Effects of ε-Viniferin and ε-Viniferin-Enriched Extract from Vitis labruscana B. ‘Campbell Early’ Cell Cultures on Wound Healing and Epidermal Barrier Restoration in Human Skin Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ε-Viniferin and Vino Chocolate™
2.2. Cell Culture
2.3. Cell Viability
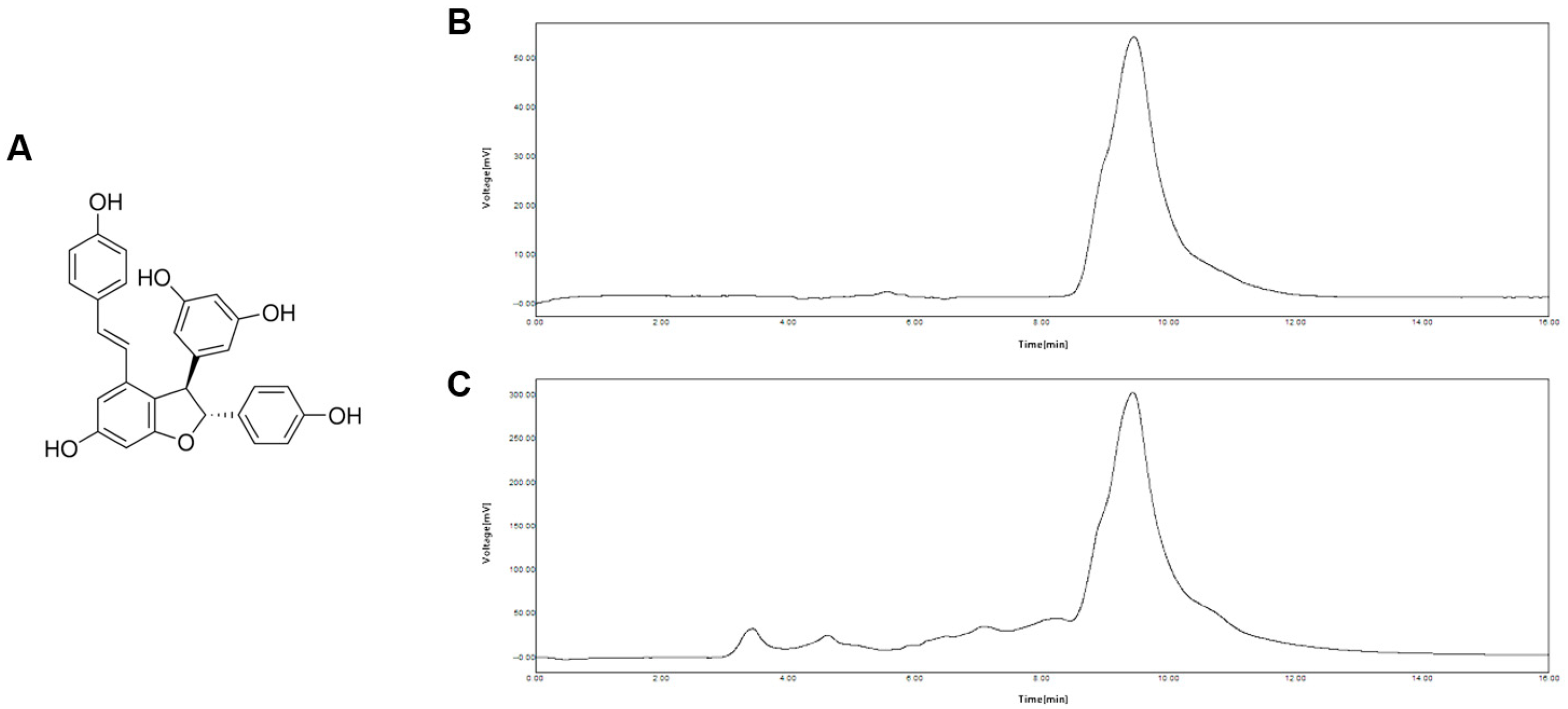
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. Wound Healing Assay
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Identification of ε-Viniferin in Vino Chocolate™ by HPLC
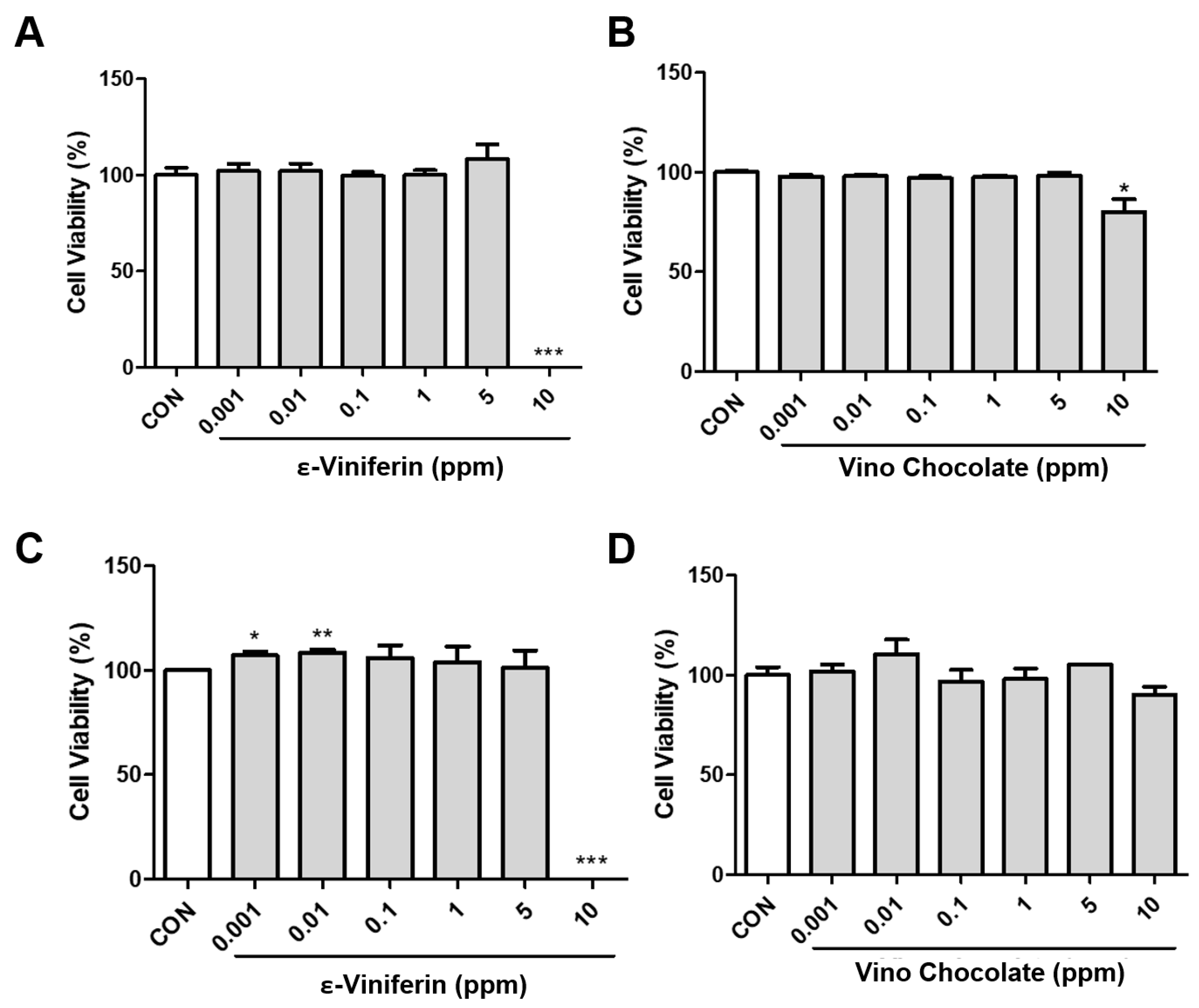
3.2. Effect of ε-Viniferin and Vino Chocolate™ on Cell Viability in HaCaT and HDFn Cells
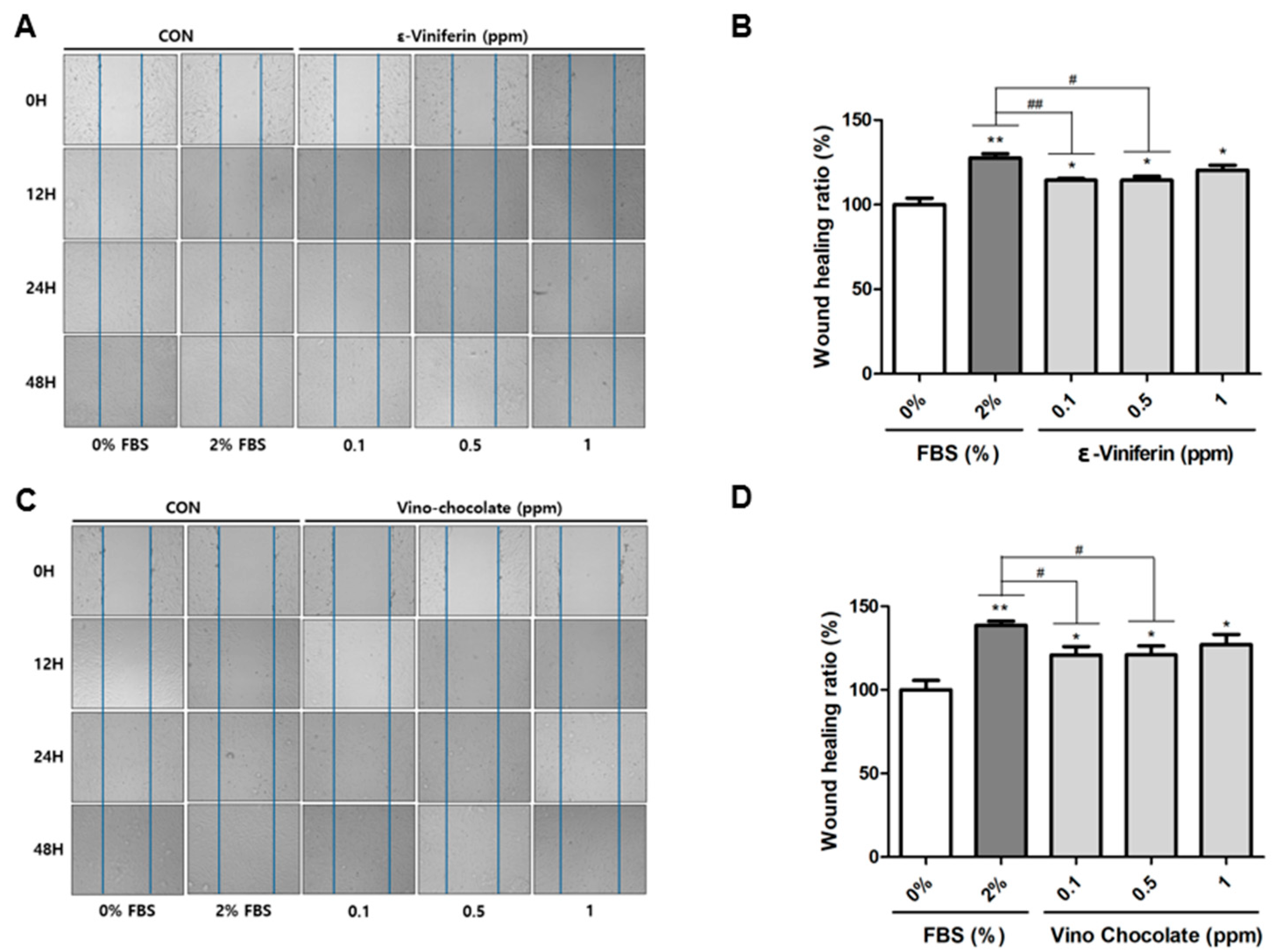
3.3. ε-Viniferin and Vino Chocolate™ Promote Wound Healing in Human Dermal Fibroblasts
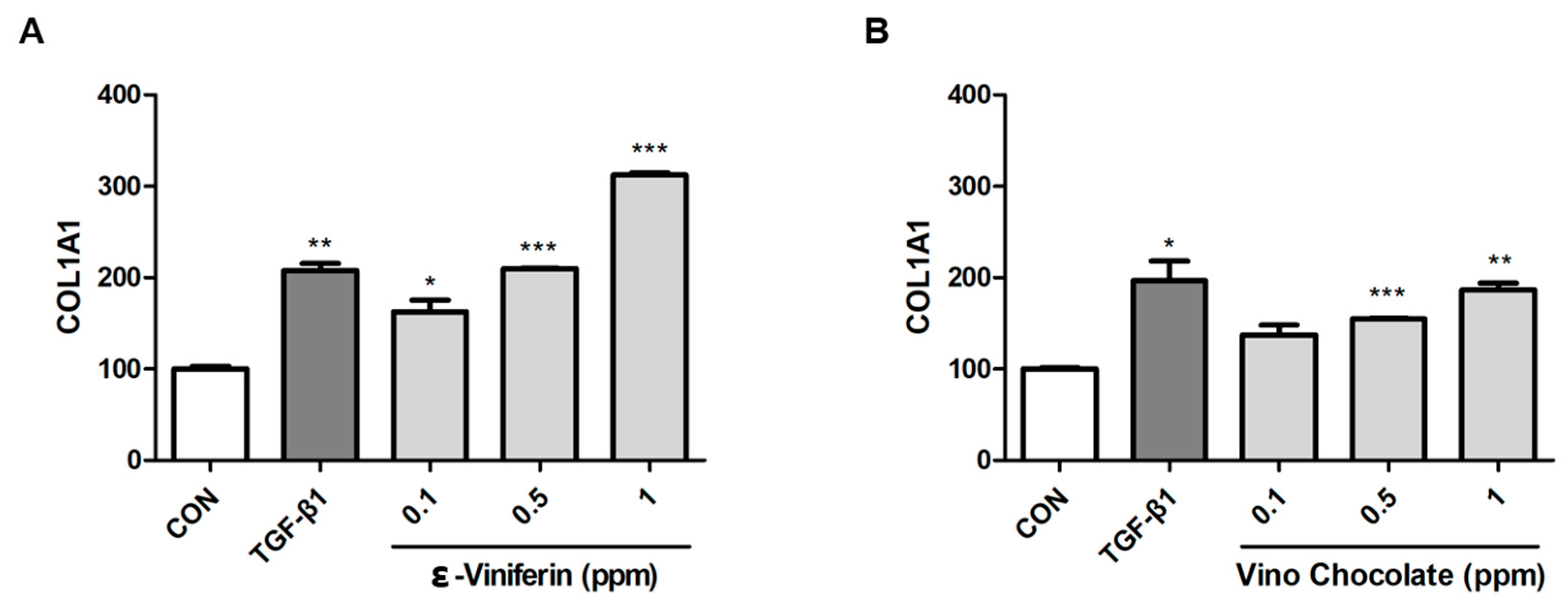
3.4. ε-Viniferin and Vino Chocolate™ Enhance Pro-Collagen Type I Synthesis in Human Dermal Fibroblasts
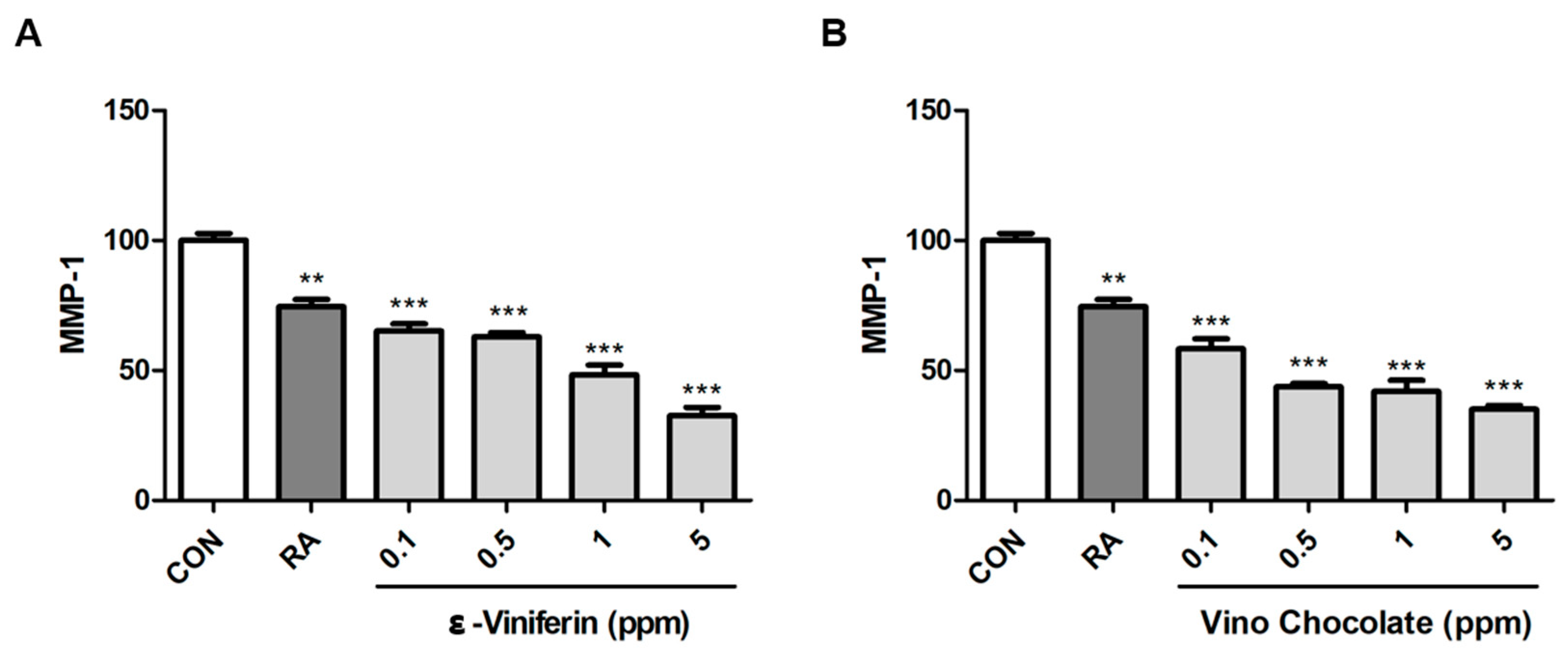
3.5. ε-Viniferin and Vino Chocolate™ Suppress MMP-1 Expression in Human Dermal Fibroblasts
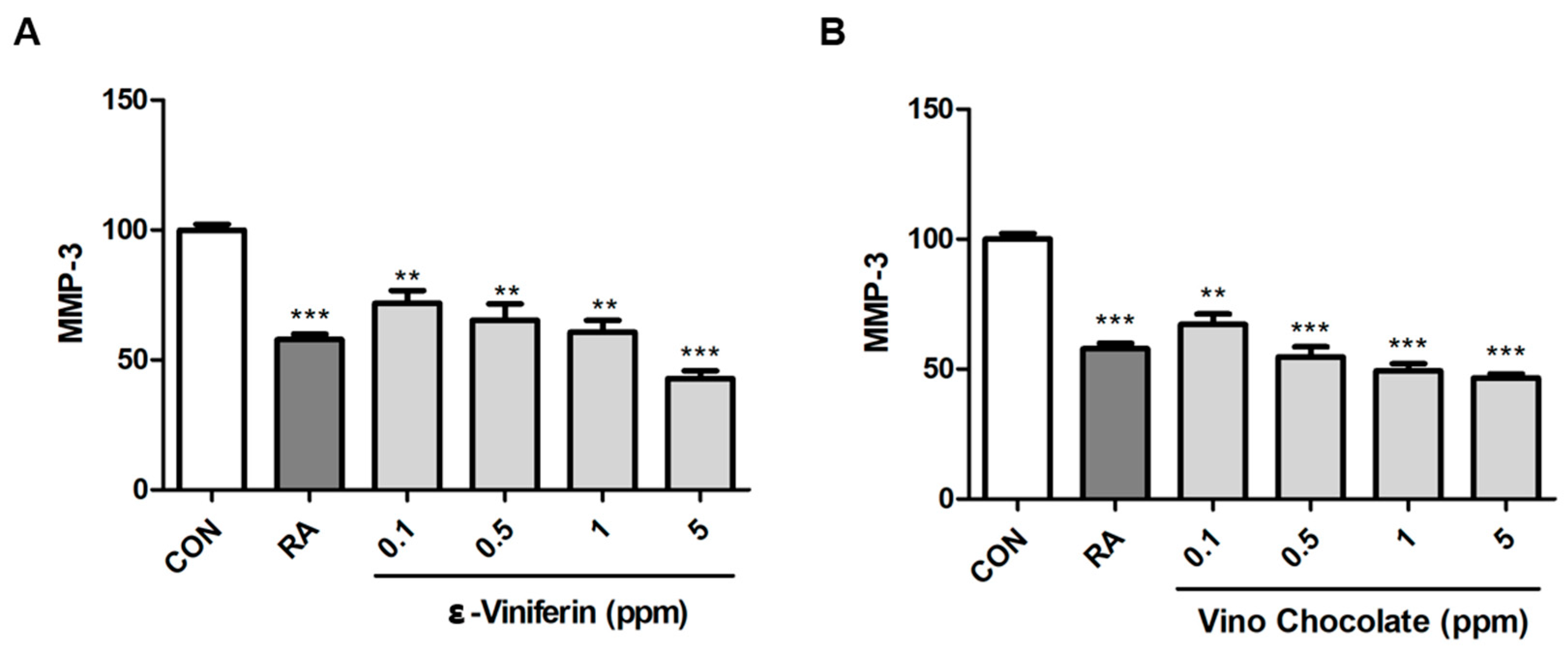
3.6. ε-Viniferin and Vino Chocolate™ Suppress MMP-3 Expression in Human Dermal Fibroblasts
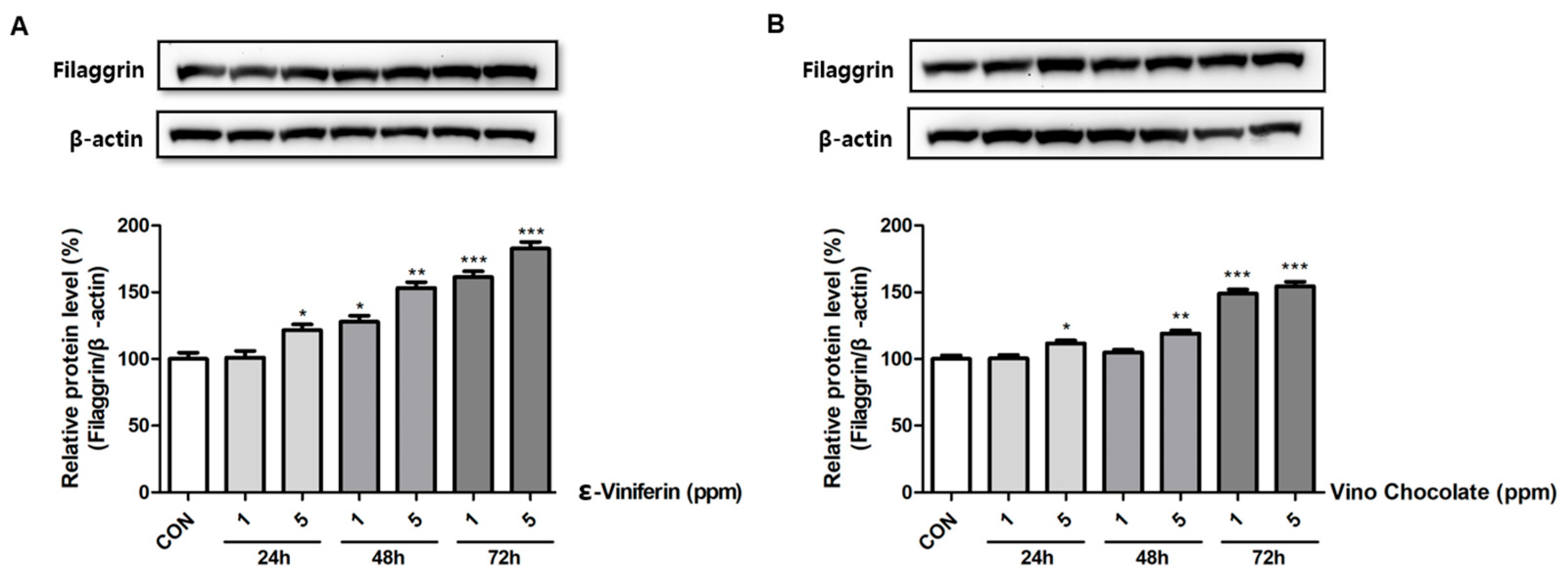
