Abstract
Rosacea is a chronic inflammatory skin condition characterized by persistent erythema and telangiectasia, often accompanied by skin barrier disruption and abnormal angiogenesis. Currently, peptide-based therapies for rosacea are limited, and existing drugs still present certain limitations and side effects. Peptides have the advantage of being relatively safe and exhibiting high target specificity, which can reduce the risk of adverse effects. Considering these points, this study aimed to explore the adhesive peptide AdhPep3 (AYDPGYK) as a potential therapeutic candidate for rosacea. AdhPep3 was designed based on protein sequences with cell junction properties and has the potential to enhance skin barrier-related protein expression by improving cell–cell adhesion and increasing adhesion-related protein levels. In LL-37-stimulated HaCaT cells, AdhPep3 effectively alleviated skin inflammation and inhibited the Toll-like receptor–nuclear factor kappa B (TLR2–NFκB) signaling pathway. Additionally, in LL-37-stimulated human umbilical vein endothelial cells (HUVECs), it reduced cell migration and the expression of angiogenesis-related proteins. Since AdhPep3 demonstrated anti-inflammatory and anti-angiogenic effects at the in vitro level, it may serve as a potential therapeutic agent for rosacea. Moreover, by increasing the expression of skin barrier and tight junction-related proteins, AdhPep3 shows potential for development as a cosmetic ingredient to improve skin health.
1. Introduction
Rosacea is a chronic inflammatory skin disease that causes erythema, inflammatory papules, and pustules on the central part of the face, affecting approximately 5.5% of the global population [1,2,3]. Rosacea is classified into four types: erythematotelangiectatic rosacea (ETR), papulopustular rosacea, phymatous rosacea, and ocular rosacea. Of these, ETR is the most predominant, characterized by persistent flushing and vasodilation [3]. Symptoms can vary from person to person. In addition to physical discomfort, patients with rosacea experience psychological stress due to the negative impact on appearance, significantly reducing their quality of life [2].
The exact pathological cause of rosacea is unclear, but various internal and external factors, such as heat, ultraviolet (UV) rays, drugs, spicy foods, and genetic factors, have been implicated in the development of this disease [2,4]. Rosacea is primarily caused by an excessive immune response to external factors, including pathogenic organisms such as Demodex, which activate the Toll-like receptor 2 (TLR2) [2,3]. This leads to the overexpression of the antimicrobial peptide LL-37 via the activation of kallikrein-5 (KLK5), stimulating the nuclear factor kappa B (NF-κB) pathway and inducing the secretion of inflammatory cytokines, such as interleukins (IL)-1β and -6, as well as tumor necrosis factor-alpha (TNF-α). These cytokines increase the expression of chemokines, such as IL-8 and vascular endothelial growth factor (VEGF), promoting inflammatory responses and angiogenesis [2,4,5,6]. Moderate levels of LL-37 protect the skin from external irritants, such as bacteria [7,8,9]. However, overexpressed LL-37 binds to TLR2 via a positive feedback loop involving mTORC1, thereby accelerating NF-κB activation downstream of TLR2 [10]. Additionally, LL-37 acts on receptors including P2X purinoceptor 7 (P2X7), epidermal growth factor receptor (EGFR), and formyl peptide receptor 2 (FPR2) through autocrine and paracrine signaling, activating the c-Jun N-terminal kinase (JNK), protein kinase B (AKT), and extracellular signal-regulated kinase (ERK) pathways. These pathways induce the release of additional proinflammatory cytokines and chemokines, amplifying the inflammatory response [8,9,11,12]. These mechanisms exacerbate skin inflammation and vasodilation, the main symptoms of rosacea, weakening the skin barrier [13]. In particular, the proliferation and differentiation of epidermal cells are accelerated, resulting in the formation of a functionally deficient stratum corneum, which impairs the ability of the skin to absorb and retain water. This decreases the moisture content of the skin, deteriorating the skin barrier [13].
Current treatments, primarily focused on inhibiting inflammation and vasodilation, have several limitations. For example, doxycycline, metronidazole, and tetracycline are effective in treating papules and pustules but have limited efficacy in reducing flushing [14]. Furthermore, as antibiotic-based therapies, their long-term use may cause antibiotic resistance and disrupt the skin microbiome [15,16,17,18]. Additionally, light-based treatments such as pulsed dye laser and intense pulsed light are effective for managing flushing and telangiectasia but show limited efficacy for papules and pustules [14]. Topical retinoid medications also reduce inflammation but can cause skin irritation and dryness, making them unsuitable for patients with sensitive skin [18]. Therefore, it would be highly beneficial to develop an effective therapeutic agent that can simultaneously suppress inflammation to treat papules and pustules, inhibit angiogenesis to manage flushing, and minimize potential side effects. Recently, biologically active peptide-based drugs have attracted increasing attention in areas such as cosmetics and immunology for their ability to target processes like inflammation and angiogenesis [19,20]. These peptides offer high target specificity, which helps reduce side effects [21], and exhibit good biocompatibility and low immunogenicity, making them safer than some existing topical treatments [22].
In this study, we evaluated the therapeutic properties of an adhesive peptide, AdhPep3 (AYDPGYK), as a potential candidate for rosacea treatment. This peptide was designed based on the sequence of MFP1 (Mussel foot protein-1), a well-known mussel adhesive protein found in approximately 20 species of mussels. MFP1, which has a molecular weight of approximately 100–130 kDa [23], is the key protein that imparts strong adhesive properties to mussels even in extreme marine environments [24,25]. After careful analysis, we observed that the AYDPGYK sequence is repeated multiple times in the MFP1 of Geukensia demissa, a type of mussel. Given the important role this sequence is expected to play in the adhesive properties of MFP1, we used it as the basis for designing an adhesive peptide [23,26]. Based on our previous research demonstrating that AdhPep3 increases tight junction proteins in keratinocytes, we hypothesized that this peptide could also alleviate inflammation and inhibit angiogenesis, thereby improving rosacea symptoms while restoring the impaired skin barrier. Therefore, in the following experiments, we investigated whether AdhPep3 could alleviate inflammation and angiogenesis in keratinocytes and vascular endothelial cells induced by LL-37.
2. Materials and Methods
2.1. Reagents
Human cathelicidin LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) was purchased from Selleckchem (Houston, TX, USA), with a purity of 99%, as confirmed by high-performance liquid chromatography (HPLC) analysis.
2.2. Sample Preparation (Peptide Synthesis)
Adhesive peptide 1, Adhesive peptide 2, and Adhesive peptide 3 were synthesized and purchased from Peptron (Daejeon, Republic of Korea). AdhPep1 and AdhPep2 were prepared at a concentration of 1 mg/mL, with a purity of 97% and 96%, respectively, as confirmed by HPLC. AdhPep3 was prepared at a concentration of 10 mg/mL with a purity of 97%, also confirmed by HPLC. The sequence of the Adhesive peptide is shown in Table 1. Detailed information on each peptide’s molecular weight, solubility, HPLC analysis, and mass spectrometry analysis is provided in the Supplementary Materials.

