Abstract
Alopecia areata (AA) is an autoimmune disorder causing non-scarring hair loss, which is often triggered by psychological stress. Conventional treatments, such as corticosteroids and immunotherapy, show variable efficacy and can cause side effects like hair discoloration. Exosome therapy, utilizing extracellular vesicles, presents a promising alternative, though its use in stress-related AA remains underexplored. A 39-year-old male with unifocal AA on the right parietal scalp developed hair loss following emotional distress after his fiancée’s death. Methotrexate and prednisolone were ineffective, prompting a bioregenerative approach using rose stem cell-derived exosomes (RSCEs) combined with thulium laser therapy. Six monthly sessions of RSCEs (20 mg/vial, 10 billion exosomes) were administered, with laser pre-treatment enhancing absorption. Within one month, vellus hair regrowth appeared, progressing to an increased density and pigmentation at three months. By six months, complete regrowth and natural pigmentation were achieved, with reduced inflammation confirmed by trichoscopy. The therapy was well-tolerated, with no adverse effects. This case highlights RSCE therapy as a promising treatment for stress-induced AA, achieving significant regrowth without corticosteroid-related side effects. Further studies are needed to validate its efficacy and refine protocols for broader clinical applications.
1. Introduction
Alopecia areata (AA) is an autoimmune disorder characterized by non-scaring hair loss, often presenting in distinct, round patches on the scalp. While the exact etiology remains unclear, the condition is known to involve a complex interplay of genetic, environmental, and immunological factors [1,2]. AA affects approximately 2% of the global population with a higher prevalence in children than in adults, increasing over time and varying significantly by region [3]. Among the various triggers, psychological stress has been widely recognized as a significant precipitant, often exacerbating the onset and progression of the AA disease [4,5].
In recent years, the role of psychological disorders in the pathogenesis of AA has garnered considerable attention [6]. Stress and anxiety are known to alter the immune response, potentially triggering autoimmune reactions that target hair follicles. The bidirectional relationship between psychological stress and AA underscores the need for a holistic treatment approach that addresses both the dermatological and psychological aspects of the disorder [4,7]. Traditional treatments for alopecia areata, including corticosteroids and immunotherapy, often provide variable results and may be associated with significant side effects [8,9]. One notable side effect of corticosteroid use is hair discoloration, where regrown hair can turn white, significantly affecting the cosmetic outcome for patients [10]. This has led to an exploration of novel therapeutic options that offer a more favorable safety profile and enhanced efficacy. One such promising treatment of AA is exosome therapy, which leverages the regenerative potential of extracellular vesicles to promote hair regrowth and modulate immune responses [11].
Exosomes are nano-sized vesicles secreted by various cell types, playing a crucial role in intercellular communication. They are rich in bioactive molecules, such as proteins, lipids, and nucleic acids, which can influence cellular processes and promote tissue regeneration [12]. In the context of AA, exosome therapy may have the potential to modulate the immune response and stimulate hair follicle activity, offering a novel and less invasive treatment option. However, there are currently no studies specifically investigating the use of exosomes in AA, particularly in cases associated with psychological stress. Importantly, exosome therapy allows hair regrowth with the original color, addressing a significant limitation associated with corticosteroid treatments [13].
Here, we present, for the first time, a patient with a history of psychological disorders who developed unifocal AA, which was successfully treated with the application of rose stem cell-derived exosomes (RSCEs) (Exocobio®) [14,15], resulting in complete hair regrowth and the restoration of the natural hair color. This study was conducted in accordance with CARE guidelines [16] (Supplementary File S1), emphasizing the importance of an interdisciplinary approach in managing complex cases of alopecia areata, particularly when associated with psychological disorders.
2. Case Report
A 39-year-old male presented with progressive hair loss localized in the right parietal region of the scalp. The onset of alopecia began in July 2022, approximately 11 months following significant emotional distress due to the death of his fiancée from cancer in August 2021. The patient initially consulted a dermatologist in August 2022 and was prescribed methotrexate (2.5 mg), prednisolone (20 mg), folic acid (5 mg), and esomeprazole (20 mg). Despite adhering to the prescribed treatment regimen until February 2023, no notable clinical improvement was observed.
