Cosmeceutical and Dermatological Potential of Olive Mill Wastewater: A Sustainable and Eco-Friendly Source of Natural Ingredients
Abstract
1. Introduction
2. Methods
3. Market Trends and Technical Advances
3.1. Leveraging Phytocompounds in Cosmeceutical Formulations
- Penetration of Active Ingredients: The active compound must penetrate the stratum corneum in sufficient concentration to reach its target site within the skin.
- Known Mechanism of Action: The compound must have a clearly understood mechanism by which it achieves its effect, such as promoting collagen synthesis, inhibiting pigmentation, or reducing inflammation.
- Clinical efficacy: The product must demonstrate measurable results consistent with its claims, through well-designed studies.
3.2. Overview of Skin Care-Product Delivery Technologies
4. Cosmeceutical and Dermatological Applications of Olive Oil Byproducts
4.1. Historical and Modern Applications of Olive-Based Skin Care and the Economic Valorization of Olive Oil Byproducts
4.2. Characterization of Olive Oil and Olive Oil-Preparation Byproduct Composition: Applications in Dermatology
4.3. Anti-Aging Properties of OMWW
4.4. Photoprotective Properties of OMWW
4.5. Anti-Inflammatory Effects and Pathway Modulation
4.6. Antimicrobial Properties and Skin-Barrier Restorative Properties of OMWW
4.7. Skin Cancer Prevention and Selective Antiproliferative Effects
4.8. Hair Health and Follicular Stimulation by OMWW
4.9. Formulations for the Delivery of OMWW Compounds
4.10. Regulatory Landscape and Research Initiatives on OMWW in the EU
4.11. Green Extraction Techniques for OMWW Valorization
4.12. Sustainability and Economic Value Addition
5. Future Perspectives
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, K.J.; Price, K.; Rando, T.L.; Boylan, J.; Dyer, A.R. Ensuring healthy skin as part of wound prevention: An integrative review of health professionals’ actions. J. Wound Care 2018, 27, 707–715. [Google Scholar] [CrossRef]
- Ibrahim, A.A.E.; Bagherani, N.; Smoller, B.R.; Reyes-Baron, C.; Bagherani, N. Functions of the Skin. In Atlas of Dermatology, Dermatopathology and Venereology; Springer: Cham, Germany, 2021. [Google Scholar] [CrossRef]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef] [PubMed]
- Urban, K.; Chu, S.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, M.E.; Delost, G.R. Burden of skin disease and associated socioeconomic status in Asia: A cross-sectional analysis from the Global Burden of Disease Study 1990–2017. JAAD Int. 2020, 2, 40–50. [Google Scholar] [CrossRef]
- Flohr, C.; Hay, R. Putting the burden of skin diseases on the global map. Br. J. Dermatol. 2021, 184, 189–190. [Google Scholar] [CrossRef] [PubMed]
- World Health Assembly (WHA) (n.d.) Resolution on Skin Diseases. Available online: https://globalskin.org/component/content/article/101-advocacy/641-wha-resolution-on-skin-diseases?Itemid=1710 (accessed on 20 March 2025).
- Saluja, S.S.; Fabi, S.G. A Holistic Approach to Antiaging as an Adjunct to Antiaging Procedures: A Review of the Literature. Dermatol. Surg. 2017, 43, 475–484. [Google Scholar] [CrossRef]
- Lodén, M. Effect of moisturizers on epidermal barrier function. Clin. Dermatol. 2012, 30, 286–296. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Cleansers. J. Cosmet. Dermatol. 2018, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- González-Acedo, A.; Ramos-Torrecillas, J.; Illescas-Montes, R.; Costela-Ruiz, V.J.; Ruiz, C.; Melguizo-Rodríguez, L.; García-Martínez, O. The Benefits of Olive Oil for Skin Health: Study on the Effect of Hydroxytyrosol, Tyrosol, and Oleocanthal on Human Fibroblasts. Nutrients 2023, 15, 2077. [Google Scholar] [CrossRef]
- Sciubba, F.; Chronopoulou, L.; Pizzichini, D.; Lionetti, V.; Fontana, C.; Aromolo, R.; Socciarelli, S.; Gambelli, L.; Bartolacci, B.; Finotti, E.; et al. Olive Mill Wastes: A Source of Bioactive Molecules for Plant Growth and Protection against Pathogens. Biology 2020, 9, 450. [Google Scholar] [CrossRef]
- Carrara, M.; Kelly, M.T.; Roso, F.; Larroque, M.; Margout, D. Potential of Olive Oil Mill Wastewater as a Source of Polyphenols for the Treatment of Skin Disorders: A Review. J. Agric. Food Chem. 2021, 69, 7268–7284. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kligman, D. Cosmeceuticals. Dermatol. Clin. 2000, 18, 609–615. [Google Scholar] [CrossRef]
- Rivers, J.K. The role of cosmeceuticals in antiaging therapy. Ski. Ther. Lett. 2008, 13, 5–9. [Google Scholar]