Table 1.
Adhesive peptide family sequences.
2.3. Cell Culture and Treatment
The human immortalized keratinocyte cell line HaCaT was purchased from CLS Cell Lines Service GmbH (Eppelheim, Heidelberg, Baden-Württemberg, Germany). HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Welgene, Daegu, Republic of Korea) supplemented with 10% fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin/streptomycin (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified incubator at 37 °C with 5% CO2 (BB150, Thermo Fisher Scientific, Inc., Waltham, MA, USA). These cells were used for cell viability assays, ELISA, RT-qPCR, and Western blotting. For each experiment, cells were pretreated with an adhesive peptide (1 nM–10 µM), doxycycline (Sigma-Aldrich, Saint Louis, MO, USA), or metronidazole (Sigma-Aldrich, MO, USA) for 2 h, followed by treatment with LL-37 (8 µM). The cells were then incubated for 24 h under standard culture conditions (37 °C, 5% CO2).
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Basel, Switzerland). HUVECs were cultured in Endothelial Cell Growth Medium-2 BulletKit™ (Lonza, Switzerland), which consists of the EBM-2 basal medium supplemented with FBS, hydrocortisone, human fibroblast growth factor-B (hFGF-B), vascular endothelial growth factor (VEGF), R3-insulin-like growth factor-1 (R3-IGF-1), ascorbic acid, human epidermal growth factor (hEGF), GA-1000, and heparin. The cells were incubated at 37 °C and 5% CO2. HUVECs were used for cell viability assays, an enzyme-linked immunosorbent assay (ELISA), Western blotting, immunocytochemistry (ICC), and other assays, including scratch migration and tube formation. In each experiment, HUVECs were pretreated with AdhPep3 (1 nM–10 µM) for 2 h, followed by treatment with LL-37 (4 µM), and then incubated for 24 h at 37 °C in a 5% CO2 incubator.
2.4. Cell Viability Assay
The cytotoxic effects of LL-37 and AdhPep3 on the HaCaT cells and HUVECs were assessed using cell viability assays. Both cell types were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated for 24 h. The cells were then treated with either 1–20 µM LL-37 or 1 nM–10 µM AdhPep3 and then incubated for 24 h. Then, the Ez-Cytox reagent (DoGenBio, Seoul, Republic of Korea) was added to each well, and the cells were incubated for an additional 2 h. Absorbance was measured at 450 nm using a Synergy H1 microplate reader (Bio Tek, Agilent, Santa Clara, CA, USA) to evaluate cell viability.
2.5. ELISA
HaCaT cells (1 × 105 cells/well) were seeded in six-well plates and incubated for 24 h. After incubation, the cells were pretreated with AdhPep1,2 (100 nM), AdhPep3 (1 nM–10 µM), doxycycline (2 µg/mL, 20 µg/mL), and metronidazole (2 µg/mL, 20 µg/mL) for 2 h, followed by LL-37 (8 µM) and incubated for 24 h. After incubation, the cell supernatants were harvested and centrifuged at 3000 RPM for 7 min at 4 °C. The clarified supernatants were used to quantify the TNF-α, IL-1β, IL-6, VEGF, and IL-8 levels using the respective ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
HUVECs (1 × 105 cells/well) were seeded in six-well plates and incubated for 24 h. The cells were pretreated with AdhPep3 (100 nM, 300 nM) for 2 h and then treated with LL-37 (4 µM) for 24 h. After incubation, the supernatants were collected and processed under the same conditions (3000 RPM, 7 min, 4 °C). The VEGF, IL-6, and IL-8 levels in the supernatants were measured using ELISA kits (R&D Systems, USA) according to the manufacturer’s instructions.
2.6. Real-Time Quantitative PCR Analysis
HaCaT cells (1 × 105 cells/well) were seeded in six-well plates and incubated for 24 h. After incubation, the cells were treated with AdhPep3 for 2 h, followed by LL-37 (8 µM) for 24 h. Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and its concentration was determined using a microspectrophotometer. Reverse transcription was performed using a first-strand cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA) to generate cDNA templates. For qRT-PCR, 10 μL of 5X HOT FIREPol EvaGreen qPCR Supermix (SolisBioDyne, Tartu, Estonia) was used for amplification in the StepOnePlus real-time PCR system. Each reaction contained 1 μL each of the forward and reverse primers, 1 μL of the cDNA template, and 7 μL of nuclease-free water. The amplification protocol was as follows: initial denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 15 s. The final melting curve was analyzed using the following conditions: 95 °C for 30 s, 60 °C for 30 s, and 95 °C for 15 s. The relative expression levels of the target genes were calculated using the 2−ΔΔCT method, with GAPDH serving as the internal reference gene. The primers used for RT-PCR are listed in Table 2.

Table 2.
Primer sequences of the genes encoding the rosacea-related cytokine and protein.
2.7. Western Blot Analysis
The HaCaT cells (1 × 105 cells/well) and HUVECs (1 × 105 cells/well) were seeded in six-well plates and incubated for 24 h. After incubation, the cells were pretreated with AdhPep3 for 2 h. For HaCaT cells, doxycycline (2 µg/mL, 20 µg/mL) and metronidazole (2 µg/mL, 20 µg/mL) were also added during the pretreatment. This was followed by treatment with LL-37 (8 µM for HaCaT and 4 µM for HUVECs). In HaCaT cells, Western blot analysis was performed at multiple time points (10 min, 30 min, 1 h, 2 h, and 4 h) after LL-37 treatment. Following the treatment, the cells were washed twice with DPBS (Welgene, Daegu, Republic of Korea) and lysed using RIPA buffer (Biosesang, Seongnam, Republic of Korea) supplemented with a protease inhibitor cocktail (Roche, Basel, Switzerland) to extract the proteins. The cell lysates were centrifuged at 13,000 RPM for 20 min at 4 °C. The total protein concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
For Western blotting, 40 µg of total protein was heated at 95 °C for 10 min and then loaded onto vertical 12% Mini-PROTEAN® TGX™ Precast Protein gels (BIO-RAD, Hercules, CA, USA) along with a prestained protein ladder for electrophoresis. The proteins were transferred to a polyvinylidene difluoride (PVDF) membrane using the iBlot™ transfer stack and the iBlot™ 2 gel transfer device (Invitrogen, Carlsbad, CA, USA) at 25 V for 5 min. The PVDF membrane was blocked with TBS-T buffer containing 0.1% Tween-20 and 5% skim milk for 1 h and then washed thrice using TBS-T and once with TBS. Subsequently, the membrane was incubated overnight at 4 °C with primary antibodies against TLR2, KLK5, MMP9, NF-κB (p65), p-NF-κB (p-p65), NF-κB (p50), p-NF-κB (p-p50), IκB-α, p-IκB-α, AKT, p-AKT, ERK1/2, p-ERK1/2, JNK, p-JNK, Involucrin, Claudin-4, Claudin-1, Filaggrin, Occludin, VEGF-A, transforming growth factor beta (TGF-β), PECAM (CD31), and β-actin (Santa Cruz, Dallas, Texas, USA). After washing, the membranes were incubated with secondary antibodies for 2 h at room temperature and then treated with the Clarity™ Western ECL substrate (BIO-RAD, Hercules, CA, USA) for 30 s. Images were obtained using the Amersham Imager 680 system (GE Healthcare Life Sciences, Chicago, IL, USA).
