud, A. Hypertrophic scars: Laser-assisted corticosteroid vs 5-fluorouracil delivery. Dermatol. Surg. 2019, 45, 423–430. [Google Scholar] [CrossRef]
- Truong, K.; Prasidha, I.; Wain, T. Laser-assisted drug delivery for keloid/hypertrophic scars: Systematic review of RCTs. Lasers Med. Sci. 2022, 37, 47–59. [Google Scholar] [CrossRef]
- Steeb, T.; Schlager, J.G.; Kohl, C.; Ruzicka, T.; Heppt, M.V.; Berking, C. Laser-assisted photodynamic therapy for actinic keratosis: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 947–956. [Google Scholar] [CrossRef]
- Haykal, D.; Cartier, H.; Goldberg, D.; Gold, M. Advancements in laser technologies for skin rejuvenation: Efficacy and safety. J. Cosmet. Dermatol. 2024, 23, 3078–3089. [Google Scholar] [CrossRef]
- Khedr, M.M.; Elhefnawy, A.A.; Mahmoud, W.H.; Mostafa, I.M.E.; Hantash, S.A. Laser-assisted delivery of tranexamic acid for facial post-burn hyperpigmentation. Eur. J. Plast. Surg. 2023, 46, 1349–1356. [Google Scholar] [CrossRef]
- Wenande, E.; Olesen, U.H.; Nielsen, M.M.B.; Janfelt, C.; Hansen, S.H.; Anderson, R.R.; Haedersdal, M. Fractional laser-assisted topical delivery enhances cutaneous 5-fluorouracil uptake. Expert Opin. Drug Deliv. 2017, 14, 307–317. [Google Scholar] [CrossRef]
- Meesters, A.A.; Nieboer, M.J.; Kezic, S.; de Rie, M.A.; Wolkerstorfer, A. Parameters in fractional laser-assisted delivery of topical anesthetics: Role of laser type/settings. Lasers Surg. Med. 2018, 50, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Cameli, N.; Abril, E.; Mariano, M.; Berardesca, E. Monopolar radiofrequency and transdermal drug delivery in melasma. Dermatol. Surg. 2014, 40, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. Advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Muskat, A.; Kost, Y.; Balazic, E.; Cohen, J.L.; Kobets, K. Laser-assisted drug delivery in scars, rhytids, and melasma: Literature review. Aesthet. Surg. J. 2023, 43, NP181–NP198. [Google Scholar] [CrossRef]
- Ibrahim, O.; Wenande, E.; Hogan, S.; Arndt, K.A.; Haedersdal, M.; Dover, J.S. Challenges to laser-assisted drug delivery: From theory to clinical practice. Lasers Surg. Med. 2018, 50, 20–27. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. Lessons learned from the first decade of laser-assisted drug delivery. Dermatol. Ther. Heidelb. 2021, 11, 93–104. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, S.; Chen, N.; Liao, J.; Zeng, L. Stem cell-derived exosomes: A supernova in cosmetic dermatology. J. Cosmet. Dermatol. 2021, 20, 3812–3817. [Google Scholar] [CrossRef]
- Dal’Forno-Dini, T.; Birck, M.S.; Rocha, M.; Bagatin, E. Exploring the reality of exosomes in dermatology. An. Bras. Dermatol. 2025, 100, 121–130. [Google Scholar] [CrossRef]
- McBride, J.D.; Rodriguez-Menocal, L.; Badiavas, E.V. Extracellular vesicles as biomarkers and therapeutics in dermatology: A focus on exosomes. J. Invest. Dermatol. 2017, 137, 1622–1629. [Google Scholar] [CrossRef]
- Bin Dayel, S.; Hussein, R.S. Exosomes in dermatology: Emerging roles in skin health and disease. Pharmaceutics 2025, 17, 600. [Google Scholar] [CrossRef]
- Kose, O.; Botsali, A.; Caliskan, E. Role of exosomes in skin diseases. J. Cosmet. Dermatol. 2022, 21, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Nahm, W.J.; Nikas, C.; Goldust, M.; Horneck, N.; Cervantes, J.A.; Burshtein, J.; Tsoukas, M. Exosomes in dermatology: Current applications, clinical evidence, and future directions. Int. J. Dermatol. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Quiñones-Vico, M.I.; Sanabria-de la Torre, R.; Sánchez-Díaz, M.; Sierra-Sánchez, Á.; Montero-Vílchez, T.; Fernández-González, A.; Arias-Santiago, S. Exosomes derived from mesenchymal stromal cells in dermatology. Front. Cell Dev. Biol. 2021, 9, 647012. [Google Scholar] [CrossRef]
- Adriano, B.; Cotto, N.M.; Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Milk exosomes: Nature’s abundant nanoplatform for theranostics. Bioact. Mater. 2021, 6, 2479–2490. [Google Scholar] [CrossRef]
- DEKA M.E.L.A. Exolight C & M; DEKA M.E.L.A.: Florence, Italy, 2025; Available online: https://www.dekalaser.com (accessed on 9 September 2025).