3.7. ε-Viniferin and Vino Chocolate™ Enhanced Filaggrin Protein Levels in Human Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HDFn | Human Dermal Fibroblast, Neonatal |
| HaCaT | High Sensitivity of Human Epidermal Keratinocytes |
| ECM | Extracellular Matrix |
| MMPs | Matrix Metalloproteinases |
| COL1A1 | Pro-collagen Type I Alpha 1 |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HPLC | High-Performance Liquid Chromatography |
| RA | Retinoic Acid |
| TGF-β1 | Transforming Growth Factor-Beta 1 |
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Kim, E.K.; Lee, S.J.; Park, N.H.; Kim, H.S.; Kim, H.K.; Char, K.H.; Jang, Y.P.; Kim, J.W. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef]
- Vičanová, J.; Boelsma, E.; Mommaas, A.M.; Kempenaar, J.A.; Forslind, B.; Pallon, J.; Egelrud, T.; Koerten, H.K.; Ponec, M. Normalization of epidermal calcium distribution profile in reconstructed human epidermis is related to improvement of terminal differentiation and stratum corneum barrier formation. J. Investig. Dermatol. 1998, 111, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Moloney, S.J.; Edmonds, S.H.; Giddens, L.D.; Learn, D.B. The hairless mouse model of photoaging: Evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem. Photobiol. 1992, 56, 505–511. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Verpoorte, R.; Suresh, B. Evaluation of in-vivo wound healing activity of Hypericum patulum leaf extract on different wound model in rats. J. Ethnopharmacol. 2000, 70, 315–321. [Google Scholar] [CrossRef]