- Barone, M.; De Bernardis, R.; Persichetti, P. Aesthetic Medicine Across Generations: Evolving Trends and Influences. Aesthetic Plast. Surg. 2024, 48, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Choi, D.K. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int. J. Nanomed. 2016, 11, 1987–2007. [Google Scholar] [CrossRef]
- Morávková, T.; Stern, P. Rheological and Textural Properties of Cosmetic Emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar] [CrossRef]
- Sambhakar, P.; Malik, S.; Bhatia, R.; Al Harrasi, S.; Rani, A.; Saharan, R.C.; Kumar, R.; Suresh, G.; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. J. Pharm. Sci. 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Pérez-Pérez, V.; Jiménez-Martínez, C.; González-Escobar, J.L.; Corzo-Ríos, L.J. Exploring the impact of encapsulation on the stability and bioactivity of peptides extracted from botanical sources: Trends and opportunities. Front. Chem. 2024, 12, 1423500. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, B.; Liu, T.W.; Mei, H.C.; Kuo, I.C.; Surboyo, M.D.C.; Lin, H.M.; Lee, C.K. Herbal nanoemulsions in cosmetic science: A comprehensive review of design, preparation, formulation, and characterization. J Food Drug Anal. 2024, 15, 428–458. [Google Scholar] [CrossRef]
- Sharma, N.; Sarangdevot, K. Nanoemulsions: A new topical drug delivery system for the treatment of acne. J. Res. Pharm. 2012, 27, 1–11. [Google Scholar]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Litchman, G.; Nair, P.A.; Badri, T.; Kelly, S.E. Microneedling. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464/ (accessed on 16 April 2025).
- Aiello, A.; Calabrone, L.; Noonan, D.M.; Corradino, P.; Nofri, S.; Cristoni, S.; Accardi, G.; Candore, G.; Caruso, C.; Zinellu, A.; et al. Effect of a Phytochemical-Rich Olive-Derived Extract on Anthropometric, Hematological, and Metabolic Parameters. Nutrients 2024, 16, 3068. [Google Scholar] [CrossRef]
- Gorini, I.; Iorio, S.; Ciliberti, R.; Licata, M.; Armocida, G. Olive oil in pharmacological and cosmetic traditions. J. Cosmet. Dermatol. 2019, 18, 1575–1579. [Google Scholar] [CrossRef]
- Nomikos, N.N.; Nomikos, G.N.; Kores, D.S. The use of deep friction massage with olive oil as a means of prevention and treatment of sports injuries in ancient times. Arch. Med. Sci. 2010, 6, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From antiquity to contemporary times: How olive oil by-products and wastewater can contribute to health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef]
- Janakat, S.; Al-Nabulsi, A.; Allehdan, S.; Olaimat, A.; Holley, R. Antimicrobial activity of amurca (olive oil lees) extract against selected foodborne pathogens. Food Sci. Technol. 2015, 35, 259–265. [Google Scholar] [CrossRef]
- Phelps, A.H., Jr. Air pollution aspects of soap and detergent manufacture. J. Air Pollut. Control Assoc. 1967, 17, 505–507. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Valorization of lyophilized olive mill wastewater: Chemical and biological properties for functional applications. Sustainability 2023, 15, 3360. [Google Scholar]
- Ruggeri, M.; De Luca, F.; Ungolo, A.; Vigani, B.; Paredes, A.J.; Russo, E.; Bottone, M.G.; Bianchi, E.; Ferrari, F.; Rossi, S.; et al. Olive mill wastewater: From by-product to smart antioxidant material. Int. J. Pharm. X 2024, 7, 100301. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M. Olive mill wastewater: Phenolic composition and antioxidant activity. J. Environ. Manag. 2016, 170, 1–9. [Google Scholar]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Env. Sci. Pollut. Res. Int. 2024, 31, 20853–20880. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, C.; Santoro, I.; Nardi, M.; Cassano, A.; Sindona, G. Eco-Friendly Extraction and Characterisation of Nutraceuticals from Olive Leaves. Molecules 2019, 24, 3481. [Google Scholar] [CrossRef]
- CYCLOLIVE Consortium. From Waste to Resource: ReCYCLing OLIVE Oil Extraction Byproducts for Sustainable Agricultural Practices in the Mediterranean Region. Project ID: 1977. p. 86. Available online: https://prima-med.org/wp-content/uploads/2024/11/PRI_booklet_2023_EXE_eng-2.pdf (accessed on 25 March 2025).
- 3D-STELLAR: 3D Solar disTillEr and Flash Pyrolysis for Recycling oLive Mill Wastewater into Irrigation Water and Biochar, Stesso Opuscolo di Prima. p. 72. Available online: https://prima-med.org/wp-content/uploads/2024/11/PRI_booklet_2023_EXE_eng-2.pdf (accessed on 16 May 2025).
- European Commission LIFE Project, 2012. RE-WASTE Final Report. LIFE07 ENV/IT/000421—RE-WASTE. Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE07-ENV-IT-000421/recovery-recycling-resource-valorisation-of-olive-mill-effluents-by-recovering-high-added-value-bio-products (accessed on 8 May 2025).
