Abstract
Waste products from agricultural crops can become valuable if their benefits are discovered. Mangosteen, known as the “queen of fruits”, has a pericarp extract that has been reported to possess various biological activities, including antioxidation, anti-inflammation, antimicrobial activity, and UVB protection (in vitro and in vivo). In this work, we revealed that mangosteen pericarp extract (MPE) exhibits photoprotective properties in primary human dermal fibroblasts (PHDFs) exposed to ultraviolet A (UVA). The α-mangostin content, a major compound in MPE, was determined to be 60.9 ± 1.2% using HPLC. In an in vitro, cell-based assay, we first assessed the cytotoxicity of MPE on PHDFs using the MTT assay. The highest concentration of MPE that showed no cytotoxicity was 50.0 µg/mL. For antioxidative effects, MPE reduced intracellular ROS levels induced by H2O2, compared to H2O2-treated PHDFs. To assess the photoprotective effect of MPE, cells were pretreated with MPE for 24 h before exposure to UVA at an intensity of 5 J/cm2. Our data demonstrated that MPE pretreatment reduced the accumulation of senescent cells compared to UVA-induced senescent cells (7.1 ± 2.4% vs. 12.0 ± 0.2%, respectively). In addition, we examined key aging-related markers, including matrix metalloproteinase 1 (MMP-1) and collagen type I. The expression level of MMP-1 levels was 23,873.4 ± 5498.1 pg/mL in MPE-treated, UVA-induced PHDFs, compared to 38,929.1 ± 6971.4 pg/mL in untreated UVA-induced PHDFs. Meanwhile, procollagen type I in MPE-pretreated PHDFs was 56,443.3 ± 3623.8 pg/mL, compared to 37,137.4 ± 4614.8 pg/mL in UVA-induced PHDFs. These experimental results highlight the photoprotective properties of Garcinia mangostana peel extract, which contains α-mangostin as a major compound, and suggest its potential as an active ingredient in cosmeceuticals for protecting against UVA-induced aging. To the best of our knowledge, this is the first study to report the photoprotective effects of MPE on UVA-induced senescent cells.
Keywords:
Garcinia mangostana; UVA; fibroblasts; antioxidation; anti-senescence; anti-aging; MMP-1; procollagen type I 1. Introduction
The skin is a crucial organ that covers the body, regulates temperature, and protects the internal environment from extrinsic factors, including chemical substances, pollution, and radiation. A key function of the skin is to prevent damage from harmful exposures. Acute exposure to ultraviolet (UV) irradiation, particularly UVA (315–400 nm), leads to various skin alterations, including tanning. Chronic UVA exposure predominantly contributes to wrinkle formation [1]. UVA, the major type of UV radiation (90–95%), not only penetrates the Earth’s atmosphere but also deeply infiltrates into the dermis [2]. Several studies have reported biomolecular and cellular changes associated with UVA-mediated skin photoaging [3,4,5]. The toxic effects of UVA exposure destroy healthy connective tissues, such as collagen and elastin fibers, leading to the structural deformation of the dermis. In response to UV exposure, skin fibroblasts are activated through the MAPK/AP-1 signaling pathways, which are mediated by free radicals, primarily reactive oxygen species (ROS). This cascade alters collagen metabolism [6,7]. ROS interfere with the activation of tissue inhibitors of metalloproteinases (TIMPs), rendering them inactive. Under normal conditions, TIMPs regulate the function of metalloproteinases (MMPs) to maintain skin integrity. However, UVA exposure upregulates MMP-1 (collagenase), leading to collagen fiber degradation [5]. Simultaneously, the expression of collagen type I is downregulated, impairing dermal support. Without proper protection, prolonged UVA exposure results in fine lines, skin sagging, and deep wrinkles, all of which are characteristic signs of skin aging [5,8]. This phenomenon is known as photoaging or premature aging. Importantly, controlling MMP levels is a key strategy to mitigate UV-induced premature aging or photoaging [9].
Not only does UVA-induced collagen degradation contribute to wrinkle formation, but the impaired ability of senescent fibroblasts to produce extracellular matrix proteins also affects the density of healthy dermal tissue [6]. A hallmark of cellular senescence is the cessation of cell proliferation and an increase in senescence-associated β-galactosidase (SA-β-Gal) activity. Several studies have reported that UVA exposure promotes fibroblast senescence [10,11,12,13]. Consequently, the accumulation of senescent fibroblasts, including both UV-exposed and chronologically aged cells, in the dermal layer, leads to aged skin. Interestingly, studies have suggested that treatment with plant extracts may retard senescence-related phenotypes [14]. In recent years, herbal and natural extracts have gained increasing attention in the fields of cosmeceuticals. Their antioxidant, anti-inflammatory, anti-senescence, and anti-photoaging properties make them promising candidates as active ingredients for protecting and treating photodamaged skin.
Garcinia mangostana Linn (mangosteen) is an economically significant fruit in Thailand, particularly in the eastern region. Utilizing agricultural crops for cosmeceutical applications could enhance their value and increase income for farmers and extraction facilities. α-Mangostin, the major bioactive compound in ethanolic mangosteen pericarp extract (MPE), has been reported to exhibit various biological activities, including antioxidation [15,16,17], anti-tyrosinase [18] anti-inflammatory [19,20,21], and antibacterial effects against Cutibacterium acnes and Staphylococcus epidermidis [22]. Additionally, its anti-genotoxic properties [23] have been documented. In an in vivo study, the collagen fibers of rat skin treated with a mask gel containing MPE were protected from UVB-induced damage [24]. Another study demonstrated that a cream containing MPE exhibited a photoprotective effect by reducing 8-hydroxy-2′-deoxyguanosine (8-OHdG) formation after UVB exposure in mice [25]. In addition, the protective effects of compounds from Garcinia mangostana L. (mangosteen) against UVB-induced damage have been reported in both in vitro (HaCaT cells) and in vivo (hairless mice) models [26]. However, to date, the preventive effects of MPE on UVA-induced aging in adult human dermal fibroblasts have not been widely investigated.
This study aims to explore the potential of MPE in preventing photoaging by assessing its impact on the expression of MMP-1 and pro-collagen type I in UVA-irradiated fibroblasts. Furthermore, we evaluated its inhibitory activity on intracellular ROS formation in H2O2-induced fibroblasts. Additionally, we investigated the anti-senescence effects of MPE by assessing SA-β-Gal activity in UVA-aged fibroblasts.