By late February 2023, the patient sought specialized consultation at the Trichology Center, Dr. Forjaz de Sampaio Medical Center in Ponta Delgada, Azores, Portugal. A thorough clinical assessment, including a detailed history, physical examination, and trichoscopy, was performed. The physical examination revealed a positive pull test and visible signs of scalp inflammation (erythema) in the affected area. Trichoscopic findings were consistent with AA, maintaining hair loss in the right parietal region of the scalp, approximately 5 cm in diameter, even after the use of conventional medication.
An innovative bioregenerative trichology protocol was proposed, involving the application of plant-derived exosomes (ExoCoBio Inc., Seoul, Republic of Korea) [15]. Exosomes from the ExoCoBio Inc. brand, Scalp Care-HRLV type®, were administered at 20 mg/vial, containing 10 billion pure exosomes. Treatment sessions included the prior use of a thulium laser (1927 nm, 5 W energy, 3 mJ power) applied once per month, totaling six sessions. An initial clinical documentation, including photographs and trichoscopic imaging from February 2024, was recorded. Follow-up evaluations at one, three, and six months post-treatment were performed.
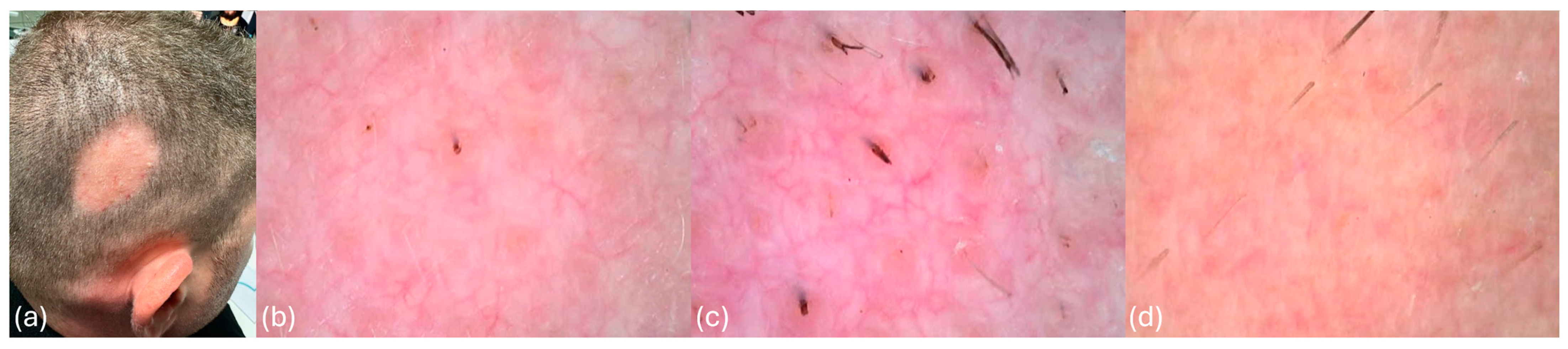
At diagnosis, the patient presented with unifocal AA, localized to the right parietal region of the scalp, measuring approximately 5 cm in diameter. Clinical and trichoscopic findings at diagnosis revealed characteristic features of AA, including erythema, arborizing vessels, yellow dots, black dots, and flame-shaped hairs, as documented in Figure 1a–d. The pull test was positive, and the scalp exhibited visible signs of inflammation. These findings confirmed a persistent, active inflammatory process resistant to previous conventional therapies.
Figure 1.
(a) Patient demonstrating unifocal alopecia areata at diagnosis, characterized by an oval-shaped patch approximately 5 cm in diameter. (b) Trichoscopy image showing erythema, arborizing vessels, yellow dots, and black dots, characteristic of alopecia areata. (c) Trichoscopy image revealing erythema, arborizing vessels, yellow dots, black dots, and flame-shaped hairs. (d) Trichoscopy image showing erythema, yellow dots, black dots, and exclamation-mark-shaped hairs.