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Rosillo, M.Á.; Castejón, M.L.; Alarcón-de-la-Lastra, C. Extra virgin olive oil: A key functional food for prevention of immune-inflammatory diseases. Food Funct. 2019, 10, 3805–3824. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Vassallo, A.; La Carrubba, V. Valorization of olive mill wastewater: Recovery of bioactive compounds for food applications. Sustainability 2023, 15, 1234. [Google Scholar]
- Cardinali, A.; De Marco, E.; De Santis, G. Phenolic compounds in olive mill wastewater: Analysis and characterization. Food Chem. 2010, 120, 690–695. [Google Scholar]
- El-Abbassi, A.; Fadhl, B.M.; Khaireddine, A. Olive mill wastewater: A review on its composition and treatment methods. J. Environ. Manag. 2012, 95, S1–S15. [Google Scholar]
- Di Mauro, M.D.; Tomasello, B.; Giardina, R.C.; Dattilo, S.; Mazzei, V.; Sinatra, F.; Caruso, M.; D’Antona, N.; Renis, M. Sugar and mineral enriched fraction from olive mill wastewater for promising cosmeceutical application: Characterization, in vitro and in vivo studies. Food Funct. 2017, 8, 4713–4722. [Google Scholar] [CrossRef] [PubMed]
- Peri, C. The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2014. [Google Scholar]
- Fotib, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Mill Wastewater as Renewable Raw Materials to Generate High Added-Value Ingredients for Agro-Food Industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Sciurba, L.; Indelicato, S.; Gaglio, R.; Barbera, M.; Marra, F.P.; Bongiorno, D.; Davino, S.; Piazzese, D.; Settanni, L.; Avellone, G. Analysis of Olive Oil Mill Wastewater from Conventionally Farmed Olives: Chemical and Microbiological Safety and Polyphenolic Profile for Possible Use in Food Product Functionalization. Foods 2025, 14, 449. [Google Scholar] [CrossRef]
- Li, H.; He, H.; Liu, C.; Akanji, T.; Gutkowski, J.; Li, R.; Ma, H.; Wan, Y.; Wu, P.; Li, D.; et al. Dietary polyphenol oleuropein and its metabolite hydroxytyrosol are moderate skin permeable elastase and collagenase inhibitors with synergistic cellular antioxidant effects in human skin fibroblasts. Int. J. Food Sci. Nutr. 2022, 73, 460–470. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lecci, R.; Romani, A.; Ieri, F.; Mulinacci, N.; Pinelli, P.; Bernini, R.; Caporali, A. Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes. Antioxidants 2021, 10, 2153. [Google Scholar] [CrossRef]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage: A review. Biomed. Pharmacother. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Perugini, P.; Vettor, M.; Rona, C.; Troisi, L.; Villanova, L.; Genta, I.; Conti, B.; Pavanetto, F. Efficacy of oleuropein against UVB irradiation: Preliminary evaluation. Int. J. Cosmet. Sci. 2008, 30, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Galletti, F.; Peluso, G.; Bifulco, M.; Russo, G.L. Biological effects of the olive tree and its derivatives on the skin. Food Funct. 2022, 13, 5952–5970. [Google Scholar]
- Visioli, F.; Galli, C.; Caruso, D. Antioxidant properties of olive oil phenols. J. Nutr. Biochem. 1999, 10, 305–310. [Google Scholar]
- Carrara, M.; Beccali, M.; Cellura, M.; Pipitone, F. Olive mill wastewater: A source of biologically active compounds. J. Clean. Prod. 2021, 279, 123841. [Google Scholar]
- Wang, H.; Syrovets, T.; Kess, D.; Büchele, B.; Hainzl, H.; Lunov, O.; Simmet, T. Targeting NF-κB with a natural triterpenoid alleviates skin inflammation in a mouse model of psoriasis. J. Immunol. 2009, 183, 4755–4763. [Google Scholar] [CrossRef]
- Zhou, Q.; Mrowietz, U.; Rostami-Yazdi, M. Oxidative stress in the pathogenesis of psoriasis. Free Radic. Biol. Med. 2009, 47, 891–905. [Google Scholar] [CrossRef]
