Skin Aging and Type I Collagen: A Systematic Review of Interventions with Potential Collagen-Related Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion Criteria
- -
- Clinical studies assessing the impact of type I collagen on skin aging.
- -
- Published between 2014 and 2025.
- -
- Full-text availability in English.
- -
- Investigated collagen-related changes in skin elasticity, hydration, or wrinkle formation in human participants.
2.4. Exclusion Criteria
- -
- Were not clinical studies (e.g., in vitro, ex vivo, or animal studies);
- -
- Did not specifically evaluate type I collagen’s role in skin aging;
- -
- Were literature reviews, meta-analyses, or opinion pieces;
- -
- Were duplicates or superseded by more recent research.
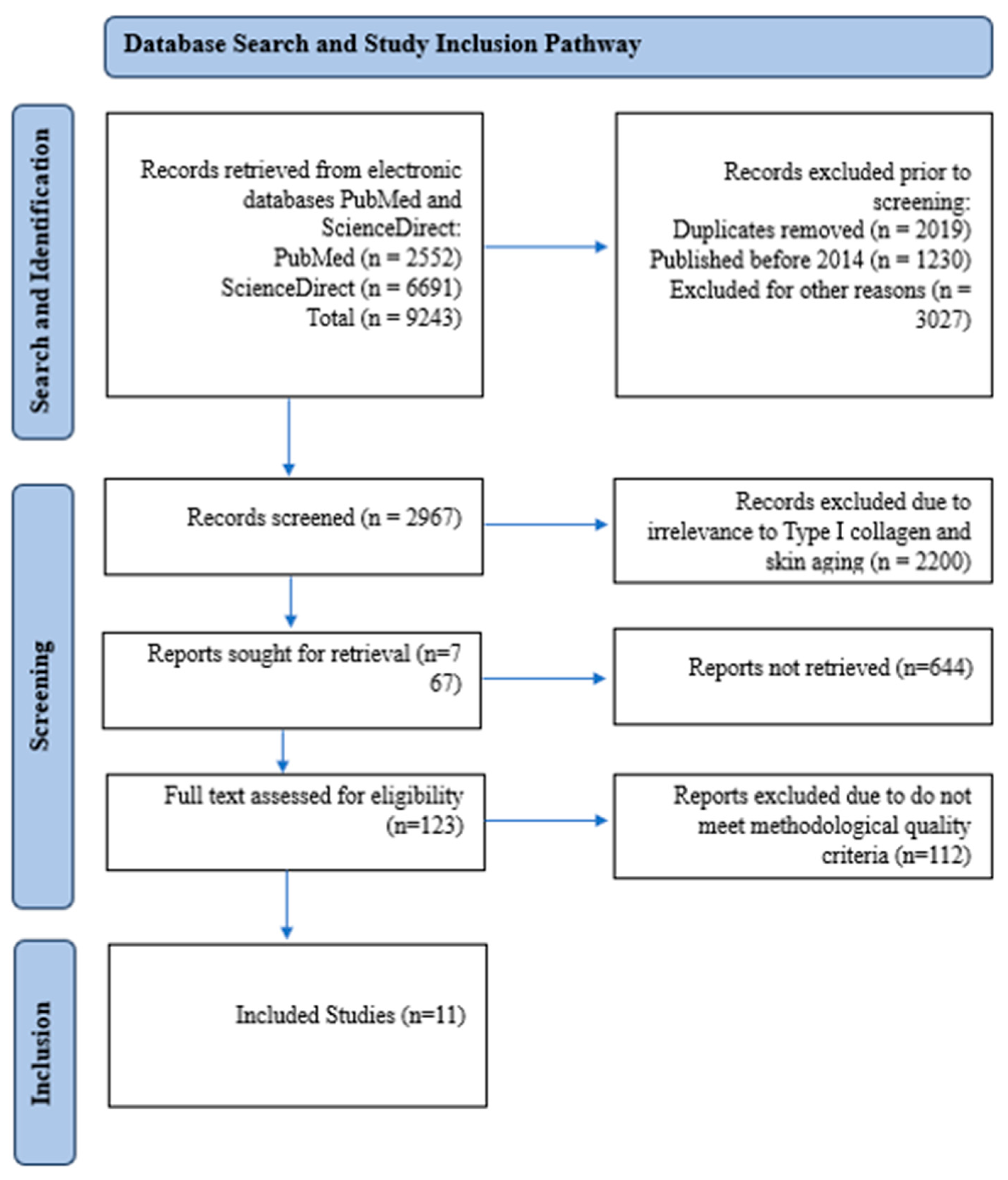
2.5. Study Selection and PRISMA Framework
2.6. Data Extraction and Analysis
2.7. Ethical Considerations
3. Results and Discussion
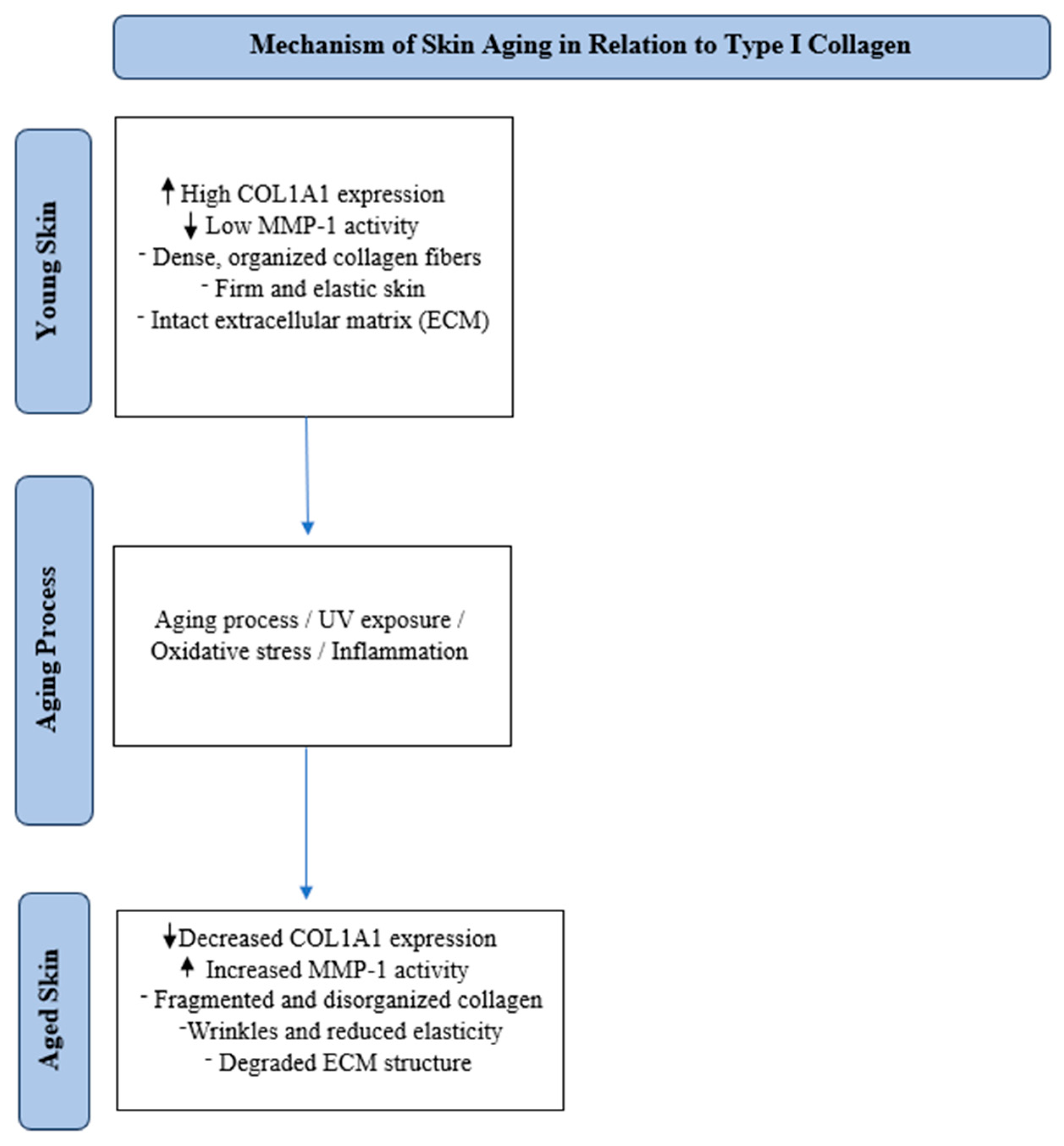
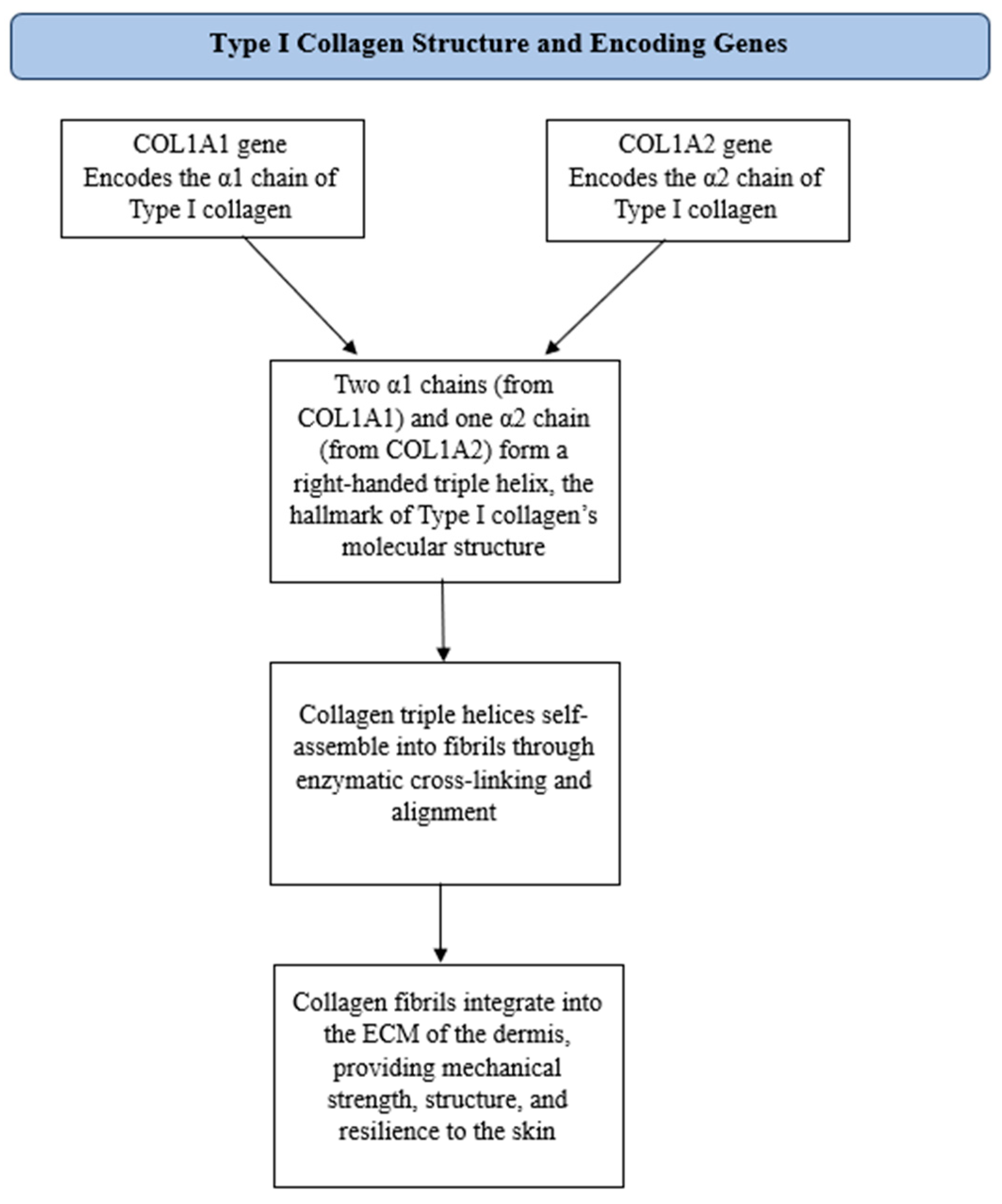
3.1. Type I Collagen and Its Impact on Skin Aging
3.2. Individual Study Analysis
3.3. Mechanisms Involved in Type I Collagen Degradation
3.4. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARE | Angelica gigas Nakai root extracts |
| COL1A1 | Collagen type I alpha 1 chain |
| COL1A2 | Collagen type I alpha 2 chain |
| ECM | Extra cellular matrix |
| HA2k | hyaluronan oligosaccharides |
| HAS | Hydroxystearic acid |
| LMWCP | low-molecular-weight collagen peptides |
| MMP | Matrix metalloproteinase |
| MOLE | Melissa officinalis Lemon |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROS | Reactive oxygen species |
| TGF β | Transforming Growth Factor Beta |
| TIMP | Tissue inhibitors of metalloproteinase |
| TβRII | Transforming Growth Factor Beta Type II receptor |
References
- Csekes, E.; Aging, L.R.S. Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef]
- Chin, L.-C.; Kimura, K.; Nakahata, Y.; Huang, L. Human Placental Extract Delays In Vitro Cellular Senescence through the Activation of NRF2-Mediated Antioxidant Pathway. Antioxidants 2022, 11, 1545. [Google Scholar] [CrossRef]
- Panichakul, T.; Srisanga, K.; Tupchiangmai, W.; Haritakun, W.; Ponnikorn, S. Skin Anti-Aging Potential of Ipomoea pes-caprae Ethanolic Extracts on Promoting Cell Proliferation and Collagen Production in Human Fibroblasts (CCD-986sk Cells). Pharmaceuticals 2022, 15, 969. [Google Scholar] [CrossRef]
- Boismal, F.; Serror, K.; Dobos, G.; Zuelgaray, E.; Bensussan, A.; Michel, L. Skin aging: Pathophysiology and innovative therapies. Med. Sci. 2020, 36, 1163–1172. [Google Scholar] [CrossRef]
- Wang, S.; Belmonte, J.C.I.; Esteban, C.R.; Long, X.; Yu, N.; Song, M.; He, Y.; Huang, J.; Zheng, Y.; Zou, Z.; et al. A Single-Cell Transcriptomic Atlas of Human Skin Aging. Dev. Cell 2021, 56, 383–397.e8. [Google Scholar] [CrossRef]
- Jourdain, E.; Morgado-Carrasco, D.; Piquero-Casals, J.; Gil-Lianes, J. Oral Supplementation and Systemic Drugs for Skin Aging: A Narrative Review. Actas Dermo-Sifiliogr. 2022, 114, T114–T124. [Google Scholar] [CrossRef]
- Rorteau, J.; Chevalier, F.P.; Fromy, B.; Lamartine, J. Functional integrity of aging skin, from cutaneous biology to an-ti-aging strategies. Med. Sci. 2020, 36, 1155–1162. [Google Scholar] [CrossRef]
- Pelletier, M.; Le Fur, M.; Granotier, F.; Couturaud, V. Reverse skin aging signs by red light photobiomodulation. Ski. Res. Technol. 2023, 29, e13391. [Google Scholar] [CrossRef]
- Kang, H.Y.; Park, T.J.; Kim, J.C. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef]
- Ge, C.; Xiao, Z.; Cao, C.; Wu, Y. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Aguilera-Aguirre, L.; Sreedhar, A.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Damiani, E.; Brugè, F.; Cirilli, I.; Marcheggiani, F.; Olivieri, F.; Armeni, T.; Cianfruglia, L.; Giuliani, A.; Orlando, P.; Tiano, L. Modulation of Oxidative Status by Normoxia and Hypoxia on Cultures of Human Dermal Fibroblasts: How Does It Affect Cell Aging? Oxid. Med. Cell. Longev. 2018, 2018, 5469159. [Google Scholar] [CrossRef]
