1. Introduction
In recent years, changes in human consumption habits have driven the demand for products that have less impact on the environment and are eco-friendly. This growing environmental concern offers new opportunities for the development of solutions that are both innovative and sustainable.
Due to their chemical composition and physical characteristics, cosmetics provide a favorable environment for the growth of microorganisms such as bacteria and fungi. This occurs due to the presence of water, suitable pH, and organic ingredients that are prone to degradation, making these products susceptible to microbial contamination. To minimize this risk, synthetic preservatives are widely used, playing an essential role in ensuring the safety and stability of products throughout their lifecycle. However, synthetic preservatives have significant limitations. They often have restricted action against specific microorganisms, operate within narrow pH ranges, and can bioaccumulate in the environment [
1]. Additionally, they are increasingly associated with adverse effects such as skin irritations and allergies, cytotoxicity, and environmental impacts. Examples include parabens and formaldehyde releasers, which are extensively criticized in the literature for their potential risks to human health and the environment [
2]. In recent years, reports of antimicrobial resistance to these compounds have also increased, emphasizing the need for safer and more effective alternatives.
In this context, essential oils (EOs) emerge as promising options. EOs comprise complex mixtures of substances produced by the plants’ secondary metabolism, and include substances such as monoterpenes, sesquiterpenes, phenolic and polyphenolic compounds [
3]. EOs are synthesized by various plant organs and possess remarkable biological properties, including antimicrobial, antifungal, and antimutagenic activities [
4]. The antimicrobial activity of EOs occurs primarily through damage to the membranes and cell walls of microorganisms, causing permeabilization, ion loss, macromolecule leakage, and cell lysis [
4]. Although these effects do not automatically classify an agent as antimicrobial, they can enhance the action of other compounds, such as antibiotics and antifungals. The literature indicates that the effectiveness of EOs often results from the synergy between their major and minor constituents. This interaction enables different components to target distinct sites in microorganisms, enhancing their antimicrobial effects [
4,
5]. Furthermore, EOs are often seen as safer and more cost-effective options, making them a viable alternative for application as natural preservatives in cosmetic formulations.
Additionally, EOs have intrinsic properties that enable their application as antioxidant compounds, making them a good alternative to commercial antioxidants in cosmetic, pharmaceutical, and food formulations. The literature defines an antioxidant compound as one that can slow down the oxidation of an oxidizable material, even in minimal quantities (<1%, typically 1000 mg/L), compared to the amount of material it is intended to protect [
6]. Antioxidants that can prevent the oxidation chain reaction from occurring are called direct antioxidants and are classified into two types based on their interference mechanism. Preventive antioxidants act at the initial stage of radical species formation, delaying the onset of oxidation. Meanwhile, chain-breaking antioxidants reduce the rate or block the propagation of reactions by competing and undergoing autooxidation [
6]. Since preventive antioxidants are ineffective after the reaction has started, chain-breaking antioxidants are the most suitable for the various necessary applications. In the cosmetic context, the incorporation of EOs with antioxidant properties offers significant benefits, such as protecting the skin from damage caused by free radicals, which can lead to premature aging and other dermatological conditions. Studies have shown that EOs derived from cinnamon, thyme, clove, lavender, and peppermint exhibit remarkable antioxidant activities, being effective in neutralizing free radicals and reducing cellular oxidative stress [
7]. EOs can also play an essential role in preventing the oxidation of emulsions themselves. Emulsions, commonly used in cosmetic formulations, are susceptible to oxidation, which can lead to degradation, loss of efficacy, and changes in the appearance of the product. This oxidation process typically occurs when the emulsion is exposed to light, air, or high temperatures [
8]. Incorporating EOs with strong antioxidant characteristics into emulsions can help to protect the active ingredients from oxidative damage. These EOs are capable of neutralizing free radicals and slowing down the oxidation process by either preventing the formation of these radicals or breaking the oxidative chain reactions once they have started [
6]. Moreover, EO not only help to protect the emulsion itself but also enhance the stability and shelf life of the cosmetic product. Their natural ability to act as preservatives is particularly valuable in formulations aimed at reducing reliance on synthetic preservatives, which may not align with consumer preferences for clean-label or natural products. In this sense, EOs can act as both active ingredients, providing benefits to the skin, and stabilizers, maintaining the integrity of the formulation over time [
7,
9].
However, the efficacy of EOs is limited due to their volatility, lipophilicity, and lack of specific targets, which can cause damage to human cells. Such damage includes alterations in cell membrane fluidity and chain reactions leading to macromolecule leakage and lysis. The safety of their use heavily depends on the concentrations employed; for instance, the cytotoxicity of thymol in fibroblasts has been reported at concentrations above 200 μg/mL [
10]. Therefore, the use of these oils must be carefully planned to ensure the safety of the final product.
To address these challenges, techniques such as the microencapsulation of EOs have shown to be advantageous. This approach not only protects the bioactive compounds from degradation but also enables controlled and targeted release, enhancing the efficacy of EOs as natural preservatives. Microencapsulation is a technique that involves coating EOs with biocompatible materials, such as biopolymers or β-cyclodextrin. β-cyclodextrin forms inclusion complexes that stabilize the volatile EOs compounds. The encapsulation can improve the stability and efficacy of essential oils in cosmetic formulations.
Lippia origanoides Kunth (syn.
Lippia sidoides Cham.), from the botanical family Verbenaceae J.St.-Hil. [
11], popularly known as “Alecrim Pimenta,” is a plant species native to Brazil that has been extensively studied for its medicinal properties. Its essential oil is rich in phenolic compounds, such as thymol and carvacrol [
12], and is renowned for its potent antimicrobial and antioxidant activities. This composition makes it a promising candidate for cosmetic applications that can act as a preservative or antioxidant ingredient.
