Unlocking Nature’s Anti-Aging Secrets: The Potential of Natural Mineral Waters Combined with Plant Extracts in Cosmetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Market Study
2.2. Formulation Development
2.3. Stability
2.4. Rheological Characterization
2.5. Skin Irritation Test
2.5.1. Reconstructed Human Epidermis EpiSkin®
2.5.2. Treatment Conditions
2.5.3. MTT Assay
2.5.4. Data Analysis
2.5.5. Acceptance Criteria
3. Results
3.1. Market Study
3.2. Formulation’ Development
3.3. Stability
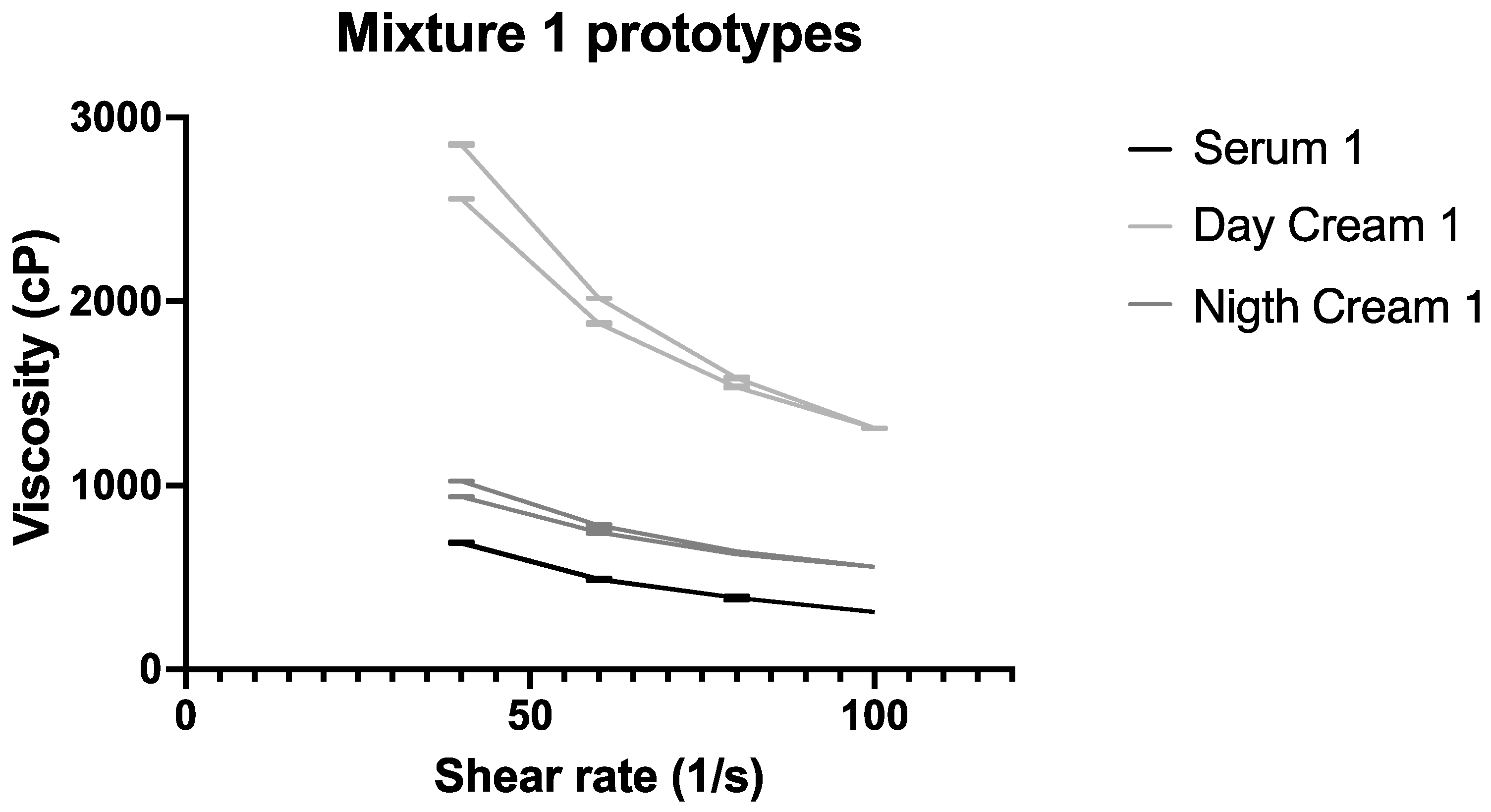
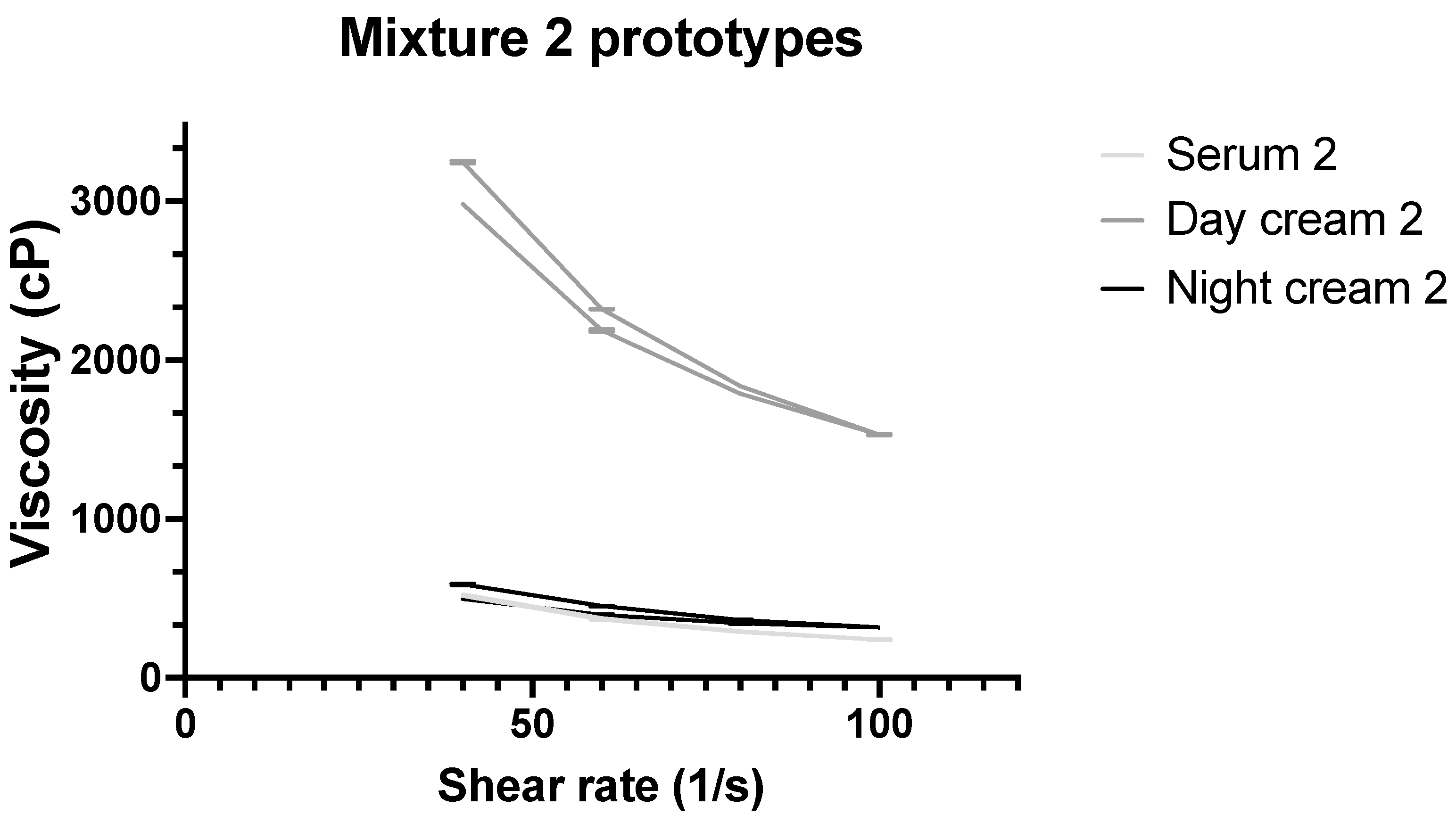
3.4. Rheological Characterization
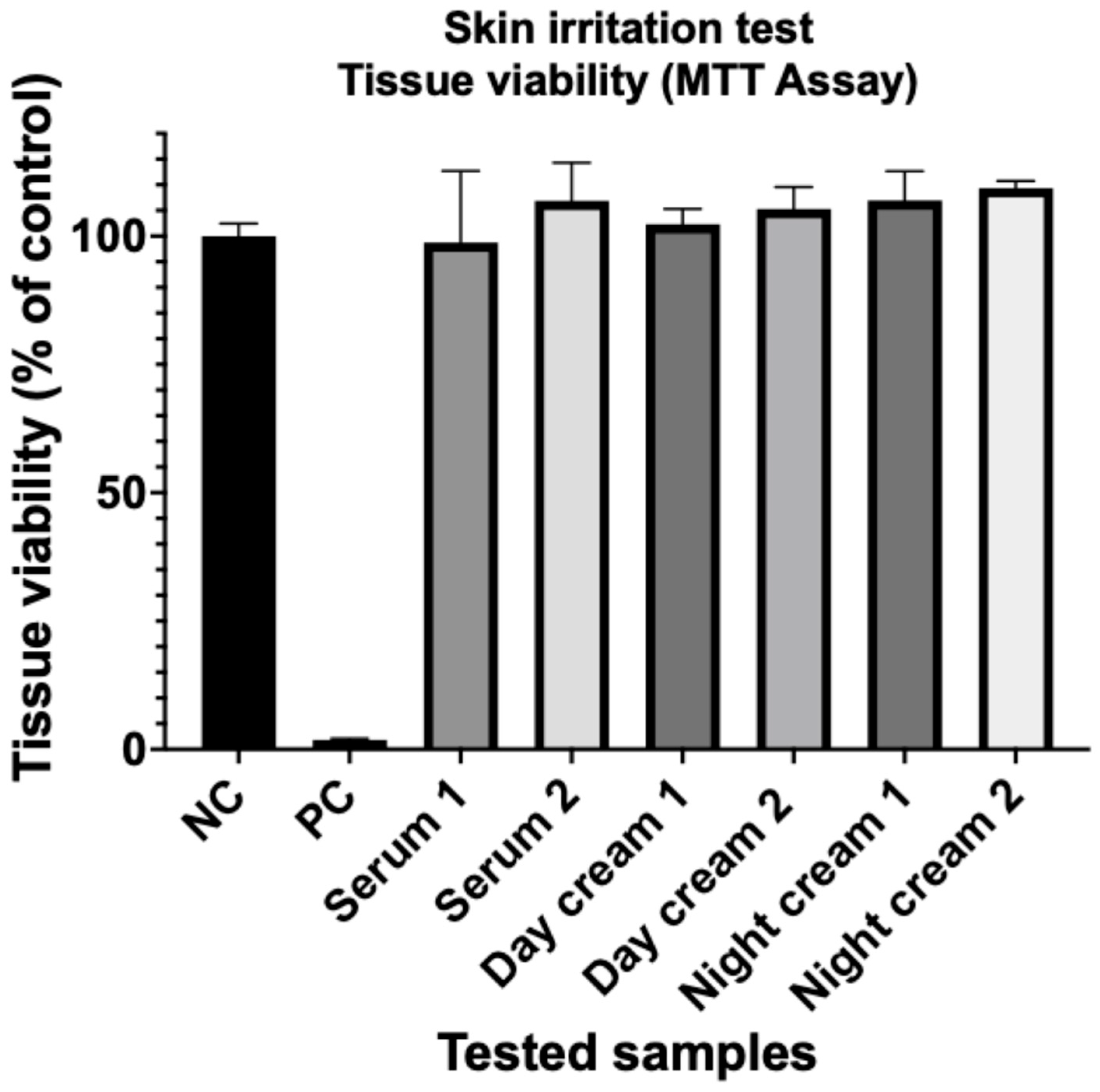
3.5. Skin Irritation Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Serum Formulation Evolution, Final Formula, and Productive Method
| Phase | Ingredient (Name/INCI) | V01 | V02 | V03 |
|---|---|---|---|---|
| % | % | % | ||
| A | NMW Unhais da Serra | 81.55 | 81.50 | 80.00 |
| Thymus × citriodorus hidrolate | 3.00 | 3.00 | 3.00 | |
| Glycerin | 5.00 | 5.00 | 5.00 | |
| Pentylene Glycol | - | - | 1.50 | |
| B | Xantan Gum | 0.80 | 0.80 | 0.80 |
| Caprylic/capric Triglycerides | 2.50 | 2,.50 | 2.50 | |
| Vitis Vinifera (Grape) Seed Oil | 1.00 | 1.00 | 1.00 | |
| C | Aqua/Water; Propanediol; Asparagopsis Armata Extractt | 2.00 | 2.00 | 2.00 |
| Niacinamide | 3.00 | 3.00 | 3.00 | |
| Tocopherol | - | 0.05 | 0.05 | |
| Phenoxyethanol | 1.00 | 1.00 | 1.00 | |
| D | Citric acid | 0.15 | 0.15 | 0.15 |
| Phase | Ingredient (Name/INCI) | V01 | V02 |
|---|---|---|---|
| % | % | ||
| A | NMW Chaves | 84.30 | 82.75% |
| Aqueous Vaccinium myrtillus (blueberry) extract | 0.25 | 0.25 | |
| Glycerin | 5.00 | 5.00 | |
| Pentylene Glycol | - | 1.50 | |
| B | Xantan Gum | 0.80 | 0.80 |
| Caprylic/capric Triglycerides | 2.50 | 2.50 | |
| Vitis Vinifera (Grape) Seed Oil | 1.00 | 1.00 | |
| C | Aqua/Water; Propanediol; Asparagopsis Armata Extractt | 2.00 | 2.00 |