- Park, E.-Y.; Oh, Y.S.; Kim, K.; Baek, D.-J.; Kim, C.-E. Prevention of UVB-Induced Photoaging by an Ethyl Acetate Fraction from Allomyrina dichotoma Larvae and Its Potential Mechanisms in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2024, 25, 7850. [Google Scholar] [CrossRef]
- Park, J.; Jang, Y.-P.; Lee, C.; Cho, H.; Kim, M.; Kim, B. Evaluating the Dermatological Benefits of Snowberry (Symphoricarpos albus): A Comparative Analysis of Extracts and Fermented Products from Different Plant Parts. Int. J. Mol. Sci. 2024, 25, 9660. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, T. Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice. Nutrients 2019, 11, 2948. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-Y.; Choi, Y.-J.; Yoo, J.; Kim, E.; Jung, J. Antiphotoaging Effect of AGEs BlockerTM in UVB-Irradiated Cells and Skh:HR-1 Hairless Mice. Curr. Issues Mol. Biol. 2023, 45, 4181–4199. [Google Scholar] [CrossRef]
- Lee, S.B.; Seo, W.-Y.; Hwang, S.J.; Shin, M.C.; Jeong, Y.; Choi, Y.J.; Ryu, D.; Kim, S.H.; Kim, K. Effects of human collagen α-1 type I-derived proteins on collagen synthesis and elastin production in human dermal fibroblasts. BMB Rep. 2021, 54, 329–334. [Google Scholar] [CrossRef]
- Hu, L.; Man, M.; Yu, H.; Zhang, J. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2023, 23, e14054. [Google Scholar] [CrossRef]
- Young, K.; Trowbridge, J.J.; SanMiguel, J.M. Hand in hand: Intrinsic and extrinsic drivers of aging and clonal hematopoiesis. Exp. Hematol. 2020, 91, 1–9. [Google Scholar] [CrossRef]
- Mysliwiec, H.; Kakareko, K.; Hryszko, T.; Rydzewska-Rosolowska, A.; Bielach-Bazyluk, A.; Zbroch, E.; Flisiak, I. Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging—A Systematic Review. Cells 2021, 10, 813. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, H.; Jo, Y.; Choi, S. Aging of hair follicle stem cells and their niches. BMB Rep. 2022, 56, 2–9. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Trowbridge, J.J.; Mistry, J.J.; Díaz, P.A.C. Hematopoietic stem cell aging and leukemia transformation. Blood 2023, 142, 533–542. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2020, 18, 58–68. [Google Scholar] [CrossRef]
- Lee, Y.H.; Shin, S.H.; Park, K.Y.; Rho, N.-K. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Ratanapokasatit, Y.; Laisuan, W.; Rattananukrom, T.; Petchlorlian, A.; Thaipisuttikul, I.; Sompornrattanaphan, M. How Micro-biomes Affect Skin Aging: The Updated Evidence and Current Perspectives. Life 2022, 12, 936. [Google Scholar] [CrossRef]
- Puzzi, M.B.; Lago, J.C. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Petrova, M.M.; Gavrilyuk, O.A.; Karpova, E.I.; Trefilova, V.V.; Demina, O.M.; Popova, T.E.; Shnayder, N.A. Genetic and Epigenetic Aspects of Skin Collagen Fiber Turnover and Functioning. Cosmetics 2021, 8, 92. [Google Scholar] [CrossRef]
- Seino, S.; Kurihara, H.; Kage, M.; Abe, Y.; Tokudome, Y. 2-kDa hyaluronan ameliorates human facial wrinkles through increased dermal collagen density related to promotion of collagen remodeling. J. Cosmet. Dermatol. 2022, 22, 320–327. [Google Scholar] [CrossRef]
- Gupta, J.; Repici, M.; Gross, S.R.; Lancaster, T.; Tabrizi, M.E.A. An Extracellular/Membrane-Bound S100P Pool Regulates Motility and Invasion of Human Extravillous Trophoblast Lines and Primary Cells. Biomolecules 2023, 13, 1231. [Google Scholar] [CrossRef]
- Liang, L.; Yang, C.; Zhou, Y.; Liu, L.; Chen, Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes 2021, 13, 1902718. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef]
- Greiner, M.A.; Jiao, C.; Pouw, A.E.; Coussa, R.G.; Skeie, J.M.; Fingert, J.H.; Sohn, E.H.; Mullins, R.F.; Han, I.C. Cell–Matrix Interactions in the Eye: From Cornea to Choroid. Cells 2021, 10, 687. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, J.; Wu, Z.; Li, J.; Wang, Z.; Yao, Y.; Tao, S.; Xiao, Y.; Wei, H.; Liu, X. Extracellular vesicles derived from Lactobacillus johnsonii promote gut barrier homeostasis by enhancing M2 macrophage polarization. J. Adv. Res. 2025, 69, 545–563. [Google Scholar] [CrossRef]
