Beauty’s Blind Spot: Unmasking the Ocular Side Effects and Concerns of Eye Cosmetics
Abstract
1. Introduction
2. Methodology of Search
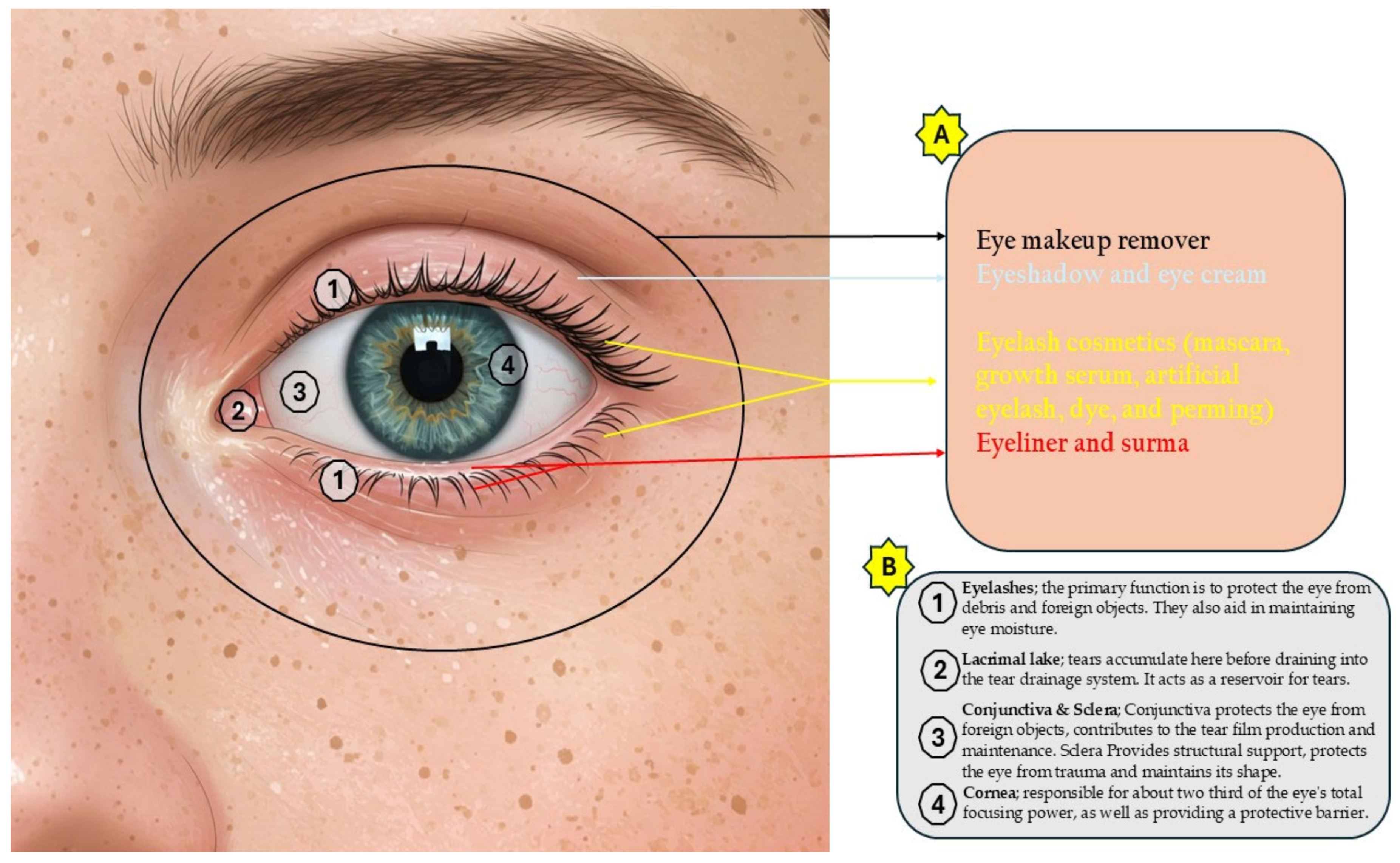
3. Eye Cosmetics
3.1. Eyelash Cosmetics
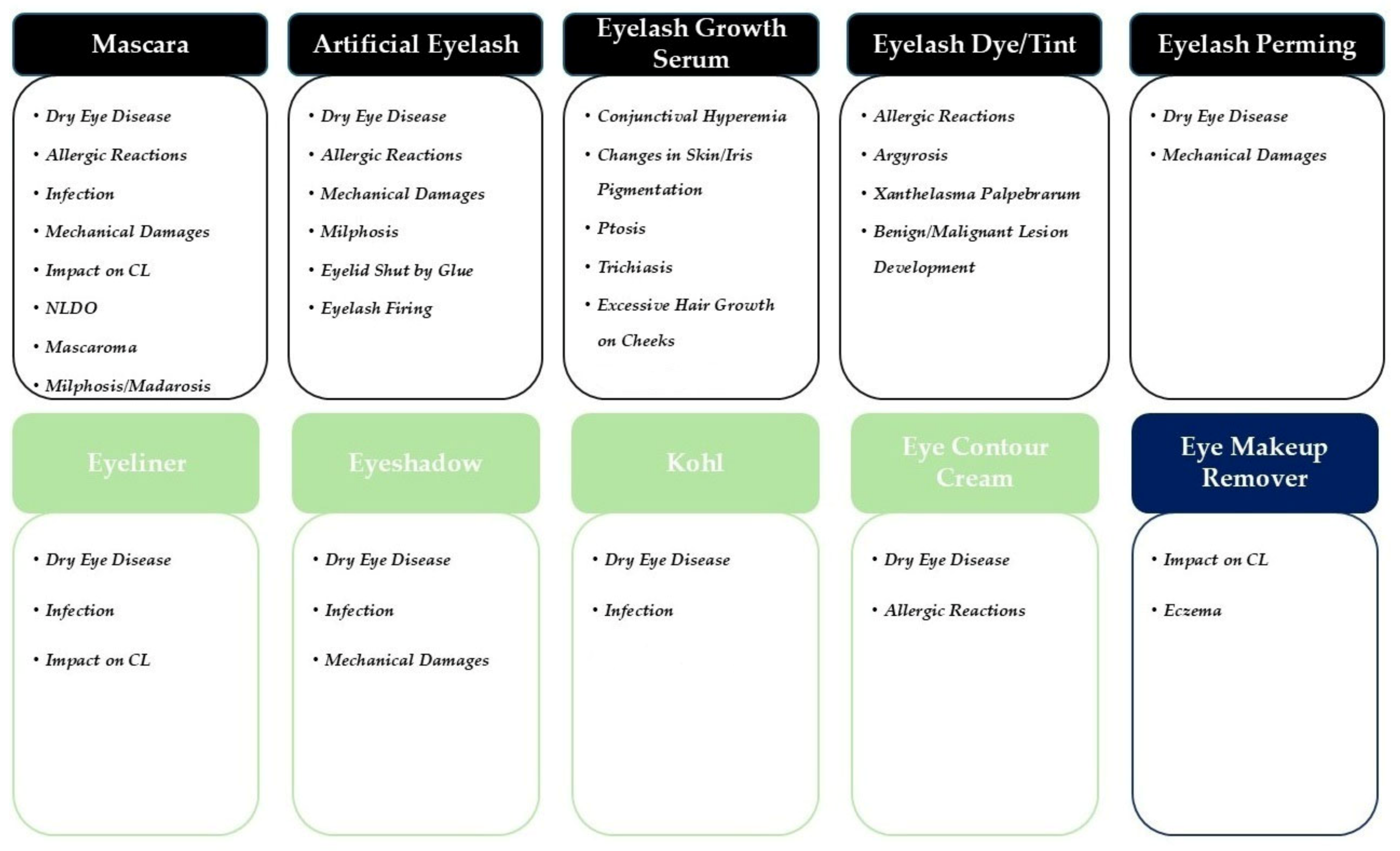
3.1.1. Mascara
3.1.2. Artificial Eyelashes
3.1.3. Eyelash Growth Serum
3.1.4. Eyelash Dye and Tint
3.1.5. Eyelash Perming
3.2. Eyelid Cosmetics
3.2.1. Eyeliner
3.2.2. Eyeshadow
3.2.3. Kohl (Surma/Kajal)
3.2.4. Eye Contour Cream
3.3. Eye Makeup Remover
4. Side Effects and Concerns
4.1. Dry Eye Disease
4.2. Allergic Events
4.3. Microbial Contamination
4.4. Mechanical Injuries
4.5. Alterations in Contact Lenses
4.6. Others
5. Can “Do-It-Yourself” Approach Solve the Problems?
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sullivan, D.A.; da Costa, A.X.; Del Duca, E.; Doll, T.; Grupcheva, C.N.; Lazreg, S.; Liu, S.H.; McGee, S.R.; Murthy, R.; Narang, P.; et al. TFOS Lifestyle: Impact of cosmetics on the ocular surface. Ocul. Surf. 2023, 29, 77–130. [Google Scholar] [CrossRef] [PubMed]
- Murube, J. Ocular cosmetics in ancient times. Ocul. Surf. 2012, 11, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Nam, C.; Hong, S.; Park, B.; Kim, H.; Lee, T.; Kim, K.; Lee, J.H.; Kim, M.H. Socioeconomic factors influencing cosmetic usage patterns. J. Expo Sci. Environ. Epidemiol. 2018, 28, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Biesterbos, J.W.; Dudzina, T.; Delmaar, C.J.; Bakker, M.I.; Russel, F.G.; von Goetz, N.; Scheepers, P.T.; Roeleveld, N. Usage patterns of personal care products: Important factors for exposure assessment. Food Chem. Toxicol. 2013, 55, 8–17. [Google Scholar] [CrossRef]
- Hamilton, T.; de Gannes, G.C. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Dermatitis 2011, 14, 16. [Google Scholar]
- Okereke, J.N.; Udebuani, A.C.; Ezeji, E.U.; Obasi, K.O.; Nnoli, M.C. Possible health implications associated with cosmetics: A review. Science 2015, 3, 58–63. [Google Scholar]
- Zulaikha, S. Hazardous ingredients in cosmetics and personal care products and health concern: A review. J. Public Health Res. 2015, 5, 7. [Google Scholar]
- Banerjee, K. Cosmetics–care, concerns and caution. Int. J. Innov. Pharm. Sci. 2018, 6, 14–31. [Google Scholar]
- Khan, A.D.; Alam, M.N. Cosmetics and their associated adverse effects: A review. J. Appl. Pharm. Sci. Res. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Yazdani, M.; Elgstøen, K.B.; Utheim, T.P. Eye make-up products and dry eye disease: A mini review. Curr. Eye Res. 2022, 47, 1–11. [Google Scholar] [CrossRef]
- Baki, G. Introduction to Cosmetic Formulation and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Draelos, Z.D. Cosmetics and Dermatologic Problems and Solutions; CRC press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Abah, E.R.; Oladigbolu, K.K.; Rafindadi, A.L.; Audu, O. Eyelash extension use among female students in a Tertiary Institution in Nigeria: A study of kaduna polytechnic, Kaduna. Niger. J. Clin. Pract. 2017, 20, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Coroneo, M.T.; Rosenberg, M.L.; Cheung, L.M. Ocular effects of cosmetic products and procedures. Ocul. Surf. 2006, 4, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Special considerations in eye cosmetics. Clin. Dermatol. 2001, 19, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Begoun, P. The Complete Beauty Bible: The Ultimate Guide to Smart Beauty; Rodale: Emmaus, PA, USA, 2004. [Google Scholar]
- Ng, A.; Evans, K.; North, R.V.; Jones, L.; Purslow, C. Impact of eye cosmetics on the eye, adnexa, and ocular surface. Eye Contact Lens 2016, 42, 211–220. [Google Scholar] [CrossRef]