Currently, there are few studies validating the use of EOs as preservatives in different matrices, with only three known commercial products based on EOs for cosmetics preservation: DMC Base Natural
®, Protecta One
®, and Protecta Two
® [
13].
The potential of essential oils in the cosmeceutical field is widely acknowledged in the scientific literature due to their antioxidant, antimicrobial, and anti-inflammatory properties. This study explores the potential of Lippia origanoides EO (registered in SisGen AD6FB6F), which contains well-documented bioactive compounds and can be regarded as a promising candidate for multifunctional cosmetic applications. However, to the best of the authors’ knowledge, no previous studies have investigated its use, either in its pure form or encapsulated in β-cyclodextrin microparticles, as a substitute or adjuvant for synthetic antioxidants and preservatives in cosmetic formulations.
This study introduces an innovative approach by exploring Lippia origanoides EO as a multifunctional cosmetic additive in topical formulations. This approach not only aligns with current market trends towards sustainability and clean-label products but also offers a natural and eco-friendly alternative to synthetic preservatives, enhancing product safety and stability.
In addition, this approach meets the growing consumer demand for environmentally responsible and sustainable cosmetics, while also supporting the valorization of Brazilian biodiversity. The use of encapsulation technology enhances the bioavailability and enables controlled release of the essential oil, contributing to the development of more effective, stable, and ecologically conscious formulations based on natural bioactive compounds.
2. Materials and Methods
2.1. Materials and Chemicals
The commercial essential oil of
Lippia origanoides used in this study was obtained from PRONAT (Horizonte, CE, Brazil). The essential oil (EO) was previously characterized and reported by our group [
12]. GC–MS analysis identified 23 compounds, with the five major constituents being thymol (76.8%), p-cymene (10.38%), caryophyllene (4.73%), β-myrcene (1.81%), and γ-terpinene (1.51%), which together accounted for 95.23% of the total EO composition [
14].
The reagents for the antioxidant capacity assays, including 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and Folin–Ciocalteu reagent, were sourced from Sigma Aldrich (St. Louis, MO, USA). For the microencapsulated process, the β-cyclodextrin utilized was obtained from Sigma Aldrich, and ultrapure water was used as a solvent. For cosmetic formulations, the products utilized were glycerine (Ref. COSM-01216), xanthan gum, sweet almond oil, and betaine purchased from GranVelada (Zaragoza, Spain), and lecithin acquired from TCI (Tokyo, Japan). Ethanol (used as the extraction solvent) was purchased from VWR (Rosny-sous-Bois, France). The commercial antioxidant, butylated hydroxytoluene (BHT), was obtained from Sigma Aldrich (St. Louis, MO, USA). The culture mediums used in the microbiological assays were Rose-Bengal Chloramphenicol Agar (RBC), Lauryl Sulfate Agar (LSA), and Plate Count Agar (PCA) from Sigma Aldrich (St. Louis, MO, USA).
2.2. Characterization of the Essential Oil
2.2.1. DPPH and ABTS Assays
The antioxidant activity of the EO was assessed using both the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. In the DPPH assay, 20 μL of the sample or Trolox standard was mixed with 180 μL of DPPH solution (150 μmol/L), and the mixture was allowed to incubate in the dark for 40 min. The absorbance was measured at 515 nm using the Gen5 2.00.18 (Agilent BioTek, Santa Clara, CA, USA). As a blank, a solution consisting of 20 μL of water and 180 μL of ethanol was used, while for the control, 20 μL of water was combined with 180 μL of DPPH solution [
15]. The DPPH inhibition percentage was calculated according to Equation (1):
For the ABTS assay, the method followed the protocol outlined by Xiao et al. [
16]. In this assay, essential oil solutions were prepared in ethanol, with concentrations varying from 5 to 150 mg/L. A volume of 20 μL from these solutions or Trolox standards was added to 180 μL of ABTS solution, followed by 15 min incubation in the dark to ensure complete reaction. After incubation, absorbance was measured at 734 nm using Gen5 software (Agilent BioTek). As a control, a solution consisting of 20 μL of 0.05 M acetic acid buffer (pH 4.6) and 180 μL of ABTS solution was used. The percentage of ABTS inhibition was calculated using Equation (2):
The results were expressed as the Trolox equivalent (TE), which allows for a standardized comparison of antioxidant capacity. To determine the TE, a Trolox calibration curve was constructed based on the percentage of DPPH inhibition by Trolox standards, with concentrations ranging from 25 to 250 mg/L. This curve was used to correlate the absorbance values obtained from the DPPH assay to Trolox equivalents.
Additionally, the concentration of the extract required to inhibit 50% of DPPH and ABTS (IC50) was determined. The IC50 value provides an estimate of the antioxidant potency of the extract, which is a widely accepted metric in antioxidant research.
Finally, the Antioxidant Activity Index (AAI) was calculated using Equation (3):
2.2.2. Antimicrobial Properties
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests were conducted to evaluate the antimicrobial activity of the EO against selected bacterial strains. Three species were tested:
Escherichia coli,
Staphylococcus aureus, and
Staphylococcus epidermidis. As described in previous studies [
17], all bacterial strains were retrieved from a −80 °C freezer, streaked onto Mueller–Hinton agar plates, and incubated overnight. Subsequently, colonies were transferred to Mueller–Hinton broth and incubated at 37 °C with continuous shaking at 150 rpm for inoculum preparation. Six predetermined concentrations of the oil were prepared in 50% aqueous DMSO (dimethyl sulfoxide): 2000 μg/mL, 1000 μg/mL, 750 μg/mL, 500 μg/mL, 250 μg/mL, and 100 μg/mL.