2. Materials and Methods
2.1. Extraction of Mangosteen Pericarp
The pericarps of Garcinia mangostana (mangosteen) were collected between June and July 2023 from a garden in Rayong province, Thailand. The fresh pericarps were cleaned and cut into small pieces. These small pieces were then dried at 50 °C for 48 h or until completely dry using a hot air oven (KH-100A, Kenton, Foshan, China). These dried peels were subsequently pulverized into a fine powder. Mangosteen pericarp extract (MPE) was prepared using a simple maceration method in 95% ethanol with a ratio of 1:2 w/v (dried powder to ethanol) for two extraction cycles (48 h per cycle). Ninety-five percent ethanol was selected based on the recommendation by Pothitirat et al. [27], as it yields the highest content of α-mangostin. The ethanolic extract was filtered using filter paper (Whatman No. 1, 90 mm diameter, Cytiva, Amersham, UK) to remove any residuals. The solvent was then evaporated using a rotary evaporator (RV 8 V, IKA, Staufen, Germany). The extract obtained was weighed to calculate the percent yield (% yield) and stored at 4 °C with light protection for further studies.
2.2. Quantification of α-Mangostin in MPE
The amount of α-mangostin in MPE was determined using high-performance liquid chromatography (HPLC) [28]. A C18 column (250 mm × 4.6 mm, 5 μm; Luna® C18, Phenomenex, CA, USA) was used as the stationary phase. The mobile phase consisted of (A) 0.2% formic acid in water and (B) methanol, eluted in a gradient mode, as follows: 75% to 90% B in A for 10 min, 90% B in A to 100% B for 5 min, and finally, 100% B for 10 min. A standard α-mangostin with ≥98.0% purity was purchased from ChemFaces Biochemical Co., Ltd. (Lot no. CFN97050, Chem faces, Wuhan, China). The injection volume and flow rate were set at 5 µL and 1.0 mL/min, respectively. Absorbance was measured at a wavelength of 245 nm. The α-mangostin content in MPE was calculated using a standard calibration curve. The limit of detection (LOD) and the limit of quantification (LOQ) were calculated based on the standard error (σ) and the slope (S) of the calibration curve, as expressed by the following equations: LOD = 3.3 × σ/S and LOQ = 10 × σ/S. All experiments were performed in triplicate.
2.3. UV Determination of MPE and α-Mangostin
Methanol was used as both the blank and the solvent to dissolve MPE and α-mangostin standard. Each sample was prepared at a final concentration of 20 µg/mL. The solutions were scanned over a wavelength range of 200 to 500 nm using a spectrophotometer (U-2900, Hitachi High-Technologies Corporation, Tokyo, Japan).
2.4. Evaluation of Cytotoxicity of PHDFs Treated with MPE
Primary human dermal fibroblasts (PHDFs) were purchased from ATCC (Lot No. 81201212, Manassas, VA, USA). To expand the PHDF population, cells were cultured in DMEM-high glucose (GibcoTM, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (GibcoTM, Brazil), 1% penicillin/streptomycin (GibcoTM, NY, USA), and 1 mg/mL amphotericin B (GibcoTM, Lod, Israel). The cultures were maintained in a CO2 incubator (CB170, BINDER GmbH, Tuttlingen, Germany), at 37 °C with 5% CO2 and high humidity. Subsequently, PHDFs at passage 3–5 were trypsinized and seeded into 96-well plates at a density of 1.0 × 104 cells/well. The cells were cultured in DMEM supplemented with 10% FBS and antibiotics for 24 h. Afterward, the medium was removed, and the cells were washed with sterile phosphate-buffered saline (PBS, pH 7.4). They were then incubated in serum-free medium with or without various concentrations of MPE (1.6–50 µg/mL) for 24 h. Cell viability was assessed using an MTT assay (Invitrogen, Thermo Fisher Scientific, New York, NY, USA). A total of 50 µL of MTT solution (1000 µg/mL) and 50 µL of serum-free medium were added to each well to yield a final MTT concentration of 500 µg/mL, equivalent to 0.05% w/v, followed by incubation at 37 °C in 5% CO2 for 4 h. DMSO was added to solubilize the formazan. Absorbance was measured at a wavelength of 470 nm using a microplate reader (FLUOstar® Omega, BMG LABTECH, Ortenberg, Germany). The viability of untreated control cells was set as 100%, and relative cell viability was calculated. All experiments were performed in triplicate.
2.5. Evaluation of UVA-Induced Fibroblast Senescence Using the Senescence-Associated β-Galactosidase (SA-β-Gal) Assay
PHDFs at passage 5–9 were seeded into 96-well plates at a density of 1.0 × 104 cells/well. The cells were cultured in DMEM supplemented with 10% FBS and antibiotics in a CO2 incubator for 24 h. After washing the cells twice with sterile PBS, the PHDFs were covered with a thin layer of PBS and exposed to UVA irradiation (FL8BLB UVA lamp, Toshiba, Japan) at various intensity (0–10 J/cm2) to determine the threshold that significantly induces cellular senescence compared to non-irradiated cells. Following UVA exposure, PBS was removed, and the cells were incubated in serum-free medium for 24 h. The cells were then fixed with a fixing solution for 5–10 min at room temperature and washed three times with PBS. Afterward, cells were stained overnight with the SA-β-Gal staining solution (ab65351, Abcam, Cambridge, UK). The plate was then washed three times with PBS, and a brightfield microscope was used to observe cells SA-β-Gal-positive cells. The percentage of SA-β-Gal-positive cells was calculated, and all experiments were performed in triplicate.
2.6. Evaluation of Antioxidant Effect of MPE on H2O2-Induced Intracellular ROS Generation
To assess the ability of MPE to inhibit H2O2-induced intracellular ROS generation, 2′,7′-dichlorofluorescin diacetate staining (Merck KGaA, Darmstadt, Germany) was performed. PHDFs at passage 3–6 were seeded in 96-well plates at a density of 1.5 × 104 cells/well and cultured in DMEM supplemented with 10% FBS and antibiotics in a CO2 incubator for 24 h. The cells were then washed twice with sterile PBS and treated with serum-free medium containing MPE at a final concentration of 50 µg/mL and H2O2 at a final concentration of 50 µM for 2 h. L-ascorbic acid (50 µg/mL) was used as a positive control. Following treatment, the cells were washed twice with PBS and incubated with ROS staining solution for 30 min. After another two PBS washes, a thin layer of PBS was added to cover the cells. Intracellular ROS formation was visualized using a fluorescence microscope (ECLIPSE Ti2-E, Nikon corporation, Kanagawa, Japan).