- Choi, S.W.; Son, B.W.; Son, Y.S.; Park, Y.I.; Lee, S.K.; Chung, M.H. The wound-healing effect of a glycoprotein fraction isolated from aloe vera. Br. J. Dermatol. 2001, 145, 535–545. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Park, S.H.; Park, S.-C.; Kim, S.; Kim, Y.-G.; Kim, S.W.; Lee, J.; Ryu, Y.B.; Jeong, J.C.; Kim, C.Y. Induced Extracellular Production of Stilbenes in Grapevine Cell Culture Medium by Elicitation with Methyl Jasmonate and Stevioside. Bioresour. Bioprocess. 2020, 7, 38. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, Y.J.; Park, S.-C.; Kim, S.; Kim, Y.-G.; Shin, G.; Jeong, H.J.; Ryu, Y.B.; Lee, J.; Lee, O.R.; et al. Highly Efficient Bioconversion of trans-Resveratrol to δ-Viniferin Using Conditioned Medium of Grapevine Callus Suspension Cultures. Int. J. Mol. Sci. 2022, 23, 4403. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.A.; Kishor, P.B.K.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Albergamo, A.; Dugo, G.; Navarra, M.; Cirmi, S. Protective Effects of a Red Grape Juice Extract against Bisphenol A-Induced Toxicity in Human Umbilical Vein Endothelial Cells. Toxics 