The MIC of the EO was determined using the microdilution method in sterile 96-well microtiter plates, following CLSI guidelines [
18]. Bacterial cells of
E. coli,
S. aureus, and
S. epidermidis were grown overnight (16 h incubation) and harvested during the exponential growth phase. The cell density was adjusted to 1 × 10
6 cells/mL, at OD (optical density) = 0.132 ± 0.02 at 600 nm, and added to sterile 96-well polystyrene microplates, containing 20 μL of the essential oil and 180 μL of the bacteria cells, with a final volume of 200 μL per well. DMSO was used as a negative control. The plates were incubated for 24 h at 37 °C, and the OD was analyzed before and after the incubation period [
19]. The MIC was defined as the lowest concentration of the EO at which the final OD was lower than or equal to the initial OD [
20].
To determine the MBC, 10 µL aliquots were taken from three wells corresponding to concentrations at and above the MIC. These were plated onto Plate Count Agar and incubated at 37 °C for 24 h. The MBC was defined as the lowest concentration at which no bacterial colonies were observed, indicating a total bacterial kill rather than merely inhibiting growth. From the plate used for MIC determination, 10 μL aliquots were taken from three wells for each concentration and plated on a nutrient-rich medium, Plate Count Agar. The plates were then incubated at 37 °C overnight. Afterwards, the colony growth was observed [
16,
19].
2.3. Synthesis of Microparticles
The synthesis methodology for β-cyclodextrin (β-CD) microparticles was adapted from previous studies [
14]. Initially, 60 g of β-CD (Cavamax
® W7 Pharma—Wacker Chemie AG, Munich, Germany) was weighed and dissolved in 150 g of distilled water at 50 °C. The mixture was maintained under continuous stirring and temperature control at 55 °C for 5 h to ensure complete polymer hydration. Subsequently, the system temperature was raised to 65 °C, and the essential oil was incorporated at a 1:10 (
v/
v) oil-to-β-CD mass ratio. Heating was then discontinued, and the formulation was left stirring overnight (18 h) to allow for gradual cooling and the formation of the inclusion complex.
Although the solubility of β-CD is approximately 2% at 25 °C, it significantly increases during the molecular inclusion process, reaching 5% (
w/
w) at 50 °C, which is sufficient to emulsify the EOs [
14]. This methodology has been previously validated for
Lippia origanoides (syn.
Lippia sidoides) essential oil. Thermal characterization studies, including thermogravimetric analysis (TG), evolved gas detection (EGD), and TG–mass spectrometry (TG–MS), have demonstrated that the main bioactive constituents of the essential oil, such as thymol and carvacrol, are thermally stable up to at least 120 °C, with volatilization being the predominant phenomenon and no evidence of chemical degradation [
20].
The molecular inclusion complex suspensions were spray-dried using the benchtop spray dryer SD-05 (Lab Plant, Huddersfield, UK) with a two-fluid atomizer (orifice diameter of 1.0 mm) under the operating conditions pre-defined in the preliminary tests, namely the following: drying air inlet temperature = 160 °C; atomizing air pressure = 2.0 bar; atomization air flow rate = 17 L/min; feed flow rate of the MIC slurry = 4 g/min; drying air flow rate = 60 m
3/h. Previous studies using the same essential oil and drying conditions reported only minor changes in the volatile profile, attributed to the intrinsic complexity of the essential oil composition [
14], supporting the thermal robustness of the formulation.
A full physicochemical characterization of the microparticles, including the encapsulation efficiency, total oil load, molar ratio of key EO constituents, relative composition of volatiles, residual moisture, water activity, particle size distribution (by laser diffraction), and crystallinity (XRD), was previously reported in detail [
14]. These analyses confirmed the successful formation and stability of the β-CD microparticles.
2.4. Cosmetic Cream Formulation
To evaluate the applicability of microparticles and essential oil as natural preservatives in cosmetic formulations, six different oil-in-water (O/W) emulsions were produced.
Table 1 provides details of the different raw materials used and their respective concentrations (wt %). The base formulation consisted of two phases: phase A (aqueous phase), comprising ultrapure water, glycerin, and xanthan gum, and phase B (oil phase), containing coconut oil, sweet almond oil, lecithin, and betaine.
Each phase was heated separately to 70 °C and mixed until complete melting and homogenization. The essential oil was then added to phase B and stirred to ensure uniform incorporation. Subsequently, phase B was then gradually incorporated into phase A under continuous stirring and a controlled temperature. The final emulsion was homogenized using an Ultra-Turrax T-25 homogenizer (IKA Works, Wilmington, DE, USA) at 12,000 rpm for 2 min [
21].
Compared to spray drying, this emulsification step involved considerably milder thermal exposure. As discussed in
Section 2.3, the essential oil was stable at this temperature range. Additionally, the emulsification process was performed under continuous stirring in a semi-closed system, minimizing EO volatilization.
For the incorporation of the additives (microparticles and positive control agents), emulsions were also prepared without the addition of essential oil. These blank formulations were cooled to 40 °C after homogenization, and the respective additives were incorporated under continuous stirring to ensure uniform distribution. The negative control received no additives.
The selected concentrations of 0.85% and 1.5%
Lippia origanoides essential oil (EO), as well as 2% and 4% β-cyclodextrin-based microparticles, were determined based on evidence from prior studies and formulation feasibility. A recent study by Figueiredo et al. [
22] demonstrated that 1.0%
L. origanoides EO in topical nanoemulsions exhibited both antimicrobial efficacy and an absence of cytotoxicity in keratinocyte cultures. Similarly, another study reported effective antimicrobial activity at concentrations below 2% in in vitro assays [
23]. These data support the use of concentrations ≤ 2% as both efficacious and safe for dermocosmetic applications. Furthermore, an encapsulation efficiency close to 80% in our microparticle system ensured that 2–4% of microparticles delivered bioactive levels of EO, in line with regulatory recommendations for leave-on cosmetic products, which typically restrict EO content to below 2%. The chosen concentrations also respected formulation stability constraints while enabling functional evaluation across a realistic and regulatory-compliant range.