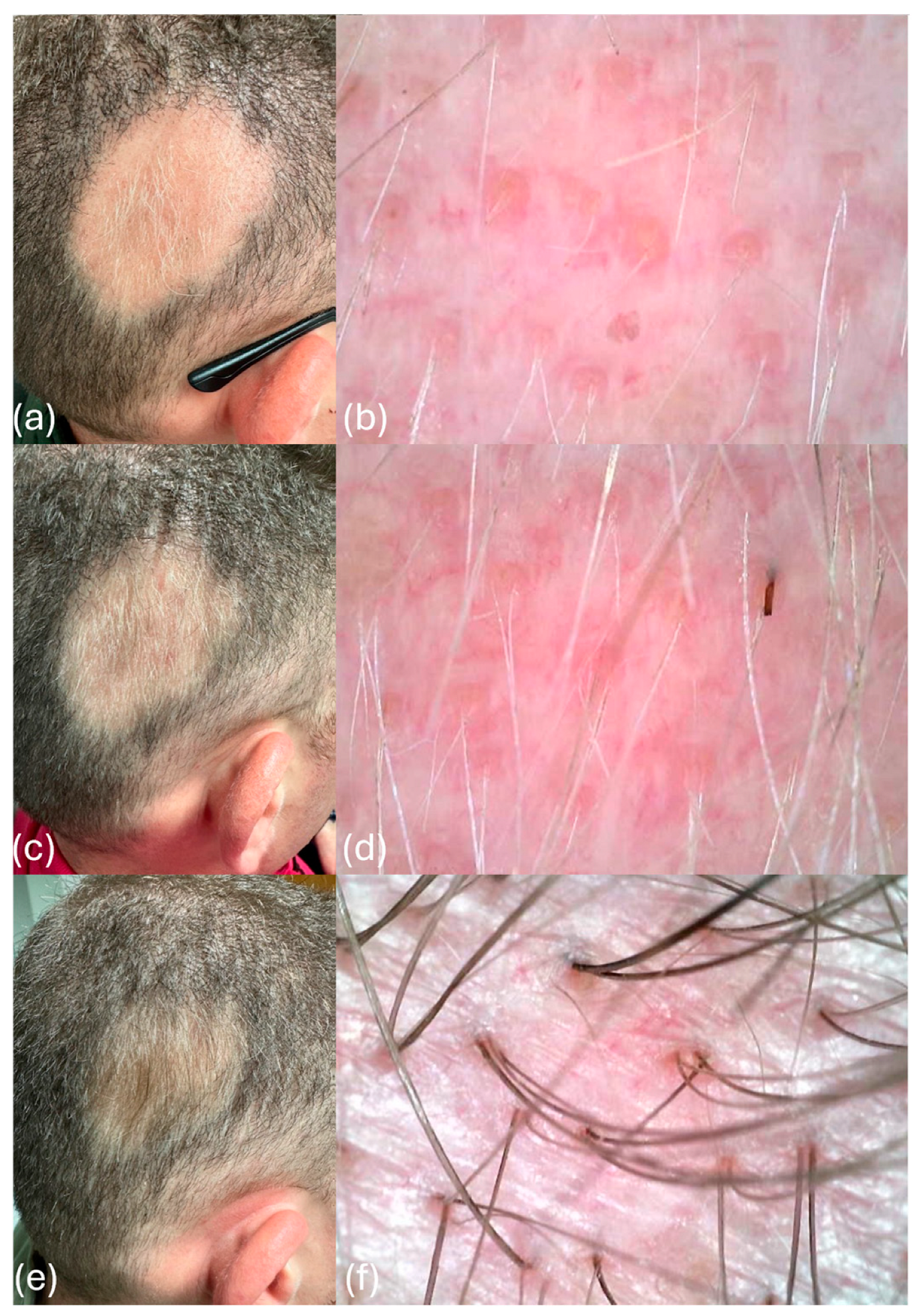
After the first month of treatment, initial signs of hair regrowth were observed. The patient exhibited central regrowth characterized by the presence of vellus hairs approximately 1 cm in length, as shown in Figure 2a. Trichoscopy revealed a mild inflammatory process, with single and double vellus hairs per follicular unit (Figure 2b). These findings indicated a promising response to the bioregenerative protocol. After the third month of treatment, further clinical improvement was evident. The diffuse regrowth of vellus hairs was observed, with the central region beginning to show signs of repigmentation (Figure 2c). A trichoscopic examination demonstrated increased hair density and diameter, with mild erythema persisting, indicative of a reduced inflammatory process (Figure 2d). The combination of hair regrowth and repigmentation marked a significant progression in the treatment response.