2023, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, M.J.; Choi, Y.H.; Chung, Y.S. Antioxidant and antiobesity activities of seed extract from Campbell Early grape (Vitis labrusca × Vitis vinifera). J. Food Biochem. 2011, 35, 1201–1209. [Google Scholar] [CrossRef]
- Sy, B.; Krisa, S.; Richard, T.; Courtois, A. Resveratrol, ε-Viniferin, and Vitisin B from Vine: Comparison of Their In Vitro Antioxidant Activities and Study of Their Interactions. Molecules 2023, 28, 7521. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.-F.; Richard, T.; Mérillon, J.-M. Stilbenes from Vitis vinifera L. Waste: A Sustainable Tool for Controlling Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxid. Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso-González, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; El Alami, M.; Borras, C.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Morazzoni, P.; Vanzani, P.; Santinello, S.; Gucciardi, A.; Zennaro, L.; Miotto, G.; Ursini, F. Grape Seeds Proanthocyanidins: Advanced Technological Preparation and Analytical Characterization. Antioxidants 2021, 10, 418. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Jiang, L.; Jeon, D.; Kim, J.; Lee, C.W.; Peng, Y.; Seo, J.; Lee, J.H.; Paik, J.H.; Kim, C.Y.; Lee, J. Pyomelanin-Producing Brevundimonas vitisensis sp. nov., Isolated from Grape (Vitis vinifera L.). Front. Microbiol. 