- Diamandopoulos, A.A. Organic and inorganic cosmetics in the preclassical Eastern Mediterranean. Int. J. Dermatol. 1996, 35, 751–756. [Google Scholar] [CrossRef]
- Mohta, A. Kajal (Kohl)–A dangerous cosmetic. Oman J. Ophthalmol. 2010, 3, 100–101. [Google Scholar] [CrossRef]
- Patel, D.K.; Prasad, S.; Tripathi, R.; Behari, J.R. The level of polyaromatic hydrocarbons in kajal and surma of major Indian brands. Int. J. Cosmet. Sci. 2009, 31, 177–182. [Google Scholar] [CrossRef]
- Hamie, H.; Yassine, R.; Shoukfeh, R.; Turk, D.; Huq, F.; Moossavi, M. A review of the efficacy of popular eye cream ingredients. Int. J. Women’s Dermatol. 2024, 10, e156. [Google Scholar] [CrossRef]
- Debbasch, C.; Ebenhahn, C.; Dami, N.; Pericoi, M.; Van den Berghe, C.; Cottin, M.; Nohynek, G.J. Eye irritation of low-irritant cosmetic formulations: Correlation of in vitro results with clinical data and product composition. Food Chem. Toxicol. 2005, 43, 155–165. [Google Scholar] [CrossRef]
- Liu, Y. Silicone Dispersions; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Suzuki, T.O.; Nakamura, M.A.; Sumida, H.; Shigeta, A. Liquid crystal make-up remover: Conditions of formation and its cleansing mechanisms. J. Soc. Cosmet. Chem. 1992, 43, 21. [Google Scholar]
- Wang, M.T.; Craig, J.P. Investigating the effect of eye cosmetics on the tear film: Current insights. Clin. Optom. 2018, 10, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS dews ii epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, F. The Human Nasolacrimal Ducts; Springer Nature: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Nelson, J.D.; Helms, H.; Fiscella, R.; Southwell, Y.; Hirsch, J.D. A new look at dry eye disease and its treatment. Adv. Ther. 2000, 17, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Irkeç, M.; Messmer, E.M.; Benítez-del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef]
- Ghach, W.; Bakkar, M.M.; Aridi, M.; Beshtawi, I.; Doughaily, R.; Al-Fayoumi, N. Prevalence and behavioral-based risk factors (eye cosmetic and tobacco use) of symptomatic dry eye disease in four Middle Eastern countries: Lebanon, Syria, Jordan, and Palestine. Clin. Ophthalmol. 2022, 16, 3851. [Google Scholar] [CrossRef]
- Ercan, Z.E. Effect of eyeliner and mascara use on tear film and meibomian glands. Saudi J. Ophthalmol. 2022, 36, 113–116. [Google Scholar] [CrossRef]
- Franck, C. Fatty layer of the precorneal film in the ‘office eye syndrome’. Acta Ophthalmol. 1991, 69, 737–743. [Google Scholar] [CrossRef]
- Franck, C.; Skov, P. Foam at inner eye canthus in office workers, compared with an average Danish population as control group. Acta Ophthalmol. 1989, 67, 61–68. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Kam, W.R.; Li, Y.; Sullivan, D.A. Toxicity of the cosmetic preservatives parabens, phenoxyethanol and chlorphenesin on human meibomian gland epithelial cells. Exp. Eye Res. 2020, 196, 108057. [Google Scholar] [CrossRef]
- Amano, Y.; Sugimoto, Y.; Sugita, M. Ocular disorders due to eyelash extensions. Cornea 2012, 31, 121–125. [Google Scholar] [CrossRef]
- Craig, J.P.; Alves, M.; Wolffsohn, J.S.; Downie, L.E.; Efron, N.; Galor, A.; Gomes, J.A.P.; Jones, L.; Markoulli, M.; Stapleton, F.; et al. TFOS Lifestyle Report Executive Summary: A Lifestyle Epidemic–Ocular Surface Disease. Ocul. Surf. 2023, 30, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Masud, M.; Shah, T.J.; Avila, M.R.; Hoopes, P.C., Sr. Chemical conjunctivitis and diffuse lamellar keratitis after removal of eyelash extensions. Am. J. Ophthalmol. Case Rep. 2018, 12, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, S.T.; Ali, M.J.; Dogru, M.; Malhotra, R. Complications and adverse effects of periocular aesthetic treatments. Surv. Ophthalmol. 2022, 67, 741–757. [Google Scholar] [CrossRef]