Figure 2.
(a) One month after treatment, the patient exhibits central regrowth, characterized by the presence of vellus hairs approximately 1 cm in length. (b) On trichoscopy analysis, the patient displays a mild inflammatory process, with single and double vellus hairs per follicular unit one month after treatment. (c) Three months after treatment, there is an increased presence of vellus hairs, indicating diffuse regrowth and the beginning of repigmentation in the central region. (d) The patient presents mild erythema, suggestive of an ongoing inflammatory process, with an increased density and diameter of vellus hairs and initial repigmentation at the base of the hair shaft three months after treatment. (e) Six months after treatment, complete regrowth is observed, with a significant increase in hair density and pigmentation. (f) Improvement in the inflammatory process, with an increased hair diameter and density (presence of 2–3 hairs per follicular unit), along with complete pigmentation of the hair shaft six months after treatment.
After the 6-month follow-up, the patient exhibited the complete regrowth of hair in the previously affected area. A notable increase in hair density and pigmentation was observed, with a significant improvement in the inflammatory process. Trichoscopy findings revealed the presence of 2–3 hairs per follicular unit, complete pigmentation of the hair shafts, and a substantial reduction in inflammatory markers (Figure 2e,f). After undergoing the bioregenerative trichology protocol, the patient noted significant improvements, including visible hair regrowth and reduced inflammation. He described feeling optimistic and grateful for the innovative approach, which not only restored his hair but also positively impacted on his self-esteem and overall well-being.
These results highlight the effectiveness of the bioregenerative trichology protocol, showcasing a promising therapeutic approach for resistant cases of alopecia areata. The six-month post-treatment follow-up showed significant hair regrowth and follicular repigmentation. The improvements observed underscored the regenerative potential of the exosome-based therapy, achieved without additional pharmacological interventions.
3. Discussion
Psychological stress is a well-documented trigger for AA, with a stress-induced dysregulation of the hypothalamic–pituitary–adrenal axis contributing to immune system activation and the inflammation of the hair follicles [4]. This bidirectional relationship between stress and AA has been reported in multiple studies, emphasizing the importance of integrating psychosocial management into treatment plans [17,18]. The patient’s history of significant emotional distress aligns with findings that stress-related immune dysregulation can exacerbate AA [16], suggesting that addressing the underlying psychological factors is critical to achieving sustained remission.
Regarding the RSCEs, Won et al. [14] described the isolation and characterization of exosome-like particles derived from rose stem cell culture supernatants, highlighting their structural and functional similarities to mammalian exosomes. These RSCEs exhibited multiple beneficial biological activities, including promoting human dermal fibroblast proliferation, enhancing collagen synthesis, and accelerating wound closure in vitro. Furthermore, the RSCEs demonstrated anti-inflammatory properties by reducing the IL-6 secretion in macrophages and showed potential in pigmentation modulation by decreasing the melanin accumulation in melanocytes. The study suggests that RSCEs have promising applications in esthetic medicine and cosmeceuticals due to their regenerative and anti-inflammatory properties, while also emphasizing their non-toxic nature and potential for sustainable skincare development [14].