- Albanesi, C.; De Pità, O.; Girolomoni, G. Resident skin cells in psoriasis: A special look at the pathogenetic functions of keratinocytes. Clin. Dermatol. 2005, 25, 581–588. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Schlupp, P.; Schmidts, T.M.; Pössl, A.; Wildenhain, S.; Lo Franco, G.; Lo Franco, A.; Lo Franco, B. Effects of a Phenol-Enriched Purified Extract from Olive Mill Wastewater on Skin Cells. Cosmetics 2019, 6, 30. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Mastracci, L.; Grillo, F.; Cornara, L.; Shirooie, S.; Nabavi, S.M.; Trombetta, D. Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: In vitro evaluation on reconstructed human epidermis model. DARU J. Pharm. Sci. 2019, 27, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Pojero, F.; Poma, P.; Spanò, V.; Montalbano, A.; Barraja, P.; Notarbartolo, M. Targeting senescence with olive oil polyphenols as a novel therapeutic strategy for chronic diseases. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar]
- Chinembiri, T.N.; du Plessis, L.H.; Gerber, M.; Hamman, J.H.; du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Olive Leaf Extract Inhibits Proliferation, Epithelial-Mesenchymal Transition and Metastatic Potential of Human Melanoma Cells. Antioxidants 2022, 11, 263. [Google Scholar]
- Mijatović, S.; Timotijević, G.; Miljković, Đ.; Radović, J.; Maksimović-Ivanić, D.; Dekanski, D.; Stošić-Grujičić, S. Multiple antimelanoma potential of dry olive leaf extract. Int. J. Cancer 2011, 128, 1955–1965. [Google Scholar] [CrossRef]
- Gallazzi, M.; Festa, M.; Corradino, P.; Sansone, C.; Albini, A.; Noonan, D.M. An Extract of Olive Mill Wastewater Downregulates Growth, Adhesion and Invasion Pathways in Lung Cancer Cells: Involvement of CXCR4. Nutrients 2020, 12, 903. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Al-Natsheh, M.S.; Yaghmoor, R.; Al-Rimawi, F. Enrichment of Phenolic Compounds from Olive Mill Wastewater and In Vitro Evaluation of Their Antimicrobial Activities. Evid.-Based Complement. Altern. Med. 2017, 2017, 3706915. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; la Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Petrotos, K.; Stagos, D.; Gerasopoulos, K.; Maimaris, A.; Makris, H.; Kafantaris, I.; Makri, S.; Kerasioti, E.; Halabalaki, M.; et al. Enhancement of Antioxidant Mechanisms and Reduction of Oxidative Stress in Chickens after the Administration of Drinking Water Enriched with Polyphenolic Powder from Olive Mill Waste Waters. Oxidative Med. Cell. Longev. 2017, 2017, 8273160. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, N.; Calabrone, L.; Gutmańska, K.; Macrì, N.; Cerrito, M.G.; Ricotta, R.; Pelosi, G.; Bruno, A.; Noonan, D.M.; Albini, A. An Olive Oil Mill Wastewater Extract Improves Chemotherapeutic Activity Against Breast Cancer Cells While Protecting From Cardiotoxicity. Front. Cardiovasc. Med. 2022, 14, 867867. [Google Scholar] [CrossRef]
- Baci, D.; Gallazzi, M.; Cascini, C.; Tramacere, M.; De Stefano, D.; Bruno, A.; Noonan, D.M.; Albini, A. Downregulation of Pro-Inflammatory and Pro-Angiogenic Pathways in Prostate Cancer Cells by a Polyphenol-Rich Extract from Olive Mill Wastewater. Int. J. Mol. Sci. 2019, 20, 307. [Google Scholar] [CrossRef]
- Bassani, B.; Rossi, T.; De Stefano, D.; Pizzichini, D.; Corradino, P.; Macrì, N.; Noonan, D.M.; Albini, A.; Bruno, A. Potential Chemopreventive Activities of a Polyphenol Rich Purified Extract from Olive Mill Wastewater on Colon Cancer Cells. J. Funct. Foods 2016, 27, 236–248. [Google Scholar] [CrossRef]
- Albini, A.; Festa, M.M.G.; Ring, N.; Baci, D.; Rehman, M.; Finzi, G.; Sessa, F.; Zacchigna, S.; Bruno, A.; Noonan, D.M. A Polyphenol-Rich Extract of Olive Mill Wastewater Enhances Cancer Chemotherapy Effects, While Mitigating Cardiac Toxicity. Front. Pharmacol. 2021, 3, 694762. [Google Scholar] [CrossRef]
- Albini, A.; Bassani, B.; Baci, D.; Dallaglio, K.; Gallazzi, M.; Corradino, P.; Bruno, A.; Noonan, D.M. Nutraceuticals and “Repurposed” Drugs of Phytochemical Origin in Prevention and Interception of Chronic Degenerative Diseases and Cancer. Curr. Med. Chem. 2019, 26, 973–987. [Google Scholar] [CrossRef]
- Zhang, H.L.; Qiu, X.X.; Liao, X.H. Dermal Papilla Cells: From Basic Research to Translational Applications. Biology 2024, 13, 842. [Google Scholar] [CrossRef]