- Han, J.; Xie, Z.; Zhu, X.; Ruan, W.; Lin, M.; Xu, Z.; Miao, L.; Zhong, J.; Lu, F.; Hu, L. The effects of eyelash extensions on the ocular surface. Contact Lens Anterior Eye 2024, 47, 102109. [Google Scholar] [CrossRef]
- Grupcheva, C.N.; Grupchev, D.I.; Usheva, N.; Grupcheva, L.O. Beauty versus Health—How Eyelash Extensions May Affect Dry Eye Disease? J. Clin. Med. 2024, 13, 3101. [Google Scholar] [CrossRef]
- Ullrich, K.; Saha, N. Semipermanent eyelash extensions causing bacterial keratitis: A case report. Can. J. Ophthalmol. 2013, 48, e50–e51. [Google Scholar] [CrossRef]
- Zhang, X.; Jeyalatha, M.V.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; et al. Dry eye management: Targeting the ocular surface microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef]
- Norris, M.R.; Bielory, L. Cosmetics and ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 404–410. [Google Scholar] [CrossRef]
- Adams, R.M.; Maibach, H.I.; Clendenning, W.E.; Fisher, A.A.; Jordan, W.J.; Kanof, N.; Larsen, W.; Mitchell, J.C.; Rudner, E.J.; Schorr, W.; et al. A five-year study of cosmetic reactions. J. Am. Acad. Dermatol. 1985, 13, 1062–1069. [Google Scholar] [CrossRef]
- Zirwas, M.J. Contact dermatitis to cosmetics. Clin. Rev. Allergy Immunol. 2019, 56, 119–128. [Google Scholar] [CrossRef]
- Goossens, A. Contact-allergic reactions to cosmetics. J. Allergy 2011, 2011, 467071. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, L.; Goossens, A. Cosmetic components causing contact urticaria: A review and update. Contact Dermat. 2016, 75, 333–344. [Google Scholar] [CrossRef]
- Chowdhury, M.M. Allergic contact dermatitis from prime yellow carnauba wax and coathylene in mascara. Contact Dermat. 2002, 46, 244. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.E.; Silverberg, J.I.; Rizk, C.; Silverberg, N. Nickel ferrule applicators: A source of nickel exposure in children. Pediatr. Dermatol. 2015, 32, e62–e63. [Google Scholar] [CrossRef] [PubMed]
- Vestey, J.P.; Buston, P.K.; Savin, J.A. Eyelash curler dermatitis. Contact Dermat. 1985, 13, 274–275. [Google Scholar] [CrossRef]
- Schalock, P.C.; Dunnick, C.A.; Nedorost, S.; Brod, B.; Warshaw, E.; Mowad, C.; Scheman, A.; American Contact Dermatitis Society Core Allergen Series Committee. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis 2020, 31, 279–282. [Google Scholar] [CrossRef]
- Pesonen, M.; Kuuliala, O.; Henriks-Eckerman, M.L.; Aalto-Korte, K. Occupational allergic contact dermatitis caused by eyelash extension glues. Contact Dermat. 2012, 67, 307–308. [Google Scholar] [CrossRef]
- Symanzik, C.; Weinert, P.; Babić, Ž.; Hallmann, S.; Havmose, M.S.; Johansen, J.D.; Kezic, S.; Macan, M.; Macan, J.; Strahwald, J.; et al. Allergic contact dermatitis caused by 2-hydroxyethyl methacrylate and ethyl cyanoacrylate contained in cosmetic glues among hairdressers and beauticians who perform nail treatments and eyelash extension as well as hair extension applications: A systematic review. Contact Dermat. 2022, 86, 480–492. [Google Scholar]
- Xiong, M.; Shaik, J.A.; Hylwa, S. Formaldehyde Release From Eyelash Glues: Analysis Using the Chromotropic Acid Method. Dermatitis 2022, 33, 442–446. [Google Scholar] [CrossRef]
- Lindström, I.; Suojalehto, H.; Henriks-Eckerman, M.L.; Suuronen, K. Occupational asthma and rhinitis caused by cyanoacrylate-based eyelash extension glues. Occup. Med. 2013, 63, 294–297. [Google Scholar] [CrossRef]
