Abstract
This paper presents a literature review on the potential of jaboticaba (Myrciaria cauliflora) peel extracts for application in multifunctional dermocosmetic formulations, particularly as natural antioxidants and photoprotective agents. Utilizing the Methodi Ordinatio methodology, of a total of 1226, 90 scientific articles were selected from six major databases and analyzed through bibliometric mapping (VOSviewer) and qualitative data processing (MAXQDA). The results highlight research concentration in three key areas: (1) extraction methodologies for bioactive compounds, (2) identification and quantification techniques, and (3) biological activities (antioxidant and photoprotective effects). The most frequent compounds reported were anthocyanins (cyanidin-3-glucoside and delphinidin-3-glucoside), quercetin-derived flavonoids (rutin and myricetin), and phenolic acids (ellagic, gallic, and ferulic acids), which exhibit synergistic effects with conventional UV filters. Ultrasound-assisted extraction (UAE) using ethanol and emerging green solvents, like glycerol and deep eutectic solvents (DESs), was identified as an effective, sustainable alternative. Despite increasing evidence supporting the dermocosmetic potential of jaboticaba peel, studies remain scarce, with only one identified investigation using it in a topical formulation. This review provides a structured scientific foundation to encourage research aimed at developing multifunctional, eco-friendly, plant-based cosmetics aligned with the principles of the circular economy.
1. Introduction
Jaboticaba is a Brazilian fruit found in the central, southern, and southeastern regions of the country. It belongs to the Myrtaceae family, also known as Plinia, with the largest production in the states of Minas Gerais, Goiás, and São Paulo [1]. The fruit exhibits various species, emphasizing M. jaboticaba (Vell.) O. Berg and M. cauliflora (Mart.) O. Berg, both in terms of productivity and the number of published studies [2]. Among the research, Schulz et al. [3] performed a literature review on seven species of fruits and highlighted that jaboticaba fruits are sources of nutrients, including vitamins, minerals, dietary fibers, and substances with biological activities (anthocyanins, flavonoids, and tannins). They emphasized their biological properties, such as antioxidant, anti-inflammatory, and antiproliferative effects, with significant potential for economic exploitation in the food, pharmaceutical, and cosmetic industries. In the food industry, jaboticaba is used to produce beverages such as syrups, juices, and fermentations. To manufacture these products, the peels and seeds, which represent 40 to 50% of the fruit weight, are discarded [4]. Nevertheless, according to Sanches et al. [5], jaboticaba by-products are a source of bioactive compounds that can be obtained sustainably using a variety of technological routes. Neves et al. [6] analyzed the phenolic compound profile of the jaboticaba species Plinia trunciflora, P. cauliflora, P. jaboticaba, and P. phitrantha. A total of 28 phenolic compounds were identified, with emphasis on anthocyanins, cyanidin-3-glucoside, and delphinidin-3-glucoside, flavonols derived from quercetin and myricetin, and derivatives of ellagic acid and methyl ellagic acid. A similar phenolic profile was found in the species Plinia cauliflora in the studies where different extraction processes were evaluated [7,8].
The jaboticaba fruit peel has a high content of dietary fiber and anthocyanins, which indicates its potential use to enrich food products. Research in animal models has demonstrated the benefits of using the whole fruit and parts of the fruit on lipid and glycemic metabolism, with glucose regulation, weight control, and insulin resistance [9]. However, information is scarce regarding the application of extracts obtained from jaboticaba peel in cosmetic formulations. In this context, we pose the question: Is there potential for developing dermocosmetic formulations from jaboticaba peel extracts to protect skin tissue against damage induced by ultraviolet radiation (UVR)?
Thus, to learn about the use of bioactive compounds and extracts produced from jaboticaba peel in dermocosmetics with antioxidant and photoprotective activities, a systematic review of the specialized literature was carried out using the Methodi Ordinatio [10,11]. A bibliometric analysis was performed using VOSviewer software, followed by a qualitative analysis of the data from the selected articles with the aid of MAXQDA software, focusing on the methodologies used in the processes of extraction, separation, identification, and quantification of bioactive compounds, as well as analyses to evaluate biological efficacy, such as antioxidant, photoprotective, and antiaging activities.
2. Materials and Methods
This research was conducted from December 2024 to March 2025 and carried out in three steps: (i) development of a portfolio of scientific articles aligned with the research topic using the Methodi Ordinatio methodology; (ii) bibliometric analysis of the scientific articles using VOSviewer software (version 1.6.20); and (iii) extraction, systematic reading, and data analysis of the articles using MAXQDA software (version 24.2).
2.1. Applying the Methodi Ordinatio
This step involved the development of a portfolio of scientific articles following a nine-step protocol. During steps 1 to 4, six databases were selected—Science Direct, Scopus, PubMed, Web of Science, SciELO, and ACS Databases—accessed through the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) journal portal, Brazil. The time frame was set for articles published from 2010 onwards. The Mendeley Reference Manager was used for step 5. During the screening procedures, duplicate articles and those unrelated to the topic were excluded after reviewing titles, abstracts, and keywords. To avoid selection bias, the step of defining inclusion/exclusion criteria for articles outside the research topic was carried out collectively by the authors and applied individually by two reviewers.
Step 6 was carried out using JabRef, version 5.11, JabRef e.V.: Sindelfingen, Germany, 2023, which identified the impact factor, year of publication, and number of citations retrieved from Google Scholar, highlighting the multicriteria nature of Methodi Ordinatio as an SLR methodology. In Step 7, the articles were ranked in the RankIn spreadsheet based on the index (InOrdinatio 2.0) obtained through Equation (1), which considers three variables: the number of citations, the impact factor (a journal metric), and the year of publication.
Here, Δ is the value from 0 to 10 that the researcher attributes to the importance of the metrics in the study, IF is the impact factor (a journal metric), λ is the value from 0 to 10 that the researcher attributes to the importance of the portfolio age in the study, Research Year is the year in which the study is being conducted (2025), Pub Year is the year of publication of the article, Cited Half Life is the median value of the Cited Half-Life for JCR journals (7.6), Ω is the value from 0 to 10 that the researcher attributes to the importance of the average annual citation count of the publications, and Ci is the total number of citations found in Google Scholar.
Using the equity criterion for the variable weights, Δ, λ, and Ω were given values of 10. After completing Step 8 with the retrieval of full-text articles, Step 9 involved subjecting the portfolio articles to bibliometric analysis (quantitative) using VOSviewer software, as well as data and information extraction with MAXQDA software (qualitative).
2.2. Applying VOSviewer Software
VOSviewer is a tool for constructing and visualizing bibliometric maps derived from the scientific literature. It employs the VOS (Visualization of Similarities) methodology to define nodes and connections within a network, allowing for in-depth analysis of co-authorship, co-citation, and keyword relationships. The software is intuitive, user friendly, and freely available, with the added advantage of exporting processed data for use in other applications [12].
Based on the analysis of metadata extracted from the articles, a bibliometric analysis was conducted, including network mapping and analysis of keywords, leading authors, journals, institutions, and countries, along with the temporal evolution and research trends within the studied topic.
2.3. Applying MAXQDA Software
MAXQDA is applied to examine qualitative data and mixed methods, using tools capable of analyzing different types of data, such as texts, images, videos, and online data [13]. Using MAXQDA, information and data were extracted from the articles and organized into spreadsheets generated within the software. The MAXDictio tool was employed, following specific steps for coding: the dictionary function was used (which enables the creation of search items for category assignment); keywords were used to scan and identify relevant search items.
The auto-coding command using the dictionary was executed. This function creates a coding system for each dictionary category and assigns newly created codes to the words identified within each category. The information generated was useful for understanding the relationships between the themes addressed in the articles.
Next, the data (article, material analyzed, extraction process, photoprotective efficacy method, antioxidant activity method, and methods for identifying bioactive compounds) were summarized in Excel spreadsheets.
3. Results and Discussion
3.1. Portfolio Developed Using the Methodi Ordinatio
The combination of keywords and Boolean operators used is described in Table 1, which also presents the number of articles found in each of the six databases for each keyword combination.

Table 1.
Combination of keywords and Boolean operators used across the six databases searched.
The final search and filtering process across the six databases is summarized in Table 2, resulting in a total of 1226 retrieved articles. After the removal of duplicates (764) and exclusion of studies that did not meet the eligibility criteria based on title, keywords, and abstract (372), a final portfolio of 90 articles was selected. These were classified into original research articles (n = 76) and literature reviews (n = 14) and ranked using the InOrdinatio index, as detailed in Appendix A.

Table 2.
Filtering procedure performed in Mendeley Reference Manager.
3.2. Main Research Areas
A bibliometric analysis using VOSviewer software allowed for the identification of thematic groups and trends of co-occurrence of keywords within the portfolio. Terms with at least four occurrences are presented in Figure 1, while Figure 2a,b illustrate the relationships between the terms and the dominant research areas over time.
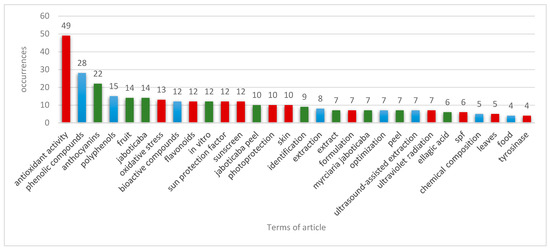
Figure 1.
Number of occurrences of terms in the articles of the portfolio. Red color: most common terms among photoprotection studies; blue color: most common terms among studies on the extraction of bioactive compounds; green color: most common terms among studies on bioactive compounds in jaboticaba peel.
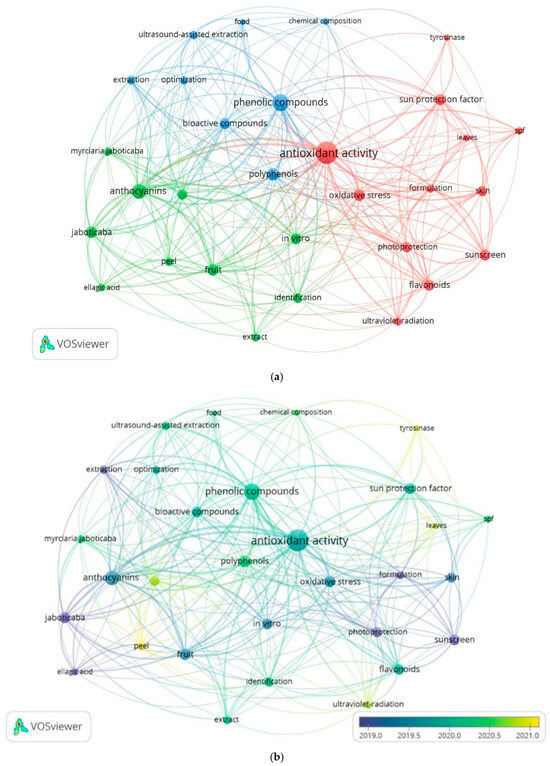
Figure 2.
Connections between terms with 4 or more occurrences in the articles. (a) Map of connections between terms and groups; (b) Trend map over the years.
The term “antioxidant activity” is the most frequently occurring (49) and is located at a central position in Figure 2a, showing a strong correlation with terms from all three groups. This biological activity is prominently addressed in most of the articles in the portfolio. In the blue group, considering “phenolic compounds” and “polyphenols” as synonyms, the second most frequent term (43) was identified, which also shows a significant correlation with the term “sun protection factor”.