- Kumar, A.; Shipman, T.; Le, L.Q.; Xing, C.; Yu, Z.; Wang, Y.; McKay, R.M.; Jiang, C. Basement membrane proteins in extracellular matrix characterize NF1 neurofibroma development and response to MEK inhibitor. J. Clin. Investig. 2023, 133, e168227. [Google Scholar] [CrossRef]
- Baldomà, L.; Díaz-Garrido, N.; Badia, J. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Mira, E.; Mañes, S.; Carmona-Rodríguez, L.; Fernández-Aceñero, M.J.; Martínez-Rey, D. Extracellular Superoxide Dismutase, the Endothelial Basement Membrane, and the WNT Pathway: New Players in Vascular Normalization and Tumor Infiltration by T-Cells. Front. Immunol. 2020, 11, 579552. [Google Scholar] [CrossRef]
- Chai, P.; Lebedenko, C.G.; Flynn, R.A. RNA Crossing Membranes: Systems and Mechanisms Contextualizing Extracellular RNA and Cell Surface GlycoRNAs. Annu. Rev. Genom. Hum. Genet. 2023, 24, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Kaji, I.; Goldenring, J.R.; Engevik, A.C. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef]
- Martínez-Puig, D.; Gálvez-Martín, P.; Costa-Larrión, E.; Rubio-Rodríguez, N. Collagen Supplementation for Joint Health: The Link between Composition and Scientific Knowledge. Nutrients 2023, 15, 1332. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Lee, S.-Y.; Liu, S.-H.; Pei, D.; Lin, W.-C.; Wang, C.-S.; Liao, E.-C.; Hsu, C.-H. Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging. Open Life Sci. 2024, 19, 20220828. [Google Scholar] [CrossRef]
- Thorseth, M.-L.; Madsen, D.H.; Rømer, A.M.A. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef]
- Wei, M.; Yu, L. Biomineralization of Collagen-Based Materials for Hard Tissue Repair. Int. J. Mol. Sci. 2021, 22, 944. [Google Scholar] [CrossRef]
- Huang, L.; Sun, J.; Wen, D.; Zhang, Y.; Liu, Y.; Ho, C.; Gao, Y.; Li, Q. Targeting the stem cell niche: Role of collagen XVII in skin aging and wound repair. Theranostics 2022, 12, 6446–6454. [Google Scholar] [CrossRef]
- Kokkonen, N.; Tuusa, J.; Tasanen, K. BP180/Collagen XVII: A Molecular View. Int. J. Mol. Sci. 2021, 22, 12233. [Google Scholar] [CrossRef]
- Haverkamp, R.G.; Sizeland, K.H.; Wells, H.C.; Kamma-Lorger, C. Collagen dehydration. Int. J. Biol. Macromol. 2022, 216, 140–147. [Google Scholar] [CrossRef]
- Li, Q.; Hsu, J.J.; Demer, L.L.; Vazquez-Padron, R.I.; Bendeck, M.P.; Tintut, Y. Collagen VIII in vascular diseases. Matrix Biol. 2024, 133, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Halfter, N.; Schnabelrauch, M.; Anderegg, U. Collagen/glycosaminoglycan-based matrices for controlling skin cell responses. Biol. Chem. 2021, 402, 1325–1335. [Google Scholar] [CrossRef]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In Vitro Mineralization of Collagen. Adv. Mater. 2021, 33, 2004418. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, I.S.; Krigman, H.R.; Weiss, M.C.; Sun, L.; Chapagain, U. COL1A1::PDGFB fusion-associated uterine sarcoma and response to Imatinib: A case report. Gynecol. Oncol. Rep. 2023, 49, 101270. [Google Scholar] [CrossRef]
- Szoka, L.; Karna, E.; Palka, J.A.; Huynh, T.Y.L. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef]
- González, S.; Dierckx, S.; Mullor, J.L.; Nergiz-Unal, R.; Merino, M.; Patrizi, M. Collagen peptides affect collagen synthesis and the expression of collagen, elastin, and versican genes in cultured human dermal fibroblasts. Front. Med. 2024, 11, 1397517. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Fauzi, M.B.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Reichetzeder, C.; Irawati, F.; Putra, S.E.D.; Kok, T.; Sulistomo, H.W.; Mulyanata, L.T.; Humardani, F.M.; Liamry, J.N.; Chandra, G. Exploring the impact of diabetes on aging: Insights from TERT and COL1A1 methylation. Turk. J. Biol. 2024, 48, 257–266. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.J.; Jeong, D.H.; Kim, S.H.; Jung, M.S.; Lee, K.H. Minimally invasive skin sampling and transcriptome analysis using microneedles for skin type biomarker research. Ski. Res. Technol. 2022, 28, 322–335. [Google Scholar] [CrossRef]
- Guilbert, M.; Manfait, M.; Terryn, C.; Garnotel, R.; Dinten, J.-M.; Koenig, A.; Sockalingum, G.D.; Jeannesson, P.; Roig, B.; Piot, O.; et al. Highlighting the impact of aging on type I collagen: Label-free investigation using confocal reflectance microscopy and diffuse reflectance spectroscopy in 3D matrix model. Oncotarget 2016, 7, 8546–8555. [Google Scholar] [CrossRef] [PubMed]