The application of exosomes in AA therapy is a rapidly emerging field, offering a novel mechanism to modulate immune responses and promote tissue regeneration. Exosomes are enriched with anti-inflammatory and regenerative bioactive molecules, such as growth factors, cytokines, and miRNAs, which play key roles in restoring follicular health. Recently, Norooznezhad et al. [19] reported the successful treatment of persistent chemotherapy-induced alopecia (PCIA) in a 36-year-old woman using human mesenchymal stromal cell (MSC)-derived exosome-enriched extracellular vesicles (EVs); the patient achieved complete scalp hair regrowth after three monthly subcutaneous injections of EVs, administered 18 months post-chemotherapy [19]. This suggests MSC-derived EVs as a potential treatment for PCIA, although further studies are needed. Additionally, Lee et al. demonstrated the efficacy of adipose-derived stem cell (ASC)-derived exosomes in hair regeneration through a combination of preclinical and clinical studies. They found that ASC-derived exosomes significantly enhanced hair follicle dermal papilla cell proliferation, upregulated hair growth-related genes, and activated the Wnt/β-catenin signaling pathway. In a clinical study involving 30 patients with androgenetic alopecia (AGA), the treatment led to a statistically significant increase in hair density and improved global photographic assessments over a 24-week period, without severe adverse reactions [20]. On the other hand, studies on plant-derived exosomes, such as RSCEs, have demonstrated their ability to suppress inflammatory pathways, stimulate follicular stem cells, and improve the hair regrowth process [21,22]. This aligns with the case’s outcome, where a significant hair regrowth and follicular repigmentation were observed after six months. However, there are no reports on the use of exosomes for hair regrowth in patients with AA diagnosed with psychological disorders [18].
Here, we report a case of a 39-year-old male with AA resistant to conventional treatment who underwent a bioregenerative protocol using RSCEs and thulium laser therapy. Within six months, he achieved complete hair regrowth, follicular repigmentation, and reduced inflammation. This case highlights the potential of exosome-based therapy as an effective treatment for refractory AA. The successful treatment of AA using RSCEs, as presented in this case report, underscores the therapeutic potential of exosome-based therapy in addressing both the regrowth and repigmentation challenges commonly associated with this autoimmune condition.
The hair repigmentation, a frequent limitation of corticosteroid therapies due to melanocyte suppression, represents a significant achievement in this case. The observed restoration of the natural hair color in our case aligns with findings suggesting that exosomes may enhance the melanocyte activity and melanogenesis pathways [23]. This unique advantage not only improves cosmetic outcomes but also addresses a common concern for AA patients undergoing treatment. Additionally, the adjunctive use of a thulium laser to enhance the penetration and efficacy of the exosome therapy represents another innovative aspect of the treatment protocol [24,25]. Laser therapy has been shown to improve the delivery of active compounds and stimulate local blood flow, creating a conducive environment for follicular regeneration in AA patients [24,25]. This synergistic approach likely contributed to the rapid and sustained improvement observed in our case.
Traditional AA treatments, including corticosteroids and immunotherapy, often carry risks of systemic side effects and incomplete responses [8]. The case aligns with studies suggesting that exosome-based therapies offer a safer alternative with fewer side effects and better patient adherence [21,22]. Moreover, the absence of additional pharmacological interventions post-treatment highlights the self-sustaining regenerative potential of exosomes. While this case demonstrates the potential of plant-derived exosome therapy in treating AA, larger-scale studies are needed to establish its efficacy and safety across diverse populations. Additionally, the exact molecular mechanisms underlying the observed hair regrowth and repigmentation remain to be elucidated. Long-term follow-up studies could further validate the durability of these outcomes and explore their generalizability to multifocal or extensive AA cases.