- Kwon, H.H.; Yang, S.H.; Lee, J.; Park, B.C.; Park, K.Y.; Jung, J.Y.; Bae, Y.; Park, G.H. Adipose-derived exosomes plus fractional CO2 laser for acne scars: A 12-week double-blind split-face RCT. Acta Derm. Venereol. 2020, 100, adv00147. [Google Scholar] [CrossRef] [PubMed]
- Fusco, I.; Zingoni, T.; Madeddu, F.; Colombo, C.; Amato, S.; Greco, M.E.; Rizzuto, G.; Zappia, E.; Bonan, P.; Nisticò, S. Preclinical results on Exolight exosomes as adjuvant to CO2 laser for skin aging management. JOJ Dermatol. Cosmet. 2025, 6, 555694. [Google Scholar] [CrossRef]
- Cassalia, F.; Ciolfi, C.; Scolaro, F.; Danese, A.; Lunardon, A.; Caroppo, F.; Belloni Fortina, A. Public use of social media on skin health: Effects on awareness and preventive behaviours. Acta Derm. Venereol. 2023, 103, adv15341. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Hashesmi, T.; Jafari, A.M.; Hamidnia, L.; Ansari, A.; Ayazi, M.; Harandi, N.T.; Mehran, Y.Z. Low-level