- Paolini, B.; Casali, P.; Sanfilippo, R.; Alessi, A.; Padovano, B.; Greco, F.G.; Franza, A.; Fabbroni, C.; Rota, S. COL1A1::PDGFB fusion-associated uterine fibrosarcoma: A case report and review of the literature. Cancer Rep. 2024, 7, e1969. [Google Scholar] [CrossRef]
- Xia, M.; Wang, X.-H.; Li, X.-M.; Yao, M.-D.; Li, D.; Yao, J.; Tong, M.; Jiao, L.; Zhao, P.-Q.; Yan, B. Single-cell RNA sequencing reveals a unique pericyte type associated with capillary dysfunction. Theranostics 2023, 13, 2515–2530. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Wu, X.; Wang, W.; Wang, Q.; Lin, W.; Zhang, Z.; Li, M. The COL1A1 rs1800012 polymorphism is associated with osteoporosis or fracture risk: A meta-analysis of 30 studies. Int. J. Burns Traum. 2024, 14, 148–159. [Google Scholar] [CrossRef]
- Chen, W.; Wu, X.; Hu, J.; Liu, X.; Guo, Z.; Wu, J.; Shao, Y.; Hao, M.; Zhang, S.; Hu, W.; et al. The translational potential of miR-26 in atherosclerosis and development of agents for its target genes ACC1/2, COL1A1, CPT1A, FBP1, DGAT2, and SMAD7. Cardiovasc. Diabetol. 2024, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, J.; Chrzanowska, N.M.; Lewandowska, M.A. Use of Fluorescence In Situ Hybridization (FISH) in Diagnosis and Tailored Therapies in Solid Tumors. Molecules 2020, 25, 1864. [Google Scholar] [CrossRef]
- Liu, L.; Huang, W. hsa_circ_0020378 regulating miR-339-3p/COL1A1 promotes osteosarcoma progression. Cancer Biol. Ther. 2023, 24, 2274120. [Google Scholar] [CrossRef]
- Harsanyi, S.; Zamborsky, R.; Krajciova, L.; Kokavec, M.; Danisovic, L. Developmental Dysplasia of the Hip: A Review of Eti-opathogenesis, Risk Factors, and Genetic Aspects. Medicina 2020, 56, 153. [Google Scholar] [CrossRef]
- Ren, J.; Da, J.; Hu, N. Identification of COL1A1 associated with immune infiltration in brain lower grade glioma. PLoS ONE 2022, 17, e0269533. [Google Scholar] [CrossRef]
- Steiner, R.D.; Basel, D. COL1A1/2 Osteogenesis Imperfecta. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK1295/ (accessed on 9 March 2025). [PubMed]
- Vankevičienė, R.; Matulevičienė, A.; Ramašauskaitė, D.; Mazgelytė, E.; Vankevičienė, K.; Paliulytė, V. A Sporadic Case of COL1A1 Osteogenesis Imperfecta: From Prenatal Diagnosis to Outcomes in Infancy—Case Report and Literature Review. Genes 2023, 14, 2062. [Google Scholar] [CrossRef]
- Nishikawa, S.; Hosoi, J.; Amano, S.; Ogura, Y.; Iriyama, S. Regeneration of collagen fibrils at the papillary dermis by reconstructing basement membrane at the dermal–epidermal junction. Sci. Rep. 2022, 12, 795. [Google Scholar] [CrossRef]
- Hasebe, Y.; Hasegawa, S.; Akamatsu, H.; Iwata, Y.; Arima, M.; Ishii, Y.; Sugiura, K.; Yamada, T.; Sanada, A. Enhanced Type I Collagen Synthesis in Fibroblasts by Dermal Stem/Progenitor Cell-Derived Exosomes. Biol. Pharm. Bull. 2022, 45, 872–880. [Google Scholar] [CrossRef]
- Cheong, H.; Park, C.; Park, J.; Kim, W.-J.; Kim, W.; Kim, S.-J. Malonic Acid Isolated from Pinus densiflora Inhibits UVB-Induced Oxidative Stress and Inflammation in HaCaT Keratinocytes. Polymers 2021, 13, 816. [Google Scholar] [CrossRef]
- Kim, S.-O.; Yun, S.-J.; Lee, J.-B.; Choi, J.-Y.; Lee, J.-A.; Cho, K.-A.; Choi, D.-I.; Lee, S.-C. Methyl-β-cyclodextrin up-regulates collagen I expression in chronologically-aged skin via its anti-caveolin-1 activity. Oncotarget 2015, 6, 1942–1953. [Google Scholar] [CrossRef]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef]
- Shim, J.H. Prostaglandin E2 Induces Skin Aging via E-Prostanoid 1 in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5555. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.J.; Choi, Y.M.; Park, C.O.; Jang, E.Y.; Jeong, D.H.; Kim, S.H.; Jung, Y.W.; Roh, Y.; Lee, K.H.; et al. Development of a biomarker-based platform for comprehensive skin characterization using minimally invasive skin sampling and quantitative real-time PCR. Ski. Res. Technol. 2024, 30, e13908. [Google Scholar] [CrossRef]