- Ali, L.; Foulds, J.S.; Abdul Ghaffar, S. Severe eyelid allergic contact dermatitis secondary to eyelash tint: Two case reports. Contact Dermat. 2017, 77, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Kaiserman, I. Severe allergic blepharoconjunctivitis induced by a dye for eyelashes and eyebrows. Ocul. Immunol. Inflamm. 2003, 11, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Pas-Wyroślak, A.; Wiszniewska, M.; Kręcisz, B.; Świerczyńska-Machura, D.; Pałczyński, C.; Walusiak-Skorupa, J. Contact blepharoconjunctivitis due to black henna—A case report. Int. J. Occup. Med. Environ. Health 2012, 25, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.A.; Coenraads, P.J.; Schuttelaar, M.L. Allergic contact dermatitis presenting as severe and persistent blepharoconjunctivitis and centrofacial oedema after dyeing of eyelashes. Contact Dermat. 2014, 71, 304–306. [Google Scholar] [CrossRef]
- Masud, M.; Moshirfar, M.; Tirth, J.S.; T Gomez, A.; Avila, M.R.; Ronquillo, Y.C. Eyelid cosmetic enhancements and their associated ocular adverse effects. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 96. [Google Scholar]
- Mselle, J. The role of eyelash dyes in allergic eye diseases. Trop. Dr. 2004, 34, 235–236. [Google Scholar] [CrossRef]
- Gallo, R.; Russo, R.; Trave, I.; Murgioni, F.; Parodi, A. Allergic contact dermatitis to pentylene glycol in an eye contour cream. Contact Dermat. 2020, 82, 254–255. [Google Scholar] [CrossRef]
- Pack, L.D.; Wickham, M.G.; Enloe, R.A.; Hill, D.N. Microbial contamination associated with mascara use. Optom.-J. Am. Optom. Assoc. 2008, 79, 587–593. [Google Scholar] [CrossRef]
- Wilson, L.A.; Ahearn, D.G. Pseudomonas-induced corneal ulcers associated with contaminated eye mascaras. Am. J. Ophthalmol. 1977, 84, 112–119. [Google Scholar] [CrossRef]
- Alshehrei, F.M. Isolation and Identification of Microorganisms associated with high-quality and low-quality cosmetics from different brands in Mecca region—Saudi Arabia. Saudi J. Biol. Sci. 2023, 30, 103852. [Google Scholar] [CrossRef]
- Huang, J.; Hitchins, A.D.; Tran, T.T.; McCarron, J. Bacteriological Analytical Manual Chapter 23: Methods for Cosmetics; FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Giacomel, C.B.; Dartora, G.; Dienfethaeler, H.S.; Haas, S.E. Investigation on the use of expired make-up and microbiological contamination of mascaras. Int. J. Cosmet. Sci. 2013, 35, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.A.; Julian, A.J.; Ahearn, D.G. The survival and growth of microorganisms in mascara during use. Am. J. Ophthalmol. 1975, 79, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Sędzikowska, A.; Bartosik, K.; Przydatek-Tyrajska, R.; Dybicz, M. Shared makeup cosmetics as a route of Demodex folliculorum infections. Acta Parasitol. 2021, 66, 631–637. [Google Scholar] [CrossRef]
- Naz, S.; Iqtedar, M.; Ul Ain, Q.; Aftab, K. Incidence of Human Skin Pathogens from Cosmetic Tools used in Beauty Saloons in Different Areas of Lahore, Pakistan. J. Sci. Res. 2012, 4, 523. [Google Scholar] [CrossRef]
- Dawson, N.L.; Reinhardt, D.J. Microbial flora of in-use, display eye shadow testers and bacterial challenges of unused eye shadows. Appl. Environ. Microbiol. 1981, 42, 297–302. [Google Scholar] [CrossRef]
- Corazza, M.; Carla, E.; Rossi, M.R.; Pedna, M.F.; Virgili, A. Face and body sponges: Beauty aids or potential microbiological reservoir? Eur. J. Dermatol. 2003, 13, 571–573. [Google Scholar]
- Rastogi, S.; Patel, K.R.; Singam, V.; Lee, H.H.; Silverberg, J.I. Associations of nickel co-reactions and metal polysensitization in adults. Dermatitis 2018, 29, 316–320. [Google Scholar] [CrossRef]
- Maeda, I.; Miyazaki, D.; Shimizu, Y.; Takeda, S.; Inoue, Y.; Shimizu, M. Eye-shadow particles under a laser in situ keratomileusis flap following corneal trauma. Jpn. J. Ophthalmol. 2009, 53, 64. [Google Scholar] [CrossRef]