2.5. Stability of the Cream Formulations
To assess the stability of the produced formulations, the following tests were conducted.
2.5.1. Organoleptic Properties
To analyze the organoleptic properties, all formulations were evaluated after emulsion stabilization for color, consistency, odor, and texture.
2.5.2. Centrifuge Test
The centrifugation test is a well-established method employed in the evaluation of cosmetic formulations to assess their physical stability by simulating gravitational stress. This accelerated test serves as a predictive tool for determining the likelihood of phase separation and contributes valuable insights into the product’s shelf life and long-term behavior. To assess the physical stability of the formulations, the samples were subjected to stress testing in a centrifuge in duplicate, following a 30 min cycle at 3000 rpm. After the experiment, phase separation was evaluated [
21].
2.5.3. Thermal Stability Test
Thermal resistance analysis was performed for all formulations. In duplicate, 5 g of each formulation was weighed and exposed to extreme temperature conditions: 24 h at −4 °C, 24 h at room temperature (±20 °C), and 24 h at 50 °C. At the end of the analysis, phase separation was assessed to determine the stability of the formulations.
2.5.4. pH
The pH of the formulations was measured over time at predetermined intervals: immediately after emulsion stabilization, and after two weeks, four weeks, and six weeks. For each formulation, 1 g of the sample was weighed in duplicate and mixed with 9 mL of ultrapure water. The mixture was homogenized by subjecting it to an ultrasonic bath for 5 min, followed by 5 min of mechanical stirring. After homogenization, the duplicates were analyzed using a digital pH meter, ensuring continuous agitation during measurement.
2.6. Antioxidant Potential of the Cream Formulations
All formulations were evaluated for their antioxidant potential over time to assess the ability of the essential oil and microparticles to act as antioxidants within the emulsion. Additionally, the study determined how this activity changed compared to what was observed under the action of the commercial antioxidant used as a positive control in cosmetic creams. Four time points were selected for analysis: one day after formulation stabilization, two weeks, four weeks, and six weeks. At each evaluation, 2 g of each formulation was weighed and mixed with 8 mL of ethanol to extract the phenolic compounds. The extraction process followed a three-step cycle: 5 min of ultrasound treatment (VWR USC, Radnor, PA, EUA), 1 min of mechanical agitation, and 10 min of centrifugation at 3000 rpm. The antioxidant capacity was then analyzed using ABTS and DPPH assays, as previously described in
Section 2.2.1.
2.7. Microbiological Contamination Tests
To evaluate the ability of the formulation components to prevent microbial growth over time and to detect potential contamination in the emulsions, microbiological analysis tests were performed. At predetermined time points—immediately after formulation stabilization, two weeks, four weeks, and eight weeks—the formulations were examined for bacterial or fungal growth. For this purpose, 800 μL of each cosmetic formulation was directly plated, in duplicate, onto Lauryl Sulfate Agar (LSA) to assess bacterial presence and onto Rose Bengal Chloramphenicol Agar (RBC) to detect yeast and/or mold growth. LSA plates were incubated at 37 °C for 24 h, while RBC plates were incubated at room temperature (±25 °C) for one week. After incubation, colony-forming unit (CFU) growth was assessed and quantified.
3. Results
3.1. Characterization of the Essential Oil
3.1.1. Antioxidant Potential
To assess the antioxidant potential of the essential oil (EO), the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) methods were employed. DPPH is a nitrogen-containing free radical with maximum absorbance at 517 nm. When it reacts with antioxidant compounds, its molecule undergoes reduction, causing a color change from purple to yellow [
24]. In the ABTS method, the ABTS radical cation (ABTS•
+), which is a blue–green substance that absorbs light most strongly at 734 nm, reacts with antioxidants. This reaction causes the substance to lose its color, resulting in a decrease in absorbance. These methods allow the determination of the IC
50 value, a key parameter that indicates the antioxidant capacity of the analyzed sample [
25]. The IC
50 represents the concentration of a given compound required to inhibit 50% of the activity of a specific target, in this case, the scavenging of the DPPH or ABTS radical. Higher IC
50 values indicate lower antioxidant capacity, as a greater sample concentration is needed to achieve 50% inhibition. Conversely, lower IC
50 values suggest higher antioxidant capacity, as a smaller sample concentration is sufficient to reach the same level of inhibition. Thus, the essential oil’s antioxidant capacity was characterized to evaluate its potential application as a natural antioxidant in formulations. The results obtained are presented in
Table 2.
In the literature, the reported antioxidant activity of
Lippia origanoides EO varies depending on the assay and experimental conditions. For the ABTS assay, an IC
50 value of 5.22 ± 0.08 µg/mL has been documented [
26], which is consistent with the results obtained in the present study. For the DPPH assay, values of 377.0 ± 34.04 µg/mL were reported in the same study, which are considerably higher and therefore indicate weaker antioxidant activity than that found in the present work [
26]. But variations are expected because factors like geographical origin, cultivation conditions, extraction methods, and chemical composition can profoundly influence the antioxidant activity of EOs, even within the same plant species.
Additionally, the Antioxidant Activity Index (AAI) was calculated according to the method proposed by Scherer and Godoy [
27], using the DPPH concentration of 150 µmol/L (equivalent to 59.15 µg/mL) and the IC
50 value of 2.0 µg/mL for the essential oil. The resulting AAI was 29.6, which classifies the antioxidant activity of
Lippia origanoides essential oil as very strong, supporting its potential as a powerful natural antioxidant.
The results obtained indicate that the EO, according to both methodologies, functions as a highly potent antioxidant agent. This classification is based on IC
50 criteria, which can be categorized into four levels: very strong (IC
50 < 50 µg/mL), strong (IC
50: 50–100 µg/mL), moderate (IC
50: 101–150 µg/mL), and weak (IC
50: 250–500 µg/mL) [
28].