- Kishi, K.; Asou, T.; Takaya, K. Aging Fibroblasts Adversely Affect Extracellular Matrix Formation via the Senescent Humoral Factor Ependymin-Related Protein 1. Cells 2022, 11, 3749. [Google Scholar] [CrossRef]
- Gecyte, E.; Gecys, D.; Sepetiene, R.; Patamsyte, V.; Skipskis, V.; Stanioniene, Z.; Barakauskas, S.; Valiukevicius, P. Genetical Signature—An Example of a Personalized Skin Aging Investigation with Possible Implementation in Clinical Practice. J. Pers. Med. 2023, 13, 1305. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, D.; Wang, M.; Fu, M.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal Transd. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Lee, J.H.; Massagué, J. TGF-β in developmental and fibrogenic EMTs. Semin. Cancer Biol. 2022, 86, 136–145. [Google Scholar] [CrossRef]
- Orwoll, E.; Esposito, P.; Jiang, M.-M.; Munivez, E.; Gannon, F.H.; Song, I.-W.; Grafe, I.; Huang, S.; Lee, B.; Nagamani, S.C.; et al. Targeting TGF-β for treatment of osteogenesis imperfecta. J. Clin. Investig. 2022, 132, e152571. [Google Scholar] [CrossRef]
- Lou, J.; Campbell, M.G.; Jespersen, J.M.; Yu, Z.; Nishimura, S.L.; Ito, S.; Cossio, P.; Baron, J.L.; Marks, J.; Wen, W.; et al. Dynamic allostery drives autocrine and paracrine TGF-β signaling. Cell 2024, 187, 6200–6219.e23. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Wan, Y.Y. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell. Mol. Immunol. 2023, 20, 1002–1022. [Google Scholar] [CrossRef]
- Seoane, J.; Lan, Y.; Wang, X.; Dussault, I.; Barcellos-Hoff, M.H.; Schlom, J.; Audhuy, F.; Gulley, J.L.; Moustakas, A. Dual inhibition of TGF-β and PD-L1: A novel approach to cancer treatment. Mol. Oncol. 2022, 16, 2117–2134. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, S.; Xu, H.; Wu, D.; Shi, X.; Wu, F.; Zhou, H.; Yang, J.; Deng, S.; Hu, T. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Gough, N.R.; Mishra, L.; Xiang, X. TGF-β Signaling in Liver, Pancreas, and Gastrointestinal Diseases and Cancer. Gastroenterology 2021, 161, 434–452.e15. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lee, S.; Chung, J.; Yoon, H. Long-term Topical Oestrogen Treatment of Sun-exposed Facial Skin in Post-menopausal Women Does Not Improve Facial Wrinkles or Skin Elasticity, But Induces Matrix Metalloproteinase-1 Expression. Acta Dermato-Venereol. 2014, 94, 4–8. [Google Scholar] [CrossRef]
- Humbert, P.; Fanian, F.; Lihoreau, T.; Jeudy, A.; Elkhyat, A.; Robin, S.; Courderot-Masuyer, C.; Tauzin, H.; Lafforgue, C.; Haftek, M. Mécano-StimulationTM of the skin improves sagging score and induces beneficial functional modification of the fibro-blasts: Clinical, biological, and histological evaluations. Clin. Interv. Aging 2015, 10, 387–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakai, Y.; Kim, D.-U.; Chung, H.-C.; Choi, J.; Lee, B.-Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef]
- Schütz, R.; Rawlings, A.V.; Wandeler, E.; Jackson, E.; Trevisan, S.; Monneuse, J.; Bendik, I.; Massironi, M.; Imfeld, D. Bio-derived hydroxystearic acid ameliorates skin age spots and conspicuous pores. Int. J. Cosmet. Sci. 2019, 41, 240–256. [Google Scholar] [CrossRef]
- Kang, J.-W.; Cho, H.-E.; Choi, H.-M.; Lee, I.-C. Anti-wrinkle properties of Angelica gigas Nakai root extracts using miner-al-rich water. J. Cosmet. Dermatol. 2023, 22, 328–334. [Google Scholar] [CrossRef]
- Guthrie, N.; Zakaria, N.; Pelipyagina, T.; Evans, M.; Lewis, E.D. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2020, 20, 825–834. [Google Scholar] [CrossRef]
- Laing, S.; Wilhelm, K.-P.; Bielfeldt, S.; Ehrenberg, C. A Dermonutrient Containing Special Collagen Peptides Improves Skin Structure and Function: A Randomized, Placebo-Controlled, Triple-Blind Trial Using Confocal Laser Scanning Microscopy on the Cosmetic Effects and Tolerance of a Drinkable Collagen Supplement. J. Med. Food 2020, 23, 147–152. [Google Scholar] [CrossRef]
- Campos, P.M.B.G.M.; Franco, R.S.B.; Kakuda, L.; Cadioli, G.F.; Maria, D.G.; Bouvret, E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Mor-phological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Molecules 2021, 26, 4880. [Google Scholar] [CrossRef]