2021, 12, 733612. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Loizzo, M.R.; Ademiluyi, A.O.; Sharifi-Rad, R.; et al. Plants of the genus Zingiber as potential sources of bioactive phytochemicals: From tradition to pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, Y.; Wang, Y.; Zhang, Y.; Wang, Y. Angelica dahurica Ethanolic Extract Improves Impaired Wound Healing by Activating Angiogenesis in Diabetes. PLoS ONE 2017, 12, e0177862. [Google Scholar] [CrossRef] [PubMed]
- Lateef, H.; Stevens, M.J.; Varani, J. All-trans-retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture. Am. J. Pathol. 2004, 165, 167–174. [Google Scholar] [CrossRef]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic acid inhibits UV-induced MMP-1 production in human skin in vivo. J. Clin. Investig. 1998, 101, 1432–1440. [Google Scholar] [CrossRef]
- Madlener, M. Differential expression of matrix metalloproteinases and their physiological inhibitors in acute murine skin wounds. Arch. Dermatol. Res. 1998, 290 (Suppl. 1), S24–S29. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Madlener, M.; Parks, W.C.; Werner, S. Matrix metalloproteinases and their physiological inhibitors are differentially expressed during excisional skin wound repair. Exp. Cell Res. 1998, 242, 201–210. [Google Scholar] [CrossRef]