- Lasisi, T.; Smallcombe, J.W.; Kenney, W.L.; Shriver, M.D.; Zydney, B.; Jablonski, N.G.; Havenith, G. Human scalp hair as a thermoregulatory adaptation. Proc. Natl. Acad. Sci. USA 2023, 120, e2301760120. [Google Scholar] [CrossRef]
- Agramunt, J.; Parke, B.; Mena, S.; Ubels, V.; Jimenez, F.; Williams, G.; Rhodes, A.D.; Limbu, S.; Hexter, M.; Knight, L.; et al. Mechanical stimulation of human hair follicle outer root sheath cultures activates adjacent sensory neurons. Sci. Adv. 2023, 9, eadh3273. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef]
- Halloy, J.; Bernard, B.A.; Loussouarn, G.; Goldbeter, A. The follicular automaton model: Effect of stochasticity and of synchronization of hair cycles. J. Theor. Biol. 2002, 214, 469–479. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Samra, T.; Lin, R.R.; Maderal, A.D. The Effects of Environmental Pollutants and Exposures on Hair Follicle Pathophysiology. Ski. Appendage Disord. 2024, 10, 262–272. [Google Scholar] [CrossRef]
- Urysiak-Czubatka, I.; Kmieć, M.L.; Broniarczyk-Dyła, G. Assessment of the usefulness of dihydrotestosterone in the diagnostics of patients with androgenetic alopecia. Postępy Dermatol. I Alergol. 2014, 31, 207–215. [Google Scholar] [CrossRef]
- Kische, H.; Arnold, A.; Gross, S.; Wallaschofski, H.; Völzke, H.; Nauck, M.; Haring, R. Sex Hormones and Hair Loss in Men From the General Population of Northeastern Germany. JAMA Dermatol. 2017, 153, 935–937. [Google Scholar] [CrossRef]
- Sittek, L.-M.; Schmidts, T.M.; Schlupp, P. Polyphenol-Rich Olive Mill Wastewater Extract and Its Potential Use in Hair Care Products. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 356–370. 87. [Google Scholar] [CrossRef]
- Zhao, J.; Harada, N.; Okajima, K. Dihydrotestosterone inhibits hair growth in mice by inhibiting insulin-like growth factor-I production in dermal papillae. Growth Horm. IGF Res. 2011, 21, 260–267. [Google Scholar] [CrossRef]
- López-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A. Anatomy, Skin (Integument). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://europepmc.org/abstract/MED/28723009 (accessed on 11 April 2025).
- Mărănducă, A.; Mărănducă, L.; Simionescu, R. The structure and function of the skin. Rom. J. Morphol. Embryol. 2020, 61, 7–14. [Google Scholar]
- Pegoraro, C.; MacNeil, S.; Battaglia, G. Transdermal drug delivery: From micro to nano. Nanoscale 2012, 4, 1881. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Meß, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Seah, B.C.-Q.; Teo, B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef]
- Ponphaiboon, J.; Limmatvapirat, S.; Limmatvapirat, C. Development and Evaluation of a Stable Oil-in-Water Emulsion with High Ostrich Oil Concentration for Skincare Applications. Molecules 2024, 29, 982. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, P.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef]
- Koutsos, T.M.; Chatzistathis, T.; Balampekou, E.I. A new framework proposal, towards a common EU agricultural policy, with the best sustainable practices for the re-use of olive mill wastewater. Sci. Total Environ. 2018, 622–623, 942–953. [Google Scholar] [CrossRef]
- European Commission. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Waste Framework Directive). Official Journal of the European Union, L 312, 22 November 2008, pp. 3–30. Official Website (EUR-Lex). Available online: https://eur-lex.europa.eu/eli/dir/2008/98/oj/eng (accessed on 15 May 2025).
- Gikas, G.D.; Tsakmakis, I.D.; Tsihrintzis, V.A. Hybrid natural systems for treatment of olive mill wastewater. J. Chem. Technol. Biotechnol. 2018, 93, 800–809. [Google Scholar] [CrossRef]
- PROSODOL Project. “Analysis of National and EU Legislative Frameworks on Olive Oil Waste.”(LIFE07 ENV/GR/000280). Available online: https://www.ims.forth.gr/en/project/view?id=56%2012 (accessed on 15 May 2025).
- Rhodolive Project. “Biotechnological Valorization of Olive Mill Wastewater.” 2021. Available online: https://www.cobiotech.eu/lw_resource/datapool/systemfiles/elements/files/CF2C7290EA262123E0537E695E86F43D/current/document/RHODOLIVE_brochure.pdf (accessed on 15 May 2025).