laser therapy plus autologous exosomes in hair growth: Case series. Sch. J. Med. Case Rep. 2024, 12, 1250–1256. [Google Scholar] [CrossRef]
- Clementi, A.; Cannarozzo, G.; Amato, S.; Zappia, E.; Bennardo, L.; Michelini, S.; Morini, C.; Sannino, M.; Longo, C.; Nisticò, S.P. Dye laser applications in cosmetic dermatology: Efficacy and safety for vascular lesions and scars. Cosmetics 2024, 11, 227. [Google Scholar] [CrossRef]
- Wang, C.K.; Tsai, T.H.; Lee, C.H. Regulation of exosomes as biologic medicines: Development and manufacturing challenges. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef]
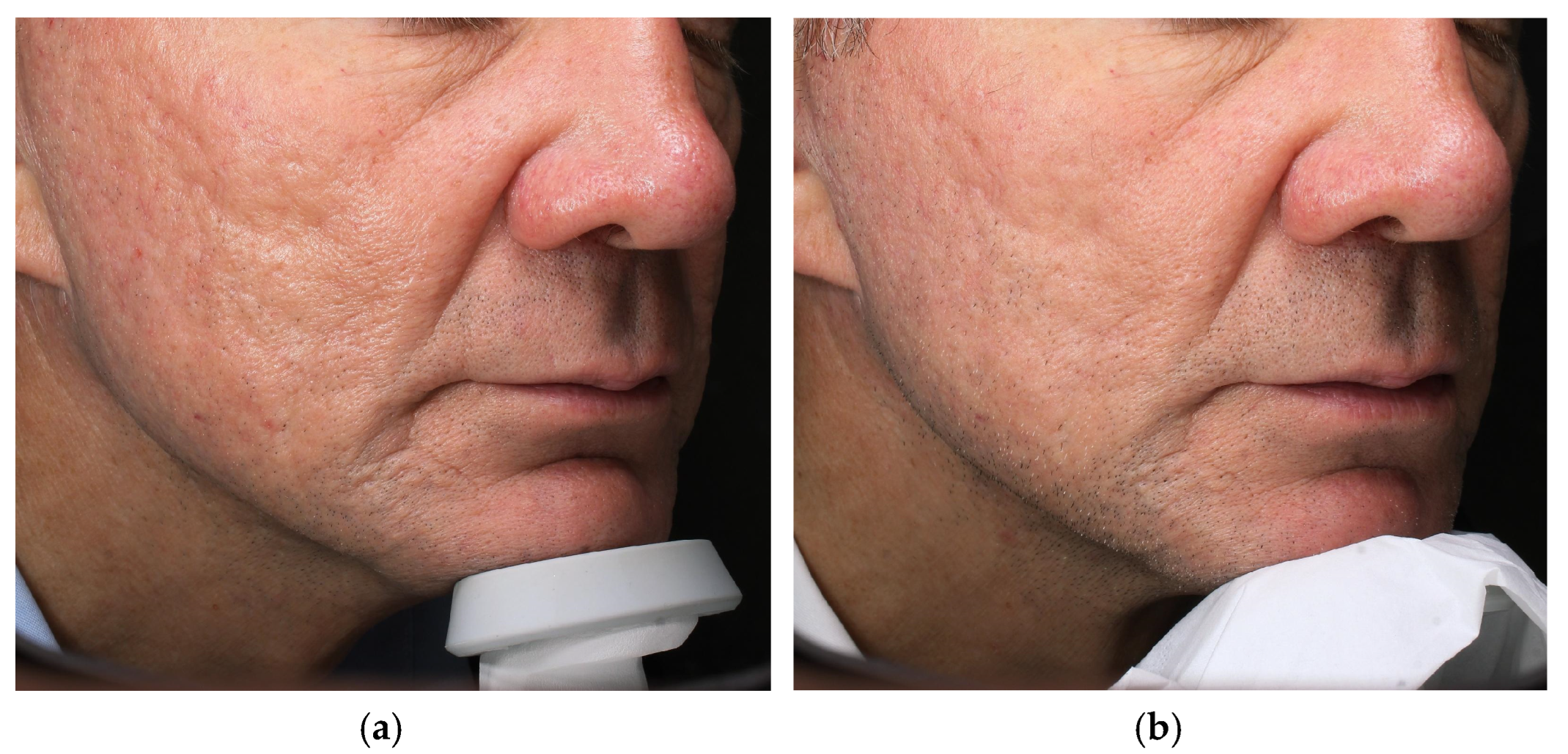
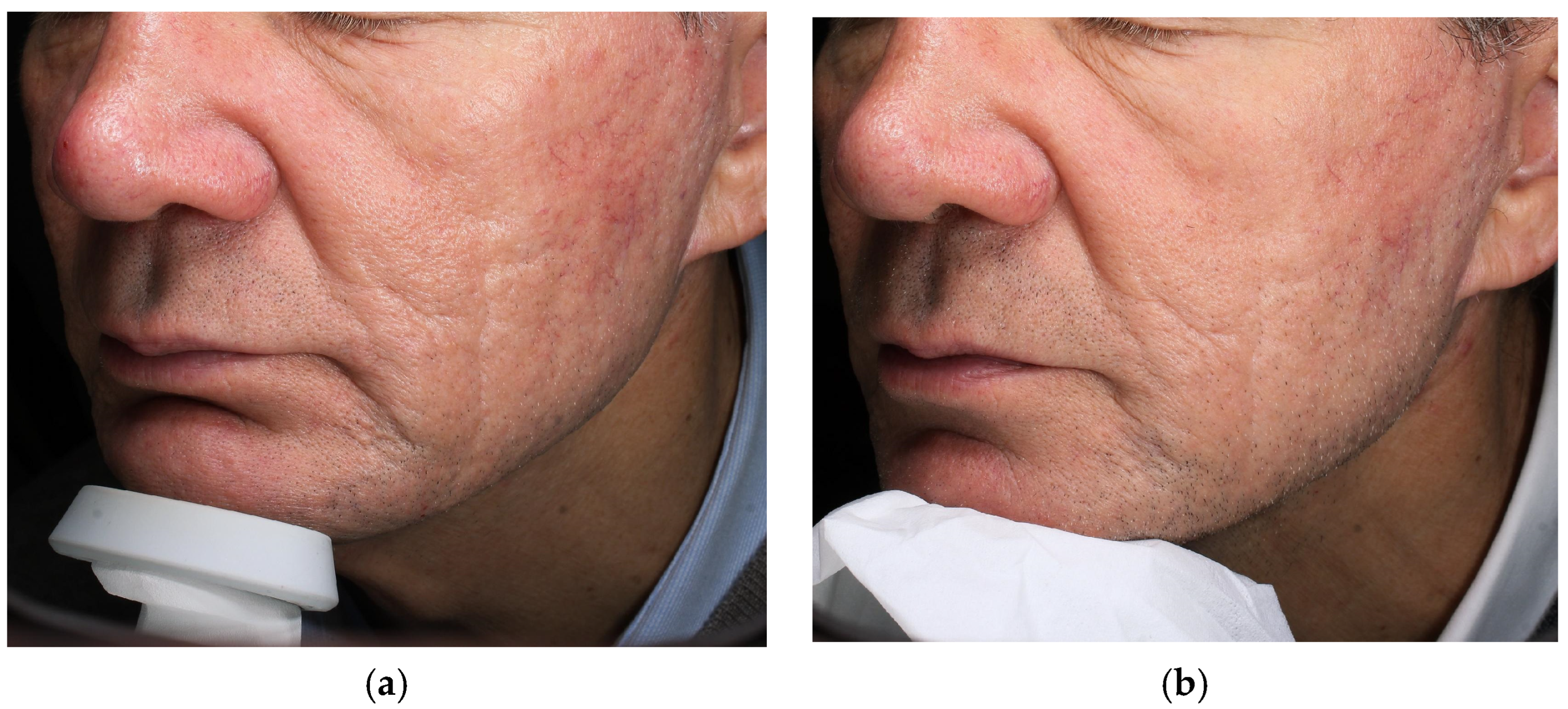

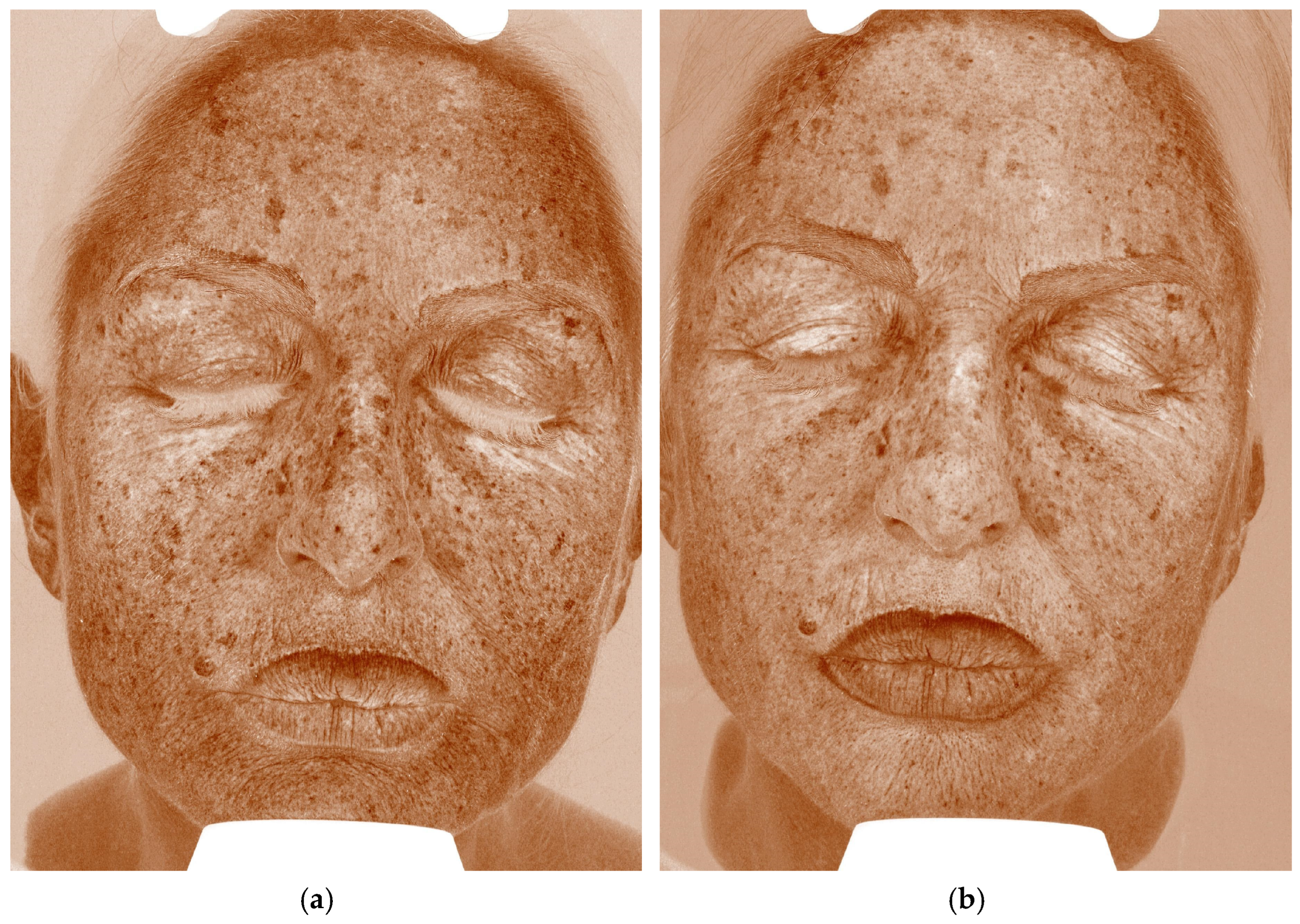
| Case | Age/Sex | Indication | Laser—Mode and Parameters | Exosome Serum | Downtime * | Clinical Improvement | Patient Self-Evaluation (Day 7) ‡ | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| 