- Kurokawa, I.; Mizutani, H.; Kusumoto, K.; Nishijima, S.; Tsujita-Kyutoku, M.; Shikata, N.; Tsubura, A. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair Regen. 2006, 14, 38–45. [Google Scholar] [CrossRef]
- Kaleci, B.; Koyuturk, M. Efficacy of Resveratrol in the Wound Healing Process by Reducing Oxidative Stress and Promoting Fibroblast Cell Proliferation and Migration. Dermatol. Ther. 2020, 33, e14357. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hung, C.-F.; Sung, H.-C.; Yang, S.-C.; Yu, H.-P.; Fang, J.-Y. The Bioactivities of Resveratrol and Its Naturally Occurring Derivatives on Skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chang, E.J.; Cho, S.H.; Chung, S.K.; Park, H.D. Protective Effects of Vitis amurensis Rupr. Stem Extract against UVB-Induced Damage in Human Dermal Fibroblasts via Regulation of the TGF-β/Smad Pathway. J. Food Biochem. 2021, 45, e13597. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limón, H.; Hidalgo, D.; Moyano, E.; Goleniosiewski, M.; Cusidó, R.M.; Palazón, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added-Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Pignet, A.-L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.-P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Jia, Y.; Shao, J.-H.; Zhang, K.-W.; Zou, M.-L.; Teng, Y.-Y.; Tian, F.; Chen, M.-N.; Yuan, Z.-D.; Wu, J.-J.; Guo, Z.-R. Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review. Molecules 2022, 27, 6736. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B.; Liu, X.; Zhang, X.; Wu, J. Interplay between MAPK signaling pathway and autophagy in skin aging: Mechanistic insights and therapeutic implications. Front. Cell Dev. Biol. 2025, 13, 1625357. [Google Scholar] [CrossRef] [PubMed]
- Pohlers, D.; Brenmoehl, J.; Löffler, I.; Müller, C.K.; Leipner, C.; Schultze-Mosgau, S.; Stallmach, A.; Kinne, R.W.; Wolf, G. TGF-β and fibrosis in different organs—Molecular pathway imprints. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2009, 1792, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, A.O.; Prendergast, C.M.; Salvatore, M.M.; Capaccione, K.M. TGF-β signaling: Critical nexus of fibrogenesis and cancer. J. Transl. Med. 2024, 22, 594. [Google Scholar] [CrossRef] [PubMed]

| Step | Time (min) | Flow Rate (mL/min) | %A | %B |
|---|---|---|---|---|
| 1 | 0 | 0.5 | 58 | 42 |
| 2 | 11 | 0.5 | 58 | 42 |
| 3 | 12 | 0.5 | 0 | 100 |
| 4 | 14 | 0.5 | 0 | 100 |
| 5 | 15 | 0.5 | 58 | 42 |