2.7. Evaluation of Photoprotective Effects of MPE on UVA-Induced Fibroblasts Aging
2.7.1. SA-β-Gal Assay
To assess the effect of MPE on SA-β-Gal activity in UVA-induced PHDF senescence, an SA-β-Gal staining kit (ab65351, Abcam, Cambridge, UK) was used. PHDFs (passage 5–9) were seeded into 96-well plates at a density of 1.0 × 104 cells/well and cultured in DMEM supplemented with 10% FBS and antibiotics in a CO2 incubator for 24 h. After washing twice with sterile PBS, the PHDFs were treated with serum-free medium containing MPE (50 µg/mL) for 24 h. The cells were washed twice with sterile PBS, and a thin layer of PBS was added before UVA exposure at an intensity of 5 J/cm2. After UVA irradiation, the cells were incubated for 24 h. Following incubation, the cells were fixed with a fixing solution for 5–10 min at room temperature and washed three times with PBS. The cells were then stained overnight with the SA-β-Gal staining solution. After staining, the plate was washed three times with PBS, and SA-β-Gal-positive cells were visualized using a brightfield microscope. The percentage of SA-β-Gal-positive cells was calculated. All experiments were in triplicate.
2.7.2. MMP-1 ELISA Assay
PHDFs (passage 5–9) were seeded into 6-well plates at a density of 3.0 × 105 cells/well and cultured in DMEM supplemented with 10% FBS and antibiotics in a CO2 incubator for 24 h. After washing twice with sterile PBS, the PHDFs were treated with serum-free medium containing MPE (50 µg/mL) for 24 h. The cells were then washed twice with PBS, covered with a thin layer of PBS, and exposed to UVA at an intensity of 5 J/cm2. After UVA irradiation, the cells were incubated for 24 h. The cell-free supernatants were collected to quantify MMP-1 levels using MMP-1 ELISA kit (ab215082, Abcam, Cambridge, UK). Absorbance was measured at 450 nm using a microplate reader (FLUOstar® Omega, BMG LABTECH, Ortenberg, Germany). The MMP-1 content in the supernatant was determined using a standard calibration curve. All experiments were performed in triplicate.
2.7.3. Pro-Collagen Type I ELISA Assay
PHDFs (passage 5–9) were seeded into 6-well plates at a density of 3.0 × 105 cells/well and cultured in DMEM supplemented with 10% FBS and antibiotics in a CO2 incubator for 24 h. After washing twice with sterile PBS, the PHDFs were treated with serum-free medium containing MPE (50 µg/mL) for 24 h. The cells were then washed twice with PBS, covered with a thin layer of PBS, and exposed to UVA at an intensity of 5 J/cm2. After UVA irradiation, the cells were incubated for 24 h. The cell-free supernatants were collected to quantify the pro-collagen I levels using a pro-collagen type I ELISA kit (ab210966, Abcam, Cambridge, UK). Absorbance was measured at 450 nm using a microplate reader (FLUOstar® Omega, BMG LABTECH, Ortenberg, Germany). The pro-collagen type I content in the supernatant was determined using a standard calibration curve. All experiments were performed in triplicate.
3. Results
3.1. Characteristics of MPE and Quantification of α-Mangostin
The MPE appeared as a dark brown paste with a viscous texture (Figure 1). The percent yield of MPE was 10.0 ± 0.8%. The major bioactive compound in MPE is α-mangostin. HPLC chromatograms of the α-mangostin standard (≥98.0%) and MPE are shown in Figure 2. After generating the HPLC calibration curve using the α-mangostin standard (≥98.0%), the α-mangostin content in MPE was determined to be 60.9 ± 1.2%. LOD and LOQ were determined to be 9.0 μg/mL and 27.3 μg/mL, respectively.

Figure 1.
The appearance of mangosteen pericarp extract (MPE).
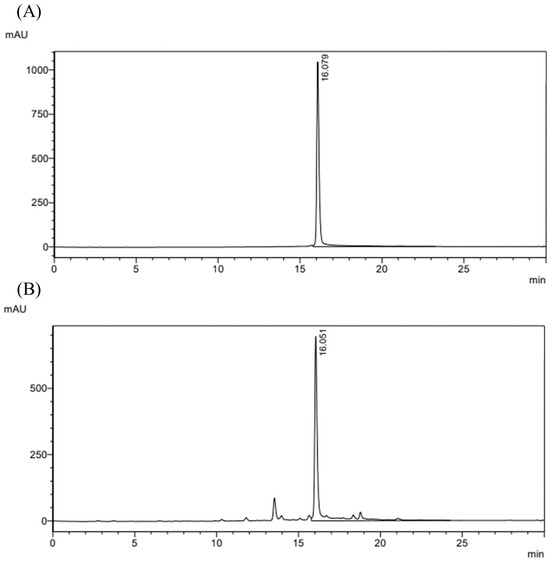
Figure 2.
HPLC chromatogram of (A) α-mangostin standard (≥98.0%) and (B) mangosteen pericarp extract (MPE). Absorbance was measured at 245 nm.
3.2. UV-Vis Absorption Profile of MPE and α-Mangostin
The UV–Vis spectra of MPE and α-mangostin are overlaid in Figure 3. Both α-mangostin-enriched MPE and α-mangostin standard showed maximum absorption peaks at 243 and 316 nm, which corresponded with the UV–Vis spectra of α-mangostin in other studies [29,30]. This result indicates that MPE has UVA-absorbing ability.
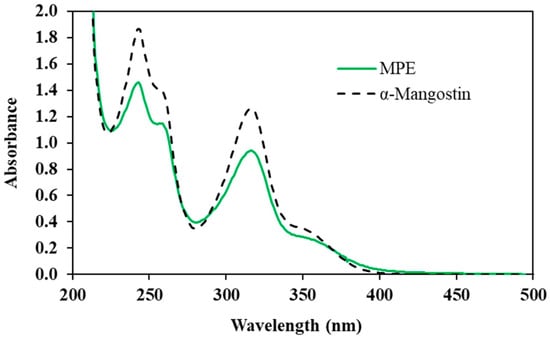
Figure 3.
