Oxidative-Inflammatory Modulation of Skin Lipid Metabolism by Squalane, Oleic Acid, and Linoleic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV Irradiation and Substance Administration Regimen
2.2.1. Histological Analysis
2.2.2. Transcriptomics Analysis
2.3. Lipid Droplet Staining of SZ95 Cells
2.4. Detection of Free Fatty Acids in SZ95 Cells
2.5. LC-MS/MS Analysis of Oxidized Lipid in SZ95 Cells
2.6. Expression of Genes Involved in sz95 Lipid Secretion
2.7. Statistical Analysis
3. Results
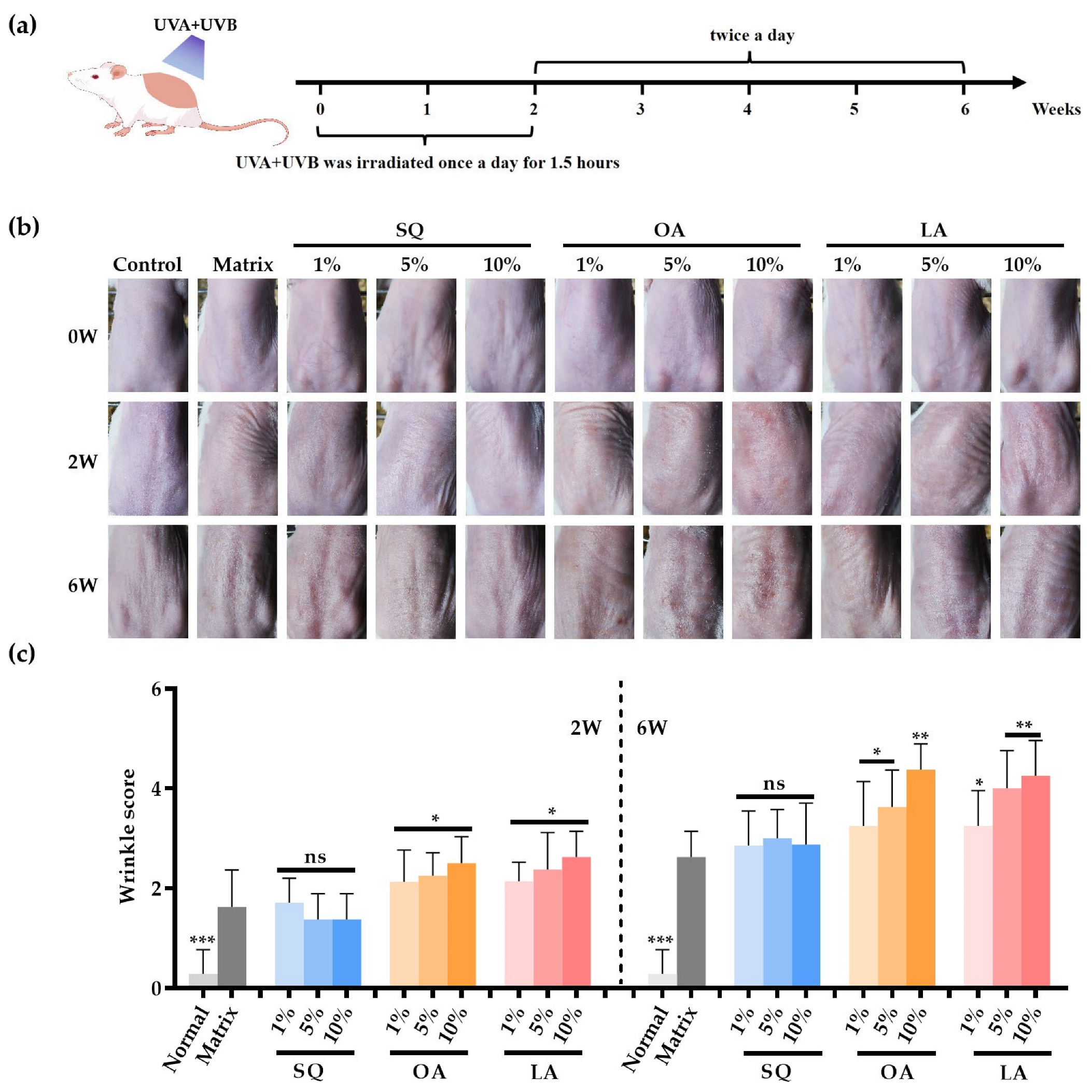
3.1. Apparent Characteristics of Mouse Skin After Ultraviolet Irradiation
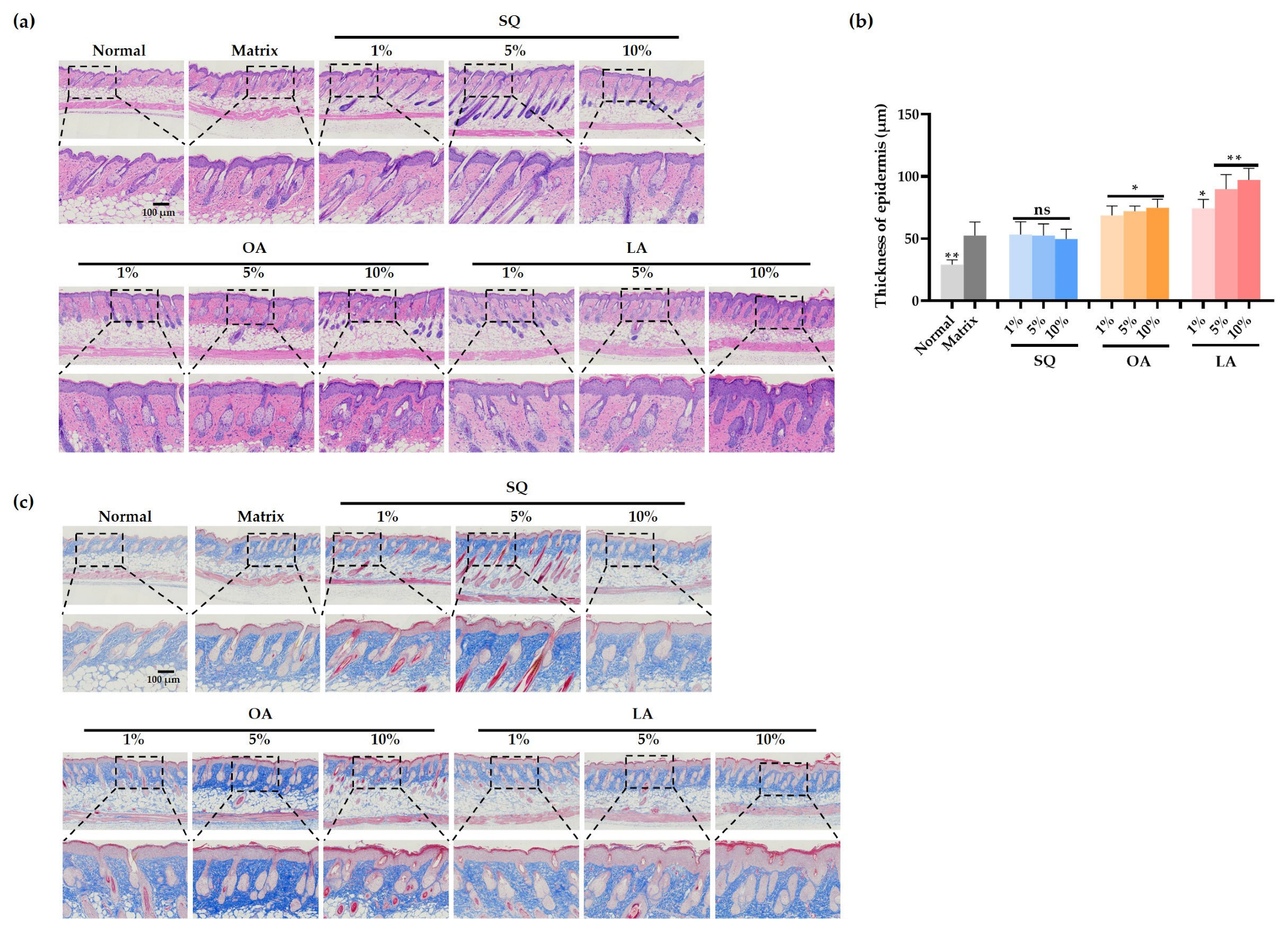
3.2. Changes in Skin Pathology Content in Mice After Ultraviolet Irradiation
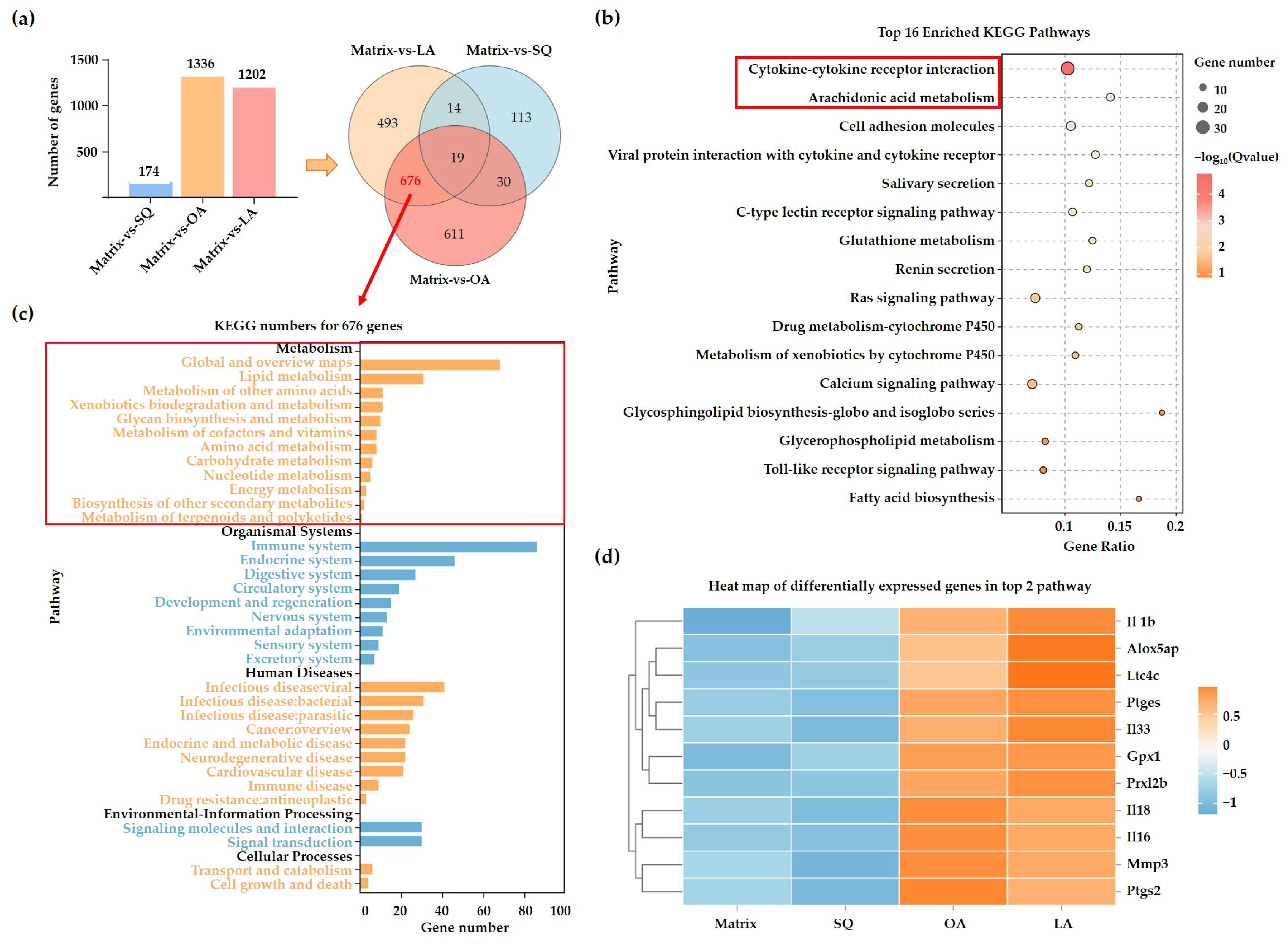
3.3. Transcriptome Analysis
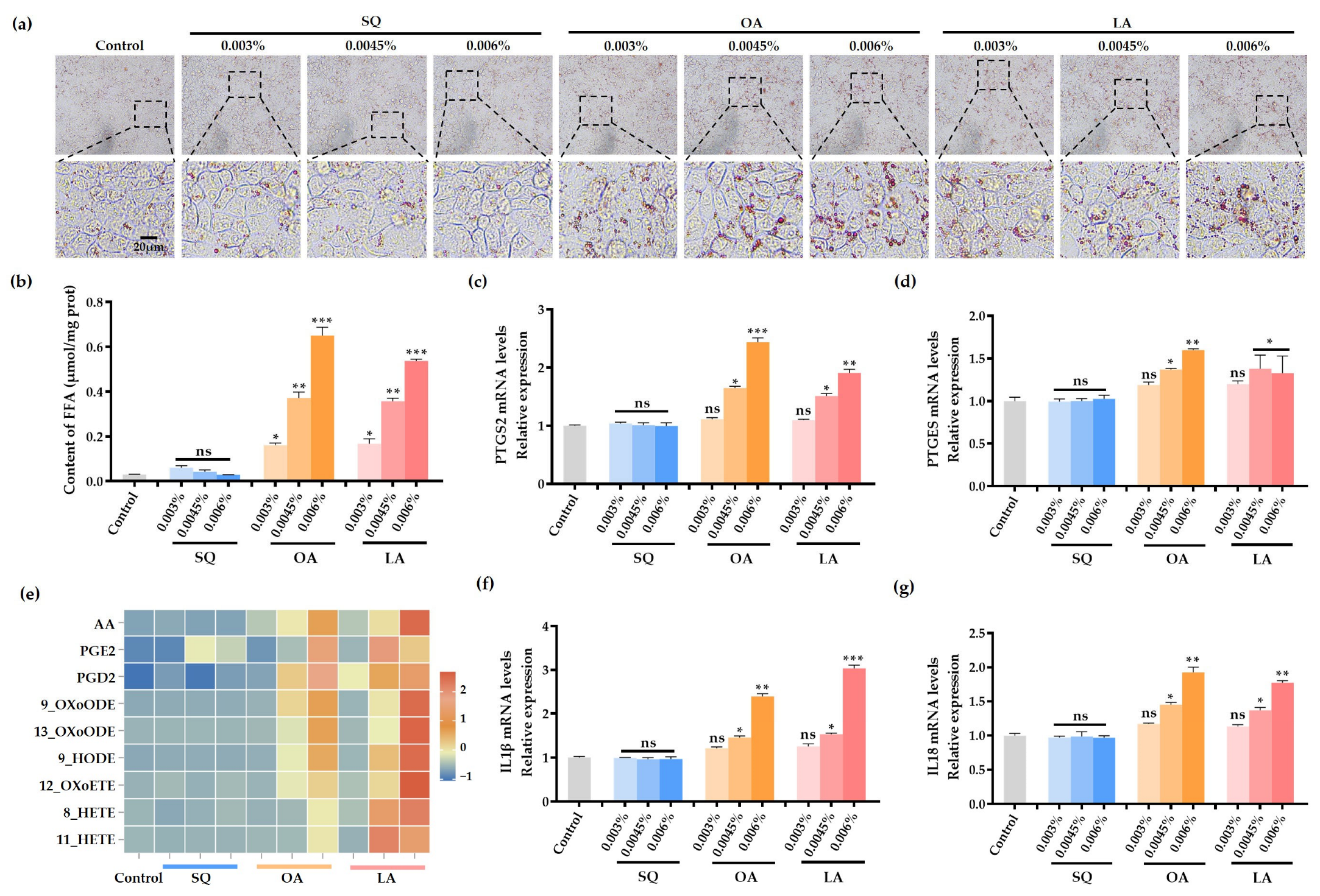
3.4. SQ, OA and LA on Lipid Droplet Synthesis in SZ95 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SQ | squalane |
| OA | oleic acid |
| LA | linoleic acid |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 |
| IL-1β | Interleukin-1 beta |
| FFAs | Free Fatty Acids |
| UV | ultraviolet |
| ALA | α-linolenic acid |
| NF-κB | Nuclear factor kappa B |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor alpha |
| PDMS | polydimethylsiloxane |
| HDA | hexyldecyl alcohol |
| KM | Kunming |
| H&E | hematoxylin and eosin |
| PTGES | Prostaglandin E Synthase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| DEGs | differentially expressed genes |
| AA | Arachidonic Acid |
| PGE2 | Prostaglandin E2 |