2.8. Immunocytochemistry
HUVECs (5 × 104 cells per well) were seeded on disk inserts (SPL Life Sciences, Gyeonggi-do, Republic of Korea) in 24-well plates. After 24 h of incubation at 37 °C, the cells were treated with LL-37 (4 µM) and AdhPep3 (100 nM and 300 nM) for 16 h. Then, the cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, MO, USA) for 10 min.
The cells were blocked in 1% BSA (Thermo Fisher Scientific, Inc., Waltham, MA, USA) in PBS for 1 h. Then, the cells were treated with the CD31 primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h at room temperature. After washing with PBS, the cells were incubated with the Alexa Fluor 488-conjugated secondary antibody (Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2 h in the dark. Nuclei were counterstained with DAPI (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The fluorescence images of the CD31-expressing cells were captured using a Nikon fluorescence microscope (Nikon, Tokyo, Japan).
2.9. Scratch Migration Assay
HUVECs (2 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h. Once the cells reached 100% confluence, a straight scratch was made across the cell monolayer using a 100 µL pipette tip. The wells were washed thrice with DPBS to remove cell debris. Then, the cells were pretreated with AdhPep3 (100 nM and 300 nM) for 2 h, followed by LL-37 (4 µM) and then incubated at 37 °C for 16 h. After incubation, the extent of cell migration was assessed by capturing random images of the scratch areas using a microscope. The migration distance was quantified using the ImageJ version 1.3 software.
2.10. Tube Formation Assay
The effect of AdhPep3 on angiogenesis in HUVECs was assessed using a tube formation assay. A 96-well plate was first coated with 50 µL of Geltrex (Thermo Fisher Scientific, Inc., Waltham, MA, USA) per well and incubated at 37 °C for 30 min to allow gel polymerization. HUVECs (2 × 104 cells/well) were seeded in the medium containing LL-37 (4 µM) or AdhPep3 (100 nM and 300 nM) and then incubated for 16 h at 37 °C. After incubation, tube formation, including the structure and amount of tube-like networks, was evaluated using a microscope. Randomly selected images were used to analyze and quantify the number of nodes and master junctions with the ImageJ software.
2.11. Statistical Analysis
All statistical analyses and figure generation were conducted using GraphPad Prism 9 (GraphPad Software, Boston, MA, USA). Data are presented as the mean ± the standard deviation (SD) from at least three independent experiments. Statistical significance between groups was assessed using one-way ANOVA. A p-value < 0.05 was considered statistically significant and was indicated using asterisks (*) in the figures for group comparisons (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). The #, ##, ###, and #### signs indicate comparisons against the non-treated (NT) group.
3. Results
3.1. Screening and Selection of Adhesive Peptides and Cytotoxicity Assessment in HaCaT Cells
We designed three adhesive peptides (Table 1) and screened them using HaCaT cells. The cells were pretreated with three peptides at the same concentration of 100 nM and then induced with LL-37 (8 μM). Among them, only AdhPep 3 (ADYPGYK) reduced skin inflammation-related cytokines and angiogenesis factors, including VEGF, IL-6, and TNF-α. (Figure 1A) Therefore, AdhPep3 was selected as the candidate therapeutic peptide for rosacea. Its structure is shown in Figure 1B.
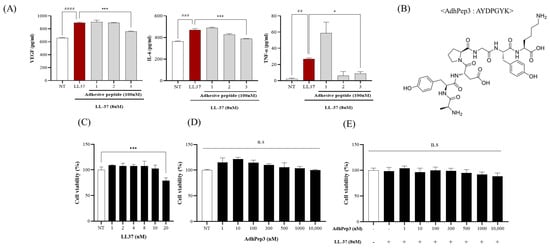
Figure 1.
Screening and selection of adhesive peptides and cytotoxicity assessment in HaCaT cells. (A) HaCaT cells were pretreated with the designed adhesive peptides (100 nM) for 2 h and then treated with LL-37 (8 µM) for 24 h. Subsequently, the rosacea inflammation markers (VEGF, IL-6, and TNF-α) were measured by ELISA. (B) The structure of AdhPep3. Cell viability of HaCaT cells treated with different concentrations of (C) LL-37 (1 µM to 20 µM), (D) AdhPep3 (1 nM to 10 µM), and (E) LL37 (8 µM) in combination with different concentrations of AdhPep3 (1 nM to 10 µM). *, p < 0.01; ***, p < 0.001; ##, p < 0.01; ###, p < 0.001; and ####, p < 0.0001 compared to the LL-37 only group, as determined by one-way ANOVA. n.s. indicates no significant difference (p > 0.05).
The cytotoxicity of LL-37 and AdhPep3 on HaCaT cells was evaluated using the WST-1 assay. Previous studies have shown that 8 µM LL-37 induces rosacea without exerting any significant toxicity [29,30]. Therefore, this concentration was used to induce the cells (Figure 1C). HaCaT cells treated with AdhPep3 (1 nM–10 µM) showed modest proliferation without any toxicity, even at concentrations as high as 10 µM (Figure 1D). Furthermore, as the treatment with LL-37 (8 µM) and AdhPep3 (1 nM to 10 µM) did not show toxicity (Figure 1E), concentrations of LL-37 and AdhPep3 up to 8 µM and 10 µM, respectively, are considered safe for treating HaCaT cells.
3.2. Effect of AdhPep3 on Inflammatory and Angiogenesis Markers Through Cytokine Release and mRNA Expression in LL-37-Induced HaCaT Cells
To study whether AdhPep3 can be used to treat rosacea, we pretreated cells with AdhPep3 for 2 h and then stimulated them with LL-37 to determine the mRNA expression and release profile of the cytokines, chemokines, and markers related to rosacea using RT-qPCR and ELISA, respectively. The results demonstrated that LL-37 (8 µM) significantly increased the secretion of VEGF, TNF-α, IL-1β, IL-6, and IL-8, whereas AdhPep3 reduced their secretion in a concentration-dependent manner (Figure 2A). We analyzed the mRNA expression in the cells treated with 100 nM, 300 nM, and 500 nM of AdhPep3, which were the most effective concentrations. LL-37 increased the mRNA expression of several rosacea markers, including VEGF, TNF-α, IL-1β, IL-6, IL-8, TLR2, and KLK5 (Figure 2B). The AdhPep3 treatment suppressed the mRNA expression of these markers. The mRNA expression levels of these markers in the AdhPep3-only group were similar to those in the NT group (Figure 2B). Based on these results, we selected 100 nM and 300 nM of AdhPep3 for the subsequent experiments.