1 | 62 M | Atrophic acne scars | Fractional CO2, Smart-Pulse 14 W · dwell 800 µs · spacing 550 µm (2 sessions, 4 weeks apart) | Exolight C (pro-collagenic) | 5–6 days (erythema + edema) | Visible scar depth/texture less marked (clinician estimate) | 5/5 | None |
| 2 | 68 F | Photo-aging dyschromia | Fractional CO2, CoolPeel 5 W · spacing 500 µm · High-Pulse (single session) | Exolight M (depigmenting) | 2 days (mild erythema/micro-peel) | UV pigment score less ~30% at 4 weeks † | 5/5 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clementi, A.; Cassalia, F.; Cannarozzo, G.; Guarino, L.; Zappia, E.; Bennardo, L.; Mazzetto, R.; Danese, A.; Longo, C.; Nisticò, S.P. Laser-Assisted Exosome Delivery (LAED) with Fractional CO2 Laser: A Pilot Two-Case Report and Narrative Review. Cosmetics 2025, 12, 199. https://doi.org/10.3390/cosmetics12050199
Clementi A, Cassalia F, Cannarozzo G, Guarino L, Zappia E, Bennardo L, Mazzetto R, Danese A, Longo C, Nisticò SP. Laser-Assisted Exosome Delivery (LAED) with Fractional CO2 Laser: A Pilot Two-Case Report and Narrative Review. Cosmetics. 2025; 12(5):199. https://doi.org/10.3390/cosmetics12050199
Chicago/Turabian StyleClementi, Alessandro, Fortunato Cassalia, Giovanni Cannarozzo, Luca Guarino, Elena Zappia, Luigi Bennardo, Roberto Mazzetto, Andrea Danese, Caterina Longo, and Steven Paul Nisticò. 2025. "Laser-Assisted Exosome Delivery (LAED) with Fractional CO2 Laser: A Pilot Two-Case Report and Narrative Review" Cosmetics 12, no. 5: 199. https://doi.org/10.3390/cosmetics12050199
APA StyleClementi, A., Cassalia, F., Cannarozzo, G., Guarino, L., Zappia, E., Bennardo, L., Mazzetto, R., Danese, A., Longo, C., & Nisticò, S. P. (2025). Laser-Assisted Exosome Delivery (LAED) with Fractional CO2 Laser: A Pilot Two-Case Report and Narrative Review. Cosmetics, 12(5), 199. https://doi.org/10.3390/cosmetics12050199