- Ramasamy, B.; Armstrong, S. Penetrating eye injury caused by eyelash curlers—A cause for concern? Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 301–303. [Google Scholar] [CrossRef]
- Koffuor, G.A.; Kyei, S.; Gyanfosu, L.; Afari, C. Effect of the working environment on oculo-visual health of some sand and stone miners in Ghana. J. Environ. Occup. Sci. 2012, 1, 83–90. [Google Scholar] [CrossRef]
- Luensmann, D.; Yu, M.; Yang, J.; Srinivasan, S.; Jones, L. Impact of cosmetics on the physical dimension and optical performance of silicone hydrogel contact lenses. Eye Contact Lens 2015, 41, 218–227. [Google Scholar] [CrossRef]
- Goto, T.; Zheng, X.; Gibbon, L.; Ohashi, Y. Cosmetic product migration onto the ocular surface: Exacerbation of migration after eyedrop instillation. Cornea 2010, 29, 400–403. [Google Scholar] [CrossRef]
- Cho, P.; Cheng, S.Y.; Chan, W.Y.; Yip, W.K. Soft contact lens cleaning: Rub or no-rub? 1. Ophthalmic Physiol. Opt. 2009, 29, 49–57. [Google Scholar] [CrossRef]
- Tsukiyama, J.; Miyamoto, Y.; Fukuda, M.; Shimomura, Y.; Tsuchiya, J.; Miura, H. Influence of eye cosmetics and cleansing products on contact lenses. J. Jpn. Contact Lens Soc. 2010, 52, 101–107. [Google Scholar]
- Tsukiyama, J.; Miyamoto, Y.; Fukuda, M.; Shimomura, Y.; Miura, H.; Tsuchiya, J. Contamination of silicone hydrogel contact lenses by cosmetics and the cleaning effect of contact lens daily cleaners. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6533. [Google Scholar]
- Scollo, P.; Davies, R.; O’Donovan, D.; Rene, C. Mascara-induced nasolacrimal duct obstruction. BMJ Case Rep. 2021, 14, e240942. [Google Scholar] [CrossRef]
- Reese, A.B. Pigmentation of the palpebral conjunctiva resulting from mascara. Am. J. Ophthalmol. 1947, 30, 1352–1355. [Google Scholar] [CrossRef]
- Shields, J.A.; Marr, B.P.; Shields, C.L.; Eagle, R.C., Jr. Conjunctival mascaroma masquerading as melanoma. Cornea 2005, 24, 496–497. [Google Scholar] [CrossRef]
- Kadri, R.; Achar, A.; Tantry, T.P.; Parameshwar, D.; Kudva, A.; Hegde, S. Mascara induced milphosis, an etiological evaluation. Int. J. Trichol. 2013, 5, 144–147. [Google Scholar] [CrossRef]
- Wachsmuth, R.; Wilkinson, M. Loss of eyelashes after use of a tinting mascara containing PPD. Contact Dermat. 2006, 54, 169–170. [Google Scholar] [CrossRef]
- Brambilla, E.; Crevani, M.; Petrolini, V.M.; Scaravaggi, G.; Di Primo, M.; Roda, E.; Locatelli, C.A. Exposure to nail and false eyelash glue: A case series study. Int. J. Environ. Res. Public Health 2020, 17, 4283. [Google Scholar] [CrossRef]
- Michaels, J.P.; Macdonald, P. Ignition of eyelash extensions during routine minor eyelid surgery. Ophthalmic Plast. Reconstr. Surg. 2014, 30, e61–e62. [Google Scholar] [CrossRef]
- Bhat, J.; Smith, A.G. Xanthelasma palpebrarum following allergic contact dermatitis from para-phenylenediamine in a black eyelash-tinting product. Contact Dermat. 2003, 49, 311. [Google Scholar] [CrossRef]
- Gallardo, M.J.; Randleman, J.B.; Price, K.M.; Johnson, D.A.; Acosta, S.; Grossniklaus, H.E.; Stulting, R.D. Ocular argyrosis after long-term self-application of eyelash tint. Am. J. Ophthalmol. 2006, 141, 198–200. [Google Scholar] [CrossRef]
- Weiler, H.H.; Lemp, M.A.; Zeavin, B.H.; Suarez, A.F. Argyria of the cornea due to self-administration of eyelash dye. Ann. Ophthalmol. 1982, 14, 822–823. [Google Scholar]
- Draelos, Z.D. Cutaneous formulation issues. In Cosmetic Formulation of Skin Care Products; CRC Press: Boca Raton, FL, USA, 2005; pp. 27–50. [Google Scholar]
- Rieger, M. (Ed.) Surfactants in Cosmetics; Routledge: Oxfordshire, UK, 2017. [Google Scholar]