UV-Vis absorption profile of mangosteen pericarp extract (MPE) and α-mangostin.
3.3. Viability of PHDFs Treated with MPE
Due to the limited non-toxic concentration of DMSO used to dissolve MPE, the highest tested concentration of MPE was 50.0 µg/mL. In this study, PHDFs were treated with MPE at concentrations ranging from 1.6 to 50.0 µg/mL for 24 h. As shown in Figure 4, no significant differences in % cell viability were observed between MPE-treated cells (1.6–50.0 µg/mL) and the control group (untreated cells). Morphological analysis under an inverted microscope (CKX41SF, Olympus Corporation, Manila, Philippines) revealed that the cell maintained normal spindle shape and that cell density remained comparable to the control group (Figure 5). Based on these results, 50 µg/mL was selected as the concentration for further studies.
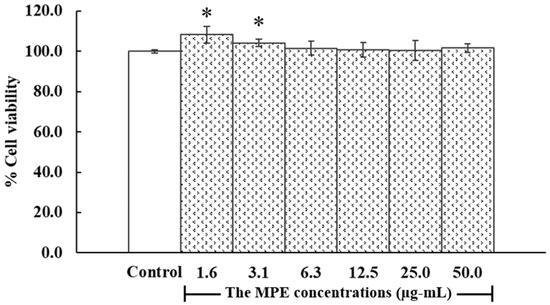
Figure 4.
Viability of primary human dermal fibroblasts (PHDFs) with and without mangosteen pericarp extract (MPE) treatment for 24 h. Results are expressed as % cell viability relative to the control group (where the OD of the control group was set to 100%). Each bar represents the mean ± S.D. of three independent experiments. * p < 0.05, compared between two groups using unpaired Student’s t-test.
Figure 5.
The cell morphology of (A) primary human dermal fibroblasts (PHDFs) without treatment (control) and (B) PHDFs treated with mangosteen pericarp extract (MPE, 50.0 µg/mL) for 24 h.
3.4. The Effect of UVA-Induced Fibroblast Senescence
According to the study on UVA exposure at various intensities (2.5, 5, and 10 J/cm2) and its effects on fibroblasts senescence, there was no significant difference in SA-β-Gal activity between PHDFs irradiated with UVA at 2.5 J/cm2 (3.3 ± 1.2%) and the control group (without UVA, 4.7 ± 0.5%) (Figure 6 and Figure 7). However, PHDFs irradiated with UVA at 5 J/cm2 (12.0 ± 0.2%) showed a significant increase in SA-β-Gal activity compared to the control group. At the highest intensity of 10 J/cm2, UVA exposure resulted in cell death. Thus, a UVA dose of 5 J/cm2 was selected for further studies.

Figure 6.
Representative phase-contrast microscopy images (200×) of (A) control (without UVA exposure) and primary human dermal fibroblasts (PHDFs) irradiated with UVA at intensities of (B) 2.5, (C) 5, and (D) 10 J/cm2, used to assess the number of SA-β-Gal-positive cells. The arrows indicate cell expressing SA-β-Gal activity.
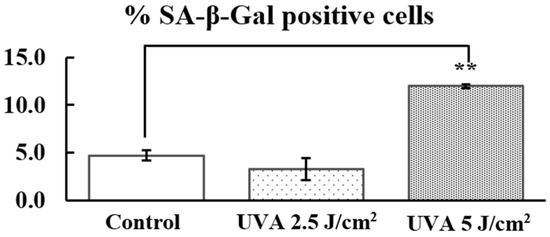
Figure 7.
Percentage of SA-β-Gal-positive cells in the control group and primary human dermal fibroblasts (PHDFs) irradiated with UVA at intensities of 2.5 and 5 J/cm2. Each bar represents the mean ± S.D. of three independent experiments. ** p < 0.01, compared between two groups using an unpaired Student’s t-test.
3.5. Antioxidant Effect of MPE on H2O2-Induced Intracellular ROS Generation
This study demonstrated the qualitative antioxidative properties of MPE. The compound 2′,7′-dichlorofluorescein-diacetate (H2DCFDA) is initially non-fluorescent. Upon penetrating the cell membrane, H2DCFDA is deacetylated by cellular esterases to form 2′,7′-dichlorodihydrofluorescein (H2DCF), which is then oxidized by intracellular ROS to produce 2′,7′-dichlorofluorescein (DCF), a fluorescent compound. As shown in Figure 8B, H2O2 exposure significantly increased intracellular ROS levels, as evidenced by strong green fluorescence, compared to the control (Figure 8A). Meanwhile, treatment with L-ascorbic acid, a positive control, effectively inhibited intracellular ROS formation (Figure 8C) compared to H2O2-induced PHDFs. Similarly, PHDFs induced by H2O2 and treated with MPE exhibited reduced ROS generation (Figure 8D), suggesting that MPE has antioxidant effects.
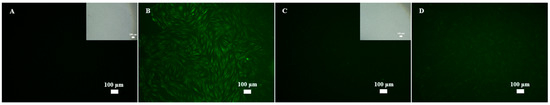
Figure 8.
Representative fluorescence microscopy images (100×) of (A) control, (B) primary human dermal fibroblasts (PHDFs) induced by H2O2 at a final concentration of 50 µM for 2 h, (C) PHDFs induced by H2O2 at final concentration of 50 µM and treated with L-ascorbic acid at final concentration of 50 µg/mL for 2 h, and (D) PHDFs induced by H2O2 at final concentration of 50 µM and treated with mangosteen pericarp extract (MPE) at a final concentration of 50 µg/mL for 2 h. After that, samples were stained with ROS solution for 30 min. The intracellular ROS formation was captured.
3.6. Photoprotective Effects of MPE on UVA-Induced Fibroblasts Aging
3.6.1. SA-β-Gal Expression
In this experiment, senescent cells were identified by purple staining, indicating SA-β-Gal activity (Figure 9). The control group (without MPE treatment and UVA exposure) exhibited SA-β-Gal activity in 4.7 ± 0.5% of cells (Figure 10). In contrast, PDHFs irradiated with UVA at 5 J/cm2 exhibited SA-β-Gal activity in 12.0 ± 0.2% of cells, confirming that UVA exposure significantly increases SA-β-Gal activity compared to the control group. However, PDHFs were pretreated with MPE treatment before UVA exposure SA-β-Gal activity in 7.1 ± 2.4% of cells, suggesting that MPE mitigates cellular senescence by reducing SA-β-Gal activity in fibroblasts.