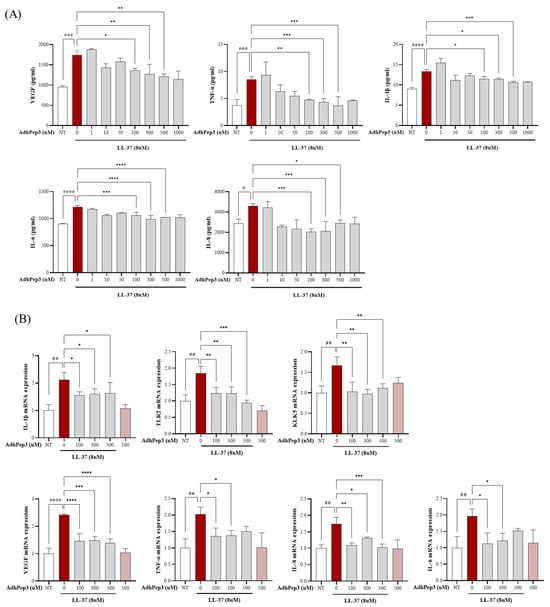
Figure 2.
Effect of AdhPep3 on mRNA expression and release of inflammatory and angiogenesis markers in LL37-induced HaCaT cells. HaCaT cells were pretreated with AdhPep3 at different concentrations for 2 h and then treated with LL37 (8 µM) to measure the rosacea markers. (A) The VEGF, TNF-α, IL-1β, IL-6, and IL-8 levels were measured using ELISA. (B) The mRNA levels of VEGF, TLR2, KLK5, IL-1β, TNF-α, IL-6, and IL-8 were measured using RT-qPCR. *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively, as determined by one-way ANOVA. #, ##, ###, and #### indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001 for comparisons with the group treated with LL-37 alone.
3.3. Effect of AdhPep3 on the Expression of Proteins Related to the TLR2-NFκB and AKT/ERK/JNK Pathways in LL-37-Induced HaCaT Cells
Previous studies have shown that TLR2 is overexpressed in the skin of patients with rosacea. TLR-2 activation upregulates LL-37 expression, which further activates the NFκB, MAPK, and PI3K-Akt signaling pathways to promote proinflammatory cytokine secretion and angiogenesis [2,11,31,32]. Therefore, we analyzed the expression of the rosacea-related proteins using Western blotting. The results showed that LL-37 (8 µM) induced the overexpression of several rosacea markers, including TLR2, MMP9, and KLK5. However, the expression of these markers was significantly suppressed in the cells pretreated with AdhPep3 (Figure 3A). Moreover, LL-37 induced excessive phosphorylation of p65, p50, and IκB-α, which was found to be significantly mitigated in the AdhPep3 pretreatment group. Furthermore, the time points at which AKT, JNK, and ERK were most actively phosphorylated following LL-37 treatment were analyzed using Western blotting. ERK and JNK were actively phosphorylated up to 4 h after LL-37 treatment. AKT phosphorylation was most active at 30 min and remained active after 4 h (Figure 3B). Therefore, the phosphorylation profile of these proteins 4 h after LL-37 and AdhPep3 treatment indicated that the phosphorylation of AKT/ERK/JNK was attenuated in the AdhPep3-treated cells compared to those treated with LL-37 only (Figure 3C).
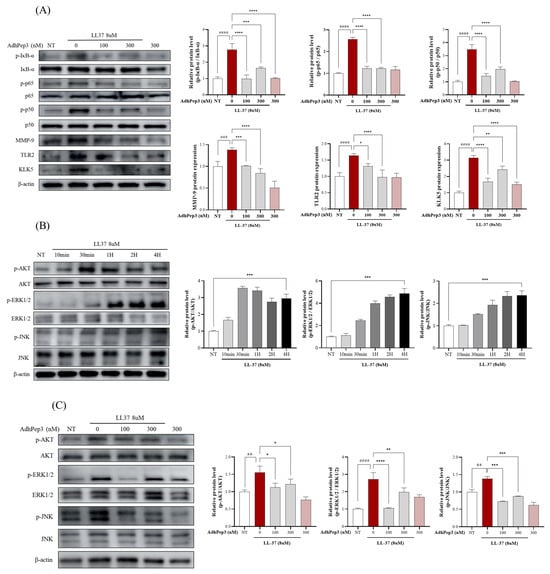
Figure 3.
AdhPep3 regulates the TLR2, AKT, and MAPK (ERK/JNK) pathways in LL37-induced HaCaT cells. HaCaT cells were treated with LL-37 (8 µM) and AdhPep3 (100 nM or 300 nM) for 10 min to 24 h and then lysed. The expression levels of the proteins involved in the (A) TLR2/NF-κB pathway, (B) the MAPK and AKT pathways (at different time intervals ranging from 10 min to 4 h), and (C) the AKT, ERK, and JNK pathways. *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively, as determined by one-way ANOVA. ##, ###, and #### indicate p < 0.01, p < 0.001, and p < 0.0001 for comparisons with the group treated with LL37 alone.
3.4. Effect of AdhPep3 on Angiogenesis Markers in LL-37-Induced HUVECs
The effect of AdhPep3 on the cell viability of HUVECs was assessed after treatment with varying concentrations of LL-37 and AdhPep3. Toxicity was observed after treatment with LL-37 at concentrations beyond 8 µM. Previous studies reported that 4 µM LL-37 can effectively induce active angiogenesis without exerting toxicity [33]. Therefore, we used this concentration for subsequent experiments (Figure 4A). Furthermore, no toxicity was observed in AdhPep3 at concentrations ranging from 1 nM to 10 µM (Figure 4A).
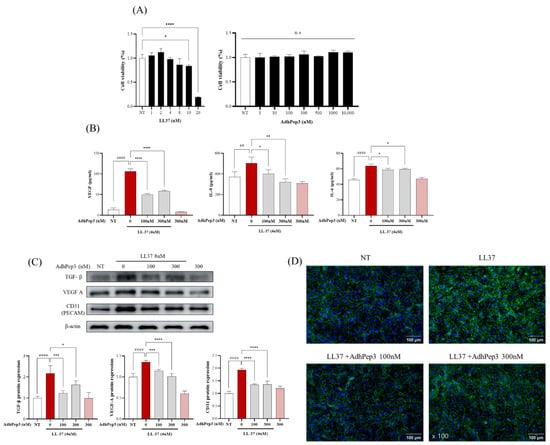
Figure 4.