The strong antioxidant potential observed, especially for the pure essential oil (AAI = 29.6), is consistent with the chemical composition of the EO used in this study. It is important to note that the essential oil was the same batch analyzed by the authors of [
14], who demonstrated that
Lippia origanoides essential oil contained thymol as the major compound (~83%), followed by p-cymene and γ-terpinene. Thymol, a phenolic monoterpene, exerts antioxidant activity primarily by donating hydrogen atoms through its hydroxyl group, stabilizing free radicals such as DPPH and ABTS•
+ and interrupting oxidative chain reactions [
29]. Additionally, thymol has been shown to inhibit lipid peroxidation and to interact with redox-sensitive biomolecular pathways. Studies also suggest that synergistic effects may occur between thymol and minor EO constituents. For example, p-cymene, despite its low intrinsic antioxidant activity, may enhance membrane permeability, facilitating the intracellular action of thymol [
5]. Similarly, γ-terpinene may act as a co-antioxidant, capable of regenerating thymol radicals or stabilizing reactive intermediates, thereby enhancing the overall antioxidant efficacy [
30].
Therefore, the antioxidant activity observed is not only supported by the IC50 values but also consistent with known chemical composition of thymol-rich oils, providing mechanistic plausibility to the functional role of Lippia origanoides EO in topical formulations.
Thus, the potential application of this oil, either in its pure form or encapsulated, as an effective antioxidant agent in various formulations, can be inferred. This study focuses on incorporating this active ingredient into cosmetic formulations, aiming to provide an alternative to commercially available antioxidants in the global market.
3.1.2. Antimicrobial Activity
To further characterize the essential oil, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against
Staphylococcus aureus,
Staphylococcus epidermidis, and
Escherichia coli were determined (
Table 3). These microorganisms were selected as models of Gram-negative and Gram-positive bacteria commonly responsible for human infections (
E. coli and
S. aureus, respectively) and opportunistic bacteria naturally present in human skin (
S. epidermidis). The MIC was defined as the lowest concentration in the microplate at which no visible bacterial growth was observed after 24 h of incubation under constant agitation and controlled temperature conditions. The MBC was defined as the lowest concentration of the EO at which no bacterial colony growth was detected on solid agar plates after incubation.
The results indicated that the essential oil exhibited lower antimicrobial activity against
S. epidermidis, whereas its highest antimicrobial activity was observed against
E. coli, suggesting its potential for clinical applications. According to the literature, strong inhibitors of microbial growth are defined as those with MIC ≤ 500 µg/mL [
17], a criterion met only against
E. coli in this study. However, phytochemicals are also considered potential antimicrobial inhibitors within the range of 100–1000 µg/mL [
31], indicating that the essential oil investigated can be classified as an antimicrobial agent against
E. coli and
S. aureus.
Determining the MBC provides important insights into the bactericidal efficacy of a compound, complementing MIC assessments. This parameter is particularly relevant in evaluating the therapeutic potential of natural or synthetic antimicrobial agents, as it helps distinguish between bacteriostatic and bactericidal effects. Regarding the MBC, the results presented in
Table 3 indicate that the tested EO exhibited bactericidal activity against all three bacterial strains analyzed. These findings suggest that the EO possesses broad-spectrum antimicrobial action, effectively eliminating the tested bacteria and reinforcing its potential for therapeutic and industrial applications.
In previous studies, the EO, when tested at absolute concentration, exhibited an MIC of 0.4 µL/mL against
S. aureus [
32], validating its potential for use in topical cosmetic formulations. This finding further supports the feasibility of incorporating the essential oil as an active ingredient in dermatological products with antimicrobial properties. Moreover, although the literature predominantly discusses a bacteriostatic mechanism of action against this bacterium, the results indicate that
Lippia origanoides EO also exerts bactericidal effects at higher concentrations. This dual mechanism of action enhances its potential applications in bacterial infection control. Additionally, studies have demonstrated the antimicrobial activity of the essential oil through the formation of inhibition halos against
S. aureus and
Escherichia coli, further reinforcing its effectiveness in combating these bacterial strains [
32].
The findings of this study highlight the significant antimicrobial potential of Lippia origanoides EO. Through MIC and MBC assays, the oil demonstrated both bacteriostatic and bactericidal effects, with notable efficacy against E. coli and S. aureus. Previous studies corroborate these results, reporting inhibition halos and low MIC values, reinforcing its potential for topical applications in cosmetic formulations. The dual mechanism of action observed suggests that this EO could serve as a natural alternative to conventional antimicrobial agents, broadening its scope for industrial applications.
3.2. Synthesis of Microparticles
The essential oil (EO) of
Lippia origanoides Kunt was microencapsulated using β-cyclodextrin (β-CD) as the encapsulating agent. Several studies have demonstrated that the encapsulation of active ingredients is an effective strategy to protect volatile and unstable compounds, thereby enhancing their physicochemical stability and prolonging their bioactivity in cosmetic formulations [
14,
32]. Given the high susceptibility of
L. origanoides EO to volatilization, its encapsulation in β-CD represents a promising approach to preserve its antioxidant and antimicrobial properties during storage and application.
Following the microencapsulation process, the encapsulation efficiency was determined to be 77%, enabling the calculation of both theoretical loading (TL) and actual loading content (ALC). The TL was estimated at 9.10%, while the ALC, defined as the amount of bioactive compound (major constituent, thymol) effectively retained per gram of microparticles, was 4.54%. This indicates that each gram of microparticles contains approximately 0.0454 g of encapsulated thymol. These values were used to determine the amount of microparticles incorporated into the tested cosmetic formulations.