- Butina, M.R.; Žmitek, J.; Pogačnik, T.; Hristov, H.; Žmitek, K.; Keršmanc, P. The Effects of Dietary Supplementation with Collagen and Vitamin C and Their Combination with Hyaluronic Acid on Skin Density, Texture and Other Parameters: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 1908. [Google Scholar] [CrossRef]
- Kawashima, Y.; Iwahashi, H.; Masaki, H.; Taga, A. Lemon Balm (Melissa officinalis L.) Leaf Extract Promotes Endo180 Production in Dermal Fibroblasts and has Antiwrinkle Effect on Human Skin. Photodermatol. Photoimmunol. Photomed. 2025, 41, e70006. [Google Scholar] [CrossRef]
| References (Author, Year) | Study Design | Sample Size and Population | Interventions | Key Findings | Clinical Implications |
|---|---|---|---|---|---|
| Yoon et al., 2014 [92] | Randomized, double-blind, vehicle-controlled study | 80 post-menopausal women (mean age: 55.2 ± 2.2 years) with photoaged skin | 1% estrone cream (topical estrogen) vs. vehicle, applied daily for 24 weeks | No significant increase in type 1 collagen (COL1A1) protein despite a 5.0-fold increase in mRNA. MMP-1 increased 10.3-fold, leading to collagen breakdown. No improvement in wrinkles or elasticity, with some worsening. Topical estrogen failed to protect or restore collagen in sun-exposed skin. The treatment was applied to the crow’s feet area. | Collagen synthesis alone is insufficient if MMP-1 is upregulated, causing collagen breakdown. Sun-exposed skin may respond differently to estrogen, leading to increased collagen degradation. Estrogen-based treatments may have negative effects on UV-exposed aging skin, highlighting the need for alternative collagen-preserving approaches. |
| Humbert et al., 2015 [93] | Double-blinded, controlled, and randomized study | 30 subjects (20 females, 10 males), aged 35–50, with mild-to-moderate facial sagging30 subjects (20 females, 10 males), aged 35–50, with mild-to-moderate facial sagging | 24 sessions of Mécano-StimulationTM (mechanical skin stimulation) over 8 weeks, applied to one side of the face. | Increased type 1 collagen synthesis in fibroblasts (107.8% increase, not statistically significant) and enhanced fibroblast migration and dermal remodeling. Higher MMP-9 expression facilitated collagen turnover. Ultrastructural improvements in dermal structures, with better-aligned collagen bundles. 70–73% of subjects showed improvements in skin sagging, firmness, and elasticity. Applied on one hemiface, covering multiple sun-exposed facial areas (chin, nasolabial folds, cheeks, cheekbones, temples, and forehead). Subjects had Fitzpatrick skin types I–IV. | Shows that mechanical stimulation activates fibroblasts, enhancing type 1 collagen synthesis and dermal restructuring. Supports collagen remodeling as an anti-aging target, reversing fibroblast dysfunction. A non-invasive alternative for improving collagen density and skin firmness. |
| Kim et al., 2018 [94] | Randomized, double-blind, placebo-controlled | 64 women (aged 40–60) with photoaged skin | Daily oral supplementation of 1 g low-molecular-weight collagen peptide (LMWCP) for 12 weeks vs. placebo | Significant increase in skin hydration at 6 weeks (p < 0.001) and 12 weeks (p = 0.003). Reduction in wrinkle severity (crow’s feet) after 12 weeks (p = 0.013). Improved skin elasticity (p = 0.025 for overall, p = 0.027 for net). LMWCP reduced collagen degradation, downregulating MMPs. No adverse effects; safety parameters normal. | Supports type 1 collagen peptides in improving skin structure and function. Demonstrates that collagen peptides inhibit MMPs, reducing collagen breakdown. Suggests LMWCP as an effective oral supplement for hydration, elasticity, and anti-wrinkle effects. |