In the green group, antioxidant activity is associated with bioactive compounds of the anthocyanin class, the most common term in the group (22). In the red group, antioxidant activity correlates with the terms “ultraviolet radiation”, “sunscreen”, “SPF” (sun protection factor), “photoprotection”, and “oxidative stress”, highlighting its importance in preventing and repairing damage caused by UV radiation to the skin. The flavonoid class shows the strongest correlation with research in photoprotective formulations because of its structural features.
For Oliveira et al. [14], the extract from the bark of Hymenaea martiana presented high levels of total flavonoids and total phenolic compounds, including myricetin, kaempferol, and quercetin. The incorporation of the extract into the chemical filter increased the antioxidant activity and SPF of the formulations, suggesting a synergistic effect on photoprotection.
Chaira et al. [15] studied the aerial part of the species Cytisus purgans subsp. balansae (Boiss.), where they identified four isoflavones (genistein, biochanin, isoprunetin, and daidzein), one flavone (chrysin), and one flavonol (quercetin). The extracts rich in polyphenols and flavonoids showed potent antioxidant activity and effective sun protection.
Figure 2b indicates a trend in research investigating the importance of enzyme activity and the prevention of damage caused by UV radiation, as demonstrated by the more recent relationships between the terms “tyrosinase”, “sun protection factor”, and “antioxidant activity”. According to the VOSviewer manual Eck & Waltman [12], the distance between terms represents their relationship within the studies analyzed by the software. The positioning of terms related to research on photoprotective formulations reveals a gap in studies investigating the use of extracts derived from jaboticaba peel in this type of formulation.
3.3. Authors, Countries, Journals, and Institutions
In addition to the keyword co-occurrence mapping, a bibliometric assessment was conducted to identify the most influential contributors to research on jaboticaba peel extracts. Table 3 summarizes the top authors, countries, journals, and institutions based on the number of publications and citations in the portfolio of 90 selected studies.

Table 3.
Top 10 authors, countries, journals, and institutions.
The most prolific authors were Baby A. R. (7 articles), Oliveira C. A. (6 articles), Velasco M. V. R. (5 articles), and Rosado C. (5 articles), indicating a consolidated research core focused on dermocosmetic applications. Brazil dominated the geographic distribution of publications, contributing 53 articles, followed by Spain (14) and Portugal (10), which highlights Latin America’s central role in advancing studies on native fruit-based ingredients. Among the scientific journals, Food Research International and Molecules led in publication volume, followed by Natural Product Research. These journals are prominent in the fields of food chemistry, natural product pharmacology, and biotechnological applications. The institutions with the most publications were the University of Campinas (15 articles), University of São Paulo (8), and Federal University of Santa Maria (6), all in Brazil.
3.4. Main Articles on Bioactive Compounds in Jaboticaba Peel
A qualitative analysis was conducted using MAXQDA software, with data systematically categorized into themes based on the MAXDictio dictionary. The analysis of the 90 articles portfolio revealed 28 studies dedicated to jaboticaba species, with a particular focus on the bioactive compounds in the fruit peel, including 6 literature reviews. The jaboticaba species and the fruit parts investigated in these 28 studies are presented in Table 4.

Table 4.
Jaboticaba fruit parts investigated in 28 articles from the portfolio.
Among these studies, three focused on the identification and quantification of compounds through chromatographic analyses. Neves et al. [16] analyzed peel extracts from four jaboticaba species (Plinia jaboticaba, P. phitrantha, P. cauliflora, and P. trunciflora) for the presence and identification of phenolic compounds using HPLC-DAD-ESI/MSⁿ. Seventeen flavonols derived from quercetin were identified based on fragment ion signals at m/z 300 and 301 and three myricetin derivatives at m/z 316 and 317. Eleven methyl ellagic acid and eighteen ellagic acid derivatives were also detected. The MS2 spectra of these compounds suggested the presence of ellagic acid derivatives (m/z 301 and/or 300) and methyl ellagic acid derivatives (m/z 315 and 300). The P. cauliflora varieties showed the highest number of compounds, with 40 derivatives in the “Paulista” variety and 37 in “Canaã-açu” variety, followed by P. jaboticaba with 35 and P. phitrantha with 17 derivatives.
Neves et al. [6] analyzed the phenolic compound profile of P. trunciflora, P. cauliflora, P. jaboticaba, and P. phitrantha jaboticaba species. Using HPLC-DAD-ESI-MSn for separation, identification, and quantification, they identified 28 phenolic compounds, with a particular focus on cyanidin-3-glucoside and delphinidin-3-glucoside, flavonols derived from myricetin and quercetin, as well as methyl ellagic acid and ellagic acid derivatives. Cefali et al. [17] identified the flavonoid rutin at a concentration of 2.71 ± 0.17 mg mL−1 in a ethanolic extract of Myrciaria cauliflora fruit peel. The extract was produced by magnetic stirring at 50 ± 2 °C for 3 h, using a 1:20 (w/v) ratio of dried bark powder to a 60% v/v ethanol/water solution.
In their literature review on the extraction of bioactive compounds from jaboticaba, Rosa et al. [18] identified 24 substances, including 4-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, catechin, quercetin, myricetin, p-coumaric acid, syringic acid, chlorogenic acid, ellagic acid, caffeic acid, delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, gallic acid, ferulic acid, isoquercitrin, isorhamnetin, luteolin, kaempferol, malvidin, petunidin, pelargonidin, peonidin, pinobanksin, and naringenin.
Inada et al. [19] analyzed the effects of dehydration and pressurization methods on the chemical composition of M. jaboticaba peel and seed powder. The dehydration was performed by two methods: forced-air oven drying and freeze drying. The oven drying method followed a 22 full factorial design with a central point in duplicate, totaling six trials. The independent variables were time, at the levels of 14, 18, and 22 h, and temperature, at the levels of 55, 65, and 75 °C. The response variables were antioxidant activity by FRAP and TEAC assays and the analysis of phenolic compounds by HPLC-DAD and HPLC-DAD-MS. Freeze drying was performed at −50 °C and 0.065 mbar for 72 h, with antioxidant activity measured by the same assays and phenolic compounds analyzed by HPLC-DAD, HPLC-DAD-MS, and HPLC-ESI-MS-MS. Compared to freeze drying, antioxidant activity in oven-dried samples varied, increasing by 20% or decreasing by up to 44%, depending on time and temperature conditions. In the FRAP assay, activity decreased by up to 60% under all conditions tested. The phenolic compounds detected included four flavonols, two anthocyanins, one hydroxycinnamic acid derivative, twelve ellagitannins, and four ellagic and gallic acid derivatives, in both oven-dried and freeze-dried samples. Freeze drying resulted in higher anthocyanin content, while oven drying produced higher levels of ellagitannins. Both methods produced jaboticaba powder rich in phenolic compounds, and the choice between them depends on the intended application, considering the higher cost of freeze drying [19].
The high hydrostatic pressure processing experiments were conducted in six trials, using a 22 full factorial design with a duplicated center point. The independent variables were processing time at 1, 5.5, and 10 min and pressure at 200, 350, and 500 MPa. The response variables were antioxidant activity by TEAC and FRAP assays and soluble phenolic compound contents by HPLC-DAD. Compared with the unprocessed sample, all pressurized samples showed a reduction in antioxidant activity, ranging from 32% (350 MPa for 5.5 min) to 67% (500 MPa for 1 min) for FRAP and from 39% (200 MPa for 1 min) to 64% (500 MPa for 10 min) for TEAC. Regarding phenolic compounds, both unprocessed and pressurized samples contained eight phenolic compounds, including the anthocyanins cyanidin-3-O-glucoside and delphinidin-3-O-glucoside, two hydroxybenzoic acid derivatives (ellagic acid and gallic acid), and four flavonols (quercetin-3-O-rutinoside, myricetin-3-O-rhamnoside, quercetin, and myricetin [19].
The extraction process and identification of bioactive compounds present in the jaboticaba fruit peel were the focus of studies conducted by Santos et al. [20], Barros et al. [21], Mattos et al. [22], Marsiglia et al. [23], and Pinc et al. [7]. Santos et al. [20] evaluated an extraction process combining ultrasound-assisted extraction (UAE) with stirred-bed extraction (SBE) to obtain antioxidant compounds from the peel of M. cauliflora. The combined method was compared with the traditional stirred-bed extraction and Soxhlet extraction methods using ethanol (acidified and non-acidified) in terms of yield, economic viability, and chemical composition. The antioxidant activity of the extracts was evaluated by the coupled oxidation method of β-carotene and linoleic acid. The best results were obtained with Soxhlet extraction using acidified ethanol (pH 3), followed by the combined UAE + ABE process with ethanol. The total phenolic content and composition were determined using the Folin–Ciocalteu method. The combined method (UAE + ABE) demonstrated superior performance to the Soxhlet extraction.
Barros et al. [21] evaluated the effects of acetic, phosphoric, and formic acids on the levels of bioactive compounds in Plinia cauliflora peel and antioxidant capacity, adjusting the pH to 1.0, 2.0, and 3.0, in UAE extraction. Antioxidant capacity was evaluated by FRAP and ORAC assays, total phenolic content, monomeric anthocyanins, and qualitative phenolic profile by liquid chromatography coupled to mass spectrometry (LC-MS). The type of acid used in the removal process mainly affected the recovery of anthocyanins. The acid that provided the highest anthocyanin yield (3.4 mg/g dry matter) and antioxidant capacity (ORAC) (841 μmol TE/g raw material) was formic acid at pH 1.0 when compared to acidic and phosphoric acids. The identified compounds included ellagic acid, anthocyanins, and the flavonoids myricetin and quercetin.
Mattos et al. [22] evaluated different extraction methods in relation to antioxidant activity (ABTS and Folin–Ciocalteu reducing capacity) and total monomeric anthocyanin content. Based on the results, the authors concluded that UAE is the most efficient and cost-effective method for extracting anthocyanins from jaboticaba peel on an industrial scale, with potential applications in the food, cosmetic, and pharmaceutical industries.
Marsiglia et al. [23] evaluated UAE, maceration and the impact of temperature on the degradation of phenolic compounds in jaboticaba peel powder. The thermal stability of phenolic compounds and antioxidant activity (FRAP, ABTS, and DPPH) were monitored over time (0 to 360 min), with temperatures at levels of 90, 110, and 130 °C. The UAE at a mass of dried peel powder/solvent ratio of 1:20 (w/v) was more effective than maceration for extracting total phenolic compounds from jaboticaba peel, which may be attributed to cell wall disruption caused by cavitation during the process. Regarding solvents, ethanol and methanol were more effective than water. HPLC analysis revealed concentrations of hesperidin (9.26 mg L−1) and gallic acid (16.28 mg L−1) in extracts obtained using ethanol and methanol, respectively.
Pinc et al. [7] evaluated extraction methods for the peel of Plinia cauliflora (Mart) Kausel. Vortex and infusion extraction was performed at 25, 40, and 80 °C, with and without precipitation, using a completely randomized design. The precipitated extraction method yielded a 45.9% extraction rate, with greater antioxidant capacity and higher levels of phenolic derivatives. UHPLC-MS/MS analysis in negative ion mode indicated a predominance of carboxylic acids, with citric acid as the major compound. In positive mode, there was a predominance of flavonoids, with quercetin being the most abundant.