| Niacinamide | 3.00 | 3.00 | |
| Tocopherol | 0.05 | 0.05 | |
| Phenoxyethanol | 1.00 | 1.00 | |
| D | Citric Acid | 0.10 | 0.15 |
Appendix A.2. Day Cream Formulation Evolution, Final Formula, and Productive Method
| Phase | Ingredient (Name/INCI) | V01 | V02 |
|---|---|---|---|
| % | % | ||
| A | NMW Unhais da Serra | 71.3 | 69.15 |
| Thymus × citriodorus hidrolate | 3.00 | 3.00 | |
| Pentylene Glycol | 1.00 | 1.00 | |
| Aqua/Water; Propanediol; Asparagopsis Armata Extractt | - | 2.00 | |
| B | Arachidyl Alcohol; Behenyl Alcohol; Arachidyl Glucoside | 3.00 | 3.00 |
| C15-19 Alkane | 7.50 | 7.50 | |
| Vitis Vinifera (Grape) Seed Oil | 2.00 | 2.00 | |
| Caprylic/capric Triglycerides | 5.50 | 5.50 | |
| Tocopherol | 0.05 | 0.05 | |
| C | Polyacrylate Crosspolymer-6 | 1.00 | 1.00 |
| Acacia Senegal Gum; Xanthan Gum | 0.50 | 0.50 | |
| D | Xylitylglucoside; Anhydroxylitol; Xylitol | 3.00 | 3.00 |
| E | Phenoxyethanol | 1.00 | 1.00 |
| F | Citric Acid | 0.15 | 0.30 |
| G | Allantoin | 1.00 | 1.00 |
| Phase | Ingredient (Name/INCI) | V01 | V02 |
|---|---|---|---|
| % | % | ||
| A | NMW Chaves | 74.05 | 72.90 |
| Aqueous Vaccinium myrtillus (blueberry) extract | 0.25 | 3.00 | |
| Pentylene Glycol | 1.00 | 1.00 | |
| Aqua/Water; Propanediol; Asparagopsis Armata Extractt | - | 2.00 | |
| B | Arachidyl Alcohol; Behenyl Alcohol; Arachidyl Glucoside | 3.00 | 3.00 |
| C15-19 Alkane | 7.50 | 7.50 | |
| Vitis Vinifera (Grape) Seed Oil | 2.00 | 2.00 | |
| Caprylic/capric Triglycerides | 5.50 | 5.50 | |
| Tocopherol | 0.05 | 0.05 | |
| C | Polyacrylate Crosspolymer-6 | 1.00 | 1.00 |
| Acacia Senegal Gum; Xanthan Gum | 0.50 | 0.50 | |
| D | Xylitylglucoside; Anhydroxylitol; Xylitol | 3.00 | 3.00 |
| E | Phenoxyethanol | 1.00 | 1.00 |
| F | Citric Acid | 0.15 | 0.30 |
| G | Allantoin | 1.00 | 1.00 |
Appendix A.3. Night Cream Formulation Evolution, Final Formula, and Productive Method