- Kim, H.W.; Choi, Y.J.; Lee, K.W.; Lee, M.J. Periorbital changes associated with prostaglandin analogs in Korean patients. BMC Ophthalmol. 2017, 17, 126. [Google Scholar] [CrossRef]
- Lee, T.H.; Sung, M.S.; Heo, H.; Park, S.W. Association between meibomian gland dysfunction and compliance of topical prostaglandin analogs in patients with normal tension glaucoma. PLoS ONE 2018, 13, e0191398. [Google Scholar] [CrossRef]
- Hart, J.; Shafranov, G. Hypertrichosis of vellus hairs of the malar region after unilateral treatment with bimatoprost. Am. J. Ophthalmol. 2004, 137, 756–757. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Furuta, A.; Tomidokoro, A.; Aihara, M.; Amano, S. Comparison of the long-term effects of various topical antiglaucoma medications on meibomian glands. Cornea 2012, 31, 1229–1234. [Google Scholar] [CrossRef]
- Couteau, C.; Girard, E.; Coiffard, L. Analysis of 275 DIY recipes for eye cosmetics and their possible safety issues. Int. J. Cosmet. Sci. 2022, 44, 403–413. [Google Scholar] [CrossRef]
- Sanchez, N.; Fayne, R.; Burroway, B. Charcoal: An ancient material with a new face. Clin. Dermatol. 2020, 38, 262–264. [Google Scholar] [CrossRef]
- Saadawi, R.; Hachmoeller, O.; Winfough, M.; Hanley, T.; Caruso, J.A.; Figueroa, J.A. The hookah series part 2: Elemental analysis and arsenic speciation in hookah charcoals. J. Anal. At. Spectrom. 2014, 29, 2146–2158. [Google Scholar] [CrossRef]
- Chen, C.C.; Jiang, S.J.; Sahayam, A.C. Determination of trace elements in medicinal activated charcoal using slurry sampling electrothermal vaporization inductively coupled plasma mass spectrometry with low vaporization temperature. Talanta 2015, 131, 585–589. [Google Scholar] [CrossRef]
- Vennam, S.; Georgoulas, S.; Khawaja, A.; Chua, S.; Strouthidis, N.G.; Foster, P.J. Heavy metal toxicity and the aetiology of glaucoma. Eye 2020, 34, 129–137. [Google Scholar] [CrossRef]
- Goto, E. The brilliant beauty of the eye: Light reflex from the cornea and tear film. Cornea 2006, 25, S78–S81. [Google Scholar] [CrossRef]
- Jordan, D.; Mawn, L.; Anderson, R.L. Surgical Anatomy of the Ocular Adnexa: A Clinical Approach; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Amador, G.J.; Mao, W.; DeMercurio, P.; Montero, C.; Clewis, J.; Alexeev, A.; Hu, D.L. Eyelashes divert airflow to protect the eye. J. R. Soc. Interface 2015, 12, 20141294. [Google Scholar] [CrossRef]
- Guillon, J.P. Abnormal lipid layers: Observation, differential diagnosis, and classification. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2: Basic Science and Clinical; Springer: Boston, MA, USA, 1998; pp. 309–313. [Google Scholar]
- Sabhahit, S.V.; Babu, M.V.D. Ocular effects of eye cosmetic formulations. Cutan. Ocul. Toxicol. 2024, 43, 154–160. [Google Scholar] [CrossRef]
- Alghonaim, Y.; Arafat, A.; Aldeghaither, S.; Alsugheir, S.; Aldekhayel, S. Social media impact on aesthetic procedures among females in Riyadh, Saudi Arabia. Cureus 2019, 11, e6008. [Google Scholar] [CrossRef]
- Sharma, G.K.; Asaria, J. The impact of COVID-19 on patient interest in facial plastic surgery. Plast. Reconstr. Surg.–Glob. Open 2021, 9, e3890. [Google Scholar] [CrossRef]
- Wesley, G.; Toyos, M.M.; DiVito, M.M.; Zirwas, M. Evaluation of the Safety and Tolerability of Lumify Eye Illuminations Cosmetic Products. Clin. Ophthalmol. 2024, 18, 3031–3042. [Google Scholar] [CrossRef]
- Chen, X.; Sullivan, D.A.; Sullivan, A.G.; Kam, W.R.; Liu, Y. Toxicity of cosmetic preservatives on human ocular surface and adnexal cells. Exp. Eye Res. 2018, 170, 188–197. [Google Scholar] [CrossRef]
- Ng, A.; Evans, K.; North, R.; Purslow, C. Eye cosmetic usage and associated ocular comfort. Ophthalmic Physiol. Opt. 2012, 32, 501–507. [Google Scholar] [CrossRef]