This case report provides compelling evidence for the use of plant-derived exosomes, such as RSCEs, as an innovative treatment for AA, particularly in cases associated with psychological stress. The observed outcomes—complete hair regrowth and the restoration of the natural hair color—highlight the potential of exosome therapy to address the limitations of conventional treatments. By integrating regenerative and immune-modulatory mechanisms, exosome-based protocols represent a promising frontier in the management of alopecia areata. Further research is warranted to confirm these findings and optimize treatment protocols for broader clinical applications.
4. Conclusions
In summary, the patient with AA was successfully treated with the plant-derived exosome therapy, resulting in significant hair regrowth after six monthly applications. This case underscores the potential of plant-derived exosome therapy as a viable treatment option for alopecia areata, particularly in patients with concomitant psychological disorders. This comprehensive approach not only ensures effective treatment but also highlights the potential of innovative therapies like exosome therapy in restoring the natural hair color and growth.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cosmetics12030097/s1: Supplementary Material S1 includes the CARE guideline checklist form.
Author Contributions
E.B.B., C.M. and H.L.R.J. have significantly contributed to the investigation, development, and writing of this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In accordance with Portuguese national legislation (Law No. 21/2014, of April 16), which regulates clinical research involving human participants, we respectfully clarify that individual case reports are not considered clinical studies under Article 2 of this law. This is because they do not involve experimental protocols, prospective data collection, or systematic investigation for the purpose of testing hypotheses. In this specific case, no clinical procedures were performed for research purposes. The patient was submitted solely to conventional trichology clinic treatment, including the use of exosomes as part of routine management for alopecia areata. All clinical decisions were made strictly for therapeutic purposes and not within the scope of a research study. The data presented in the manuscript are purely retrospective, based on medical documentation gathered during standard follow-up.
Informed Consent Statement
Written informed consent for publication was obtained from the patient involved in this study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. No additional data is available.
Acknowledgments
We would like to express our gratitude to ExoCoBio® for their support and contribution to this study, which made this successful treatment possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | alopecia areata |
| AGA | androgenetic alopecia |
| ASC | adipose-derived stem cell |
| MSC | mesenchymal stromal cell |
| PCIA | persistent chemotherapy-induced alopecia |
| RSCWs | rose stem cell-derived exosomes |
References
- Dahabreh, D.; Jung, S.; Renert-Yuval, Y.; Bar, J.; Del Duca, E.; Guttman-Yassky, E. Alopecia Areata: Current Treatments and New Directions. Am. J. Clin. Dermatol. 2023, 24, 895–912. [Google Scholar] [CrossRef]
- Rudnicka, L.; Arenbergerova, M.; Grimalt, R.; Ioannides, D.; Katoulis, A.C.; Lazaridou, E.; Olszewska, M.; Ovcharenko, Y.S.; Piraccini, B.M.; Prohic, A.; et al. European expert consensus statement on the systemic treatment of alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Iwata, M.; Kaku, Y. Alopecia areata: What’s new in the epidemiology, comorbidities, and pathogenesis? J. Dermatol. Sci. 2023, 112, 120–127. [Google Scholar] [CrossRef]
- Ahn, D.; Kim, H.; Lee, B.; Hahm, D.H. Psychological Stress-Induced Pathogenesis of Alopecia Areata: Autoimmune and Apoptotic Pathways. Int. J. Mol. Sci. 2023, 24, 11711. [Google Scholar] [CrossRef] [PubMed]