| Phase | Ingredient (Name/INCI) | V01 | V02 | V03 |
|---|---|---|---|---|
| % | % | % | ||
| A | NMW Unhais da Serra | 69.85 | 64.35 | 66.35 |
| Thymus × citriodorus hidrolate | 3.00 | 3.00 | 3.00 | |
| Pentylene Glycol | 1.00 | 1.00 | 1.00 | |
| Aqua/Water; Propanediol; Asparagopsis Armata Extractt | - | - | 2.00 | |
| Niacinamide | 1.50 | 1.50 | 1.50 | |
| B | Arachidyl Alcohol; Behenyl Alcohol; Arachidyl Glucoside | 3.00 | 3.00 | 3.00 |
| Isononyl Isononanoate | 5.00 | 5.00 | 5.00 | |
| Vitis Vinifera (Grape) Seed Oil | 3.00 | 3.00 | 3.00 | |
| Caprylic/capric Triglycerides | 6.00 | 6.00 | 6.00 | |
| Tocopherol | 0.05 | 0.05 | 0.05 | |
| C | Polyacrylate-13, Polyisobutene, Polysorbate 20 | 0.8 | 0.80 | 0.80 |
| Acacia Senegal Gum; Xanthan Gum | - | 0.50 | 0.50 | |
| D | Xylitylglucoside; Anhydroxylitol; Xylitol | 1.50 | 1.5 | 1.5 |
| Glycerin | 3.00 | 4.00 | 4.00 | |
| Sepicide LD | 1.00 | 1.00 | 1.00 | |
| E | Citric Acid | 0.30 | 0.30 | 0.30 |
| F | Allantoin | 1.00 | 1.00 | 1.00 |
| Phase | Ingredient (Name/INCI) | V01 | V02 | V03 |
|---|---|---|---|---|
| % | % | % | ||
| A | NMW Chaves | 72.60 | 67.10 | 69.10 |
| Aqueous Vaccinium myrtillus (blueberry) extract | 0.25 | 0.25 | 0.25 | |
| Pentylene Glycol | 1.00 | 1.00 | 1.00 | |
| Aqua/Water; Propanediol; Asparagopsis Armata Extractt | - | - | 2.00 | |
| Niacinamide | 1.50 | 1.50 | 1.50 | |
| B | Arachidyl Alcohol; Behenyl Alcohol; Arachidyl Glucoside | 3.00 | 3.00 | 3.00 |
| Isononyl Isononanoate | 5.00 | 5.00 | 5.00 | |
| Vitis Vinifera (Grape) Seed Oil | 3.00 | 3.00 | 3.00 | |
| Caprylic/capric Triglycerides | 6.00 | 6.00 | 6.00 | |
| Tocopherol | 0.05 | 0.05 | 0.05 | |
| C | Polyacrylate-13, Polyisobutene, Polysorbate 20 | 0.8 | 0.80 | 0.80 |
| Acacia Senegal Gum; Xanthan Gum | - | 0.50 | 0.50 | |
| D | Xylitylglucoside; Anhydroxylitol; Xylitol | 1.50 | 1.5 | 1.5 |