| PGD2 | Prostaglandin D2 |
| 12-OXO-ETE | 12-oxo-eicosatetraenoic acid |
| 8-HETE | 8-hydroxyeicosatetraenoic acid |
| 11-HETE | 11-hydroxyeicosatetraenoic acid |
| 9-OXO-ODE | 9-oxo-odecatetraenoic acid |
| 13-HETE | 13-hydroxyeicosatetraenoic acid |
| SREBP1 | sterol regulatory element-binding protein 1 |
| SCD1 | stearoyl-CoA desaturase 1 |
| ACC | acetyl-CoA carboxylase |
| PPARγ | peroxisome proliferator-activated receptor gamma |
References
- Ferrara, F.; Woodby, B.; Pecorelli, A.; Schiavone, M.L.; Pambianchi, E.; Messano, N.; Therrien, J.P.; Choudhary, H.; Valacchi, G. Additive effect of combined pollutants to UV induced skin OxInflammation damage. Evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol. 2020, 34, 101481. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef]
- Bacellar, I.O.L.; Baptista, M.S. Mechanisms of Photosensitized Lipid Oxidation and Membrane Permeabilization. ACS Omega 2019, 4, 21636–21646. [Google Scholar] [CrossRef]
- Jung, Y.R.; Shin, J.M.; Kim, C.H.; Kim, S.; Kim, C.D.; Seo, Y.J.; Lee, J.H.; Im, M.; Lee, Y.; Lee, Y.H. Activation of NLRP3 Inflammasome by Palmitic Acid in Human Sebocytes. Ann. Dermatol. 2021, 33, 541–548. [Google Scholar] [CrossRef]
- Flori, E.; Mastrofrancesco, A.; Ottaviani, M.; Maiellaro, M.; Zouboulis, C.C.; Camera, E. Desaturation of sebaceous-type saturated fatty acids through the SCD1 and the FADS2 pathways impacts lipid neosynthesis and inflammatory response in sebocytes in culture. Exp. Dermatol. 2023, 32, 808–821. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Damayanti Anggraeni, S.; Umborowati, M.A.; Sari, M.; Hendaria, M.P.; Thahir, T.F. The Role of Moisturizer Containing Anti-inflammatory on Skin Hydration in Mild-Moderate Atopic Dermatitis Patients. Dermatol. Res. Pract. 2024, 2024, 3586393. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Wojasiński, M.; Czarnomysy, R.; Dębowska, R.; Łopianiak, I.; Adasiewicz, K.; Ciach, T.; Winnicka, K. Chitosan-Enriched Solution Blow Spun Poly (Ethylene Oxide) Nanofibers with Poly (Dimethylsiloxane) Hydrophobic Outer Layer for Skin Healing and Regeneration. Int. J. Mol. Sci. 2022, 23, 5135. [Google Scholar] [CrossRef] [PubMed]
- Douguet, M.; Picard, C.; Savary, G.; Merlaud, F.; Loubat-bouleuc, N.; Grisel, M. Spreading properties of cosmetic emollients: Use of synthetic skin surface to elucidate structural effect. Colloids Surf. B Biointerfaces 2017, 154, 307–314. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. Inflammasome activity regulation by PUFA metabolites. Front. Immunol. 2024, 15, 1452749. [Google Scholar] [CrossRef]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.H. Protocatechuic Aldehyde Attenuates UVA-Induced Photoaging in Human Dermal Fibroblast Cells by Suppressing MAPKs/AP-1 and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef]
- Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- Grujić-Milanović, J.D.; Miloradović, Z.Z.; Mihailović-Stanojević, N.D.; Banjac, V.V.; Vidosavljević, S.; Ivanov, M.S.; Karanović, D.J.; Vajić, U.V.; Jovović, D.M. Excesive consumption of unsaturated fatty acids leads to oxidative and inflammatory instability in Wistar rats. Biomed. Pharmacother. 