- Cifuni, G.F.; Claps, S.; Morone, G.; Sepe, L.; Caparra, P.; Benincasa, C.; Pellegrino, M.; Perri, E. Valorization of Olive Mill Byproducts: Recovery of Biophenol Compounds and Application in Animal Feed. Plants 2023, 12, 3062. [Google Scholar] [CrossRef]
- Leone, R.; La Scalia, G.; La Fata, C.M.; Micale, R. Analysis of Processes for Olive Oil Mill Wastewater Treatment. J. Inf. Syst. Eng. Manag. 2025, 10, 531–537. [Google Scholar] [CrossRef]
- Dutournié, P.; Jeguirim, M.; Khiari, B.; Goddard, M.L.; Jellali, S. Olive mill waste water: From a pollutant to green fuels, agricultural water source, and bio-fertilizer. Part 2: Water recovery. Water 2019, 11, 768. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef]
- Schievano, A.; Adani, F.; Buessing, L.; Botto, A.; Casoliba, E.N.; Rossoni, M.; Goldfarb, J.L. An integrated biorefinery concept for olive mill waste management: Supercritical CO2 extraction and energy recovery. Green. Chem. 2015, 17, 2874–2887. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 1, 126218. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N. Microwave-assisted Extraction of Phenolic Compounds from Olive By-products. Chem. Eng. Trans. 2022, 91, 613–618. [Google Scholar] [CrossRef]
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Lozano Pérez, A.S.; Lozada Castro, J.J.; Guerrero Fajardo, C.A. Application of Microwave Energy to Biomass: A Comprehensive Review of Microwave-Assisted Technologies, Optimization Parameters, and the Strengths and Weaknesses. J. Manuf. Mater. Process. 2024, 8, 121. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Evangelina, P.-S.; García-Moreno, A.J.; Martínez-García, M.; Pérez-Colodrero, L.; García-Zapata, L.; García-Ruiz, R. Bio-based solvents for polyphenol recovery: Transforming olive mill wastewater into high-value resources. J. Water Process Eng. 2022, 47, 102769. [Google Scholar]
- Yang, J.; Hamid, M.B.B. Sustainable Beauty: A Conceptual Paper of How Sustainable Marketing Impacts Consumer Behaviour in the Cosmetic Industry. In Proceedings of the 2nd International Conference on Management Research and Economic Development, Peking University, Beijing, China, 30 May 2024. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of olive leaf extract-containing cream for facial rejuvenation: A pilot study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Dauber, C.; Parente, E.; Zucca, M.P.; Gámbaro, A.; Vieitez, I. Olea europea and By-Products: Extraction Methods and Cosmetic Applications. Cosmetics 2023, 10, 112. [Google Scholar] [CrossRef]
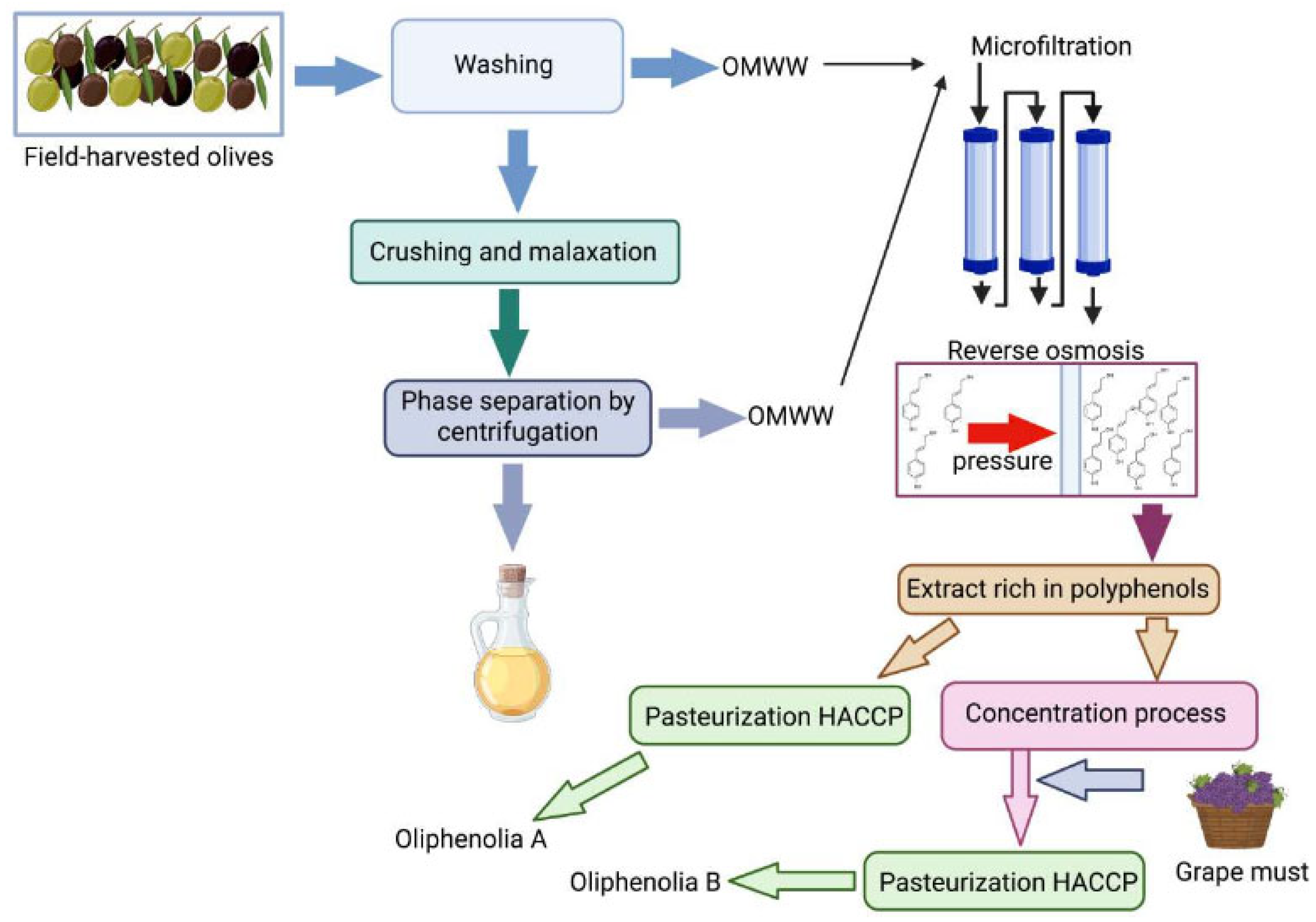

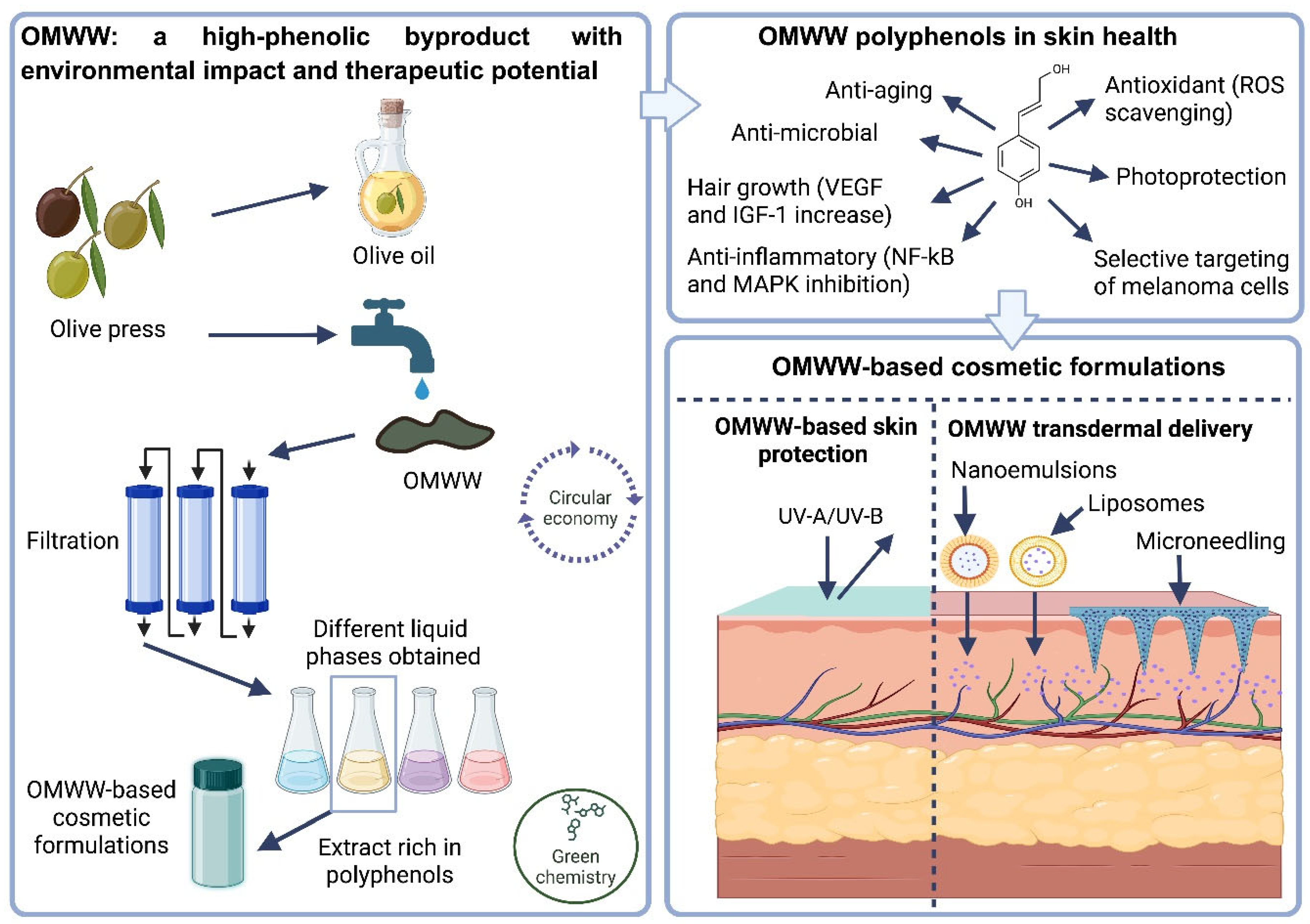