Figure 9.
Representative phase-contrast microscopy images (200×) of (A) control, (B) primary human dermal fibroblasts (PHDFs) irradiated with UVA at an intensity of 5 J/cm2, and (C) PHDFs treated with mangosteen pericarp extract (MPE) and irradiated with UVA. These images were used to assess the number of SA-β-Gal-positive cells. The arrows indicate cell expressing SA-β-Gal activity.

Figure 10.
Percentage of SA-β-Gal-positive cells in the control group, primary human dermal fibroblasts (PHDFs) irradiated with UVA at an intensity of 5 J/cm2, and PHDFs treated with mangosteen pericarp extract (MPE) and irradiated with UVA. Each bar represents the mean ± S.D. of three independent experiments. * p < 0.05 and ** p < 0.01, compared between two groups using an unpaired Student’s t-test.
3.6.2. MMP-1 Expression
The control group (without MPE treatment and UVA exposure) exhibited MMP-1 levels of 8928.0 ± 1033.3 pg/mL (Figure 11). In contrast, PHDFs irradiated with UVA at 5 J/cm2 exhibited significantly increased MMP-1 levels of 38,929.1 ± 6971.4 pg/mL, confirming that UVA exposure stimulates MMP-1 production. MMP-1 is a metalloprotease involved in collagen degradation. However, PHDFs pretreated with MPE before UVA irradiation exhibited MMP-1 levels of 23,873.4 ± 5498.1 pg/mL, indicating that MPE significantly reduces MMP-1 expression compared to the UVA-exposed group.
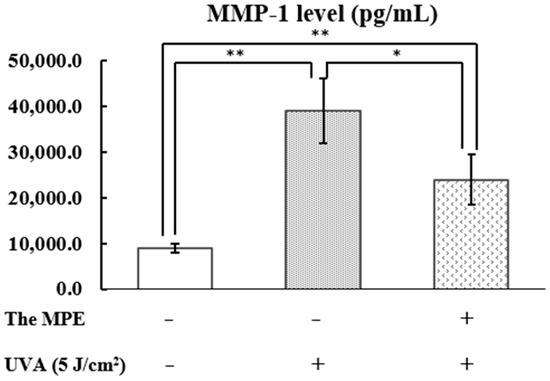
Figure 11.
Matrix metalloproteinase 1 (MMP-1) levels (pg/mL) in the supernatant, determined using an ELISA assay. The control group, primary human dermal fibroblasts (PHDFs) irradiated with UVA at an intensity of 5 J/cm2, and PHDFs pretreated with mangosteen pericarp extract (MPE, 50 µg/mL) for 24 h before UVA irradiation. Supernatant was collected at 24 h after UVA exposure. Each bar represents the mean ± S.D. of three independent experiments. * p < 0.05 and ** p < 0.01, compared between two groups using an unpaired Student’s t-test.
3.6.3. Pro-Collagen Type I Expression
The control group (without MPE treatment and UVA irradiation) exhibited pro-collagen type I levels of 102,041.4 ± 8721.8 pg/mL (Figure 12). PHDFs irradiated with UVA at 5 J/cm2 exhibited significantly reduced pro-collagen type I levels of 37,137.4 ± 4614.8 pg/mL, confirming that UVA exposure significantly decreases pro-collagen type I production compared to the control group. However, PHDFs pretreated with MPE before UVA exposure exhibited pro-collagen type I levels of 56,443.3 ± 3623.8 pg/mL, suggesting that MPE helps preserve pro-collagen type I production and secretion in UVA-induced fibroblasts compared to the UVA-exposed group.
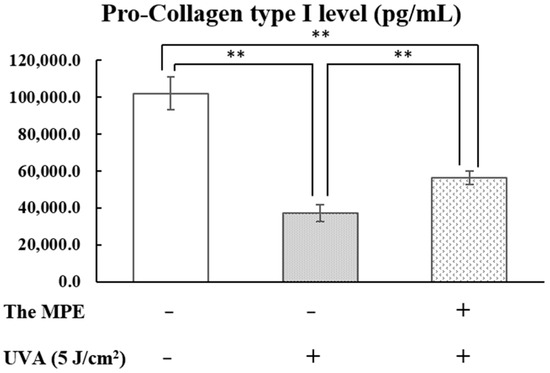
Figure 12.
Pro-collagen type I levels (pg/mL) in the supernatant, determined using an ELISA assay. The control group, primary human dermal fibroblasts (PHDFs) irradiated with UVA at an intensity of 5 J/cm2, and PHDFs pretreated with mangosteen pericarp extract (MPE, 50 µg/mL) before UVA irradiation. Supernatant was collected at 24 h after UVA exposure. Each bar represents the mean ± S.D. of three independent experiments. ** p < 0.01, compared between two groups using an unpaired Student’s t-test.
4. Discussion
Our results suggest that the mangosteen pericarp extract (MPE), which contain α-mangostin, has the potential to protect UVA-irradiated dermal fibroblasts. The beneficial effects of MPE include reducing SA-β-Gal activity in UVA-induced aged fibroblasts. Furthermore, MPE mitigates intracellular ROS-induced by H2O2. Additionally, MPE decreases MMP-1 levels, while preserving pro-collagen type I levels, which are otherwise disrupted by UVA irradiation.
Previous studies have compared various extraction methods and concluded that 95% ethanol is a recommended solvent for efficiently extracting α-mangostin from mangosteen fruit rind [27]. Ethanol is widely recognized as a green, environmentally friendly solvent and is commonly used in cosmetic formulations due to its skin-safe properties, particularly at lower concentrations. Therefore, in this study, 95% ethanol was selected as the extraction solvent in combination with a simple maceration method. We obtained MPE with an α-mangostin content yield of 60.9 ± 1.2%. Compared to a previous study [27], this high yield may be attributed to several factors, including the source of raw material and differences in extraction methods. The α-mangostin content can vary significantly depending on the geographical origin and cultivation conditions of mangosteen. Additionally, our extraction protocol was optimized to increase α-mangostin yield. We used a 1:2 ratio of mangosteen peel powder to 95% ethanol, replaced the solvent every 48 h, and repeated the extraction for two full cycles (48 h per cycle) with continuous shaking.