| Glycerin | 3.00 | 4.00 | 4.00 | |
| Sepicide LD | 1.00 | 1.00 | 1.00 | |
| E | Citric Acid | 0.30 | 0.30 | 0.30 |
| F | Allantoin | 1.00 | 1.00 | 1.00 |
References
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Shai, A. (Ed.) Handbook of Cosmetic Skin Care, 2nd ed.; Series in cosmetic and laser therapy; Informa Healthcare: London, UK, 2009. [Google Scholar]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Krutmann, J. Skin Aging; Springer: Berlin, Germany, 2006. [Google Scholar]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal Extracts as Potential Antioxidant, Anti-Aging, Anti-Inflammatory, and Whitening Cosmeceutical Ingredients. Chem. Biodivers. 2021, 18, e2100245. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Budzianowska, A.; Banaś, K.; Budzianowski, J.; Kikowska, M. Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Appl. Sci. 2025, 15, 2571. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, Y.J.; Kim, C.M.; Lee, Y.-M. Revolutionizing Cosmetic Ingredients: Harnessing the Power of Antioxidants, Probiotics, Plant Extracts, and Peptides in Personal and Skin Care Products. Cosmetics 2024, 11, 157. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Rodrigues, M.; Mourelle, M.L.; Araujo, A.R.T.S. Thermal Spring Waters as an Active Ingredient in Cosmetic Formulations. Cosmetics 2023, 10, 27. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Hydrobiome of Thermal Waters: Potential Use in Dermocosmetics. Cosmetics 2023, 10, 94. [Google Scholar] [CrossRef]
- Silva, A.; Oliveira, A.S.; Vaz, C.V.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.M.F.; Palmeira-de-Oliveira, A.; et al. Anti-Inflammatory Potential of Portuguese Thermal Waters. Sci. Rep. 2020, 10, 22313. [Google Scholar] [CrossRef] [PubMed]
- ISO 17516:2014; Cosmetics—Microbiology—Microbiological Limits. International Organization for Standardization: Geneva, Switzerland, 2014.