| Component Category | Percentage | Reference |
|---|---|---|
| Water | 83–94% | [41] |
| Organic Matter | 4–16% | |
| Mineral Salts | 0.4–2.5% |
| Component Category | Biological Role | Reference |
|---|---|---|
| Sugars | Energy supply and osmotic balance (glucose, fructose, and mannitol). | [44] |
| Nitrogenous Compounds | Cell repair and signaling (proteins and amino acids). | [45] |
| Organic Acids | pH regulation and antimicrobial action (acetic, malic, and citric acids). | [46] |
| Lipids | Skin barrier support (residual olive oil, essential fatty acids). | [46] |
| Phenolic Compounds | Antioxidant and anti-inflammatory activity (hydroxytyrosol, tyrosol, oleuropein, caffeic acid, and verbascoside). | [13,29] |
| Flavonoids | Anti-inflammatory and photoprotective effects (luteolin, apigenin, and glycosides) | [29,47] |
| Lignans | Antioxidant and anti-cancer properties (Pinoresinol and acetoxypinoresinol). | [46] |
| Vitamins | Skin protection and antioxidant defense (mainly vitamin E). | [46] |
| Minerals | Cellular hydration and function (potassium, sodium, calcium, and magnesium) | [45] |
| Dietary Fibers | Moisturizing and protective effects (mucilage and pectin) | [46] |
| Sugars | Energy supply and osmotic balance (glucose, fructose, and mannitol). | [44] |
| Type of Study | Cells/Tissue | Effect |
|---|---|---|
| In vitro | Keratinocyte [60] | Antibacterial; antioxidant (decreased ROS); anti-inflammatory (decreased IL-8); photoprotection. |
| Human follicle dermal papilla [87] | Increased proliferation and IGF-1; decreased ROS; antioxidant protection. | |
| HaCaT cells (keratinocytes) [60] | Decreased IL-8 after TNF-α; anti-inflammatory effect comparable to hydrocortisone. | |
| Normal human epidermal keratinocytes [50] | Increased growth and migration; antioxidant activity; improved barrier function. | |
| A375 melanoma cells [60] | Selective cytotoxicity to melanoma cells; no toxicity for normal cells. | |
| HFDPCs (human follicle dermal papilla cells) [87] | Increased IGF-1, VEGF and proliferation; antioxidant action. | |
| In vivo | Human skin (clinical) [44] | Increased hydration and elasticity; decreased erythema index. |
| Bioactivity | Experimental Model | Intervention | Outcome Parameters | Key Findings | References |
|---|---|---|---|---|---|
| Antioxidant Activity | HaCaT keratinocytes | OMWW extract (phenol-enriched); 100 µM ascorbic acid control | ROS levels | Decreased ROS > 60%; superior to ascorbic acid | [60] |
| Anti-inflammatory Effects | HaCaT keratinocytes | OMWW extract; TNF-α stimulation | IL-8, iNOS, COX-2 | Decreased IL-8 expression, comparable to hydrocortisone | [60,61] |