| Schütz et al., 2019 [95] | Double-blind, vehicle-controlled, parallel-group study (in vivo) | 37 Caucasian women (aged 38–65) with visible signs of skin aging and 38 in the placebo group (aged 34–65) | Topical application of 1% 10-hydroxystearic acid (HSA) twice daily for 8 weeks | Significant increase in type 1 collagen (+96%). HSA inhibited UVB-induced MMP-1 expression by 83% (p < 0.01) and reduced collagen degradation. Mitigated UVB-induced stress markers (p53 −46%, sunburn cells −34%, p < 0.01). Reduced pore size and age spot pigmentation after 8 weeks (p < 0.05). | HSA stimulates collagen synthesis and prevents degradation, showing potential as an anti-aging agent. Inhibiting MMP-1 protects type 1 collagen from UV-induced breakdown, supporting skin structure. Potential for topical HSA as a non-invasive alternative to preserve dermal integrity. |
| Kang et al., 2023 [96] | Double-blind, randomized, placebo-controlled, in vivo clinical study | This clinical test was conducted on volunteers who met the inclusion criteria and not the exclusion criteria. | Topical application of Angelica gigas Nakai root extract (ARE) in mineral-rich water vs. placebo for 8 weeks | ARE increased type 1 collagen production by 40% (p < 0.05). Significantly reduced crow’s feet wrinkles and inhibited photodamage (p < 0.05). Suppressed MMP-3 expression, reducing collagen breakdown. Improved skin roughness parameters (R1–R5) after 8 weeks (p < 0.05). | Confirms that ARE stimulates type 1 collagen synthesis and prevents degradation. Demonstrates anti-aging effects, reducing wrinkles and photodamage. Suggests botanical extracts may enhance collagen production and protect against ECM degradation. |
| Evans et al., 2020 [97] | Randomized, triple-blind, placebo-controlled, parallel study | 50 women (aged 45–60) with visible signs of natural and photoaging | Daily oral supplementation of 10 g freshwater marine collagen (VWC) vs. placebo for 12 weeks | 35% reduction in wrinkles after 12 weeks (p = 0.035). 24% greater reduction on the right side vs. placebo. Improved elasticity and hydration, with self-reported benefits in radiance (+22%), firmness (+25%), and wrinkles (+15%). Well tolerated with no adverse events. Anatomical locations: Cheeks and nasolabial folds. | Oral collagen supplementation enhances collagen integrity and elasticity in aging skin, providing essential amino acids for dermal repair and stimulating collagen synthesis. The study highlights marine collagen as a potential nutritional intervention for skin aging. More research is needed to determine long-term effects and optimal dosing strategies. |
| Laing et al., 2020 [98] | Randomized, placebo-controlled, triple-blind trial | 60 healthy female participants (aged 40–70) | Daily oral supplementation of 2.5 g collagen peptides (with acerola, vitamin C, E, biotin, and zinc) vs. placebo for 12 weeks. | Significant improvement in collagen structure (p < 0.05), reduced fragmentation, and increased fiber organization, enhancing dermal integrity. Better subjective ratings in elasticity, firmness, hydration, and skin appearance. No adverse effects; supplement well tolerated. | Confirms that collagen supplementation supports type 1 collagen structure, reduces fragmentation, and improves ECM integrity. Suggests collagen supplementation as a safe, effective anti-aging strategy, especially with essential micronutrients. |
| Campos et al., 2021 [99] | Randomized, double-blind, placebo-controlled study | 46 women (aged 45–59) with visible signs of aging | Daily oral supplementation of 500 mg hydrolyzed fish cartilage collagen peptides for 90 days vs. placebo | Significant increase in dermis echogenicity (p < 0.05), indicating improved collagen density. RCM showed better collagen morphology and reduced elastosis. Wrinkle reduction: Nasolabial (−31%, p < 0.05), periorbital (−26%, p < 0.05), and frontal (−14%, NS). Increased dermis thickness (p < 0.05), suggesting better hydration and collagen regeneration. Participants reported improvements in firmness, hydration, and skin tone. | Oral collagen supplementation improves skin structure, reduces wrinkles, and increases dermal density. Supports collagen supplementation as a non-invasive anti-aging strategy. Suggests low-dose hydrolyzed fish collagen enhances collagen integrity and reduces aging signs. |