3.5. Extraction Methods
Extraction processes were applied to 55 plant species, utilizing various parts including leaves, stems, peels, pulps, seeds, and agro-industrial solid wastes. Ten distinct extraction methods were identified, each cited at least once in 56 articles from the portfolio. The most commonly used methods were ultrasound-assisted extraction (UAE) with 23 occurrences (41%), maceration with 8 (14%), agitated bed extraction (ABE) with 7 (13%), and Soxhlet with 6 (11%) (Figure 3a). The results reported in this review on ultrasound-assisted extraction with the solvent ethanol in dried jaboticaba peel corroborate the conclusions of Rosa et al. [18], who highlighted it as one of the most efficient and viable non-conventional techniques from both ecological and economic perspectives.
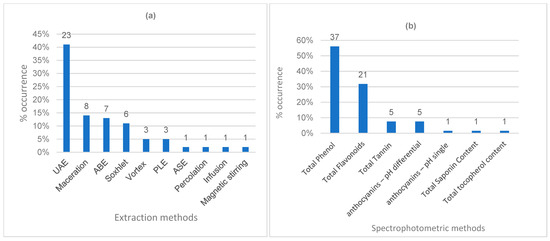
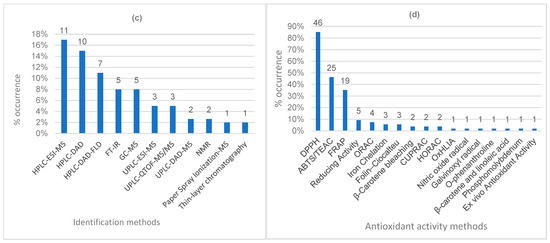
Figure 3.
Number of methods used in the research articles from the portfolio. (a) Extraction methods; (b) spectrophotometric methods; (c) structural identification methods; (d) antioxidant activity evaluation methods. UAE: ultrasound-assisted extraction; ABE: agitated bed extraction; PLE: pressurized liquid extraction; ASE: accelerated solvent extraction; HPLC-ESI-MS: high-performance liquid chromatography/electrospray ionization–mass spectrometry; DAD: diode array detector; FLD: fluorescence detector; FT-IR: Fourier-transform infrared spectroscopy; GC: gas chromatography; UPLC: ultra-performance liquid chromatography; QTOF: quadrupole time of flight; NMR: nuclear magnetic resonance spectroscopy.
The UEA method was the most used one, due to its advantages compared to other conventional extraction methods, including greater efficiency and yield in the recovery of bioactive compounds from plant matrices, lower solvent consumption, shorter extraction time, and high reproducibility, which allows for obtaining standardized extracts [8,24,25]. However, during ultrasonic extraction, it is advisable that the temperature remains below 40 °C and the extraction time does not exceed 60 min, thus avoiding the degradation of bioactive compounds [24].
Another factor to consider during the extraction of phenolic compounds is the acidification of the extraction solution. Acidic conditions can promote cell wall disruption, thereby improving extraction yields. For the extraction of anthocyanins such as cyanidin-3-glucoside and delphinidin-3-glucoside, higher yields are achieved at pH 1 due to the predominance of the flavylium cation form in contrast to a pH above 3, where the cationic form is significantly reduced. Among the acids tested, formic acid shows superior performance compared to phosphoric acid [21]. Regarding the extraction of the flavonol quercetin, the best results are obtained using acetic acid at pH 3, whereas both formic acid and acetic acid at pH 1 yield lower concentrations. In the case of ellagic acid, the highest extraction yields are achieved with phosphoric acid at pH 3, while significantly lower levels are observed with acetic acid at pH 1 [21]. Therefore, in future studies investigating the applicability of antioxidant and photoprotective compounds from plant matrices in dermocosmetic samples, the choice of acid and pH value or range in the extraction solution must be carefully optimized.
The levels of bioactive compounds present in the industrial residue of Prinsepia utilis were investigated in two studies. Kewlani et al. [26] optimized the extraction of phenolic compounds from oil industry waste using UAE and response surface methodology (RSM). The extracts, rich in phenols, flavonoids, tannins, and antioxidant activity, exhibited notable antiaging effects through the inhibition of tyrosinase and hyaluronidase, along with photoprotective properties. Santos et al. [27] identified that the most effective solvent for the extraction of bioactive compounds from P. utilis residues was 80% v/v ethanol. The optimal extraction conditions were 41 min at 45 °C for dynamic maceration extraction (DME) and 15 min at 45 °C for UAE. This last one showed higher antioxidant activity and yield than DME, in addition to antiproliferative effects. The estimated sun protection factor (SPF) was determined using the Mansur method, which provided results of 18.22 for DME and 11.68 for UAE. However, despite its operational convenience due to its speed and reduced costs, this spectrophotometric method can be considered relevant only in the pre-selection of plant species that present SPF potential. Another limitation is that the Mansur method cannot be used to evaluate photoprotectors containing physical filters due to their low solubility in methanol, ethanol, or isopropanol solvents [28,29].
Regarding the extracting solution, we observed the highest occurrence of binary ethanol/water mixtures, in proportions ranging from 50 to 90% v/v. This fact can be explained by the occurrence of hydrophilic anthocyanins, such as cyanidin-3-glucoside and delphinidin-3-glucoside, petunidin, pelargonidin, and peonidin, relatively soluble in aqueous solutions, especially under acidic conditions (typically pH ~1–3). On the contrary, compounds such as ellagic acid, quercetin, myricetin derivatives, and phenolic acids have low water solubility, which can be improved in hydroalcoholic media. Particularly, the use of hydroethanolic solutions has the advantage of lower toxicity of ethanol when compared to solvents, such as methanol and propanone [27,30].
Wawoczny & Gillner [31] reviewed recent advances in environmentally friendly and efficient extraction methods, specifically regarding the use of deep eutectic solvents (DESs) for extracting bioactive compounds. They discussed extraction systems and key variables influencing efficiency, such as water content and the nature of hydrogen bond donors and acceptors. The review also highlighted a growing research trend toward the use of hydrophobic DESs for extracting less polar compounds, the application of ternary solvents (choline chloride, a diol or polyol), and citric acid for acidity adjustment.
Lim et al. [32] analyzed the application of glycerol as a solvent for the extraction of bioactive compounds from Hesperethusa crenulata peel as an alternative to organic solvents, which pose risks to both human health and the environment. Extracts were prepared using ethanol, water, glycerol, glycerol/ethanol (1:1, v/v), and glycerol/water (1:1, v/v) as solvents. Among the five extracts, glycerol yielded the highest content of bioactive compounds and flavonoids. The glycerol/ethanol extract showed the highest total phenolic content and the greatest antioxidant activity in both ABTS and FRAP assays. The authors identified that glycerol was more efficient than water and ethanol in extracting bioactive compounds, representing a promising green solvent alternative to replace toxic solvents.
The use of chemometric experimental design methods in extraction processes was a trend in eight more recent studies. Methods, such as fractional factorial design [33], Box–Behnken experimental design [34], and central composite design combined with response surface methodology [22,26,35] can provide time and resource savings by identifying the effects of predictor variables and their interactions, optimizing the ideal conditions to maximize efficiency and yield.
3.6. Analytical Methods
For the quantification of bioactive compounds using spectrophotometric methods, total phenolic content was assessed in 37 studies, 21 of which also investigated photoprotective activity by determining the sun protection factor (SPF), on 18 occasions using the in vitro UV/VIS spectrophotometry methodology based on the Mansur method and on 3 occasions using the in vitro diffuse reflectance spectrophotometry methodology with an integrating sphere. Total flavonoid content was the second most frequently used method, reported in 21 studies, 14 of which also evaluated SPF. The total anthocyanin content was determined in six studies, five using the differential pH method and one using the single pH method. Total tannins were quantified in five studies, while total saponins and total tocopherol were each reported in one study (Figure 3b).
Among the methods used for the separation and structural identification of bioactive compounds, 42 chromatographic techniques were reported: 28 by high-performance liquid chromatography (HPLC), 8 by ultra-performance liquid chromatography (UPLC), 5 by gas chromatography (GC), and 1 by thin-layer chromatography (TLC). Furthermore, Fourier-transform infrared spectroscopy (FT-IR) was cited in five studies, nuclear magnetic resonance (NMR) in two, and paper spray ionization mass spectrometry (PSI-MS) in one (Figure 3c).
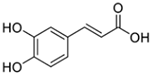
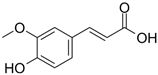
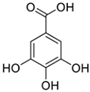
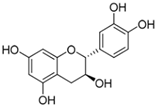
The primary phenolics identified in the jaboticaba peel were anthocyanins (cyanidin-3-glucoside and delphinidin-3-glucoside), quercetin and myricetin-derived flavonoids (rutin), and phenolic acids (gallic, ferulic, and ellagic acid) [2,7,16]. These molecules may act synergistically with UV filters, enhancing their efficacy and stability, and contribute to the development of multifunctional cosmetic products aimed at preventing and repairing UV-induced damage, particularly that related to free radical generation [14,15,17].
3.7. Antioxidant Activity Assessment Methods
Seventeen antioxidant activity assays were identified across 54 articles in the portfolio (Figure 3d). Regarding the number of methods used in each study, 38 (68.5%) employed two or more methods, while 17 articles (31.5%) reported using only one method.
Most of the methods identified (15) were based on chemical assays that measure antioxidant capacity through free radical scavenging, metal chelation, or reducing power. Two of the cited methods show greater biological correlation. Lekmine et al. [36] employed the Oxidative Hemolysis Inhibition Assay (OxHLIA), evaluating the inhibition of damage to cell membranes, in which peroxyl radicals act as pro-oxidants and human erythrocytes serve as oxidizable targets. Peres et al. [37] assessed antioxidant activity using the DPPH method, applying cosmetic formulations to human skin and analyzing the stratum corneum extract collected with adhesive tape through the ex vivo antioxidant activity method.
Gadjalova & Mihaylova [38] evaluated the total phenolic content and antioxidant activity using the DPPH, FRAP, CUPRAC, and ABTS methods of eight medicinal species. Correlation analysis revealed a tendency to employ at least three distinct assays, considering the differences in antioxidant mechanisms of action. However, in early research stages, such as screening studies, the use of a single method may be well justified, particularly when combined with other approaches, such as total phenolic content determination. Of the studies that employed two or more methods, only four did not use DPPH, the most common method, which was the only assay used in thirteen of them.
However, the lack of standardization of extraction methods and antioxidant activity assessment makes it difficult to compare the results obtained.
3.8. Photoprotective Efficacy Assessment Methods
In 39 articles in the portfolio, at least one method was used to determine the efficiency of photoprotective activity, with the in vitro UV/VIS spectrophotometry methodology based on the Mansur method being used on 26 occasions. The in vitro diffuse reflectance spectrophotometry methodology with an integrating sphere was used on 13 occasions. In vivo SPF determination was performed in four studies. Among these studies, the authors evaluated the incorporation of isolated compounds into topical formulations.
Tomazelli et al. [39] evaluated the SPF in vivo, with results suggesting that rutin is a promising candidate for use in multifunctional sunscreens to improve photoprotection, with a pronounced antioxidant effect, which can help protect the skin from damage caused by UV radiation.
Ferulic acid was evaluated by Peres et al. [37], who concluded that the formulations presented good skin biocompatibility and a satisfactory safety profile, as well as a synergistic effect with synthetic UV filters, increasing the SPF in vivo by 37% and the FPUVA by 26%. Rao et al. [40] evaluated the use of quercetin and curcumin by optimizing the preparation of a phytodermal gel (PDG), applying in vitro and in vivo methods. The polymeric mixed micelles (PMMs) of quercetin and curcumin effectively improved the antioxidant and photoprotective effects.