Effect of AdhPep3 on alleviating angiogenesis in LL-37-induced human umbilical vein endothelial cells (HUVECs). (A) Cell viability of HUVECs treated with different concentrations of LL-37 (1 uM to 20 uM) and AdhPep3 (1 nM to 10 uM). HUVECs were pretreated with 100 nM and 300 nM AdhPep3 for 2 h and then incubated with LL-37 (4 μM) for 24 h to measure the angiogenesis markers. (B) VEGF, IL-8, and IL-6 levels were measured by ELISA. (C) The TGF-β, VEGF-A, and CD31 (PECAM) levels were measured by Western blotting. (D) CD31 expression in HUVECs was assessed using immunocytochemistry. *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively, as determined by one-way ANOVA. ## and #### indicate p < 0.01 and p < 0.0001 for comparisons with the group treated with LL-37 alone.
The anti-angiogenic effect of AdhPep3 in the LL-37-stimulated HUVECs was analyzed by evaluating the cytokines, chemokines, and angiogenesis markers using ELISA, Western blotting, and ICC. The ELISA results showed that cells treated with 4 µM LL-37 exhibited significantly increased levels of VEGF, IL-8, and IL-6, which are angiogenesis-related cytokines and chemokines. Their levels were mitigated after pretreatment with AdhPep3 (100 nM and 300 nM) (Figure 4B). Western blotting results showed that LL-37-treated cells overexpressed the angiogenesis markers TGF-β, VEGF-A, and CD31, while the pretreatment with AdhPep3 significantly reduced their expression levels (Figure 4C). Additionally, ICC analysis revealed that CD31 was overexpressed at the junctions between the LL-37-treated HUVECs, as shown by the strong fluorescence intensity. However, cells pretreated with AdhPep3 exhibited lower fluorescence intensity, indicating that CD31 expression was significantly reduced (Figure 4D). These results indicate that AdhPep3 attenuates angiogenesis in LL-37-induced HUVECs.
3.5. Effect of AdhPep3 on Migration and Tube Formation in LL-37-Induced HUVECs
Angiogenesis is mediated through active migration and tube formation in HUVECs. Therefore, the anti-angiogenic effect of AdhPep3 in LL-37-induced HUVECs was evaluated using the scratch migration and tube formation assays. HUVECs treated with LL-37 (4 µM) showed excessive cell migration compared to the NT group, but the cell migration of HUVECs treated with 100 nM and 300 nM AdhPep3 was reduced (Figure 5A). Similarly, in the tube formation assay, LL-37-treated HUVECs exhibited increased tube forming ability, thereby inducing excessive angiogenesis. However, treatment with AdhPep3 (100 nM and 300 nM) significantly suppressed tube formation in HUVECs (Figure 5B). AdhPep3 alleviated angiogenesis by reducing the number of tubes, master junctions, and nodes, which are indicators of tube formation (Figure 5B), demonstrating its anti-angiogenic effects in LL-37-induced HUVECs.
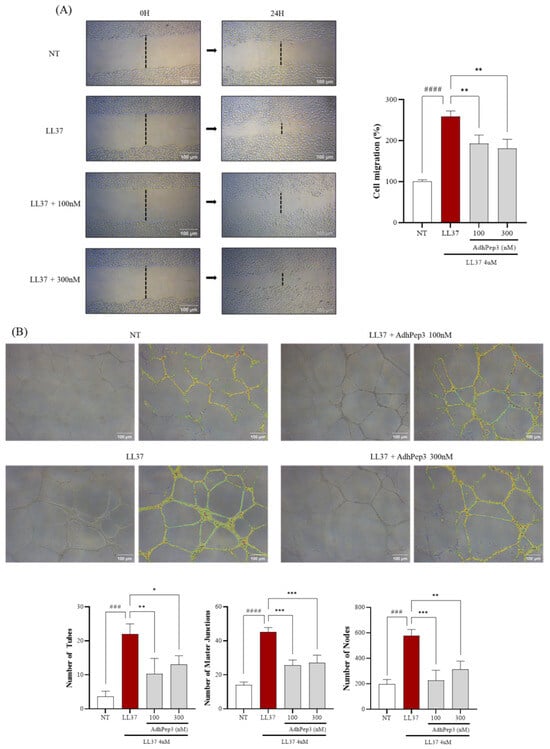
Figure 5.
Effect of AdhPep3 on migration and tube formation in LL37-induced human umbilical vein endothelial cells. To evaluate migration and tube formation, HUVECs were treated with LL-37 (4 μM) and AdhPep3 (100 nM, 300 nM) and observed for 16 h to 24 h. The cell migration and tube formation ability of the HUVECs were evaluated using the (A) scratch migration and (B) tube formation assays, respectively. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively, as determined by one-way ANOVA. ### and #### indicate p < 0.001 and p < 0.0001 for comparisons with the group treated with LL-37 alone.
3.6. Effect of AdhPep3 on the Expression of Proteins Related to Tight Junctions and the Skin Barrier in HaCaT Cells
To investigate whether AdhPep3 has the potential to strengthen the skin barrier and tight junctions, HaCaT cells were treated with various concentrations of AdhPep3 and analyzed using Western blotting. AdhPep3 increased the expression of skin barrier-related proteins (Filaggrin and Involucrin) and tight junction-related proteins (Claudin-1, Claudin-4, and Occludin) in a concentration-dependent manner starting from a low concentration of 10 nM. Notably, at both 100 nM and 300 nM, the expression of these proteins was consistently increased (Figure 6A,B). These results suggest that AdhPep3 has the potential to strengthen the skin barrier in rosacea.
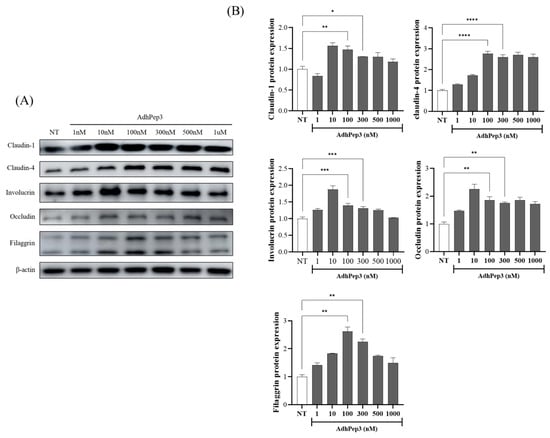
Figure 6.
Effect of AdhPep3 on the expression of proteins related to tight junctions and the skin barrier in HaCaT cells. HaCaT cells were treated with AdhPep3 (1 nM to 1 µM), and 24 h later, the expression of tight junction- and skin barrier-related proteins was measured. (A) Western blotting analysis and (B) quantification of the Claudin-1, Claudin-4, Involucrin, Filaggrin, and Occludin levels. *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively, as determined by one-way ANOVA.