Physicochemical characterization confirmed the successful formation and stability of the microparticles. Laser diffraction analysis (Beckman Coulter LS 13 320—Brea, CA, USA) revealed an average particle diameter of 16.7 ± 1.5 μm, with a narrow size distribution suitable for cosmetic applications. The residual moisture content was 2.8%, and the water activity (Aw) was 0.22, both indicative of good storage stability. X-ray diffraction (XRD) analysis showed a partially amorphous structure, consistent with the formation of molecular inclusion complexes between β-CD and EO constituents. These results, reported in detail by Oliveira and Miguel [
14], reinforce the suitability of the microparticles for incorporation into emulsified cosmetic systems.
3.3. Cosmetic Cream Formulation
Six oil-in-water (O/W) emulsion formulations (
Figure 1) were developed for comparative analysis. One of them was formulated without the addition of any active compounds, serving as the negative control (NC). The positive control (PC) contained 0.05% of butylated hydroxytoluene (BHT), a synthetic antioxidant commonly used to prevent oxidative degradation, and 0.80% of phenoxyethanol, a widely accepted preservative in cosmetic formulations, with a maximum authorized concentration of 1.0% according to international regulatory standards [
33]. To evaluate the antioxidant and preservative potential of
Lippia origanoides EO, both in its free and encapsulated forms, different concentrations were selected based on preliminary data and encapsulation efficiency. For the formulations containing the free EO, two concentrations were tested: 0.85% and 1.5%. These concentrations were chosen to assess whether increasing the content of free EO would enhance its functional efficacy in stabilizing the emulsion and inhibiting microbial or oxidative degradation. For the formulations containing the microencapsulated EO, the incorporation was performed using microparticles previously characterized for their encapsulation efficiency (77%) and actual loading content (4.54%). Based on these parameters, the amounts of 2 g and 4 g of microparticles per 100 g of total formulation were selected. This allowed for a direct comparison not only between different concentrations but also between the free and encapsulated forms in terms of their effectiveness in maintaining the physicochemical and microbiological stability of the cosmetic emulsions over time.
This experimental design enabled the assessment of dose-dependent effects as well as the impact of the encapsulation process on the functional properties of L. origanoides EO when applied to cosmetic formulations.
3.4. Stability Test
3.4.1. Organoleptic Properties
All tested formulations exhibited organoleptic properties consistent with those expected for cosmetic creams in emulsion form (
Table 4). The samples presented a uniform light-yellow color, attributed to their shared oily phase composition. In addition, all formulations showed a homogeneous consistency and pleasant odor, although formulations containing pure EO, in varying concentrations, displayed a more distinctive scent profile. Particularly, the texture of the formulations containing microparticles exhibited a mildly exfoliating effect, which can be attributed to the incomplete solubilization of the microparticles within the emulsion system developed for this study. Interestingly, the exfoliating characteristic observed in certain formulations is not considered a drawback. Instead, it presents an opportunity to repurpose the product prototype as a body or facial scrub. The presence of microparticles contributes to a gentle abrasive effect, which can aid in the removal of dead skin cells, promoting smoother and more radiant skin. This aligns with the benefits of physical exfoliation, which include enhancing skin texture and appearance.
3.4.2. Centrifuge Test
Centrifugation tests were conducted to evaluate whether the formulations in this study were stable or not concerning phase separation. As observed in
Figure 2, all tested samples exhibited no signs of phase separation following centrifugation, except the NC and PC, which did not emulsify as good as the formulations with oil active compounds. This centrifuge stability indicates satisfactory physical stability under stress conditions. These findings support the continuation of the study, as physical instability in emulsified systems may interfere with the assessment of other critical characteristics over time.
3.4.3. Thermal Stability Test
Another critical test to ensure the shelf life and long-term stability of the formulations is the assessment of thermal stability under accelerated conditions, exposing the samples to extreme temperature variations. The results demonstrated that all tested formulations exhibited satisfactory thermal stability, further supporting the progression of the study into the evaluation of antioxidant and preservative potential. Moreover, to ensure consumer acceptance, it is essential that the developed formulation not only incorporates innovative active ingredients but also maintains the physicochemical stability typically expected of emulsion-based cosmetic products.
Although the thermal resistance assay used in this study served as a preliminary accelerated stress test—simulating temperature fluctuations that may occur during storage and transport—the authors acknowledge that more standardized protocols are necessary to ensure robust and reproducible shelf-life assessments. Following ICH guidelines for accelerated stability studies (e.g., storage at 40 ± 2 °C and 75% RH ± 5% for six months) would enable a more comprehensive evaluation of long-term physicochemical and functional stability.
3.4.4. pH
The pH is a crucial parameter in the context of cosmetic formulations. It can indicate whether a product remains stable over time, must be compatible with the skin’s natural pH at the site of application, and can provide functional benefits when properly regulated. Currently, skin pH is recognized as a regulatory factor in the homeostasis of the stratum corneum and the integrity of the skin barrier [
34]. Human skin is slightly acidic, with a pH typically ranging from 4.1 to 5.8, depending on the anatomical region [
34].
The acidified pH of the skin plays a vital role and has been the subject of extensive research. Its most critical functions include supporting keratinocyte differentiation, promoting the formation and organization of epidermal lipids, and maintaining microbial balance, particularly under conditions of cutaneous disorders or disruptions [
35]. Maintaining the skin’s physiological pH is essential for preserving barrier function, enzymatic activity, and microbial homeostasis. Therefore, the pH of cosmetic formulations must be carefully adjusted to remain within a range that supports skin health and compatibility.