Due to the high costs, demand for volunteers, and the time required to perform in vivo tests, in vitro methods are alternatives for screening tests and preliminary evaluations of natural extracts and prototypes of cosmetic formulations. They present advantages such as speed of execution, affordable cost, reproducibility, ease of operator training, and no exposure of volunteers to risk.
3.9. Evidence of the Use of Jaboticaba Peel Extracts in Photoprotective Dermocosmetics
Table 5 summarizes the main phenolic compounds identified in jaboticaba peel, their UVR absorption wavelengths, biological activities, and potential dermocosmetic applications. The meta-analysis suggested that extracts from jaboticaba peel hold promising potential for the development of dermocosmetics aimed at mitigating UVR-induced damage to the cutaneous tissue.

Table 5.
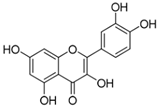
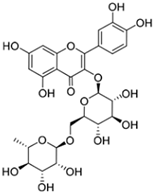
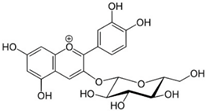
Chemical structures of phenolic compounds present in jaboticaba peel with skin protection action against damage caused by UVR.
3.10. Perspectives for the Development of Jaboticaba Peel Extracts with Antioxidant and Photoprotective Activities
Based on the bibliometric analysis, we propose a methodological approach to extract jaboticaba peels and evaluate their antioxidant activity and photoprotective performance against UVR-induced damage (Figure 4). The scheme outlines five steps of the first phase of in vitro testing. It includes the production of jaboticaba peel flour and the extraction process optimization for phenolic compounds with antioxidant and photoprotective potential.
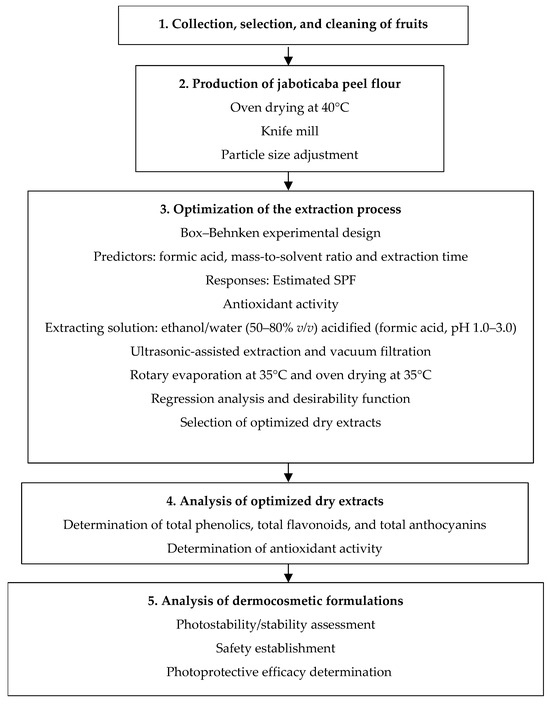
Figure 4.
Perspectives for the evaluation of the antioxidant and photoprotective performances of jaboticaba peel extracts and the development of a dermocosmetic formulation.
In a second phase, pre-clinical and in vivo safety and efficacy establishment must be performed after the production, characterization, and incorporation of the optimized extracts into a cosmetic vehicle. Studies may involve cytotoxicity [50,54,55], antiproliferative and antimutagenic activities [53,56], evaluation of the inhibition of enzymes responsible for skin hyperpigmentation [57,58] and aging prevention [59,60,61], and evaluation of the combat against oxidative stress [62], in addition to stability tests of the samples [63,64,65]. The determination of in vivo SPF [39,66], skin compatibility [67,68], photo irritation potential, photosensitivity, and skin surficial hydration tests [39] are analyses that may prove the potential use of jaboticaba peel extract in multifunctional dermocosmetics.
4. Conclusions
One of the primary strengths of this systematic review lies in its methodological rigor, incorporating the Methodi Ordinatio protocol for article selection, which combines citation metrics, impact factor, and publication year to ensure a high-quality and relevant portfolio. The use of integrated tools such as VOSviewer and MAXQDA for bibliometric and qualitative analyses enabled a comprehensive exploration of research trends, methodological approaches, and compound characterization. Moreover, the review synthesizes multidisciplinary findings spanning phytochemistry, pharmacology, and cosmetic science, offering valuable insights into the dermocosmetic potential of jaboticaba peel extracts. Despite these strengths, certain limitations warrant consideration.
The inclusion criteria, specifically the restriction to articles published from 2010 onwards, may have inadvertently excluded earlier foundational studies that could offer historical context or unique insights. A significant limitation also arises from the current state of research: while this review provides a robust analysis of in vitro evidence, the infrequency of clinical studies on jaboticaba-derived formulations inherently constrains the direct generalizability of our conclusions to real-world dermocosmetic applications.
Until rigorous formulation development and clinical testing are carried out, definitive conclusions about the use of jaboticaba peel in dermocosmetics may not be drawn. Given these findings, future research should aim to (i) standardize extraction and quantification methods to enable cross-study comparisons; (ii) expand biological assays to include validated in vitro and in vivo photoprotective tests; (iii) develop and evaluate dermocosmetic formulations using jaboticaba peel extracts; and (iv) investigate clinical safety, efficacy, and user acceptability. By bridging the gap between phytochemical research and formulation science, the valorization of jaboticaba peel can contribute meaningfully to the development of sustainable, effective, and environmentally responsible cosmetic products.
Nonetheless, the potential advantages of using natural extracts—especially those derived from agricultural residues—should not be overlooked. Further research on bioactive-rich food residues is essential for advancing sustainable practices in the cosmetic, pharmaceutical, and food industries.
Author Contributions
Conceptualization, Y.R.T. and J.S.S.; methodology, C.P.d.S. and J.S.S.; formal analysis, M.G.d.S., C.R., C.P.d.S. and J.S.S.; investigation, Y.R.T., M.G.d.S. and J.S.S.; resources, Y.R.T., C.R., A.R.B. and J.S.S.; data curation, M.G.d.S., C.P.d.S. and J.S.S.; writing—original draft preparation, A.R.B., C.R., F.R.S.A. and J.S.S.; writing—review and editing, Y.R.T., A.R.B., C.R., F.R.S.A. and J.S.S.; visualization, Y.R.T., C.R., A.R.B. and J.S.S.; supervision, Y.R.T., A.R.B. and J.S.S.; project administration, Y.R.T. and J.S.S.; funding acquisition, C.R. and A.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq (grant number 303862/2022-0); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES), grant number 001; Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant number 2024/01920-0); and FCT—Foundation for Science and Technology, I.P. [DOI 10.54499/UIDP/04567/2020; DOI 10.54499/UIDB/04567/2020].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or developed in this study. Data sharing does not apply to this article.
Acknowledgments
The authors would like to thank the University of São Paulo (USP), State University of Midwest (UNICENTRO), and the Federal Institute of Paraná (IFPR). F.R.S.A. is grateful to CAPES for the doctorate scholarship. A.R.B. is grateful to CNPq, for the Research Productivity Grant, and to FAPESP.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Portfolio of 90 articles developed from Methodi Ordinatio.
Table A1.
Portfolio of 90 articles developed from Methodi Ordinatio.
| Nº | Reference | Article | InOrdinatio |
|---|---|---|---|
| 1 | [44] | Coffee by-products in topical formulations: A review. | 412 |
| 2 | [53] | Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. | 301 |
| 3 | [19] | Effect of high hydrostatic pressure and drying methods on phenolic compounds profile of jaboticaba (Myrciaria jaboticaba) peel and seed. | 264 |
| 4 | [69] | Plant-based active photoprotectants for sunscreens. | 251 |
| 5 | [16] | Flavonols and ellagic acid derivatives in peels of different species of jaboticaba (Plinia spp.) identified by HPLC-DAD-ESI/MS. | 249 |
| 6 | [37] | Ferulic acid photoprotective properties in association with UV filters: multifunctional sunscreen with improved SPF and UVA-PF. | 238 |
| 7 | [70] | Phytochemistry and health benefits of jaboticaba, an emerging fruit crop from Brazil. | 232 |
| 8 | [33] | Techno-economic evaluation of the extraction of anthocyanins from purple yam (Dioscorea alata) using ultrasound-assisted extraction and conventional extraction processes. | 221 |
| 9 | [71] | Cyanobacteria and red macroalgae as potential sources of antioxidants and UV radiation-absorbing compounds for cosmeceutical applications. | 218 |
| 10 | [72] | Characterization and quantification of tannins, flavonols, anthocyanins and matrix-Bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. | 215 |
| 11 | [73] | Anti-photoaging and potential skin health benefits of seaweeds. | 212 |
| 12 | [20] | Extraction of antioxidant compounds from jaboticaba (Myrciaria cauliflora) skins: yield, composition and economical evaluation. | 211 |
| 13 | [74] | Development of alginate beads with encapsulated jaboticaba peel and propolis extracts to achieve a new natural colorant antioxidant additive. | 209 |
| 14 | [6] | Identification and quantification of phenolic composition from different species of jaboticaba (Plinia spp.) by HPLC-DAD-ESI/MS. | 207 |
| 15 | [75] | Comparative study of chemical and phenolic compositions of two species of jaboticaba: Myrciaria jaboticaba (Vell.) Berg and Myrciaria cauliflora (Mart.) O. Berg. | 207 |
| 16 | [2] | Jaboticaba berry: A comprehensive review on its polyphenol composition, health effects, metabolism, and the development of food products. | 197 |
| 17 | [43] | Valorization of agri-food waste through the extraction of bioactive molecules. Prediction of their sunscreen action. | 193 |
| 18 | [67] | Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. | 189 |
| 19 | [3] | Composition and potential health effects of dark-colored underutilized Brazilian fruits–A review. | 186 |
| 20 | [21] | Influence of different types of acids and pH in the recovery of bioactive compounds in Jaboticaba peel (Plinia cauliflora). | 179 |
| 21 | [76] | Ultrasound-assisted extraction of bioactive compounds from palm pressed fiber with high antioxidant and photoprotective activities. | 178 |
| 22 | [39] | SPF enhancement provided by rutin in a multifunctional sunscreen. | 174 |
| 23 | [68] | Functional photostability and cutaneous compatibility of bioactive UVA sun care products. | 169 |
| 24 | [30] | In vitro antioxidant and photoprotective activity of five native Brazilian bamboo species. | 164 |
| 25 | [31] | The Most Potent Natural Pharmaceuticals, Cosmetics, and Food Ingredients Isolated from Plants with Deep Eutectic Solvents. | 155 |
| 26 | [77] | Artemisia sieversiana Ehrhart ex Willd. Essential Oil and Its Main Component, Chamazulene: Their Photoprotective Effect against UVB-Induced Cellular Damage and Potential as Novel Natural Sunscreen Additives. | 154 |