3.7. Comparison of the Rosacea Treatment Effects of Doxycycline, Metronidazole, and AdhPep3 on LL-37-Induced HaCaT Cells
To compare the effects of AdhPep3 with those of doxycycline and metronidazole, which are currently used to treat rosacea, ELISA and Western blotting were performed. Metronidazole reduced the secretion of VEGF, IL-6, IL-8, and TNF-α, whereas doxycycline reduced the secretion of VEGF, IL-8, and TNF-α but had no significant effect on IL-6. Notably, AdhPep3 reduced VEGF, IL-6, IL-8, and TNF-α secretion, demonstrating rosacea treatment effects comparable to those of existing treatments (Figure 7A). Furthermore, similar to doxycycline and metronidazole, AdhPep3 reduced LL37-induced overexpression of TLR2, KLK5, and phosphorylated p65 (Figure 7B). These results indicate that AdhPep3 can reduce inflammation and angiogenesis in rosacea to a degree comparable to that of current treatments.
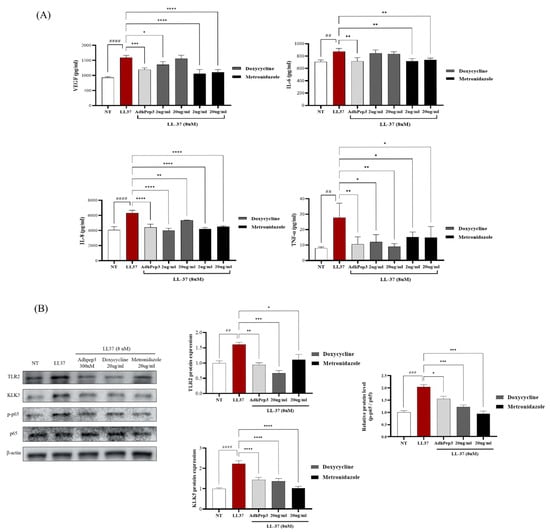
Figure 7.
Comparison of the rosacea treatment effects of doxycycline, metronidazole, and AdhPep3 on LL-37-Induced HaCaT Cells. HaCaT cells were treated with AdhPep3 (300 nM), doxycycline (2 µg/mL, 20 µg/mL), and metronidazole (2 µg/mL, 20 µg/mL) for 2 h, followed by treatment with LL37 (8 µM), and rosacea markers were measured. (A) VEGF, IL-6, IL-8, and TNF-α levels were measured by ELISA. (B) Protein expression levels of TLR2, KLK5, and NF-κB (p65, p-p65) were measured by Western blotting. *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively, as determined by one-way ANOVA. ##, ###, and #### indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001 for comparisons with the group treated with LL37 alone.
4. Discussion
Rosacea is a chronic inflammatory skin disease characterized by redness, which results from vasodilation and angiogenesis, as well as skin inflammation [1]. In this study, we demonstrated that AdhPep3 (AYDPGYK) significantly reduces LL37-induced inflammation and angiogenesis in keratinocytes and vascular endothelial cells. Specifically, AdhPep3 decreased the secretion of proinflammatory cytokines and angiogenesis-related factors while also upregulating skin barrier- and tight junction-related proteins. These findings indicate that AdhPep3 has the potential to simultaneously alleviate rosacea symptoms and improve skin barrier function.
TLR2, which is overexpressed and activated, is commonly found in the skin of rosacea patients. TLR2 activation, in turn, activates the serine protease KLK5 through MMP9-mediated regulation [4]. Activated KLK5 cleaves hCAP18, a precursor of antimicrobial peptides, leading to the excessive production of the antimicrobial peptide LL-37 [4]. Overexpressed LL-37, which is also observed in the skin of rosacea patients, stimulates the NF-κB signaling pathway. This induces the release of proinflammatory cytokines (TNF-α, IL-6, and IL-1β), chemokines (IL-8), and angiogenic factors (VEGF), ultimately resulting in excessive inflammation and angiogenesis [34]. For these reasons and based on previous studies [35], we modeled rosacea skin inflammation by stimulating human keratinocytes (HaCaT cells) with LL-37 and modeled angiogenesis by stimulating human umbilical vein endothelial cells (HUVECs) with LL-37.
Treatment with AdhPep3 in LL-37-induced keratinocytes reduced the overexpression of TLR2, MMP-9, and KLK5. This suggests that AdhPep3 downregulates TLR2, leading to decreased KLK5 production and subsequently reducing the cleavage of hCAP18 into LL-37, thereby preventing LL-37 overexpression. Since the NF-κB signaling pathway drives the release of proinflammatory cytokines, its inhibition is essential for controlling inflammation. AdhPep3 suppressed the phosphorylation of p65, p50, and IκB within the NF-κB pathway, which is activated by excessive LL-37. Moreover, AdhPep3 downregulated the positive feedback loop of LL-37 by reducing TLR2 expression, thereby inhibiting the phosphorylation of AKT, JNK, and ERK1/2. These results indicate that AdhPep3 reduces the paracrine effects of overexpressed LL-37 through other receptors, thereby alleviating additional inflammatory pathways. Increased expression of TNF-α, IL-6, IL-1β, IL-8, and VEGF activates immune cells such as neutrophils, macrophages, and T cells, inducing an excessive immune response that leads to persistent inflammation and angiogenesis [4,12]. In this study, RT-PCR and ELISA analyses showed that AdhPep3 effectively decreased both the mRNA expression and secretion levels of proinflammatory cytokines (TNF-α, IL-6, and IL-1β), chemokines (IL-8), and the angiogenesis-promoting factor VEGF in LL-37-stimulated keratinocytes. These findings indicate that AdhPep3 can effectively reduce inflammation in rosacea by targeting the TLR2-NF-κB signaling pathway in keratinocytes.
Another primary clinical feature of patients with rosacea is dilated blood vessels and active angiogenesis. Overexpressed LL-37 binds to FPRL1 on HUVECs, activating pathways such as ERK1/2, MAPK, and PI3K/AKT, which promote HUVEC proliferation and migration, thereby facilitating angiogenesis [36,37]. When stimulated by LL-37, HUVECs also secrete proangiogenic cytokines and chemokines and overexpress proteins that support blood vessel formation [5]. In this study, treatment with LL-37 increased the secretion of VEGF, IL-8, and IL-6 in HUVECs and elevated the expression of angiogenesis marker proteins including VEGF-A, CD31, and TGF-β. However, AdhPep3 treatment effectively reduced the secretion of VEGF, IL-8, and IL-6, as well as the expression of these marker proteins. This suggests that AdhPep3 can mitigate blood vessel dilation by decreasing angiogenesis-inducing factors in HUVECs. Additionally, AdhPep3 significantly reduced HUVEC migration and inhibited the formation of blood vessel-like structures and abnormal vascular networks. Since cell migration and tube formation are critical indicators of angiogenesis, as new vascular structures arise from endothelial cell movement and arrangement, the inhibition of these processes by AdhPep3 provides strong evidence supporting its angiogenesis-alleviating effect in rosacea.