2021, 139, 111691. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Saguie, B.O.; Pereira de Almeida Nogueira, N.; Paes, M.; Dos Santos Valença, S.; Atella, G.C.; Monte-Alto-Costa, A. Oleic acid and hydroxytyrosol present in olive oil promote ROS and inflammatory response in normal cultures of murine dermal fibroblasts through the NF-κB and NRF2 pathways. Food. Res. Int. 2020, 131, 108984. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.; Alestas, T.; Flori, E.; Mastrofrancesco, A.; Zouboulis, C.C.; Picardo, M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: A possible role in acne vulgaris. J. Investig. Dermatol. 2006, 126, 2430–2437. [Google Scholar] [CrossRef]
- Ishikawa, A.; Ito, J.; Shimizu, N.; Kato, S.; Kobayashi, E.; Ohnari, H.; Sakata, O.; Naru, E.; Nakagawa, K. Linoleic acid and squalene are oxidized by discrete oxidation mechanisms in human sebum. Ann. N. Y. Acad. Sci. 2021, 1500, 112–121. [Google Scholar] [CrossRef]
- Cheng, L.C.; Li, Y.T.; Ji, C.; Liu, Y.W.; Wang, L.R.; Cheng, P.; Yu, J. Determine content of linoleic acid and squalene of acne patients sebum by HPLC. Chin. J. Aesthetic Med. 2016, 25, 5. [Google Scholar]
- Kong, S.Z.; Shi, X.G.; Feng, X.X.; Li, W.J.; Liu, W.H.; Chen, Z.W.; Xie, J.H.; Lai, X.P.; Zhang, S.X.; Zhang, X.J.; et al. Inhibitory effect of hydroxysafflor yellow a on mouse skin photoaging induced by ultraviolet irradiation. Rejuvenation Res. 2013, 16, 404–413. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Hrdinová, I.; Opálka, L.; Boncheva Bettex, M.; Vávrová, K. Time-Dependent Differences in the Effects of Oleic Acid and Oleyl Alcohol on the Human Skin Barrier. Mol. Pharm. 2023, 20, 6237–6245. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; He, H. The Role of Linoleic Acid in Skin and Hair Health: A Review. Int. J. Mol. Sci. 2024, 26, 246. [Google Scholar] [CrossRef]
- Manosalva, C.; Bahamonde, C.; Soto, F.; Leal, V.; Ojeda, C.; Cortés, C.; Alarcón, P.; Burgos, R.A. Linoleic Acid Induces Metabolic Reprogramming and Inhibits Oxidative and Inflammatory Effects in Keratinocytes Exposed to UVB Radiation. Int. J. Mol. Sci. 2024, 25, 10385. [Google Scholar] [CrossRef]
- Ito, J.; Komuro, M.; Parida, I.S.; Shimizu, N.; Kato, S.; Meguro, Y.; Ogura, Y.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. Evaluation of lipid oxidation mechanisms in beverages and cosmetics via analysis of lipid hydroperoxide isomers. Sci. Rep. 2019, 9, 7387. [Google Scholar] [CrossRef] [PubMed]
- Brami-Cherrier, K.; Chernavsky, A.; You, H.; Grando, S.A.; Brideau-Andersen, A.; Sondergaard, B. Botulinum Neurotoxin Type A Directly Affects Sebocytes and Modulates Oleic Acid-Induced Lipogenesis. Toxins 2022, 14, 708. [Google Scholar] [CrossRef]
- Kovács, D.; Camera, E.; Póliska, S.; Cavallo, A.; Maiellaro, M.; Dull, K.; Gruber, F.; Zouboulis, C.C.; Szegedi, A.; Törőcsik, D. Linoleic Acid Induced Changes in SZ95 Sebocytes-Comparison with Palmitic Acid and Arachidonic Acid. Nutrients 2023, 15, 3315. [Google Scholar] [CrossRef] [PubMed]