| 27 | [18] | Sustainable production of bioactive compounds from jaboticaba (Myrciaria cauliflora): A bibliometric analysis of scientific research over the last 21 years. | 150 |
| 28 | [78] | Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. | 149 |
| 29 | [79] | Strawberry-based cosmetic formulations protect human dermal fibroblasts against UVA-induced damage. | 145 |
| 30 | [80] | Another reason for using caffeine in dermocosmetics: Sunscreen adjuvant. | 139 |
| 31 | [81] | Photoprotective, antioxidant, anticholinesterase activities and phenolic contents of different Algerian Mentha pulegium extracts. | 136 |
| 32 | [59] | Cynara scolymus L.: A promising Mediterranean extract for topical antiaging prevention. | 134 |
| 33 | [82] | Vitamin and bioactive compound diversity of seven fruit species from south Brazil. | 132 |
| 34 | [83] | Hyaluronic acid/polyphenol sunscreens with broad-spectrum UV protection properties from tannic acid and quercetin. | 116 |
| 35 | [84] | Antiproliferative activity on human colon adenocarcinoma cells and in vitro antioxidant effect of anthocyanin-rich extracts from peels of species of the Myrtaceae family. | 110 |
| 36 | [40] | Phyto-cosmeceutical gel containing curcumin and quercetin loaded mixed micelles for improved antioxidant and photoprotective activity. | 109 |
| 37 | [85] | Jaboticaba (Plinia peruviana) extract nanoemulsions: development, stability, and in vitro antioxidant activity. | 108 |
| 38 | [86] | Evaluation of Dispersive Solid-Phase Extraction (d-SPE) as a Clean-up Step for Phenolic Compound Determination of Myrciaria cauliflora Peel. | 108 |
| 39 | [87] | A novel approach in skin care: by-product extracts as natural UV filters and an alternative to synthetic ones. | 108 |
| 40 | [88] | Plinia cauliflora (Mart.) Kausel: A comprehensive ethnopharmacological review of a genuinely Brazilian species. | 107 |
| 41 | [89] | Phenolic composition of peels from different Jaboticaba species determined by HPLC-DAD-ESI/MSn and antiproliferative activity in tumor cell lines. | 106 |
| 42 | [90] | Formulation and optimization of phytosomes of ethanolic extract of Viola tricolor flowers using design of experiment (DOE) to evaluate in vitro photoprotective potential as sunscreen cream. | 106 |
| 43 | [91] | Exploring Mycosporine-like Amino Acid UV-Absorbing Natural Products for a New Generation of Environmentally Friendly Sunscreens. | 105 |
| 44 | [49] | Phenolic compounds from leaves and flowers of Hibiscus roseus: Potential skin cosmetic applications of an under-investigated species. | 102 |
| 45 | [57] | In vitro study of the antioxidant, photoprotective, anti-tyrosinase, and anti-urease effects of methanolic extracts from leaves of six Moroccan Lamiaceae. | 102 |
| 46 | [58] | Photoprotection and Antiaging Activity of Extracts from Honeybush (Cyclopia sp.)—In vitro wound healing and inhibition of the skin extracellular matrix enzymes: tyrosinase, collagenase, elastase and hyaluronidase. | 99 |
| 47 | [92] | Process optimization of phytoantioxidant and photoprotective compounds from carob pods (Ceratonia siliqua L.) using ultrasonic assisted extraction method. | 93 |
| 48 | [64] | Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. | 92 |
| 49 | [93] | Antioxidant activity, sun protection activity, and phytochemical profile of ethanolic extracts of Daemonorops acehensis resin and its phytosomes. | 90 |
| 50 | [94] | Factors affecting SPF in vitro measurement and correlation with in vivo results. | 90 |
| 51 | [95] | Brazilian berry extract (Myrciaria jaboticaba): A promising therapy to minimize prostatic inflammation and oxidative stress. | 89 |
| 52 | [96] | Brazilian agro-industrial wastes as a potential resource of bioactive compounds and their antimicrobial and antioxidant activities. | 86 |
| 53 | [62] | Jaboticaba (Myrciaria jaboticaba) peel as a sustainable source of anthocyanins and ellagitannins delivered by phospholipid vesicles for alleviating oxidative stress in human keratinocytes. | 85 |
| 54 | [97] | Milk thistle extracts could enhance the UV-protection efficiency and stability of mineral filters in sunscreen formulations. | 85 |
| 55 | [65] | Utilization of colored extracts for the formulation of ecological friendly plant-based green products. | 84 |
| 56 | [36] | Investigation of photoprotective, anti-inflammatory, antioxidant capacities and LC-ESI-MS phenolic profile of Astragalus gombiformis Pomel. | 82 |
| 57 | [98] | Efficient extraction of total polyphenols from apple and investigation of its SPF properties. | 80 |
| 58 | [99] | Potential of the ethyl acetate fraction of Padina boergesenii as a natural UV Filter in sunscreen cream formulation. | 78 |
| 59 | [100] | Efficiency of different solvents in the extraction of bioactive compounds from Plinia cauliflora and Syzygium cumini fruits as evaluated by paper spray mass spectrometry. | 78 |
| 60 | [17] | Jaboticaba, a Brazilian jewel, source of antioxidant and wound healing promoter. | 78 |
| 61 | [34] | Optimization of ultrasound-assisted extraction of bioactive compounds from jaboticaba (Myrciaria cauliflora) fruit through a Box-Behnken experimental design. | 77 |
| 62 | [26] | Sustainable extraction of phenolics and antioxidant activities from Prinsepia utilis byproducts for alleviating aging and oxidative stress. | 76 |
| 63 | [101] | Photoprotective, antioxidant potential and DNA damage protection assay of leaf methanolic extract of Holoptelea integrifolia (Roxb) Planch and determination of some bioactive phenolic compounds by RP-HPLC. | 76 |
| 64 | [27] | Valorization of wastes from the juice passion fruit production industry: extraction of bioactive compounds from seeds, antioxidant, photoprotective and antiproliferative activities. | 75 |
| 65 | [63] | In vitro solar protection factor, antioxidant activity, and stability of a topical formulation containing Benitaka grape (Vitis vinifera L.) peel extract. | 73 |
| 66 | [102] | Plinia cauliflora (Mart.) Kausel: toxicological assays, biological activities, and elemental analysis of organic compounds. | 71 |
| 67 | [103] | Labdanum resin from Cistus ladanifer L.: A natural and sustainable ingredient for skin care cosmetics with relevant cosmeceutical bioactivities. | 70 |
| 68 | [54] | Protective role of jaboticaba Plinia peruviana peel extract in copper-induced cytotoxicity in Allium cepa. | 65 |
| 69 | [22] | Anthocyanin extraction from jaboticaba skin (Myrciaria cauliflora Berg.) using conventional and non-conventional methods. | 65 |
| 70 | [104] | Freeze-dried jaboticaba (Myrciaria jaboticaba) peel powder, a rich source of anthocyanins and phenolic acids, mitigates inflammation-driven colorectal cancer in mice. | 59 |
| 71 | [7] | Extraction methods, chemical characterization, and in vitro biological activities of Plinia cauliflora (Mart.) Kausel peels. | 54 |
| 72 | [61] | A comprehensive review of the molecular mechanisms driving skin photoaging and the recent advances in therapeutic interventions involving natural polyphenols. | 53 |
| 73 | [32] | Glycerol extraction of bioactive compounds from Thanaka (Hesperethusa crenulata) bark through LCMS profiling and their antioxidant properties. | 52 |
| 74 | [105] | Spondias purpurea L. stem bark extract: antioxidant and in vitro Photoprotective Activities. | 49 |
| 75 | [14] | Photoprotective activity and HPLC-MS-ESI-IT profile of flavonoids from the barks of Hymenaea martiana Hayne (Fabaceae): development of topical formulations containing the hydroalcoholic extract. | 45 |
| 76 | [106] | The Cosmetic Potential of The Medicinal Halophyte Tamarix gallica L. (Tamaricaceae) growing in the eastern part of Algeria: photoprotective and antioxidant activities. | 44 |
| 77 | [23] | Thermal stability of total phenolic compounds and antioxidant activities of jaboticaba peel: Effect of solvents and extraction methods. | 44 |
| 78 | [55] | Industrial solid wastes from Ganoderma lucidum extract production: chemical characterization and investigation of antioxidant, photoprotective and cytotoxic activities. | 42 |
| 79 | [107] | Bioactivity profile of three types of seaweed as an antioxidant, UV-protection as sunscreen and their correlation activity. | 41 |
| 80 | [15] | Exploring the potential of Cytisus purgans as a source of bioactive molecules: In vitro pharmacological evaluation. | 37 |
| 81 | [108] | Valorization of peel-based agro-waste flour for food products: a systematic review on proximate composition and functional properties. | 34 |
| 82 | [109] | Modulating the photostability of (E)-resveratrol in grape cane extract formulations. | 33 |
| 83 | [60] | Antioxidant, UV protection, and antiphotoaging properties of anthocyanin-pigmented lipstick formulations. | 30 |
| 84 | [110] | Recovery of bioactive compounds from an agro-industrial waste: extraction, microencapsulation, and characterization of jaboticaba (Myrciaria cauliflora Berg) pomace as a source of antioxidant. | 26 |
| 85 | [35] | Optimization of phenolic compounds extraction and a study of the edaphic effect on the physicochemical composition of freeze-dried jaboticaba peel. | 24 |
| 86 | [111] | Anthocyanin extraction from jaboticaba (Myrciaria cauliflora) skins by different techniques: economic evaluation. | 20 |
| 87 | [112] | Peel of pineapple (Ananas comosus) as a potential source of antioxidants and photoprotective agents for sun protection cosmetics. | 16 |
| 88 | [113] | Jaboticaba (Myrciaria jaboticaba) peel extracts induce reticulum stress and apoptosis in breast cancer cells. | 15 |
| 89 | [114] | In vitro photoprotective, antioxidant and antibacterial activity of Vernonia squarrosa (D. Don) Less. | 12 |
| 90 | [115] | Influence of drying temperature on the chemical constituents of jaboticaba (Plinia jaboticaba (Vell.) Berg) skin. | 10 |
References
- IBGE Produção de Jabuticaba. Instituto Brasileiro de Geografia e Estatística. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/jabuticaba/br (accessed on 12 February 2025).