| 6 | 16 | 0.5 | 58 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Lim, J.; Lee, K.; Kim, G.; Pyee, J.; You, M.; Hwang, J. Effects of ε-Viniferin and ε-Viniferin-Enriched Extract from Vitis labruscana B. ‘Campbell Early’ Cell Cultures on Wound Healing and Epidermal Barrier Restoration in Human Skin Cells. Cosmetics 2025, 12, 181. https://doi.org/10.3390/cosmetics12050181
Kim D, Lim J, Lee K, Kim G, Pyee J, You M, Hwang J. Effects of ε-Viniferin and ε-Viniferin-Enriched Extract from Vitis labruscana B. ‘Campbell Early’ Cell Cultures on Wound Healing and Epidermal Barrier Restoration in Human Skin Cells. Cosmetics. 2025; 12(5):181. https://doi.org/10.3390/cosmetics12050181
Chicago/Turabian StyleKim, Daeun, Jimin Lim, Kyuri Lee, Gisol Kim, Jaeho Pyee, Minkyoung You, and Jaesung Hwang. 2025. "Effects of ε-Viniferin and ε-Viniferin-Enriched Extract from Vitis labruscana B. ‘Campbell Early’ Cell Cultures on Wound Healing and Epidermal Barrier Restoration in Human Skin Cells" Cosmetics 12, no. 5: 181. https://doi.org/10.3390/cosmetics12050181
APA StyleKim, D., Lim, J., Lee, K., Kim, G., Pyee, J., You, M., & Hwang, J. (2025). Effects of ε-Viniferin and ε-Viniferin-Enriched Extract from Vitis labruscana B. ‘Campbell Early’ Cell Cultures on Wound Healing and Epidermal Barrier Restoration in Human Skin Cells. Cosmetics, 12(5), 181. https://doi.org/10.3390/cosmetics12050181