Patients with various skin diseases, including atopic dermatitis, psoriasis, acne, eczema, and rosacea, suffer from a weakened skin barrier [13,38,39,40]. In particular, excessive inflammation and angiogenesis act synergistically in the skin of patients with rosacea, accelerating epidermal proliferation and differentiation. This leads to the formation of a functionally deficient stratum corneum, further deteriorating the skin barrier [13]. Recent studies have reported that the expression of skin barrier-related proteins, such as Claudin-1, Clauldin-4, and Filaggrin, is reduced in the skin of patients with rosacea [41,42]. We found that AdhPep3 effectively increased the expression of proteins related to the skin barrier (Filaggrin and Involucrin) and tight junction (Claudin-1, Claudin-4, and Occludin) in keratinocytes, which may have the potential to strengthen the skin barrier. Current Rosacea treatments, such as doxycycline and metronidazole, primarily aim to reduce inflammation, which helps treat papules and pustules [14]. However, our results showed that 300 nM AdhPep3 not only alleviated inflammation and angiogenesis at a similar level as 20 µg/mL doxycycline (41 µM) and 20 µg/mL metronidazole (117 µM) but also increased the expression of skin barrier-related proteins. In other words, AdhPep3 showed significant effects at a much lower concentration than existing treatments, suggesting that it has the advantage of having the same effect at a lower dose and improving overall skin health.
Recently, artesunate, artemisinin, EGCG, and melatonin have been studied as potential treatments for rosacea [30,33,35]. Similar to these studies, AdhPep3 demonstrated anti-inflammatory and anti-angiogenic effects and additionally showed potential to improve the skin barrier. Moreover, as a peptide-based material, AdhPep3 offers the advantage and creativity of allowing modification of amino acid sequences to optimize stability and cellular permeability. However, this study has some limitations. Rosacea involves the progression from innate to adaptive immune responses, engaging various immune cells such as mast cells, macrophages, and Th1 and Th17 cells [43,44]. Thus, additional studies using animal models are necessary to comprehensively evaluate the effects of AdhPep3 on rosacea. Also, due to the inherent properties of peptides, the oral administration of AdhPep3 is challenging, and topical application is expected. To be effective, the peptide must penetrate the strong skin barrier without being degraded by factors such as proteases and UV radiation [45]. Therefore, further evaluations of pharmacokinetic properties, including skin absorption, degradation, and stability, are needed. If these aspects are clarified through additional research, AdhPep3 could be a promising candidate for rosacea treatment.
5. Conclusions
In this study, we investigated the therapeutic effects and mechanisms of AdhPep3, an adhesive peptide, on rosacea. In keratinocytes, AdhPep3 attenuated the activation of the TLR2-LL-37-NFκB pathway, significantly reducing the expression and secretion of proinflammatory cytokines and chemokines. Additionally, it effectively inhibited endothelial cell migration and angiogenesis. These findings suggest that AdhPep3 modulates inflammation and angiogenesis in keratinocytes, highlighting its potential as both a therapeutic and cosmetic strategy for rosacea.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics12040143/s1, Figure S1: Adhesive Pep 1 (GRALARG) Analysis Data Sheet; Figure S2: Adhesive Pep 2 (AKPTYK) Analysis Data Sheet; Figure S3: Adhesive Pep 3 (AYDPGYK) Analysis Data Sheet.
Author Contributions
T.Y.K.: Writing—review and editing, writing—original draft, visualization, validation, methodology, investigation, formal analysis, data curation, and conceptualization. J.H.K.: Supervision, validation, and data curation. Y.-J.L.: Supervision, software, and data curation. M.J.S.: Software and formal analysis. H.H.P.: Software and formal analysis. J.H.C.: Validation, supervision, project administration, methodology, investigation, formal analysis, and conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported from the Korean Society of Cardiology (Grant No. 201201-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to express our sincere gratitude to Ji Hyung Chung at CHA University for his invaluable guidance and support throughout this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fisher, G.W.; Travers, J.B.; Rohan, C.A. Rosacea pathogenesis and therapeutics: Current treatments and a look at future targets. Front. Med. 2023, 10, 1292722. [Google Scholar] [CrossRef]
- Rainer, B.M.; Kang, S.; Chien, A.L. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinology 2017, 9, e1361574. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schauber, J.; Leyden, J.J. New insights into rosacea pathophysiology: A review of recent findings. J. Am. Acad. Dermatol. 2013, 69, S15–S26. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Zhang, L.; Liu, X.; Zhang, H.; Cao, Y.; Wang, X.; Zeng, Q. Exploring the Pathogenesis and Mechanism-Targeted Treatments of Rosacea: Previous Understanding and Updates. Biomedicines 2023, 11, 2153. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int. J. Mol. Sci. 2021, 22, 12035. [Google Scholar] [CrossRef]
- Steinhoff, M.; Buddenkotte, J.; Aubert, J.; Sulk, M.; Novak, P.; Schwab, V.D.; Mess, C.; Cevikbas, F.; Rivier, M.; Carlavan, I.; et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosaceaJ. Investig. Dermatol. Symp. Proc. 2011, 15, 2–11. [Google Scholar] [CrossRef]
- Moreno-Angarita, A.; Aragón, C.C.; Tobón, G.J. Cathelicidin LL-37: A new important molecule in the pathophysiology of systemic lupus erythematosus. J. Transl. Autoimmun. 2020, 3, 100029. [Google Scholar] [CrossRef]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef]
- Verjans, E.T.; Zels, S.; Luyten, W.; Landuyt, B.; Schoofs, L. Molecular mechanisms of LL-37-induced receptor activation: An overview. Peptides 2016, 85, 16–26. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Liu, Y.; Xu, S.; Ouyang, Y.; Shi, W.; Jian, D.; Wang, B.; Liu, F.; Li, J.; et al. A positive feedback loop between mTORC1 and cathelicidin promotes skin inflammation in rosacea. EMBO Mol. Med. 2021, 13, e13560. [Google Scholar] [CrossRef]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef]