- Inada, K.O.P.; Leite, I.B.; Martins, A.B.N.; Fialho, E.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Jaboticaba berry: A comprehensive review on Its polyphenol composition, health effects, metabolism, and the development of food products. Food Res. Int. 2021, 147, 110518. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Seraglio, S.K.T.; Brugnerotto, P.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Composition and potential health effects of dark-colored underutilized brazilian fruits—A review. Food Res. Int. 2020, 137, 109744. [Google Scholar] [CrossRef]
- Araujo, N.M.P.; Berni, P.; Zandoná, L.R.; Toledo, N.M.V.; Silva, P.P.M.; Toledo, A.A.; Maróstica Junior, M.R. Potential of brazilian berries in developing innovative, healthy, and sustainable food products. Sustain. Food Technol. 2023, 2, 506–530. [Google Scholar] [CrossRef]
- Sanches, M.A.R.; Camelo-Silva, C.; Tussolini, L.; Tussolini, M.; Zambiazi, R.C.; Becker Pertuzatti, P.B. Determination of acetylcholinesterase and alpha-glucosidase inhibition by electrophoretically-mediated microanalysis and phenolic profile by HPLC-ESI-MS/MS of fruit juices from brazilian Myrtaceae Plinia cauliflora (Mart.) Kausel and Eugenia uniflora L. Food Res. Int. 2022, 11, 154–159. [Google Scholar] [CrossRef]
- Neves, N.D.; Stringheta, P.C.; da Silva, I.F.; Garcia-Romero, E.; Gomez-Alonso, S.; Hermosin-Gutierrez, I. Identification and quantification of phenolic composition from different species of jaboticaba (Plinia spp.) by HPLC-DAD-ESI/MSn. Food Chem. 2021, 355, 129605. [Google Scholar] [CrossRef]
- Pinc, M.M.; Dalmagro, M.; da Cruz Alves Pereira, E.; Donadel, G.; Thomaz, R.T.; da Silva, C.; Macruz, P.D.; Jacomassi, E.; Gasparotto Junior, A.; Hoscheid, J.; et al. Extraction methods, chemical characterization, and In Vitro biological activities of Plinia cauliflora (Mart.) Kausel peels. Pharmaceuticals 2023, 16, 1173. [Google Scholar] [CrossRef] [PubMed]
- Dal Prá, V.; Lunelli, F.C.; Vendruscolo, R.G.; Martins, R.; Wagner, R.; Lazzaretti, A.P.; Freire, D.M.G.; Alexandri, M.; Koutinas, A.; Mazutti, M.A.; et al. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Chem. 2023, 3, 686–695. [Google Scholar] [CrossRef]
- Souza, Á.C.; Geraldi, M.V.; Marostica Junior, M.R. Jaboticaba berry: Metabolic benefits, bioactive compounds, and opportunities for commercialization. Trends Food Sci. Technol. 2025, 157, 104894. [Google Scholar] [CrossRef]
- Campos, E.A.R.; Pagani, R.N.; Resende, L.M.; Pontes, J. Construction and qualitative assessment of a bibliographic portfolio using the methodology Methodi Ordinatio. Scientometrics 2018, 116, 815–842. [Google Scholar] [CrossRef]
- Corsi, A.; Pagani, R.N.; Kovaleski, J.L.; Luiz da Silva, V. Technology transfer for sustainable development: Social impacts depicted and some other answers to a few questions. J. Clean. Prod. 2020, 245, 118522. [Google Scholar] [CrossRef]
- Eck, N.J.V.; Waltman, L.; Eck, N.J.V.; Waltman, L. Manual for VOSviewer Version 1.6.20. 2023. Available online: https://www.vosviewer.com/documentation/Manual_VOSviewer_1.6.20.pdf (accessed on 9 February 2025).
- VERBI Software. MAXQDA 2022 Online Manual. Available online: https://maxqda.com/help-max24/welcome (accessed on 10 February 2025).
- Oliveira, F.G.S.; Veras, B.O.; Silva, A.P.S.; Barbosa, D.C.S.; Silva, T.C.M.; Ribeiro, E.R.F.R.; Maia, M.M.L.; Júnior, U.P.S.; Lima, V.L.d.M.; Silva, M.V.D. Photoprotective activity and HPLC-MS-ESI-IT profile of flavonoids from the barks of Hymenaea martiana Hayne (Fabaceae): Development of topical formulations containing the hydroalcoholic extract. Biotechnol. Equip. 2021, 35, 504–516. [Google Scholar] [CrossRef]
- Chaira, S.; Bouzghaia, B.; Hanfer, M.; Kaddi, I.; Ben Moussa, M.T.; Pale, P.; Harkat, H. Exploring the potential of Cytisus purgans as a source of bioactive molecules: In Vitro pharmacological evaluation. Eur. J. Integr. Med. 2024, 67, 102349. [Google Scholar] [CrossRef]
- Neves, N.D.A.; Stringheta, P.C.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Flavonols and ellagic acid derivatives in peels of different species of Jaboticaba (Plinia spp.) identified by HPLC-DAD-ESI/MSn. Food Chem. 2018, 252, 61–71. [Google Scholar] [CrossRef]
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Santos, É.M.D.; Fava, A.L.M.; Figueiredo, M.C.; Ataide, J.A.; Foglio, M.A.; Mazzola, P.G. Jaboticaba, a brazilian jewel, source of antioxidant and wound healing promoter. Sustain. Chem. Pharm. 2021, 20, 100401. [Google Scholar] [CrossRef]
- Rosa, R.G.; Sganzerla, W.G.; Barroso, T.L.C.T.; Buller, L.S.; Berni, M.D.; Forster-Carneiro, T. Sustainable production of bioactive compounds from Jaboticaba (Myrciaria cauliflora): A bibliometric analysis of scientific research over the Last 21 Years. Sustain. Chem. Pharm. 2022, 27, 100656. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Nunes, S.; Martinez-Blazquez, J.A.; Tomas-Barberan, F.A.; Perrone, D.; Monteiro, M. Effect of high hydrostatic pressure and drying methods on phenolic compounds profile of jaboticaba (Myrciaria jaboticaba) peel and seed. Food Chem. 2020, 309, 125794. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Extraction of antioxidant compounds from jaboticaba (Myrciaria cauliflora) skins: Yield, composition and economical evaluation. J. Food Eng. 2010, 101, 23–31. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Baseggio, A.M.; Angolini, C.F.F.; Pastore, G.M.; Cazarin, C.B.B.; Marostica-Junior, M.R. Influence of different types of acids and pH in the recovery of bioactive compounds in jaboticaba peel (Plinia cauliflora). Food Res. Int. 2019, 124, 16–26. [Google Scholar] [CrossRef]
- Mattos, G.N.; Santiago, M.; Chaves, A.; Rosenthal, A.; Tonon, R.V.; Cabral, L.M.C. Anthocyanin extraction from jaboticaba skin (Myrciaria cauliflora Berg.) using conventional and non-conventional methods. Foods 2022, 11, 885. [Google Scholar] [CrossRef]
- Marsiglia, W.I.M.L.; Oliveira, L.S.C.; Almeida, R.L.J.; Santos, N.C.C.; Neto, J.M.S.; Santiago, Â.M.; Melo, B.C.A.; Silva, F.L.H. Thermal stability of total phenolic compounds and antioxidant activities of jaboticaba peel: Effect of solvents and extraction methods. J. Indian Chem. Soc. 2023, 100, 100995. [Google Scholar] [CrossRef]
- Bohn, L.R.; Mibielli, G.M.; Teleken, J.G. Optimization of ultrasound-assisted extraction of phenolic compounds from wine production waste. In Open Science Research VIII; Editora Científica Digital: São Paulo, Brazil, 2022; pp. 1082–1095. [Google Scholar] [CrossRef]
- Sumere, B.R.; de Souza, M.C.; dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef]
- Kewlani, P.; Singh, L.; Singh, B.; Bhatt, I.D. Sustainable extraction of phenolics and antioxidant activities from Prinsepia utilis byproducts for alleviating aging and oxidative stress. Sustain. Chem. Pharm. 2022, 29, 100791. [Google Scholar] [CrossRef]
- Santos, G.J.; Defendi, R.O.; Düsman, E.; Biffi, M.T.; Berton, G.H.; Tonin, A.P.P.; Meurer, E.C.; Suzuki, R.M.; Sípoli, C.C.; Tonin, L.T.D. Valorization of wastes from the juice passion fruit production industry: Extraction of bioactive compounds from seeds, antioxidant, photoprotective and antiproliferative activities. Waste Biomass Valorization 2023, 14, 1233–1250. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Balogh, T.S.; Pedriali, C.A.; Sarruf, F.D.; Pinto, C.A.S.O.; Kaneko, T.; Baby, A.R. Novas metodologias analíticas para avaliação da eficácia fotoprotetora (In Vitro)—Revisão. Rev. Cienc. Farm. Basica Apl. 2011, 32, 27–34. [Google Scholar]
- Donglikar, M.M.; Deore, S.L. Development and evaluation of herbal sunscreen. Pharmacogn. J. 2017, 9, 83–97. [Google Scholar] [CrossRef]
- Wroblewska, K.B.; Baby, A.R.; Guaratini, M.T.G.; Moreno, P.R.H. In Vitro antioxidant and photoprotective activity of five native brazilian bamboo species. Ind. Crops Prod. 2019, 130, 208–215. [Google Scholar] [CrossRef]
- Wawoczny, A.; Gillner, D. The most potent natural pharmaceuticals, cosmetics, and food ingredients isolated from plants with deep eutectic solvents. J. Agric. Food Chem. 2023, 71, 10877–10900. [Google Scholar] [CrossRef]
- Lim, M.W.; Quan Tang, Y.; Aroua, M.K.; Gew, L.T. Glycerol extraction of bioactive compounds from thanaka (Hesperethusa crenulata) bark through LCMS profiling and their antioxidant properties. ACS Omega 2024, 9, 14388–14405. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.; Durango-Zuleta, M.M.; Osorio-Tobon, J.F. Techno-economic evaluation of the extraction of anthocyanins from purple yam (Dioscorea alata) using ultrasound-assisted extraction and conventional extraction processes. Food Bioprod. Process. 2020, 122, 111–123. [Google Scholar] [CrossRef]
- Fernandez-Barbero, G.; Pinedo, C.; Espada-Bellido, E.; Ferreiro-Gonzalez, M.; Carrera, C.; Palma, M.; Garcia-Barroso, C. Optimization of ultrasound-assisted extraction of bioactive compounds from jaboticaba (Myrciaria cauliflora) fruit through a Box-Behnken experimental design. Food Sci. Technol. 2019, 39, 1018–1029. [Google Scholar] [CrossRef]
- Pereira, L.D.; Ascheri, D.P.R.; Bastos, S.M.C.; Ascheri, J.L.R.; Santos, S.D. Optimization of phenolic compounds extraction and a study of the edaphic effect on the physicochemical composition of freeze-dried jaboticaba peel. Cienc. Agrotecnologia 2018, 42, 431–440. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Akkal, S.; Martín-García, A.I.; Boumegoura, A.; Kadi, K.; Djeghim, H.; Mekersi, N.; Bendjedid, S.; Bensouici, C.; et al. Investigation of photoprotective, anti-inflammatory, antioxidant capacities and LC-ESI-MS phenolic profile of Astragalus gombiformis pomel. Foods 2021, 10, 1937. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Gadjalova, A.V.; Mihaylova, D.S. Ultrasound-assisted extraction of medicinal plants and evaluation of their biological activity. Food Res. 2019, 3, 530–536. [Google Scholar] [CrossRef]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Gaikwad, P.; Misal, P.; Gandhi, S.V. Phyto-cosmeceutical gel containing curcumin and quercetin loaded mixed micelles for improved anti-oxidant and photoprotective activity. Colloids Surf. B Biointerfaces 2024, 237, 113837. [Google Scholar] [CrossRef] [PubMed]