- Gomes, C.P.; Oliveira, A.S.; Rolo, J.; Da Silveira, T.F.F.; Palmeira De Oliveira, R.; Alves, M.J.; Plasencia, P.; Palmeira De Oliveira, A. Natural Mineral Water–Plant Extract Combinations as Potential Anti-Aging Ingredients: An In Vitro Evaluation. Cosmetics 2025, 12, 113. [Google Scholar] [CrossRef]
- Plasencia, P.; Heleno, S.A.; Finimundy, T.; Carocho, M.; Calhelha, R.C.; Añibarro-Ortega, M.; Alves, M.J.; Oludemi, T.; Quidiongo, N.; Barreiro, F.; et al. Recovery of High Valuable Bioactive Molecules from Vaccinium myrtillus L. Bioresidues. Waste Biomass Valorization 2023, 14, 2873–2884. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Scientific Committee on Consumer Safety (SCCS). SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation; SCCS/1647/22; 12th Revision; European Commission: Brussels, Belgium, 2023; Available online: https://health.ec.europa.eu/publications/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision_en (accessed on 15 May 2025).
- Regulation (EC) No 1223/2009 of the European Parliament and of The Council of 30 November 2009 on Cosmetic Products. 2009. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 5 May 2021).
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD: Paris, France, 2021. [Google Scholar] [CrossRef]
- Pinto-Ribeiro, I.; Castro, C.; Rocha, P.E.; Carvalho, M.J.; Pintado, A.; Mendes, A.; Pedrosa, S.S.; Capeto, P.; Azevedo-Silva, J.; Oliveira, A.L.S.; et al. Chaves Thermal Spring Water Impact on Skin Health: Potential Cosmetic Application. Appl. Sci. 2024, 14, 7911. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Cavaleiro, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Thymus × citriodorus: An Emerging Aromatic and Medicinal Hybrid Plant with Relevant Bioactive Potential. Rev. Bras. Farmacogn. 2023, 33, 1089–1109. [Google Scholar] [CrossRef]
- Araujo, A.R.T.S.; Sarraguça, M.C.; Ribeiro, M.P.; Coutinho, P. Physicochemical Fingerprinting of Thermal Waters of Beira Interior Region of Portugal. Environ. Geochem. Health 2017, 39, 483–496. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.; Magalhães, C.; Sousa Lobo, J.M.; Sousa, E.; Almeida, I.F. Trends in the Use of Marine Ingredients in Anti-Aging Cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- McEwen, L.; Déchelette, C.; Fauverghe, S. Anthropological Study on the Perception of Skin Aging and Aesthetic Procedures: An International, Generational Analysis. Ethics Med. Public Health 2025, 33, 101109. [Google Scholar] [CrossRef]
- Chermahini, S.H.; Majid, F.A.A.; Sarmidi, M.R. Cosmeceutical Value of Herbal Extracts as Natural Ingredients and Novel Technologies in Anti-Aging. J. Med. Plants Res. 2011, 5, 3074–3077. [Google Scholar]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of Plant Extracts Cosmetics in the Field of Anti-Aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Magalhães, M.; Oliveira, R.; Sousa-Lobo, J.; Almeida, I. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Ferraz, C.; Coelho, S.; Pastorinho, M.R.; Sousa, A.C.; et al. Chemical Characterization and Bioactive Potential of Thymus × citriodorus (Pers.) Schreb. Preparations for Anti-Acne Applications: Antimicrobial, Anti-Biofilm, Anti-Inflammatory and Safety Profiles. J. Ethnopharmacol. 2022, 287, 114935. [Google Scholar] [CrossRef]