- Dahlan, N.H.; Sitohang, I.B.S.; Indriatmi, W.; Wibowo, H.; Enggy, L.E. Correlation Between Reduced IL-1β Levels in Acne Lesions and the Decrease in Acne Inflammatory Lesions Following Topical Vitamin D Administration: A Double-Blind Randomized Controlled Trial. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2183–2195. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.H.; Liu, Y.F.; Zouboulis, C.C.; Shi, G. Emodin exhibits anti-acne potential by inhibiting cell growth, lipogenesis, and inflammation in human SZ95 sebocytes. Sci. Rep. 2023, 13, 21576. [Google Scholar] [CrossRef]
- Wan, K.; Li, J.; Ma, L.; Chen, T.; Chen, Y.; Li, Z.; Zouboulis, C.C.; Wang, G.L.; Wang, J. Camellia saponin modulates oleic acid/linoleic acid-induced lipogenesis in human sebocytes through lipophagy activation. Int. J. Cosmet. Sci. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lin, X.Y. Research progress on acne and skin barrier. Chin. J. Integr. Tradit. West. Med. Dermatol. Venereol. 2021, 20, 416–419. [Google Scholar]
- Kawamoto, A.; Kuwano, T.; Watarai, E.; Igarashi, T.; Katayama, Y.; Kushida, K.; Nakamura, S.; Murase, T.; Yoshida, H.; Ishikawa, J. Oleic acid-induced interleukin-36γ: A possible link between facial skin redness and sebum. J. Cosmet. Dermatol. 2023, 22, 2308–2317. [Google Scholar] [CrossRef]
- Condrò, G.; Sciortino, R.; Perugini, P. Squalene Peroxidation and Biophysical Parameters in Acne-Prone Skin: A Pilot “In Vivo” Study. Pharmaceuticals 2023, 16, 1704. [Google Scholar] [CrossRef]
| Primer Name | Primer Sequence |
|---|---|