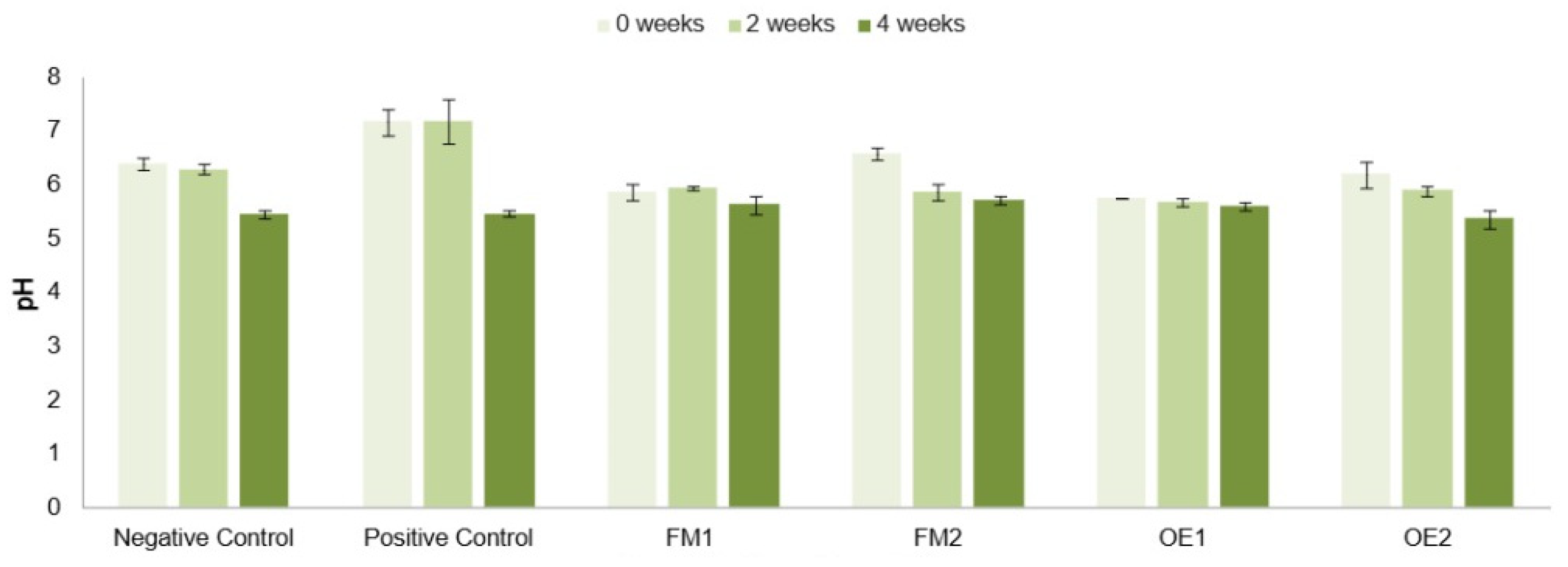
Accordingly, the pH values of the formulations developed in this study were measured to assess both their topical suitability and their stability over time (
Figure 3). An initial observation revealed a slight but consistent decrease in pH across all formulations as time progressed. Since the base formulation was a water-in-oil emulsion, oxidation reactions in the oil phase components may have gradually occurred over time, contributing to this pH decline. However, it is important to note that the NC and PC formulations displayed pH values outside the ideal range. Specifically, the NC showed values above 6 prior to the pH drop after four weeks, while the PC exhibited neutral pH values, neither of which aligns with the mildly acidic range considered optimal for topical application. In contrast, the formulations containing the selected active ingredients exhibited a reduced degree of acidification over time, suggesting greater pH stability and, consequently, enhanced formulation robustness when compared to the controls.
3.5. Antioxidant Potential of the Cream Formulations
The antioxidant potential was monitored over time to assess whether the active compounds used in this study could preserve the integrity of the formulations against oxidative stress. This is particularly relevant considering that the cosmetic formulation is primarily composed of oils, which are highly prone to oxidation. Overall, a high antioxidant capacity was observed for both the pure oil and the microparticles, especially when incorporated into the formulations.
From
Figure 4, it is possible to analyze the values of the antioxidant potential through the ABTS methodology. The positive control exhibited unexpected behavior, such as an increase in antioxidant potential over time, contrary to the expectation that this potential would decrease due to possible degradation of the commercial antioxidant. This outcome may be related to testing instabilities or even to an uneven distribution of the active compounds within the formulation at the start of the analysis.
Formulations containing microparticles demonstrated increasing antioxidant potential over time, despite initially high values. This trend supports the occurrence of sustained release, a characteristic commonly associated with microencapsulation strategies. In contrast, formulations containing non-encapsulated essential oils (EOs) exhibited high initial antioxidant activity that progressively declined throughout the study period, which is consistent with the known instability and degradation of EOs when not protected by encapsulation.
The ABTS-based methodology yielded results that diverged from those obtained using the DPPH reagent for antioxidant potential quantification. This discrepancy may be attributed to several factors. Firstly, the ABTS reagent is soluble in both organic and aqueous media, whereas DPPH is only soluble in organic solvents. Additionally, ABTS has a longer shelf life compared to DPPH when stored, which can contribute to more consistent results over time. Furthermore, there may be specific interactions between ABTS and the antioxidants present in the formulations that influence the observed outcomes [
36].
Upon analyzing the antioxidant potential quantified through the DPPH assay (
Figure 5), it was possible to better understand how the tested actives relate to the control groups. As expected, the negative control (NC), which lacked any added antioxidant agents, showed low antioxidant potential from the beginning, which continued to decline over time.
Contrary to expectations, the positive control (PC), containing commercial antioxidants, also followed a downward trend, although its antioxidant potential decreased at a slower rate compared to the NC. While the PC initially demonstrated higher antioxidant potential, its effectiveness diminished over time, indicating a gradual loss of protective capability against oxidative degradation.
These findings suggest that although the PC was designed to offer protection, its ability to sustain this protection over time was limited. In contrast, the tested actives appeared to maintain their antioxidant potential more effectively, highlighting their potential superiority in protecting cosmetic formulations from oxidative degradation over an extended period.