- Spence, C.; Zhang, T. Multisensory Contributions to Skin-cosmetic Product Interactions. Int. J. Cosmet. Sci. 2024, 46, 833–849. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.-S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, E.H.; Baik, J.H.; Park, J.D. The Role of Rheology in Cosmetics Research: A Review. Korea-Aust. Rheol. J. 2024, 36, 271–282. [Google Scholar] [CrossRef]
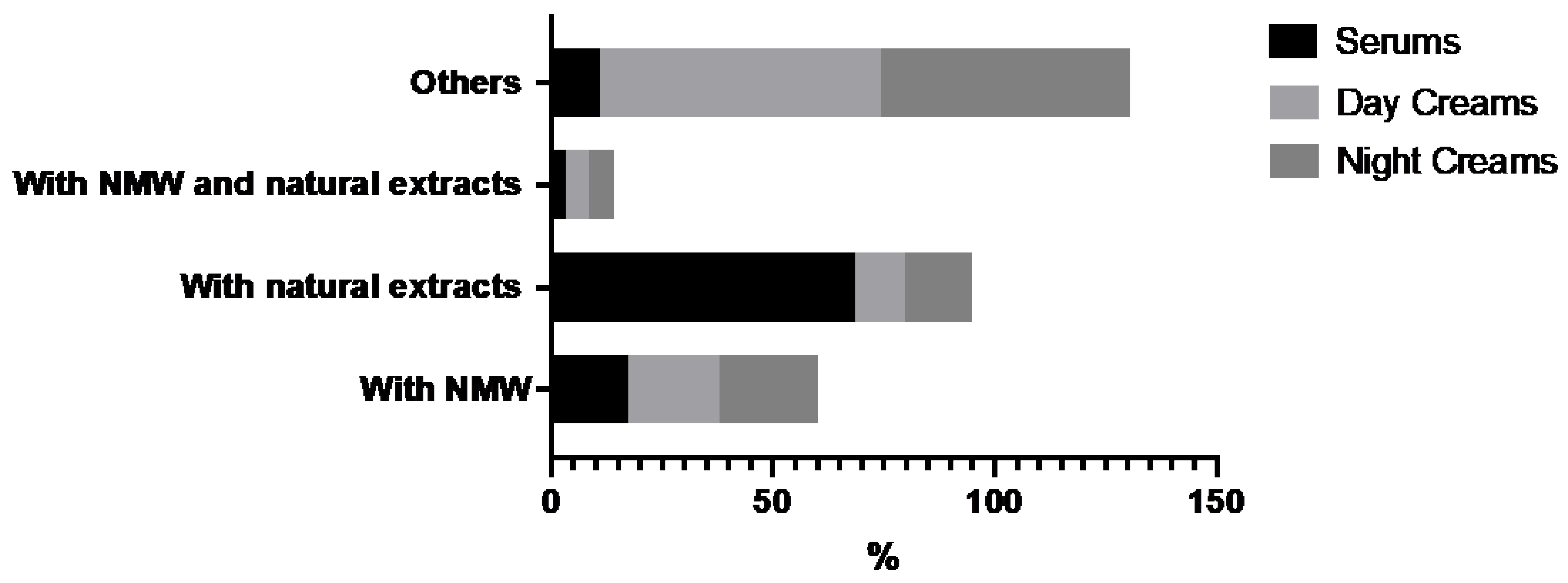



| Attribute | Recommended Specification | ||
|---|---|---|---|
| Serum | Day Cream | Night Cream | |
| Product name | Anti-aging hydrating serum | Anti-aging moisturizing day cream | Anti-aging regenerating night cream |
| Product application | Face | Face | Face |
| Galenic form and application | Gel, applied via dropper | Cream with creamy texture, applied by hand with gentle massage | Cream with soft and fluid texture, applied by hand with massage |
| Packaging | Dropper bottle (glass) | Jar (glass) | Pump bottle (glass) |
| Use (single or multiple) | Multiple use | ||
| Production method | Cold-process | Emulsion prepared at low temperatures (<60 °C) | |
| Stability | Greater than 30 months | ||
| Quality specifications | Challenge Test (CT)—Criteria A; Microbiological Quality (QM) according to ISO 17516:2014 [14]; Peroxide value: <10 meq O2/kg | ||
| Innovation | >90% natural ingredients; use of Portuguese NMW and plant extracts mixtures; | ||
| Intended claims | Hydration, antioxidant, anti-inflammatory, anti-aging (fine lines/wrinkles) | Moisturizing, protective, film forming, antioxidant, anti-aging (fine lines/wrinkles) | Moisturizing, regenerative, protective, antioxidant, anti-aging (fine lines/wrinkles) |
| Recommended market | European (Natural and sustainable skincare, anti-aging segment) | ||
| INCI Name | Trade Name | Company | Country | Formulas |
|---|---|---|---|---|
| Natural mineral water from Termas de Unhais da Serra | - | Termas de Unhais da Serra | Portugal | S 1 DC 1 NC 1 |
| Natural mineral water from Termas de Chaves | - | Termas de Chaves | Portugal | S 2 DC 2 NC 2 |
| Aqueous Vaccinium myrtillus (blueberry) extract | - | CIMO, IPB | Portugal | S 2 DC 2 NC 2 |
| Thymus × citriodorus hydrolat | - | Ervitas Catitas | Portugal | S 1 DC 1 NC 1 |
| Pentylene glycol | A-Leen 5 | Unigolden | Spain | S DC NC |
| Aqua/Water; Propanediol; Asparagopsis Armata extract | ASPAR’AGE | SEPPIC | France | |
| Xanthan gum | Goma Xantan | Acofarma | Spain | S DC NC |
| Allantoin | Alantoina | Acofarma | Spain | |
| Niacinamide | Nicotinamida EP | Escuder | Spain | S NC |
| Arachidyl alcohol; behenyl alcohol; arachidyl glucoside | Montanov 202 | SEPPIC | France | DC NC |
| Isononyl isononanoate | Lanol 99 | SEPPIC | France | NC |
| C15-19 alkane | Emogreen L15 | SEPPIC | France | DC |
| Vitis Vinifera (Grape) Seed Oil | GSO—Douro Valley Grape Seed Oil | EFP Biotek | Portugal | S DC NC |
| Caprylic/capric Triglycerides | Caprilic capric trigliceridos EP | Escuder | Spain | |
| Tocopherol | Tocomix 50 | AOM | Spain | |
| Polyacrylate-13, Polyisobutene, Polysorbate 20 | Sepiplus 400 | SEPPIC | France | NC |
| Polyacrylate Crosspolymer-6 | Sepimax Zen | SEPPIC | France | DC |
| Acacia Senegal Gum; Xanthan Gum | Solagum AX | SEPPIC | France | DC NC |
| Xylitylglucoside; Anhydroxylitol; Xylitol | Aquaxyl | SEPPIC | France | |
| Glycerin | Glicerina Vegetal EP | Escuder | Spain | S NC |
| Phenoxyethanol | Sepicide LD | SEPPIC | France | S DC NC |
| Citric Acid | Acido citrico monohidrato cristal | Acofarma | Spain |
| Class of Ingredients | Analyzed Products | Brand | |||