- Noecker, R. Effects of common ophthalmic preservatives on ocular health. Adv. Ther. 2001, 18, 205–215. [Google Scholar] [CrossRef]
- Canavez, A.D.; de Oliveira Prado Corrêa, G.; Isaac, V.L.; Schuck, D.C.; Lorencini, M. Integrated approaches to testing and assessment as a tool for the hazard assessment and risk characterization of cosmetic preservatives. J. Appl. Toxicol. 2021, 41, 1687–1699. [Google Scholar] [CrossRef]
- Scheman, A. Adverse reactions to cosmetic ingredients. Dermatol. Clin. 2000, 18, 685–698. [Google Scholar] [CrossRef]
- Andersen, F.A. Annual review of cosmetic ingredient safety assessments: 2007–2010. Int. J. Toxicol. 2011, 30, 73S–127S. [Google Scholar] [CrossRef]
- Methylparaben, E.; Propylparaben, I.; Butylparaben, I. And Benzylparaben as used in Cosmetic Products. Int. J. Toxicol. 2008, 27, 1–82. [Google Scholar]
- Lee, E.; An, S.; Choi, D.; Moon, S.; Chang, I. Comparison of objective and sensory skin irritations of several cosmetic preservatives. Contact Dermat. 2007, 56, 131–136. [Google Scholar] [CrossRef]
- Baudouin, C.; Liang, H.; Hamard, P.; Riancho, L.; Creuzot-Garcher, C.; Warnet, J.M.; Brignole-Baudouin, F. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology 2008, 115, 109–115. [Google Scholar] [CrossRef]
- Kahook, M.Y.; Noecker, R.J. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with sofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tears. Cornea 2008, 27, 339–343. [Google Scholar] [CrossRef]
- Tressler, C.S.; Beatty, R.; Lemp, M.A. Preservative use in topical glaucoma medications. Ocular Surf. 2011, 9, 140–158. [Google Scholar] [CrossRef]
- Dauda, J.S.; Friday, E.; Benjamin, B. Harmful effects of heavy metals in cosmetics. World J. Adv. Eng. Technol. Sci. 2023, 8, e374–e381. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Ng, A.; Evans, K.; North, R.V.; Purslow, C. Migration of cosmetic products into the tear film. Eye Contact Lens 2015, 41, 304–309. [Google Scholar] [CrossRef]
- Malik, A.; Claoué, C. Transport and interaction of cosmetic product material within the ocular surface: Beauty and the beastly symptoms of toxic tears. Contact Lens Anterior Eye 2012, 35, 247–259. [Google Scholar] [CrossRef]
- Lipham, W.J.; Tawfik, H.A.; Dutton, J.J. A histologic analysis and three-dimensional reconstruction of the muscle of Riolan. Ophthalmic Plast. Reconstr. Surg. 2002, 18, 93–98. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Chirapapaisan, C.; Chitkornkijsin, C.; Pinitpuwadol, W.; Saiman, M.; Veeraburinon, A. Eyeliner induces tear film instability and meibomian gland dysfunction. Cornea 2020, 39, 473–478. [Google Scholar] [CrossRef]
- O’Dell, L.E.; Sullivan, A.G.; Periman, L.M. Beauty does not have to hurt. Adv. Ocul. Care 2016, 7, 42–47. [Google Scholar]
- Driver, P.J.; Lemp, M.A. Meibomian gland dysfunction. Surv. Ophthalmol. 1996, 40, 343–367. [Google Scholar] [CrossRef]
- Cheng, A.M.; Hwang, J.; Dermer, H.; Galor, A. Prevalence of ocular demodicosis in an older population and its association with symptoms and signs of dry eye. Cornea 2021, 40, 995–1001. [Google Scholar] [CrossRef]
| First Author, Year | Type of Study | Results |
|---|---|---|
| Ghach, 2022 | Population-based cross-sectional study | * Individuals who apply cosmetics, particularly internal eyeliner and mascara, are found to have a greater prevalence and severity of symptomatic DED compared to those who do not use cosmetics. * Regularly removing eye makeup before bedtime using cleansing creams (not water or soapy water) can significantly reduce the prevalence and severity of symptomatic DED. |