| Abe et al., 2022 [33] | Randomized | 21 women participants | Topical 0.1% HA2k lotion, applied daily for 8 weeks | HA2k penetrates the stratum corneum and upregulates COL1A1 and MMP-1 promoting collagen remodeling. After 4 weeks, it increases dermal collagen density, reduces wrinkle depth, and improves skin elasticity and hydration. | Supports collagen remodeling in aging skin, offering a non-invasive anti-aging alternative to fillers/micro-needling. Has potential for use in cosmetic formulations targeting collagen synthesis and wrinkle reduction. |
| Žmitek et al., 2024 [100] | Randomized, double-blind, placebo-controlled study | 87 women (aged 40–65) | Daily oral supplementation of 5 g hydrolyzed collagen with 80 mg vitamin C (CP) vs. 5 g hydrolyzed collagen + 30 mg hyaluronic acid + 80 mg vitamin C (CPHA) vs. placebo for 16 weeks. | Significant increase in dermal density (16.3% for CP, 16.0% for CPHA, p < 0.001). Significant reduction in wrinkle volume (−13.8% for CP, −13.9% for CPHA, p < 0.001) and maximum wrinkle depth (−16.9% for CP, −19.2% for CPHA, p < 0.05). No significant impact on skin elasticity or hydration compared to placebo. No additional benefits from adding hyaluronic acid to collagen supplementation. | Collagen supplementation improves skin density and reduces wrinkles. Demonstrates collagen’s role in ECM maintenance, particularly type 1 collagen. Supports collagen supplementation as an effective anti-aging intervention. Hyaluronic acid offers no additional benefits in collagen supplementation. |
| Iwahashi et al., 2025 [101] | Double-blind, randomized, placebo-controlled clinical trial (RCT) | 20 Japanese women (mean age: 44.5 ± 3.4 years) with photoaged skin | Topical application of Lemon Balm Extract (MOLE) or placebo twice daily for 8 weeks | MOLE significantly increased type 1 collagen production in fibroblasts, improving collagen remodeling and reducing MMP-1 levels. UVB-induced collagen reduction in keratinocytes was reversed by MOLE. Wrinkle severity at the eye corner was significantly lower in the MOLE group after 8 weeks (p < 0.05). | MOLE may preserve type 1 collagen and improve skin aging signs. |
| Collagen Degradation Mechanism | Intervention | Outcome |
|---|---|---|
| Increased MMP (Matrix Metalloproteinases) Activity [72,93] | Topical collagen peptides, mechanical stimulation (e.g., Mécano-StimulationTM), oral collagen supplementation. | Reduced MMP activity, improved collagen synthesis, enhanced skin firmness and elasticity. |
| Collagen Fragmentation and Cross-link Formation [97] | Hydrolyzed collagen supplements, marine collagen peptides. | Improved collagen structure, reduction in wrinkles, increased collagen density. |
| Decreased TGF-β (Transforming Growth Factor Beta) Activity | Oral collagen supplementation, topical treatments (e.g., MOLE). | Enhanced collagen synthesis, reduced collagen breakdown, and better skin hydration and elasticity. |
| Reduced Fibroblast Attachment Sites to Collagen [101] | Mechanical stimulation, collagen supplementation. | Improved skin firmness, increased collagen synthesis, reduction in skin sagging. |
| Inflammaging (Oxidative Stress and Inflammation) [3,15,45,74] | Oral collagen supplements, topical treatments with antioxidants or hyaluronic acid. | Stabilization of collagen structure, reduced inflammation, improved skin texture and firmness. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar, O.; Valiukevičienė, S. Skin Aging and Type I Collagen: A Systematic Review of Interventions with Potential Collagen-Related Effects. Cosmetics 2025, 12, 129. https://doi.org/10.3390/cosmetics12040129
Bar O, Valiukevičienė S. Skin Aging and Type I Collagen: A Systematic Review of Interventions with Potential Collagen-Related Effects. Cosmetics. 2025; 12(4):129. https://doi.org/10.3390/cosmetics12040129
Chicago/Turabian StyleBar, Ofek, and Skaidra Valiukevičienė. 2025. "Skin Aging and Type I Collagen: A Systematic Review of Interventions with Potential Collagen-Related Effects" Cosmetics 12, no. 4: 129. https://doi.org/10.3390/cosmetics12040129
APA StyleBar, O., & Valiukevičienė, S. (2025). Skin Aging and Type I Collagen: A Systematic Review of Interventions with Potential Collagen-Related Effects. Cosmetics, 12(4), 129. https://doi.org/10.3390/cosmetics12040129