|---|---|---|---|---|---|
| Thermal Water | Natural Extract | Serums (N) | Day Creams (N) | Night Creams (N) | |
| N | Y | 4 | 3 | 2 | Caudalíe (France) |
| N | Y | 4 | 8 | 8 | Clarins (France) |
| N | Y | 3 | 9 | 4 | Eucerin (Germany) |
| N | Y | 9 | 5 | 4 | Filorga (France) |
| N | Y | 3 | 3 | 1 | Isdin (Spain) |
| N | Y | 4 | 6 | 6 | Lierac (France) |
| N | Y | - | 3 | 3 | Noreva (France) |
| N | Y | 5 | 6 | 5 | Nuxe (France) |
| N | Y | - | 1 | 1 | Rilastil (Italy) |
| N | Y | 5 | 4 | 5 | Sensilis (Spain) |
| N | Y | 21 | 11 | 4 | Sesderma (Spain) |
| N | Y | 2 | 6 | 5 | Shiseido (Japan) |
| Y | N | 2 | 4 | 5 | Bioderma (France) |
| Y | N | 4 | 5 | 3 | La Roche Posay (France) |
| Y | N | 1 | 4 | 3 | Uriage (France) |
| Y | N | 6 | 5 | 5 | Vichy (France) |
| N | N | 7 | 9 | 10 | Institut Esthederm (France) |
| N | N | 3 | 8 | 6 | Martiderm (Spain) |
| N | N | 2 | - | - | Noreva (France) |
| N | N | 3 | - | - | Rilastil (Italy) |
| N | N | 1 | 2 | 2 | Topicrem (France) |
| Y | Y | 3 | 6 | 3 | Avéne (France) |
| TOTAL | 92 | 117 | 85 | ||
| Serum (Mixture 1) Ingredients | Serum (Mixture 2) Ingredients |
|---|---|
| Aqua (Water), Glycerin, Niacinamide, Caprylic/Capric Triglyceride, Propanediol, Asparagopsis Armata Extract, Pentylene Glycol, Vitis Vinifera (Grape) Seed Oil, Phenoxyethanol, Xanthan Gum, Vaccinium myrtillus (blueberry) Extract, Citric Acid, Tocopherol. | Aqua (Water), Glycerin, Niacinamide, Propanediol, Asparagopsis Armata Extract, Caprylic/Capric Triglyceride, Thymus Citriodorus (Lemon Thyme) Hydrolat, Pentylene Glycol, Vitis Vinifera (Grape) Seed Oil, Phenoxyethanol, Xanthan Gum, Citric Acid, Tocopherol. |
| Day Cream (Mixture 1) Ingredients | Day Cream (Mixture 2) Ingredients |
|---|---|
| Aqua (Water), C15-19 Alkane, Caprylic/Capric Triglycerides, Xylitylglucoside, Anhydroxylitol, Xylitol, Arachidyl Alcohol, Behenyl Alcohol, Arachidyl Glucoside, Propanediol, Asparagopsis Armata Extract, Vitis Vinifera (Grape) Seed Oil, Thymus Citriodorus (Lemon Thyme) hydrolate, Allantoin, Pentylene Glycol, Polyacrylate Crosspolymer-6, Phenoxyethanol, Acacia Senegal Gum, Xanthan Gum, Citric Acid, Tocopherol. | Aqua (Water), C15-19 Alkane, Caprylic/Capric Triglycerides, Xylitylglucoside, Anhydroxylitol, Xylitol, Propanediol, Asparagopsis Armata Extract, Arachidyl Alcohol, Behenyl Alcohol, Arachidyl Glucoside, Vitis Vinifera (Grape) Seed Oil, Pentylene Glycol, Allantoin, Polyacrylate Crosspolymer-6, Phenoxyethanol, Acacia Senegal Gum, Xanthan Gum, Vaccinium myrtillus (blueberry) Extract, Citric Acid, Tocopherol. |
| Night Cream (Mixture 1) Ingredients | Night Cream (Mixture 2) Ingredients |
|---|---|
| Aqua (Water), Caprylic/Capric Triglycerides, Isononyl Isononanoate, Vitis Vinifera (Grape)Seed Oil, Arachidyl Alcohol, Behenyl Alcohol, Arachidyl Glucoside, Glycerin, Propanediol, Asparagopsis Armata Extract, Niacinamide, Xylitylglucoside, Anhydroxylitol, Xylitol, Thymus Citriodorus (Lemon Thyme) Water, Allantoin, Pentylene Glycol, Phenoxyethanol, Polyacrylate-13, Polyisobutene, Polysorbate 20, Acacia Senegal Gum, Xanthan Gum, Citric Acid, Tocopherol | Aqua (Water), Caprylic/Capric Triglycerides, Isononyl Isononanoate, Vitis Vinifera (Grape)Seed Oil, Arachidyl Alcohol, Behenyl Alcohol, Arachidyl Glucoside, Glycerin, Propanediol, Asparagopsis Armata Extract, Niacinamide, Xylitylglucoside, Anhydroxylitol, Xylitol, Allantoin, Pentylene Glycol, Phenoxyethanol, Polyacrylate-13, Polyisobutene, 9,Polysorbate 20, Acacia Senegal Gum, Xanthan Gum, Vaccinium myrtillus (Blueberry) Extract, Citric Acid, Tocopherol |