In contrast, formulations containing microparticles demonstrated two distinct behaviors depending on their concentration, highlighting the direct influence of active dosage on antioxidant efficacy. The FM1 formulation, which contained a lower concentration of microparticles, showed a consistent increase in antioxidant potential over time. This may suggest a sustained release of actives into the medium, resulting in enhanced protection of the formulation. Notably, its initial antioxidant potential was comparable to that of the commercial antioxidant control, supporting the potential of using microparticles as a natural source of antioxidants in cosmetic applications. Conversely, the FM2 formulation, with a higher microparticle concentration, displayed a more irregular pattern. Although FM2 initially declined in antioxidant activity, it recovered by week 6, suggesting a delayed release mechanism. This delayed but significant increase (p < 0.001) reinforces the hypothesis of prolonged functional activity provided by the encapsulation.
Finally, the OE1 formulation, containing a lower concentration of essential oil, showed a gradual decline in antioxidant potential over time, although it consistently maintained higher values than the other samples. The OE2 formulation, which contained the highest EO concentration, displayed a more variable profile, with a notable increase between weeks 4 and 6, suggesting cumulative effects or stabilization of bioactive compounds within the emulsion matrix over time.
The antioxidant potential analyses revealed high activity in all formulations containing active compounds. Although the ABTS assay presented some inconsistencies in its temporal trend, due to the sensitivity of the radical and interactions with formulation components, the general antioxidant performance remained superior in formulations containing Lippia origanoides EO, both in its pure and encapsulated forms. When compared to commercial antioxidants and preservatives at the same concentration, Lippia origanoides showed greater and more sustained antioxidant activity over time. These findings support its potential as an effective natural alternative to conventional synthetic antioxidants and preservatives or as a complementary ingredient to enhance overall formulation stability.
Formulations incorporating microparticles demonstrated significant antioxidant capacity throughout the study, which progressively increased, indicating a gradual release of active compounds into the medium. Despite this promising performance, their use may be more appropriate as preservative boosters rather than primary preservatives.
3.6. Microbiological Contamination Tests
To evaluate the extent to which the EO of Lippia origanoides, which exhibited strong antimicrobial activity in MIC and MBC tests, could protect cosmetic formulations microbiologically, compared to commercial preservatives, a microbial challenge test was performed. This assessment involved formulations containing the active compound in both the encapsulated and pure forms, analyzed over time for bacterial and fungal growth.
Given that the product is a water-in-oil (W/O) emulsion, the aqueous phase is significantly reduced after emulsification. Additionally, the slightly acidic pH values and the presence of bioactive compounds contribute to an environment that is inherently less favorable for microbial proliferation.
As observed in
Table 5, the total bacterial count results were satisfactory: none of the formulations, including the NC, showed bacterial contamination (LSA medium) throughout the study period. This indicates not only the antimicrobial effectiveness of the active compounds but also suggests that the emulsion base itself possesses intrinsic bacteriostatic properties, likely due to its composition and physical characteristics.
The analysis of fungal contamination using RBC medium revealed that, from the second week of storage onward, both the negative control formulation and those containing microparticles exhibited signs of fungal growth, primarily mold. These findings contrast the absence of bacterial contamination across all samples throughout the observation period. While ISO 17516:2014 establishes a maximum of 100 CFU/g or mL of total fungi for general-use cosmetic products [
37], the affected formulations exceeded this threshold and were therefore considered microbiologically unsatisfactory. This contamination is likely associated with the delayed release of encapsulated active compounds, which may have hindered antifungal action during the early stages of storage. Despite this, the potential of microparticles as preservative boosters remains relevant and warrants further investigation.
In contrast, formulations containing Lippia origanoides essential oil demonstrated complete microbiological stability throughout the entire study, with no fungal or bacterial growth detected. This reinforces the essential oil’s strong antimicrobial efficacy and supports its use as a natural preservative in cosmetic products.
In summary, formulations containing Lippia origanoides EO demonstrated superior microbial stability and consistently prevented contamination across all time points. These results highlight its effectiveness as a natural preservative, outperforming both the negative control and microparticle-based formulations.
4. Conclusions
This work proposed the development of a more sustainable cosmetic cream by integrating Lippia origanoides Kunt essential oil as a natural active compound. The essential oil, both in its pure and microencapsulated forms, was comprehensively analyzed and demonstrated excellent antioxidant and antimicrobial performance.
The inclusion of the active ingredients in the cosmetic emulsions resulted in formulations with high stability, desirable physicochemical characteristics, and effective protection against microbial contamination, particularly in samples with the non-encapsulated oil. Encapsulation with β-cyclodextrin allowed for the controlled release of bioactive compounds, offering an alternative for gradual and prolonged functional activity within the formulation. Importantly, the tested emulsions maintained stable pH, texture, and appearance over time, with no signs of phase separation or degradation under stress conditions. The findings support the use of Lippia origanoides essential oil as a promising alternative to conventional synthetic antioxidants and preservatives, or with commercial products as a preservative booster, contributing to the creation of cleaner, safer, and more sustainable cosmetic products.
For future research, it is advisable to conduct a microbiological challenge test following ISO 11930 [
38] guidelines to confirm the preservative efficacy of all tested actives under simulated contamination conditions and a shelf-life assessment throughout a longer period to further validate their potential for large-scale cosmetic application. Also, while this study highlights the multifunctional potential of
Lippia origanoides essential oil and its microparticles in cosmetic formulations, it is important to acknowledge that industrial application still depends on factors such as production costs, process scalability, and formulation stability over time. The development of sustainable products must go beyond efficacy, considering technical and economic feasibility to ensure that these natural alternatives can be realistically adopted at a commercial scale. Future work should aim to bridge this gap by exploring strategies that optimize both performance and process.