- Candido, T.M.; Ariede, M.B.; Pinto, C.; Lima, F.V.; Magalhaes, W.V.; Pedro, N.M.E.; Padovani, G.; Sufi, B.D.; Rijo, P.; Velasco, M.V.R.; et al. Rosmarinic acid multifunctional sunscreen: Comet assay and In Vivo establishment of cutaneous attributes. Cosmetics 2022, 9, 141. [Google Scholar] [CrossRef]
- Candido, T.M.; Ariede, M.B.; Pinto, C.; Lourenco, F.R.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Prospecting In Vitro antioxidant and photoprotective properties of rosmarinic acid in a sunscreen system developed by QbD containing octyl p-methoxycinnamate and bemotrizinol. Cosmetics 2022, 9, 29. [Google Scholar] [CrossRef]
- Martínez-Inda, B.; Esparza, I.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Valorization of agri-food waste through the extraction of bioactive molecules. prediction of their sunscreen action. J. Environ. Manag. 2023, 325, 116460. [Google Scholar] [CrossRef] [PubMed]
- Santos, É.M.d.; Macedo, L.M.d.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Morocho-Jacome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vivo SPF from multifunctional sunscreen systems developed with natural compounds-a review. J. Cosmet. Dermatol. 2021, 20, 729–737. [Google Scholar] [CrossRef]
- Zhang, K.-Q.; Lin, L.-L.; Xu, H. Research on antioxidant performance of diglucosyl gallic acid and its application in emulsion cosmetics. Int. J. Cosmet. Sci. 2022, 44, 177–188. [Google Scholar] [CrossRef]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of plant extracts cosmetics in the field of anti-aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Effect of grape seed extract on skin fibroblasts exposed to UVA light and its photostability in sunscreen formulation. J. Cosmet. Dermatol. 2021, 20, 1271–1282. [Google Scholar] [CrossRef]
- Nascimento, L.B.S.; Gori, A.; Raffaelli, A.; Ferrini, F.; Brunetti, C. Phenolic compounds from leaves and flowers of hibiscus roseus: Potential skin cosmetic applications of an under-investigated species. Plants 2021, 10, 522. [Google Scholar] [CrossRef]
- Hübner, A.A.; Demarque, D.P.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Kikuchi, I.S.; Bacchi, E.M. Phytocompounds recovered from the waste of Cabernet Sauvignon (Vitis vinifera L.) vinification: Cytotoxicity (in normal and stressful conditions) and In Vitro photoprotection efficacy in a sunscreen system. Cosmetics 2023, 10, 2. [Google Scholar] [CrossRef]
- Ruscinc, N.; Morocho-Jácome, A.L.; Martinez, R.M.; Magalhaes, W.V.; Escudeiro, C.C.; Giarolla, J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Vaccinium myrtillus L. Extract associated with octocrylene, bisoctrizole, and titanium dioxide: In Vitro and In Vivo tests to evaluate safety and efficacy. J. Cosmet. Dermatol. 2022, 21, 4765–4774. [Google Scholar] [CrossRef] [PubMed]
- Sarruf, F.D.; Sauce, R.; Candido, T.M.; Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Butyrospermum parkii butter increased the photostability and In Vivo SPF of a molded sunscreen system. J. Cosmet. Dermatol. 2020, 19, 3296–3301. [Google Scholar] [CrossRef] [PubMed]
- Leite-Legatti, A.V.; Batista, A.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Machado, A.R.T.; Carvalho-Silva, L.B.; Ruiz, A.; et al. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef]
- Franscescon, F.; Mazon, S.C.; Bertoncello, K.T.; Boligon, A.A.; Sachett, A.; Rambo, C.L.; Rosemberg, D.B.; Magro, J.D.; Siebel, A.M. Protective role of jaboticaba Plinia peruviana peel extract in copper-induced cytotoxicity in Allium cepa. Environ. Sci. Pollut. Res. 2018, 25, 35322–35329. [Google Scholar] [CrossRef]
- Veljović, S.; Petrović, M.; Jovanović, M.; Mitić-Ćulafić, D.; Semen, T.Ž.Z.; Kostić, M.; Natić, M.; Veljovic, S.; Petrovic, M.; Jovanovic, M.; et al. Industrial solid wastes from Ganoderma lucidum extract production: Chemical characterization and investigation of antioxidant, photoprotective and cytotoxic activities. J. Food Meas. Charact. 2023, 17, 3673–3682. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Mazzei, J.L.; Oliveira, C.G.; Evangelista, H.; Marques, M.R.C.; Ferraz, E.R.A.; Felzenszwalb, I. Protection against UV-induced toxicity and lack of mutagenicity of antarctic Sanionia uncinata. Toxicology 2017, 376, 126–136. [Google Scholar] [CrossRef] [PubMed]
- El Aanachi, S.; Gali, L.; Rammali, S.; Bensouici, C.; Aassila, H.; Dari, K. In Vitro study of the antioxidant, photoprotective, anti-tyrosinase, and anti-urease effects of methanolic extracts from leaves of six moroccan Lamiaceae. J. Food Meas. Charact. 2021, 15, 1785–1795. [Google Scholar] [CrossRef]
- Hering, A.; Stefanowicz-Hajduk, J.; Gucwa, M.; Wielgomas, B.; Ochocka, J.R. Photoprotection and antiaging activity of extracts from honeybush (Cyclopia sp.)-In Vitro wound healing and inhibition of the skin extracellular matrix enzymes: Tyrosinase, collagenase, elastase and hyaluronidase. Pharmaceutics 2023, 15, 1542. [Google Scholar] [CrossRef]
- Marques, P.; Marto, J.; Goncalves, L.M.; Pacheco, R.; Fitas, M.; Pinto, P.; Serralheiro, M.L.M.; Ribeiro, H. Cynara scolymus L.: A promising mediterranean extract for topical anti-aging prevention. Ind. Crops Prod. 2017, 109, 699–706. [Google Scholar] [CrossRef]
- Westfall, A.; Sigurdson, G.T.; Giusti, M.M. Antioxidant, UV protection, and antiphotoaging properties of anthocyanin-pigmented lipstick formulations. J. Cosmet. Sci. 2019, 70, 63–76. Available online: https://pubmed.ncbi.nlm.nih.gov/31125306 (accessed on 8 February 2025).
- Sharma, P.; Dhiman, T.; Negi, R.S.; OC, A.; Gupta, K.; Bhatti, J.S.; Thareja, S. A comprehensive review of the molecular mechanisms driving skin photoaging and the recent advances in therapeutic interventions involving natural polyphenols. South Afr. J. Bot. 2024, 166, 466–482. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Allaw, M.; Hellström, J.; Granato, D.; Manconi, M. Jaboticaba (Myrciaria jaboticaba) peel as a sustainable source of anthocyanins and ellagitannins delivered by phospholipid vesicles for alleviating oxidative stress in human keratinocytes. Molecules 2021, 26, 6697. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Sousa, I.M.d.O.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Foglio, M.A.; Mazzola, P.G. In Vitro solar protection factor, antioxidant activity, and stability of a topical formulation containing benitaka grape (Vitis vinifera L.) peel extract. Nat. Prod. Res. 2020, 34, 2677–2682. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.A.; de Oliveira, C.A.; da Costa, M.S.; Tokunaga, V.K.; Mota, J.P.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. Ski. Res. Technol. 2016, 22, 325–333. [Google Scholar] [CrossRef]
- Adeel, S.; Habiba, M.; Kiran, S.; Iqbal, S.; Abrar, S.; Hassan, C.M. Utilization of colored extracts for the formulation of ecological friendly plant-based green products. Sustainability 2022, 14, 1758. [Google Scholar] [CrossRef]
- Hübner, A.; Sobreira, F.; Neto, A.V.; Pinto, C.; Dario, M.F.; Diaz, I.E.C.; Lourenco, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The synergistic behavior of antioxidant phenolic compounds obtained from winemaking waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Peres, D.D.; Graziola, F.; Chacra, N.A.B.; Araujo, G.L.B.; Florido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous Biocompatible Rutin-Loaded Gelatin-Based Nanoparticles Increase the SPF of the Association of UVA and UVB Filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Peres, D.D.; Rugno, C.M.; Kojima, M.; Pinto, C.A.S.O.; Consiglieri, V.O.; Kaneko, T.M.; Rosado, C.; Mota, J.; Velasco, M.V.R.; et al. Functional photostability and cutaneous compatibility of bioactive UVA sun care products. J. Photochem. Photobiol. B Biol. 2015, 148, 154–159. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-B.; Long, C.L.; Kennelly, E. Phytochemistry and health benefits of jaboticaba, an emerging fruit crop from brazil. Food Res. Int. 2013, 54, 148–159. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and red macroalgae as potential sources of antioxidants and uv radiation-absorbing compounds for cosmeceutical applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Maróstica, M.R.; Rodrigues, E.; Bochi, V.C.; Emanuelli, T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Anti-photoaging and potential skin health benefits of seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Dallabona, I.; de Lima, G.G.; Cestaro, B.I.; Tasso, I.D.S.; Paiva, T.S.; Laureanti, E.J.G.; Jorge, L.M.D.; Silva, B.J.G.; Helm, C.V.; Mathias, A.L.L. Development of alginate beads with encapsulated jaboticaba peel and propolis extracts to achieve a new natural colorant antioxidant additive. Int. J. Biol. Macromol. 2020, 163, 1421–1432. [Google Scholar] [CrossRef]
- Alezandro, M.R.; Dubé, P.; Desjardins, Y.; Lajolo, F.M.; Genovese, M.I. Comparative study of chemical and phenolic compositions of two species of jaboticaba: Myrciaria jaboticaba (Vell.) Berg and Myrciaria cauliflora (Mart.) O. Berg. Food Res. Int. 2013, 54, 468–477. [Google Scholar] [CrossRef]
- Dal Pra, V.; Lunelli, F.C.; Vendruscolo, R.G.; Martins, R.; Wagner, R.; Lazzaretti, A.P.; Freire, D.M.G.; Alexandri, M.; Koutinas, A.; Mazutti, M.A.; et al. Ultrasound-assisted extraction of bioactive compounds from palm pressed fiber with high antioxidant and photoprotective activities. Ultrason. Sonochem. 2017, 36, 362–366. [Google Scholar] [CrossRef]
- Zhou, Y.; He, L.; Wang, W.; Wei, G.; Ma, L.; Liu, H.; Yao, L. Artemisia sieversiana Ehrhart Ex Willd. essential oil and its main component, chamazulene: Their photoprotective effect against UVB-induced cellular damage and potential as novel natural sunscreen additives. ACS Sustain. Chem. Eng. 2023, 11, 17675–17686. [Google Scholar] [CrossRef]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic contents and In Vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Reboredo-Rodriguez, P.; Cianciosi, D.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M.; Giampieri, F. Strawberry-based cosmetic formulations protect human dermal fibroblasts against UVA-induced damage. Nutrients 2017, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Rosado, C.; Tokunaga, V.K.; Sauce, R.; De Oliveira, C.A.; Sarruf, F.D.; Parise-Filho, R.; Maurício, E.; De Almeida, T.S.; Velasco, M.V.R.; Baby, A.R. Another reason for using caffeine in dermocosmetics: Sunscreen adjuvant. Front. Physiol. 2019, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Yakoubi, R.; Megateli, S.; Hadj Sadok, T.; Gali, L. Photoprotective, antioxidant, anticholinesterase activities and phenolic contents of different algerian Mentha pulegium Extracts. Biocatal. Agric. Biotechnol. 2021, 34, 102038. [Google Scholar] [CrossRef]