Wrinkle formation is influenced not only by natural aging but also by environmental factors, particularly UV exposure. Both UVB and UVA contribute to skin disorders, but through different molecular pathways. In the context of photoaging, UVA is recognized as a major contributor. Numerous studies have reported that repeated exposure to UVA leads to biomolecular modifications and cellular alterations [3,4,5,7,8]. At the molecular level, UVA-irradiated fibroblasts undergo changes in gene expression, including the upregulation of collagenase named MMP-1, a tissue inhibitor of metalloproteinases (TIMP-1), and inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [31,32,33]. In addition, senescence-associated β-galactosidase (SA-β-Gal) activity, a key biomarker of cellular senescence, is significantly increased in UVA-exposed fibroblasts [10]. UVA can directly activate cell surface receptors, such as tumor necrosis factor receptor (TNFR), and indirectly induce ROS formation, which damages cellular components, including lipids, proteins, and nucleic acids. These molecular events contribute to premature aging, inflammation, and even, skin cancer [33,34,35]. However, natural extracts and bioactive compounds with strong antioxidant properties can help prevent or mitigate these harmful effects on the skin.
A previous study reported that MPE exhibits potent DPPH radical-scavenging activity, with an IC50 value of 9.40 µg/mL, which is slightly more effective than ascorbic acid (IC50 = 10.47 µg/mL) [36,37]. Additionally, the protective effects of α-mangostin in preserving the activities of antioxidant enzymes (superoxide dismutase and catalase) by scavenging free radicals in UVB-irradiated hairless mice have also been reported [26]. In this study, we utilized ethanol-extracted MPE to evaluate its biological effects, including ROS inhibition in H2O2-induced fibroblasts, SA-β-Gal activity, MMP-1 suppression, and the modulation of collagen type I in UVA-irradiated fibroblasts. Our findings demonstrated that MPE effectively inhibited intracellular ROS production in UVA-induced aged fibroblasts. An imbalance between antioxidants and oxidants leads to oxidative stress, which amplifies UVA-induced damage. TNFR can be activated directly by UVA or indirectly by ROS, triggering TNF-α secretion via autocrine and paracrine signaling. TNF-α, in turn, induces MAPK phosphorylation, leading to increased c-Jun/AP-1 activity and MMP-1 production [33,35,38]. This results in collagen degradation, followed by the accumulation of fragmented collagen fibrils [38,39,40]. Notably, MPE suppresses MMP-1 expression through UVA absorption and antioxidant mechanisms. The UV-Vis absorption spectra of α-mangostin and MPE exhibit peaks at 243 and 316 nm, and the spectrum of α-mangostin corresponds with previous studies [29,30], demonstrating its ability to absorb UVA radiation. Therefore, MPE, which contains a high concentration of α-mangostin, may directly absorb UVA and thereby delay UVA-induced premature aging.
Another key factor associated with skin aging and UVA exposure is senescence-associated β-galactosidase (SA-β-Gal) activity, a hallmark of cellular senescence. Although senescent cells remain viable, they undergo irreversible growth arrest and are regarded as major contributors to aging-related changes. Cellular senescence can be triggered by DNA damage, ROS accumulation, chronic inflammation, and UV-activated cell surface receptors [41,42,43,44]. As previously mentioned, TNFR activation (either directly or via ROS) induces NFκB phosphorylation, leading to IκB degradation and the subsequent production of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, in UVA-irradiated fibroblasts. These cytokines, in turn, promote MMP-1 upregulation [45,46]. Furthermore, studies have shown that increased cellular senescence is associated with excessive ROS levels, transcription factor zinc finger E-box binding homeobox 1 (ZEB1) dysregulation, and DNA methyltransferase 1 (DNMT1) overexpression in UVA-induced cells. However, antioxidant treatment has been reported to inhibit UVA-induced intracellular ROS accumulation and decreases cellular senescence by enhancing ZEB1 and DNMT1 expression [11,47]. Given that MPE has been extensively studied and recognized as a potent antioxidant [20,36,37,48], and that its major compounds—including the tricyclic aromatic xanthones α-mangostin—can act as antioxidants through UVA absorption [49], it is plausible that MPE mitigates UVA-induced fibroblast senescence by reducing ROS levels and absorbing UVA radiation.
Skin inflammation appears to be linked to cellular senescence. Chronic or prolonged exposure to TNF-α triggers the phosphorylation of the p38 MAPK pathway, serving as an initial inducer of senescence. Additionally, human dermal fibroblasts senescence is characterized by reduced cell proliferation and an increase in SA-β-Gal-positive cells [43]. Several studies have reported the anti-inflammatory properties of Garcinia mangostana. For example, mangosteen extract has been shown to inhibit TNF-α release from RAW264.7 macrophage cells [50,51,52]. Furthermore, α-mangostin has been demonstrated to suppress TNF-α and IL-6 production in UVB-irradiated HaCaT cells [26,51]. As a result, α-mangostin-enriched mangosteen extract may act as a TNF-α antagonist. In our study, we investigated the effects of MPE on reducing UVA-induced fibroblast senescence by analyzing SA-β-Gal activity. The results demonstrated that pretreatment with MPE significantly suppressed the number of SA-β-Gal-positive cell. This suggests that the anti-senescent effects of MPE may involve the inhibition of the TNF-α/ MAPK signaling cascade. In addition, it is plausible that the α-mangostin/ROS/SIRT-1/NFκB signaling pathway is also involved, as reported by Franceschelli et al. [53], who found that α-mangostin inhibited SIRT-1 (a nuclear histone deacetylase) and NFκB activity in LPS-induced U937 cells, leading to reduced inflammation. However, further in-depth studies are necessary to elucidate the precise mechanisms underlying the anti-senescence and anti-photoaging properties of MPE in UVA-damaged fibroblasts. A deeper understanding of these mechanisms could have significant implications for the prevention and treatment of photoaging.
5. Conclusions
The extract from Garcinia mangostana pericarp demonstrates significant potential in preventing UVA-induced photoaging. The primary effects of MPE include antioxidation, anti-senescence, and anti-photoaging activities. MPE effectively inhibits intracellular ROS production induced by H2O2. In addition, MPE reduces the expression of MMP-1 in UVA-irradiated fibroblasts, leading to the suppression of collagen I degradation. Furthermore, MPE decreases SA-β-Gal expression in UVA-irradiated cells. As a result, fibroblasts retain collagen fibers, supporting normal cell morphology and skin structure, thereby contributing to healthier skin and delaying signs of aging.