| Preliminary Stability (Centrifugation 30 min at 3000 rpm) | Temperature Cycles (4 °C and 40 °C, 4 Weeks) | Stability (4 °C and 40 °C, 6 Months) | Organoleptic Characteristics | pH | |
|---|---|---|---|---|---|
| Serum 1 | Stable | No phase separation or color change. Maintain characteristic odor. | Stable Slight odor attenuation; no significant changes. | Texture—fluid Color—transparent Odor—mixture 1 characteristic odor | Initial pH = 5.0 pH (4 weeks) = 5.2 pH (6 months) = 5.2 |
| Serum 2 | Stable | No phase separation or color change | Stable No significant changes, maintain organoleptic characteristics. | Texture—fluid Color—transparent Odor—odorless | Initial pH = 5.0 pH (4 weeks) = 5.1 pH (6 months) = 5.1 |
| Day cream 1 | Stable | No phase separation or color change. Maintain characteristic odor. | Stable Slight odor attenuation; no significant changes. | Texture—creamy Color—white Odor—mixture 1 characteristic odor | Initial pH = 5.0 pH (4 weeks) = 5.3 pH (6 months) = 5.3 |
| Day cream 2 | Stable | No phase separation or color change. | Stable No significant changes, maintain organoleptic characteristics. | Texture—creamy Color—white Odor—odorless | Initial pH = 5.0 pH (4 weeks) = 5.2 pH (6 months) = 5.2 |
| Night cream 1 | Stable | No phase separation or color change. Maintain characteristic odor. | Stable Slight odor attenuation; no significant changes. | Texture—fluid cream Color—white Odor—mixture 1 characteristic odor | Initial pH = 5.0 pH (4 weeks) = 5.1 pH (6 months) = 5.2 |
| Night cream 2 | Stable | No phase separation or color change. | Stable No significant changes, maintain organoleptic characteristics. | Texture—fluid cream Color—white Odor—odorless | Initial pH = 5.0 pH (4 weeks) = 5.2 pH (6 months) = 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama, A.R.; Gomes, C.P.; Caetano, C.; Oliveira, A.S.; Rolo, J.; Barros, L.; Plasencia, P.; Garcia, J.; Correia, D.; Alves, M.J.; et al. Unlocking Nature’s Anti-Aging Secrets: The Potential of Natural Mineral Waters Combined with Plant Extracts in Cosmetics. Cosmetics 2025, 12, 150. https://doi.org/10.3390/cosmetics12040150
Gama AR, Gomes CP, Caetano C, Oliveira AS, Rolo J, Barros L, Plasencia P, Garcia J, Correia D, Alves MJ, et al. Unlocking Nature’s Anti-Aging Secrets: The Potential of Natural Mineral Waters Combined with Plant Extracts in Cosmetics. Cosmetics. 2025; 12(4):150. https://doi.org/10.3390/cosmetics12040150
Chicago/Turabian StyleGama, Ana Rita, Carolina P. Gomes, Cátia Caetano, Ana Sofia Oliveira, Joana Rolo, Lillian Barros, Paula Plasencia, Juliana Garcia, Daniela Correia, Maria José Alves, and et al. 2025. "Unlocking Nature’s Anti-Aging Secrets: The Potential of Natural Mineral Waters Combined with Plant Extracts in Cosmetics" Cosmetics 12, no. 4: 150. https://doi.org/10.3390/cosmetics12040150
APA StyleGama, A. R., Gomes, C. P., Caetano, C., Oliveira, A. S., Rolo, J., Barros, L., Plasencia, P., Garcia, J., Correia, D., Alves, M. J., Martinez-de-Oliveira, J., Palmeira-de-Oliveira, A., & Palmeira-de-Oliveira, R. (2025). Unlocking Nature’s Anti-Aging Secrets: The Potential of Natural Mineral Waters Combined with Plant Extracts in Cosmetics. Cosmetics, 12(4), 150. https://doi.org/10.3390/cosmetics12040150