| PTGS2 | F:5′-TCTGAAACCCACTCCAAACACA-3′ |
| R:5′-CATTTCGAAGGAAGGGAATGTTATT-3′ | |
| PTGES | F:5′-GCTGATCACACCCACAGTTG-3′ |
| R:5′-CCAGGAAAAGGAAGGGGTAG-3′ | |
| IL-6 | F:5′-CATCCTCGACGGCATCTCAG-3′ |
| R:5′-TCACCAGGCAAGTCTCCTCA-3′ | |
| IL-18 | F:5′-CAGAAGRACTGADCTCGCC-3′ |
| R:5′-CAGAAGTACCTGAGCTCGCC-3′ | |
| GAPDH | F:5′-CAGGAGGCATTGCTGATGAT-3′ |
| R:5′-GAAGGCTGGGGCTCATTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-R.; Zhang, Q.-R.; Zhou, Z.-Y.; Zhang, Y.-F.; Li, X.-W.; Shen, H.-Y.; Tang, L.-F.; Xiang, Q. Oxidative-Inflammatory Modulation of Skin Lipid Metabolism by Squalane, Oleic Acid, and Linoleic Acid. Cosmetics 2025, 12, 130. https://doi.org/10.3390/cosmetics12040130
Zhang W-R, Zhang Q-R, Zhou Z-Y, Zhang Y-F, Li X-W, Shen H-Y, Tang L-F, Xiang Q. Oxidative-Inflammatory Modulation of Skin Lipid Metabolism by Squalane, Oleic Acid, and Linoleic Acid. Cosmetics. 2025; 12(4):130. https://doi.org/10.3390/cosmetics12040130
Chicago/Turabian StyleZhang, Wen-Rong, Qi-Rong Zhang, Zi-Yan Zhou, Yi-Fan Zhang, Xue-Wan Li, Hai-Yang Shen, Li-Feng Tang, and Qi Xiang. 2025. "Oxidative-Inflammatory Modulation of Skin Lipid Metabolism by Squalane, Oleic Acid, and Linoleic Acid" Cosmetics 12, no. 4: 130. https://doi.org/10.3390/cosmetics12040130
APA StyleZhang, W.-R., Zhang, Q.-R., Zhou, Z.-Y., Zhang, Y.-F., Li, X.-W., Shen, H.-Y., Tang, L.-F., & Xiang, Q. (2025). Oxidative-Inflammatory Modulation of Skin Lipid Metabolism by Squalane, Oleic Acid, and Linoleic Acid. Cosmetics, 12(4), 130. https://doi.org/10.3390/cosmetics12040130