We suggest that MPE holds strong potential as an active ingredient in cosmeceuticals for protecting against UVA-induced skin aging. However, further research is necessary to elucidate its underlying mechanisms of action. Additionally, due to the lipophilic nature of MPE, which may limit skin penetration, the development of an effective delivery system remains a crucial area for future investigation.
Author Contributions
K.L. performed the experiment using a cell-based assay, prepared the extract, analyzed the data, interpreted the data, and revised the manuscript; P.K. performed the experiment using a cell-based assay and prepared the extract; S.Y. generated the manuscript; J.P. revised the manuscript; T.S. prepared the extract and contributed to the materials and reagents; B.V. designed the experiments, contributed to the reagents, materials, analysis tools, and data, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Research Grant of Faculty of Pharmaceutical Sciences, Burapha University (grant no. Rx 2/2566).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time, as the data also form part of an ongoing study.
Acknowledgments
The authors express their gratitude to the Faculty of Pharmaceutical Sciences at Burapha University for facility support and to Aomsin Rungjang for conducting the HPLC experiment. This work was financially supported by the Research Grant of Faculty of Pharmaceutical Sciences, Burapha University (grant no. Rx 2/2566).
Conflicts of Interest
All authors have no conflicts of interest, and they agree to submit the manuscript to Cosmetics as an original research article. This manuscript has not been published elsewhere and is not currently under consideration by another journal.
References
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Ma, W.M.; Wlaschek, I.; Tantcheva-Poor, L.A.; Schneider, L.; Naderi, Z.; Razi-Wolf, J.S.; Scharffetter-Kochanek, K. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin. Exp. Dermatol. 2001, 26, 592–599. [Google Scholar] [CrossRef]
- Wlaschek, M.I.; Tantcheva-Poor, L.; Naderi, W.; Ma, L.A.; Schneider, Z.; Razi-Wolf, J.S.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Landau, M. Exogenous factors in skin aging. In Current Problems in Dermatology; Karger: Basel, Switzerland, 2007; Volume 35, pp. 1–13. [Google Scholar]
- Liu, S.; Mohri, S.; Manabe, Y.; Ejima, A.; Sato, K.; Sugawara, T. Gly-Pro protects normal human dermal fibroblasts from UVA-induced damages via MAPK-NF-κB signaling pathway. J. Photochem. Photobiol. B Biol. 2022, 237, 112601. [Google Scholar] [CrossRef]
- Nakyai, W.; Saraphanchotiwitthaya, A.; Viennet, C.; Humbert, P.; Viyoch, J. An in vitro Model for Fibroblast Photoaging Comparing Single and Repeated UVA Irradiations. Photochem. Photobiol. 2017, 93, 1462–1471. [Google Scholar] [CrossRef]
- Jung, H.O.; Fatih, K.; Chang-Suk, K.; Youngwan, S. Antiphotoaging Effect of 3,5-Dicaeoyl-epi-quinic Acid against UVA-Induced Skin Damage by Protecting Human Dermal Fibroblasts In Vitro. Int. J. Mol. Sci. 2020, 21, 7756. [Google Scholar]
- Luangpraditkun, K.; Pimjuk, P.; Phimnuan, P.; Wisanwattana, W.; Wisespongpand, C.; Waranuch, N.; Viyoch, J. Anti-Aging Properties of Cannabis sativa Leaf Extract against UVA Irradiation. Cosmetics 2024, 11, 45. [Google Scholar] [CrossRef]
- Yi, Y.; Xie, H.; Xiao, X.; Wang, B.; Du, R.; Liu, Y.; Li, Z.; Wang, J.; Sun, L.; Deng, Z.; et al. Ultraviolet A irradiation induces senescence in human dermal fibroblasts by down-regulating DNMT1 via ZEB1. Aging 2018, 10, 212–228. [Google Scholar] [CrossRef]
- Gen-Long, B.; Ping, W.; Xin, H.; Zi-Yue, W.; Di, C.; Chuan, L.; Yi-Yi, L.; Ruo-Lin, L.; Ai-Jun, C. Rapamycin Protects Skin Fibroblasts From UVA-Induced Photoaging by Inhibition of p53 and Phosphorylated HSP27. Front. Cell Dev. Biol. 2021, 9, 633331. [Google Scholar]
- Yao, X.; Li, H.; Chen, L.; Tan, L.P. UV-induced senescence of human dermal fibroblasts restrained by low-stiffness matrix by inhibiting NF-κB activation. Eng. Regen. 2022, 3, 365–373. [Google Scholar] [CrossRef]
- Lämmermann, I.; Terlecki-Zaniewicz, L.; Weinmüllner, R.; Schosserer, M.; Dellago, H.; de Matos Branco, A.D.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. npj. Aging Mech. Dis. 2018, 4, 4. [Google Scholar] [CrossRef]
- Weecharangsan, W.; Opanosopit, P.; Sukma, M.; Ngawhirunpat, T.; Sotanaphun, U.; Siripong, P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Med Princ. Pract. 2006, 15, 281–287. [Google Scholar] [CrossRef]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, E.; Pietradewi, H. Evaluation of mangosteen (Garcinia mangostana) antioxidant activity in clinical trials and in vivo animal studies: A systematic review. J. Appl. Pharm. Sci. 2020, 10, 114–129. [Google Scholar] [CrossRef]
- Tangyuenyongwatana, P.; Gritsanapan, W. Development of sunscreen containing alpha-mangostin riched extract with anti-tyrosinase activities. Chiang Mai Univ. J. Nat. Sci. 2022, 21, e2022064. [Google Scholar] [CrossRef]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Wattanapiromsakul, C.; Mahabusarakam, W. Effects of compounds from Garcinia mangostana on inflammatory mediators in RAW264.7 macrophage cells. J. Ethnopharmacol. 2009, 121, 379–382. [Google Scholar] [CrossRef]
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-mangostin: Anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem. Toxicol. 2019, 124, 300–315. [Google Scholar] [CrossRef]