| Photoprotection | Human keratinocytes | OMWW polyphenols (hydroxytyrosol, oleuropein); UVA/UVB exposure | Inflammatory cytokines, ROS, collagen | Decreased cytokines and ROS; preserved collagen | [50,51] |
| Antimicrobial Activity | In vitro; S. aureus, P. acnes, C. albicans | OMWW extract (MIC 50–200 µg/mL) | Pathogen viability | Inhibited bacterial/fungal growth | [53,69] |
| Skin Barrier Enhancement | Human keratinocytes | OMWW extract | Cell migration, hydration markers | Increased keratinocyte migration and hydration | [50] |
| Anti-aging Effects | Fibroblasts | OMWW extract; 0.1–1 mM | Elastase, collagenase activity, ROS | Decreased collagenase/elastase activity; increased collagen matrix | [48,53] |
| Hair Growth Promotion | HFDPC (dermal papilla cells) | OMWW extract (1:250 dilution) | IGF-1, VEGF, ROS | Increased IGF-1/VEGF; decreased ROS by 60%; increased proliferation | [87] |
| Skin Cancer Prevention | A375 melanoma cells | OMWW extract, oleuropein (250 μM) | Apoptosis, viability | Decreased melanoma viability; increased apoptosis; spared normal keratinocytes | [60,70] |
| ROS Scavenging | Multiple (HaCaT, HFDPC) | OMWW extract | ROS levels | Decreased ROS in both keratinocytes and DPCs | [60,87] |
| Wound Healing Support | Human fibroblasts, in vivo mouse model | OMWW extract | Fibroblast proliferation, collagen synthesis | Increased fibroblast activity, accelerated tissue regeneration | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albini, A.; Corradino, P.; Morelli, D.; Albini, F.; Noonan, D. Cosmeceutical and Dermatological Potential of Olive Mill Wastewater: A Sustainable and Eco-Friendly Source of Natural Ingredients. Cosmetics 2025, 12, 142. https://doi.org/10.3390/cosmetics12040142
Albini A, Corradino P, Morelli D, Albini F, Noonan D. Cosmeceutical and Dermatological Potential of Olive Mill Wastewater: A Sustainable and Eco-Friendly Source of Natural Ingredients. Cosmetics. 2025; 12(4):142. https://doi.org/10.3390/cosmetics12040142
Chicago/Turabian StyleAlbini, Adriana, Paola Corradino, Danilo Morelli, Francesca Albini, and Douglas Noonan. 2025. "Cosmeceutical and Dermatological Potential of Olive Mill Wastewater: A Sustainable and Eco-Friendly Source of Natural Ingredients" Cosmetics 12, no. 4: 142. https://doi.org/10.3390/cosmetics12040142
APA StyleAlbini, A., Corradino, P., Morelli, D., Albini, F., & Noonan, D. (2025). Cosmeceutical and Dermatological Potential of Olive Mill Wastewater: A Sustainable and Eco-Friendly Source of Natural Ingredients. Cosmetics, 12(4), 142. https://doi.org/10.3390/cosmetics12040142