- Schmidt, H.D.O.; Rockett, F.C.; Pagno, C.H.; Possa, J.; Assis, R.Q.; de Oliveira, V.R.; da Silva, V.L.; Flôres, S.H.; Rios, A.D.O. Vitamin and bioactive compound diversity of seven fruit species from south Brazil. J. Sci. Food Agric. 2019, 99, 3307–3317. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.; Park, S.A.; Park, J.J.; Park, W.H. Hyaluronic Acid/Polyphenol Sunscreens with broad-spectrum UV protection properties from tannic acid and quercetin. Int. J. Biol. Macromol. 2024, 257, 128585. [Google Scholar] [CrossRef]
- Frauches, N.S.; Montenegro, J.; Amaral, T.; Abreu, J.P.; Laiber, G.; Junior, J.; Borguini, R.; Santiago, M.; Pacheco, S.; Nakajima, V.M.; et al. Antiproliferative activity on human colon adenocarcinoma cells and In Vitro antioxidant effect of anthocyanin-rich extracts from peels of species of the Myrtaceae family. Molecules 2021, 26, 564. [Google Scholar] [CrossRef]
- Mazzarino, L.; da Silva Pitz, H.; Lorenzen Voytena, A.P.; Dias Trevisan, A.C.; Ribeiro-Do-Valle, R.M.; Maraschin, M. Jaboticaba (Plinia peruviana) extract nanoemulsions: Development, stability, and In Vitro antioxidant activity. Drug Dev. Ind. Pharm. 2018, 44, 643–651. [Google Scholar] [CrossRef]
- Senes, C.E.R.; Nicácio, A.E.; Rodrigues, C.A.; Manin, L.P.; Maldaner, L.; Visentainer, J.V. Evaluation of dispersive solid-phase extraction (d-SPE) as a clean-up step for phenolic compound determination of Myrciaria cauliflora peel. Food Anal. Methods 2020, 13, 155–165. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel approach in skin care: By-product extracts as natural UV filters and an alternative to synthetic ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef]
- Gasparotto Junior, A.; de Souza, P.; Lívero, F.A.D.R.; Junior, A.G.; de Souza, P.; Livero, F.A.D. Plinia cauliflora (Mart.) Kausel: A comprehensive ethnopharmacological review of a genuinely brazilian species. J. Ethnopharmacol. 2019, 245, 112169. [Google Scholar] [CrossRef]
- Paludo, M.C.; de Oliveira, S.B.P.; de Oliveira, L.F.F.; Colombo, R.C.C.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; Prata, R.; Lima, A.F.F.; Filho, J.T.; Ballus, C.A.A.; et al. Phenolic composition of peels from different jaboticaba species determined by HPLC-DAD-ESI/MSn and antiproliferative activity in tumor cell lines. Curr. Plant Biol. 2022, 29, 100233. [Google Scholar] [CrossRef]
- Ameri, A.; Khazaeli, P.; Behnam, B.; Mehrabani, M.; Forootanfar, H. Formulation and optimization of phytosomes of ethanolic extract of Viola tricolor flowers using design of experiment (DOE) to evaluate In Vitro photoprotective potential as sunscreen cream. Ind. Crops Prod. 2024, 209, 118057. [Google Scholar] [CrossRef]
- Rosic, N.; Climstein, M.; Boyle, G.M.; Thanh Nguyen, D.; Feng, Y. Exploring mycosporine-like amino acid UV-absorbing natural products for a new generation of environmentally friendly sunscreens. Mar. Drugs 2023, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Ayad, R.; Ayad, R.; Bourekoua, H.; Lefahal, M.; Makhloufi, E.H.; Akkal, S.; Medjroubi, K.; Nieto, G. Process optimization of phytoantioxidant and photoprotective compounds from carob pods (Ceratonia siliqua L.) using ultrasonic assisted extraction method. Molecules 2022, 27, 8802. [Google Scholar] [CrossRef]
- Sari, R.K.; Prayogo, Y.H.; Rozan, S.A.; Rafi, M.; Wientarsih, I. Antioxidant activity, sun protection activity, and phytochemical profile of ethanolic extracts of Daemonorops acehensis resin and its phytosomes. Sci. Pharm. 2022, 90, 10. [Google Scholar] [CrossRef]
- Cvetkovska, A.D.; Manfredini, S.; Ziosi, P.; Molesini, S.; Dissette, V.; Magri, I.; Scapoli, C.; Carrieri, A.; Durini, E.; Vertuani, S. Factors affecting SPF In Vitro measurement and correlation with In Vivo results. Int. J. Cosmet. Sci. 2017, 39, 310–319. [Google Scholar] [CrossRef]
- Lamas, C.A.; Kido, L.A.; Hermes, T.A.; Nogueira-Lima, E.; Minatel, E.; Collares-Buzato, C.B.; Maróstica, M.R.; Cagnon, V.A. Brazilian berry extract (Myrciaria jaboticaba): A promising therapy to minimize prostatic inflammation and oxidative stress. Prostate 2020, 80, 859–871. [Google Scholar] [CrossRef]
- Valerio, A.; Avila, L.B.; Lacorte, D.H.; Martiny, T.R.; Rosseto, V.; Moraes, C.C.; Dotto, G.L.; Carreno, N.L.V.; da Rosa, G.S.; Filho, A.V.; et al. Brazilian agro-industrial wastes as a potential resource of bioative compounds and their antimicrobial and antioxidant activities. Molecules 2022, 27, 6876. [Google Scholar] [CrossRef]
- Erdoğan, Ü.; Gökçe, E.H.; Muhammed, M.T.; Önem, E.; Erten, A.A.; Süleymanoğlu, B. Milk thistle extracts could enhance the UV-protection efficiency and stability of mineral filters in sunscreen formulations. J. Photochem. Photobiol. A Chem. 2024, 450, 115460. [Google Scholar] [CrossRef]
- Opris, O.; Lung, I.L.K.; Soran, M.L.; Stegarescu, A.; Cesco, T.; Ghendov-Mosanu, A.; Podea, P.; Sturza, R. Efficient extraction of total polyphenols from apple and investigation of its SPF properties. Molecules 2022, 27, 1679. [Google Scholar] [CrossRef]
- Soleimani, S.; Yousefzadi, M.; Babaei Mahani Nezhad, S.; Pozharitskaya, O.N.; Shikov, A.N. Potential of the ethyl acetate fraction of Padina boergesenii as a natural UV filter in sunscreen cream formulation. Life 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Correia, V.T.D.; Silva, V.D.M.; Mendonca, H.D.P.; Ramos, A.; Silva, M.R.; Augusti, R.; de Paula, A.; Ferreira, R.; Melo, J.O.F.; Fante, C.A. Efficiency of different solvents in the extraction of bioactive compounds from Plinia cauliflora and Syzygium cumini fruits as evaluated by paper spray mass spectrometry. Molecules 2023, 28, 2359. [Google Scholar] [CrossRef]
- Mondal, S.; Bandyopadhyay, A. Photoprotective, antioxidant potential and DNA damage protection assay of leaf methanolic extract of Holoptelea integrifolia (Roxb) planch and determination of some bioactive phenolic compounds by RP-HPLC. Biocatal. Agric. Biotechnol. 2023, 50, 102728. [Google Scholar] [CrossRef]
- Assis, P.M.; Dutra, R.C.; Amarante, C.B.; Chaves, A.; Moreira, C.; Brandao, M.; Raposo, N.R. Plinia cauliflora (Mart.) Kausel: Toxicological assays, biological activities, and elemental analysis of organic compounds. Nat. Prod. Res. 2021, 35, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Díaz, T.S.; Silva, A.M. Labdanum resin from Cistus ladanifer L.: A natural and sustainable ingredient for skin care cosmetics with relevant cosmeceutical bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Nascimento, R.D.; Rizzato, J.S.; Polezi, G.; Moya, A.; Silva, M.F.; Machado, A.P.D.; Franchi, G.C.F.J.; Borguini, R.G.; Santiago, M.; Paiotti, A.P.R.; et al. Freeze-dried jaboticaba (Myrciaria jaboticaba) peel powder, a rich source of anthocyanins and phenolic acids, mitigates inflammation-driven colorectal cancer in mice. Food Biosci. 2023, 53. [Google Scholar] [CrossRef]
- Rodrigues, F.A.M.; Giffony, P.S.; dos Santos, S.B.F.; Guedes, J.A.C.; Ribeiro, M.E.N.P.; de Araújo, T.G.; da Silva, L.M.R.; Zocolo, G.J.; Ricardo, N.M.P.S. Spondias purpurea L. stem bark extract: Antioxidant and In Vitro photoprotective activities. J. Braz. Chem. Soc. 2021, 32, 1918–1930. [Google Scholar] [CrossRef]
- Lefahal, M.; Makhloufi, E.H.; Trifa, W.; Ayad, R.; El Hattab, M.; Benahmed, M.; Keskin, M.; Akkal, S. The cosmetic potential of the medicinal Halophyte tamarix Gallica L. (Tamaricaceae) growing in the eastern part of Algeria: Photoprotective and antioxidant activities. Comb. Chem. High Throughput Screen. 2021, 24, 1671–1678. [Google Scholar] [CrossRef]
- Sami, F.J.; Soekamto, N.H.; Firdaus; Latip, J. Bioactivity profile of three types of seaweed as an antioxidant, UV-protection as sunscreen and their correlation activity. Food Res. 2021, 5, 441–447. [Google Scholar] [CrossRef]
- Lesmana, D.; Vianney, Y.M.; Goenawan, Y.A.; Natalie, K.; Sukweenadhi, J.; Buschle-Diller, G.; Mukti, Y.P.; Erawati, C.M.; Purwanto, M.G.M. Valorization of Peel-Based Agro-Waste Flour for Food Products: A systematic review on proximate composition and functional properties. ACS Food Sci. Technol. 2022, 2, 3–20. [Google Scholar] [CrossRef]
- Besrukow, P.; Orbach, A.; Schweiggert, R. Modulating the photostability of (E)-resveratrol in grape cane extract formulations. ACS Food Sci. Technol. 2024, 4, 625–632. [Google Scholar] [CrossRef]
- Santos, S.S.D.; Paraíso, C.M.; Costa, S.C.D.A.; Ogawa, C.Y.L.; Sato, F.; Madrona, G.S. Recovery of bioactive compounds from an agro-industrial waste: Extraction, microencapsulation, and characterization of jaboticaba (Myrciaria cauliflora Berg) pomace as a source of antioxidant. Anais Acad. Bras. Cienc. 2022, 94, e20191372. [Google Scholar] [CrossRef] [PubMed]
- Veggi, P.C.; Santos, D.T.; Meireles, M.A.A. Anthocyanin extraction from jaboticaba (Myrciaria cauliflora) skins by different techniques: Economic evaluation. Procedia Food Sci. 2011, 1, 1725–1731. [Google Scholar] [CrossRef]
- Samarakoon, S.M.G.K.; Rajapakse, C.S.K. Peel of Pineapple (Ananas comosus) as a potential source of antioxidants and photo-protective agents for sun protection cosmetics. Med. Plants 2022, 14, 95–104. [Google Scholar] [CrossRef]
- da Silva-Maia, J.K.; Nagalingam, A.; Cazarin, C.B.B.; Marostica Junior, M.R.; Sharma, D. Jaboticaba (Myrciaria jaboticaba) peel extracts induce reticulum stress and apoptosis in breast cancer cells. Food Chem. Mol. Sci. 2023, 6, 100167. [Google Scholar] [CrossRef]
- Das, A.; Burman, S.; Chandra, G.; Bandyopadhyay, A. In Vitro photoprotective, antioxidant and antibacterial activity of Vernonia squarrosa (D. Don) Less. Plant Sci. Today 2021, 8, 331–339. [Google Scholar] [CrossRef]
- Alves, A.P.C.; Corrêa, A.D.; Oliveira, F.C.; Isquierdo, E.P.; Abreu, C.M.P.; Borém, F.M. Influence of drying temperature on the chemical constituents of jaboticaba (Plinia jaboticaba (Vell.) Berg) Skin. Acta Sci. Technol. 2014, 36, 721–726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).