- Torales, J.; Castaldelli-Maia, J.M.; Ventriglio, A.; Almirón-Santacruz, J.; Barrios, I.; O’Higgins, M.; García, O.; Navarro, R.; Melgarejo, O.; Jafferany, M. Alopecia areata: A psychodermatological perspective. J. Cosmet. Dermatol. 2022, 21, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef]
- Moattari, C.R.; Jafferany, M. Psychological Aspects of Hair Disorders: Consideration for Dermatologists, Cosmetologists, Aesthetic, and Plastic Surgeons. Skin. Appendage Disord. 2022, 8, 186–194. [Google Scholar] [CrossRef]
- Malhotra, K.; Madke, B. An Updated Review on Current Treatment of Alopecia Areata and Newer Therapeutic Options. Int. J. Trichol. 2023, 15, 3–12. [Google Scholar] [CrossRef]
- Lintzeri, D.A.; Constantinou, A.; Hillmann, K.; Ghoreschi, K.; Vogt, A.; Blume-Peytavi, U. Alopecia areata—Current understanding and management. J. Dtsch. Dermatol. Ges. 2022, 20, 59–90. [Google Scholar] [CrossRef]
- Stacey, S.K.; McEleney, M. Topical Corticosteroids: Choice and Application. Am. Fam. Physician 2021, 103, 337–343. [Google Scholar]
- Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin. Exp. Med. 2024, 24, 46. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, J.; Ji, Q.; Xie, S.; Jiang, J.; Ni, H.; He, X.; Yang, Y.; Wu, M. Exosomes derived from uMSCs promote hair regrowth in alopecia areata through accelerating human hair follicular keratinocyte proliferation and migration. Cell Biol. Int. 2024, 48, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.J.; Lee, E.; Min, S.Y.; Cho, B.S. Biological function of exosome-like particles isolated from rose (Rosa damascena) stem cell culture supernatant. bioRxiv 2023. [Google Scholar] [CrossRef]
- Exocobio Inc. Cosmetic Composition Comprising Rose Stem Cell-Derived Exosome as Effective Ingredient. Eur Pat EP3815675A1, 5 March 2025. [Google Scholar]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Marahatta, S.; Agrawal, S.; Adhikari, B.R. Psychological Impact of Alopecia Areata. Dermatol. Res. Pract. 2020, 2020, 8879343. [Google Scholar] [CrossRef]
- Gandhi, K.; Shy, M.E.; Ray, M.; Fridman, M.; Vaghela, S.; Mostaghimi, A. The Association of Alopecia Areata-Related Emotional Symptoms with Work Productivity and Daily Activity Among Patients with Alopecia Areata. Dermatol. Ther. 2023, 13, 285–298. [Google Scholar] [CrossRef]
- Norooznezhad, A.H.; Yarani, R.; Payandeh, M.; Hoseinkhani, Z.; Mahmoudi, H.; Kiani, S.; Mansouri, K. Treatment of persistent chemotherapy-induced hair loss (Alopecia) with human mesenchymal stromal cells exosome enriched extracellular vesicles: A case report. Heliyon 2023, 9, e15165. [Google Scholar] [CrossRef]
- Lee, E.; Choi, M.S.; Cho, B.S.; Won, Y.J.; Lee, J.H.; Kim, S.R.; Kim, M.H.; Jeon, J.H.; Park, G.H.; Kwon, H.H.; et al. The efficacy of adipose stem cell-derived exosomes in hair regeneration based on a preclinical and clinical study. Int. J. Dermatol. 2024, 63, 1212–1220. [Google Scholar] [CrossRef]
- Lueangarun, S.; Cho, B.S.; Tempark, T. Rose stem cell-derived exosomes for hair regeneration enhancement via noninvasive electroporation in androgenetic alopecia. J. Cosmet. Dermatol. 2024, 23, 3791–3794. [Google Scholar] [CrossRef]
- Lueangarun, S.; Cho, B.S.; Tempark, T. Hair repigmentation of poliosis circumscripta in androgenetic alopecia patient treated with exosomes and fractional picosecond laser. J. Cosmet. Dermatol. 2024, 23, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.B.; Goo, B.L.; Zheng, Z.; Yoo, K.H.; Kang, J.S.; Kim, H. Therapeutic efficacy and safety of a 1927-nm fractionated thulium laser on pattern hair loss: An evaluator-blinded, split-scalp study. Lasers Med. Sci. 2018, 33, 851–859. [Google Scholar] [CrossRef]
- Jedlowski, P.M.; Anthony, M. Use of fractionated laser therapy for the treatment of androgenetic alopecia: A systematic review and meta-analysis. Lasers Med. Sci. 2023, 39, 4. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).