- Chomnawang, T.M.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005, 101, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Silva, R.; Pereira, A.C.; Dos Santos Alves, R.P.; Guecheva, T.N.; Henriques, J.A.; Brendel, M.; Pungartnik, C.; Rios-Santos, F. DNA Protection against Oxidative Damage Using the Hydroalcoholic Extract of Garcinia mangostana and Alpha-Mangostin. Evid.-Based Complement. Altern. Med. 2016, 2016, 3430405. [Google Scholar] [CrossRef]
- Singh Gill, T.J.; Dewi Ratnayanti, I.G.A.; Nyoman Arijana, I.G.K. The effect of purple mangosteen (Garcinia mangostana) peel extract on collagen fiber in male Wistar rats after Ultraviolet-B (UV-B) exposure. Intisari Sains Medis 2018, 9, 131–134. [Google Scholar]
- Abdi, D.A.; Massi, N.; Djawad, K.; Vitayani, S.; Nurmadilla, N. Effect of Photoprotective Cream of Mangosteen Pericarp Extract (Garcinia Mongostana L) Against 8-OHdG After UVB Exposure On Albino Mice. Indian J. Forensic Med. Toxicol. 2021, 15, 2240–2244. [Google Scholar] [CrossRef]
- Im, A.; Kim, Y.; Chin, Y.; Chae, S. Protective effects of compounds from Garcinia mangostana L. (mangosteen) against UVB damage in HaCaT cells and hairless mice. Int. J. Mol. Med. 2017, 40, 1941–1949. [Google Scholar] [CrossRef][Green Version]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar] [CrossRef]
- Kongkiatpaiboon, S.; Vongsak, B.; Machana, S.; Weerakul, T.; Pattarapanich, C. Simultaneous HPLC quantitative analysis of mangostin derivatives in Tetragonula pagdeni propolis extracts. J. King Saud Univ.-Sci. 2016, 28, 131–135. [Google Scholar] [CrossRef]
- Madihah, A.; Bohari, Y.; Azwan, M.L. A study on dispersion and characterisation of α-mangostin loaded pH sensitive microgel systems. Chem. Cent. J. 2013, 7, 85. [Google Scholar]
- Sang-aroon, W.; Tontapha, S.; Promgool, T.; Amornkitbamrung, V.; Kanokmdehakul, S. Effects of dye-adsorption solvents, acidification and dye combination on efficiency of DSSCs sensitized by α-mangostin and anthocyanin from mangosteen pericarp. J. Mater. Sci. Mater. Electron. 2017, 337, 1419–1428. [Google Scholar]
- Wang, X.; Hong, H.; Wu, J. Hen collagen hydrolysate alleviates UVA-induced damage in human dermal fibroblasts. J. Funct. Foods 2019, 63, 103574. [Google Scholar] [CrossRef]
- Betul, K.; Isabella, Z.; Nicolle, B.; Tobias, J.; Annika, H.; Buket, A.; Schroeder, P.; Chondrogianni, N.; Gonos, E.S.; Isabelle, P.; et al. The Proteasome Is an Integral Part of Solar Ultraviolet A Radiation-induced Gene Expression. J. Biol. Chem. 2009, 284, 30076–30086. [Google Scholar]
- Tiraravesit, N.; Yakaew, S.; Rukchay, R.; Luangbudnark, W.; Viennet, C.; Humbert, P.; Viyoch, J. Artocarpus altilis heartwood extract protects skin against UVB in vitro and in vivo. J. Ethnopharmacol. 2015, 4, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Katiyar, S. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Ihsanpuro, S.I.; Gunawan, S.; Ibrahim, R.; Aparamarta, H.W. Extract with high 1,1-diphenyl-2-picrylhydrazyl (DPPH) inhibitory capability from pericarp and seed of mangosteen (Garcinia mangostana L.) using microwave-assisted extraction (MAE) two-phase solvent technique. Arab. J. Chem. 2022, 15, 104310. [Google Scholar] [CrossRef]
- Tjahjani, S.; Widowati, W.; Khiong, K.; Suhendra, A.; Tjokropranoto, R. Antioxidant Properties of Garcinia mangostana L (Mangosteen) Rind. Procedia Chem. 2014, 13, 198–203. [Google Scholar] [CrossRef]
- Qin, Z.; Robichaud, P.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts. PLoS ONE 2014, 9, e115402. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Konstantinou, A.; Kletsas, D. Long-term exposure to TNF-α leads human skin fibroblasts to a p38 MAPK- and ROS-mediated premature senescence. Biogerontology 2018, 19, 237–249. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.-H. Protocatechuic Aldehyde Attenuates UVA-induced Photoaging in Human Dermal Fibroblast Cells by Suppressing MAPKs/AP-1 and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef]
- Wang, X.; Bi, Z.; Chu, W.; Wan, Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int. J. Mol. Med. 2005, 16, 6, 1117–1124. [Google Scholar] [CrossRef]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Harada, E.; Miki, A.; Tsukamoto, K.; Liang, S.Q.; Yamahara, J.; Murakami, N. Antioxidant constituents from the fruit hulls of mangosteen (Garcinia mangostana L.) originating in Vietnam. Yakugaku Zasshi 1994, 114, 129–133. [Google Scholar] [CrossRef]
- Wittenauer, J.; Falk, S.; Schweiggert-Weisz, U.; Carle, R. Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC–DAD–MSn. Food Chem. 2012, 134, 445–452. [Google Scholar] [CrossRef]
- Aisha, A.F.; Abu-Salah, K.M.; Siddiqui, M.J.; Ismail, Z.; Majid, A.M.S.A. Quantification of α-, β-and γ-mangostin in Garcinia mangostana fruit rind extracts by a reverse phase high performance liquid chromatography. J. Med. Plant Res. 2012, 6, 4526–4534. [Google Scholar]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia 2007, 78, 401–408. [Google Scholar] [CrossRef]
- Mohan, S.; Syam, S.; Abdelwahab, S.I.; Thangavel, N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: An in silico, in vitro and in vivo approach. Food Funct. 2018, 9, 3860–3871. [Google Scholar] [CrossRef]
- Jin, J.; Bao, Y.; Wang, Y.; Zheng, H.; Guo, H.; Zhang, L.; Guo, R.; Yang, L. Protective Activity of Alpha-Mangostin against UVB-Induced Injury in HaCaT Cells by Modulating the Ceramide and MAPK and NF-κB Signaling Pathways. J. Food Biochem. 2023, 2023, 1–11. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Patruno, A.; Pasqualone, L.; Carlucci, G.; Ferrone, V.; Carlucci, M.; de Lutiis, M.A.; Grilli, A.; et al. A Novel Biological Role of α-Mangostin in Modulating Inflammatory Response Through the Activation of SIRT-1 Signaling Pathway. J. Cell. Physiol. 2016, 231, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).