- Yang, F.; Wang, L.; Song, D.; Zhang, L.; Wang, X.; Du, D.; Jiang, X. Signaling pathways and targeted therapy for rosacea. Front. Immunol. 2024, 15, 1367994. [Google Scholar] [CrossRef]
- Addor, F.A. Skin barrier in rosacea. An. Bras. Dermatol. 2016, 91, 59–63. [Google Scholar] [CrossRef]
- Thiboutot, D.; Anderson, R.; Cook-Bolden, F.; Draelos, Z.; Gallo, R.L.; Granstein, R.D.; Kang, S.; Macsai, M.; Gold, L.S.; Tan, J. Standard management options for rosacea: The 2019 update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2020, 82, 1501–1510. [Google Scholar] [CrossRef]
- Cardwell, L.A.; Alinia, H.; Moradi Tuchayi, S.; Feldman, S.R. New developments in the treatment of rosacea—Role of once-daily ivermectin cream. Clin. Cosmet. Investig. Dermatol. 2016, 9, 71–77. [Google Scholar] [CrossRef]
- Engin, B.; Özkoca, D.; Kutlubay, Z.; Serdaroğlu, S. Conventional and Novel Treatment Modalities in Rosacea. Clin. Cosmet. Investig. Dermatol. 2020, 13, 179–186. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M.; Johnson, J.L.; Graber, E.M. A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics 2021, 10, 757. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Gonçalves, T.; Peixoto, D.; Pires, P.C.; Velsankar, K.; Jha, N.K.; Chavda, V.P.; Mohammad, I.S.; Cefali, L.C.; Mazzola, P.G.; et al. Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems. Mol. Pharm. 2023, 20, 3804–3828. [Google Scholar] [CrossRef]
- Lima, T.N.; Pedriali Moraes, C.A. Bioactive Peptides: Applications and Relevance for Cosmeceuticals. Cosmetics 2018, 5, 21. [Google Scholar] [CrossRef]
- Zhang, L.; Falla, T.J. Cosmeceuticals and peptides. Clin. Dermatol. 2009, 27, 485–494. [Google Scholar] [CrossRef]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Sachdeva, S. Peptides as ‘drugs’: The journey so far. Int. J. Pept. Res. Ther. 2017, 23, 49–60. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Waite, J.H. Mussel-designed protective coatings for compliant substrates. J. Dent. Res. 2008, 87, 701–709. [Google Scholar] [CrossRef]
- Deacon, M.P.; Davis, S.S.; Waite, J.H.; Harding, S.E. Structure and Mucoadhesion of Mussel Glue Protein in Dilute Solution. Biochemistry 1998, 37, 14108–14112. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Laursen, R.A. Reflections on the structure of mussel adhesive proteins. Results Probl. Cell Differ. 1992, 19, 55–74. [Google Scholar]
- Sengupta, D.; Heilshorn, S.C. Protein-engineered biomaterials: Highly tunable tissue engineering scaffolds. Tissue Eng. Part B Rev. 2010, 16, 285–293. [Google Scholar] [CrossRef]
- Straley, K.S.; Heilshorn, S.C. Design and adsorption of modular engineered proteins to prepare customized, neuron-compatible coatings. Front. Neuroeng. 2009, 2, 9. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Wang, H.; Zhu, P.; Jiang, S.; Qi, R.; Wu, Y.; Gao, X. Activation of aryl hydrocarbon receptor ameliorates rosacea-like eruptions in mice and suppresses the TLR signaling pathway in LL-37-induced HaCaT cells. Toxicol. Appl. Pharmacol. 2022, 451, 116189. [Google Scholar] [CrossRef]
- Zhou, L.; Zhong, Y.; Wang, Y.; Deng, Z.; Huang, Y.; Wang, Q.; Xie, H.; Zhang, Y.; Li, J. EGCG identified as an autophagy inducer for rosacea therapy. Front. Pharmacol. 2023, 14, 1092473. [Google Scholar] [CrossRef]
- Ikutama, R.; Peng, G.; Tsukamoto, S.; Umehara, Y.; Trujillo-Paez, J.V.; Yue, H.; Nguyen, H.L.T.; Takahashi, M.; Kageyama, S.; Komatsu, M.; et al. Cathelicidin LL-37 Activates Human Keratinocyte Autophagy through the P2X7, Mechanistic Target of Rapamycin, and MAPK Pathways. J. Investig. Dermatol. 2023, 143, 751–761.e757. [Google Scholar] [CrossRef]
- Tang, S.; Hu, H.; Li, M.; Zhang, K.; Wu, Q.; Liu, X.; Wu, L.; Yu, B.; Chen, X. OPN promotes pro-inflammatory cytokine expression via ERK/JNK pathway and M1 macrophage polarization in Rosacea. Front. Immunol. 2023, 14, 1285951. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Tang, Y.; Zuo, Z.; Zhang, Y.; Xie, H. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed. Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, Y.; Zhang, Z.; Liu, H.; Xu, H.; Cai, W.; Gao, X.; Yang, J. Long-Term Administration of LL-37 Can Induce Irreversible Rosacea-like Lesion. Curr. Issues Mol. Biol. 2023, 45, 2703–2716. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Li, Y.; Wang, Y.; Yan, S.; Xu, S.; Deng, Z.; Yang, X.; Xie, H.; Li, J. Bioinformatics and Network Pharmacology Identify the Therapeutic Role and Potential Mechanism of Melatonin in AD and Rosacea. Front. Immunol. 2021, 12, 756550. [Google Scholar] [CrossRef]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krötz, F.; Zahler, S.; Gloe, T.; Issbrücker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, M.-S.; Lee, H.Y.; Kim, S.D.; Shim, J.W.; Jo, S.H.; Lee, J.W.; Kim, J.Y.; Choi, Y.-W.; Baek, S.-H.; et al. F2L, a peptide derived from heme-binding protein, inhibits LL-37-induced cell proliferation and tube formation in human umbilical vein endothelial cells. FEBS Lett. 2008, 582, 273–278. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Proksch, E.; Fölster-Holst, R.; Jensen, J.-M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Xie, H.; Jian, D.; Xu, S.; Peng, Q.; Sha, K.; Liu, Y.; Zhang, Y.; Shi, W.; et al. Claudin reduction may relate to an impaired skin barrier in rosacea. J. Dermatol. 2019, 46, 314–321. [Google Scholar] [CrossRef]
- Medgyesi, B.; Dajnoki, Z.; Béke, G.; Gáspár, K.; Szabó, I.L.; Janka, E.A.; Póliska, S.; Hendrik, Z.; Méhes, G.; Törőcsik, D.; et al. Rosacea Is Characterized by a Profoundly Diminished Skin Barrier. J. Investig. Dermatol. 2020, 140, 1938–1950.e1935. [Google Scholar] [CrossRef]
- Tu, K.Y.; Jung, C.J.; Shih, Y.H.; Chang, A.L.S. Therapeutic strategies focusing on immune dysregulation and neuroinflammation in rosacea. Front. Immunol. 2024, 15, 1403798. [Google Scholar] [CrossRef]
- Wladis, E.J.; Adam, A.P. Immune signaling in rosacea. Ocul. Surf. 2021, 22, 224–229. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).