| Ercan, 2022 | Cross-sectional study | * The combined use of eyeliner and mascara does not result in a synergistically detrimental effect on the ocular surface. |
| Wang, 2020 | In vitro | * Cosmetic preservatives have the potential to cause atrophy and mortality in HMGECs within 24 h after exposure, even at concentrations that are equal to or below the dosages permitted for human application. |
| Han, 2024 | Prospective study | * One-hour post-application of artificial eyelashes, the foreign body sensation is at its peak. * At the one-week mark, tear breakup time and tear meniscus height are at the lowest level. |
| Grupcheva, 2024 | Prospective study | * After a duration of four weeks following the removal of artificial eyelashes, OSDI, TBUT, corneal staining, and blink frequency exhibit considerable improvement. |
| Mselle, 2004 | Case–control study | * The most prevalent adverse effect of eyelash dying is allergic reaction. |
| Pack, 2008 | Randomized controlled trial | * After three months of individual use, up to 35% of mascaras show microbial contamination. |
| Alshehrei, 2023 | In vitro | * Microbial contamination is more evident and diverse in low-quality cosmetics when compared to high-quality products. |
| Maeda, 2009 | Case report | * Inadvertent “bumping” during the application process may lead to the entrance of powdered eyeshadow beneath the edge of a LASIK flap, even several years after surgery. |
| Ramasamy, 2010 | Case report | * Open-globe injuries can be seen by makeup tools. |
| Luensmann, 2015 | In vitro | * Makeup removers can have a pronounced effect on the diameter, base curve, and sagittal depth of lenses. * Mascara can significantly decrease the image quality of contact lens wearers. |
| Goto, 2010 | Semiquantitative study | * Cosmetics applied to the eyelash line and eyelid margins can migrate to the ocular surface within five minutes. |
| Recommendation |
|---|
| 1. Avoid using eye makeup if it is essential to apply eye drops. |
| 2. Avoid sharing cosmetics with other persons. |
| 3. Avoid using testers in beauty salons that provide open cosmetic items. |
| 4. Use of one-time, disposable applicators in beauty salons is recommended. Otherwise, disinfection strategies for contaminated applicators and sponges should be employed. |
| 5. Sponges need to be completely cleaned following each use and kept in a dry location. |
| 6. Makeup brushes should be regularly cleaned once a week. |
| 7. Avoid applying makeup while in a moving vehicle. |
| 8. Discard old eye cosmetics; for example, mascara should be thrown away three months after purchase. |
| 9. Never use saliva or water to moisten eye makeup. |
| 10. Avoid storing cosmetics at temperatures exceeding 85 degrees Fahrenheit, as this can lead to the breakdown of preservatives. |
| 11. Avoid using eye cosmetics if there is eye infection or inflammation in the surrounding area. |
| 12. Cease the use of any cosmetic that causes irritation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheraqpour, K. Beauty’s Blind Spot: Unmasking the Ocular Side Effects and Concerns of Eye Cosmetics. Cosmetics 2025, 12, 149. https://doi.org/10.3390/cosmetics12040149
Cheraqpour K. Beauty’s Blind Spot: Unmasking the Ocular Side Effects and Concerns of Eye Cosmetics. Cosmetics. 2025; 12(4):149. https://doi.org/10.3390/cosmetics12040149
Chicago/Turabian StyleCheraqpour, Kasra. 2025. "Beauty’s Blind Spot: Unmasking the Ocular Side Effects and Concerns of Eye Cosmetics" Cosmetics 12, no. 4: 149. https://doi.org/10.3390/cosmetics12040149
APA StyleCheraqpour, K. (2025). Beauty’s Blind Spot: Unmasking the Ocular Side Effects and Concerns of Eye Cosmetics. Cosmetics, 12(4), 149. https://doi.org/10.3390/cosmetics12040149






