1. Introduction
Currently, the cosmetic industry is growing rapidly, especially the natural personal care segment, through innovative strategies. Various techniques, including nanoemulsions, microemulsions, and liquid crystal emulsions, have been employed to enhance the efficacy, sensory properties, and stability of cosmetic formulations compared to conventional ones. Nanoemulsions are a type of emulsion that consists of two immiscible liquids stabilized by emulsifying agents. They are characterized by small particle sizes, typically ranging from 50 to 500 nm, and exhibit superior kinetic and thermodynamic stability compared to conventional emulsions [
1,
2]. Nanoemulsions are produced by both low-energy methods (transition phase inversion, self-emulsification) and high-energy methods (ultrasonication, high-pressure homogenization) [
1,
3]. High-energy emulsification methods are commonly employed to create homogeneous nanoemulsions by breaking down the macroemulsion into nano-sized droplets. These methods are widely utilized in the production of cosmetics, food, and pharmaceuticals. The particle size of nanoemulsions produced by high-energy methods depends on factors such as the composition of the nanoemulsion, the type of homogenizer, and the operating conditions, including time and temperature [
3,
4]. In cosmetic topical applications, particularly in skincare products, nanoemulsions are used to deliver active ingredients or natural raw materials, especially plant extracts, targeting anti-aging, anti-wrinkle, skin hydration, skin brightening, and UV protection [
5,
6,
7,
8,
9]. Furthermore, nanoemulsions can enhance the poor solubility of active ingredients, ensure the stability of natural materials used in cosmetics, and provide excellent skin penetration along with long-lasting effects [
9,
10].
Peristrophe bivalvis (L.) Merr. (see
Figure 1), commonly known as the Magenta plant, belongs to the Acanthaceae family and is widely distributed across tropical regions of Asia and Southeast Asia, including China, India, Vietnam, Indonesia, Malaysia, Cambodia, and Thailand. It is also found in Nigeria, Africa [
11,
12,
13]. This plant is an herbaceous perennial that can grow to a height of 0.3–1 m. Its green leaves have an oval-lanceolate shape, measuring 2–3 cm in width and 4–9 cm in length, and they impart a sweet taste. The flowers are rose-purple, magenta, or reddish-violet in color, composed of two lobes with a long axis of up to 5 cm. The sepals are lanceolate and 4–6 mm long, while the peduncle measures 3–4 cm in length [
13].
This plant extract has traditionally been used as a colorant in certain foods, such as dumplings, taro-filled cakes, and
banh tet in Vietnam [
13].
Peristrophe bivalvis has also been traditionally used in herbal medicine for treating diabetes, tuberculosis, hepatitis, and hypertension in China [
14,
15]. In Malaysia, its ground fresh leaves have been used to treat skin diseases, swelling, and painful sprains [
13], while in Nigeria, boiled leaves have been used as a blood tonic and for general well-being [
14]. In addition, various research on the bioactivities of
P. bivalvis extract also indicates that it has anti-hypertensive, anti-lipidemic [
14,
16], anti-microbial [
17,
18], antioxidant [
16], anti-inflammatory [
16], and anticancer [
19] properties. The main chemical constituents of
P. bivalvis leaves were anthocyanidin (pelargonidin), anthocyanins (afzelechin (4-8) pelargonidyl glucoside, epiafzelechin (4α-8) pelargonidin 3-O-β-glucopiranose), terpenoids (carotenoids), and alkaloids (peristrophine) [
13]. In addition, other phytochemical components of this plant, including cyanidin, cyanidin-3-O-β-D-glucoside, β-sitosterol, and coumarin, have also been reported [
13,
20]. The safety of the aqueous extract of
P. bivalvis leaves was assessed through acute toxicity testing in mice, which showed no recorded death in mice orally administered the extract at 6250 mg/kg body weight. The LD
50 was determined to be 9100 ± 290 mg/kg body weight [
21]. Based on its traditional use and results from acute toxicity testing, the
P. bivalvis is considered to have low toxicity and be safe. Despite its numerous health-related benefits and pharmacological properties, there are limited data on its application as a natural active ingredient in cosmetics formulations, particularly in relation to its potential to prevent skin aging through the inhibition of glycation and lipid peroxidation processes by various solvent extracts.
To enhance the value of the products based on this plant, the present study, for the first time, explores its bioactivity—specifically anti-glycation and anti-lipid peroxidation—for potential cosmetic and cosmeceutical applications, evaluates its cytotoxicity on skin cells, and investigates its incorporation into nanoemulsions. Accordingly, the purpose of this study was to investigate the potential use of P. bivalvis leaf crude extracts as a natural active ingredient for cosmetic applications. The phytochemical constituents of the crude extracts were screened, and their antioxidants, anti-glycation, anti-lipid peroxidative activities, as well as their skin cytotoxicity, were evaluated. Furthermore, the P. bivalvis leaf crude extracts as a natural active ingredient were incorporated into a nanoemulsion, and the stability of this nanoemulsion was also investigated.
2. Materials and Methods
2.1. Chemical and Reagents
The 95% ethanol, dimethyl sulfoxide (DMSO), hydrochloric acid, and sulfuric acid (H2SO4) were sourced from RCI Labscan (Bangkok, Thailand). Gallic acid, Trolox, quercetin, acetic anhydride, aluminum chloride, calcium chloride, ferric chloride (FeCl3), potassium persulphate, sodium acetate, sodium carbonate, sodium chloride, sodium hydroxide, ABTS (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), DPPH (2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl), Folin–Ciocalteu’s phenol reagent, fetal bovine serum (FBS), and Dulbecco’s Modified Eagle Medium (DMEM: high glucose) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from a supplier in Canada (Bio Basic, Inc., Markham, ON, Canada). All standards, reagents, and chemicals were of analytical grade. Propylene glycol, squalane, tocopheryl acetate, and diazolidinyl urea (and) iodopropynyl butylcarbamate (and) propylene glycol were purchased from Namsiang Group (Namsiang Co., Ltd., Bangkok, Thailand). Propanediol, PEG-30 dipolyhydroxystearate, polyglyceryl-3 diisostearate, caprylic/capric triglyceride (CCT), and jojoba oil were purchased from Chemecosmetics (Bangkok, Thailand). Magnesium sulfate was purchased from MySkinRecipes (Bangkok, Thailand).
2.2. Plant Material and Extraction
The leaves of P. bivalvis were collected from Mae Sai, Chiang Rai, Thailand, and identified by an expert. The voucher herbarium specimen (No. Wichayapreechar 002) was deposited at the School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand. The leaves were cleaned, dried at 50 °C in a hot air oven for 2 days, and then powdered using a blender.
To obtain water and acidic P. bivalvis leaf crude extracts (WE and AE), 100 g of powdered leaf material was macerated in 1 L of deionized water (DI water) for three days, while the acidic crude extract was macerated in a 1% hydrochloric acid in 50% ethanolic solution for 3 days. These extract solutions were then filtered and lyophilized using a freezer dryer. For the ethanolic P. bivalvis leaf crude extract (EE), 100 g of powdered leaf material was macerated in 1 L of 95% ethanol, filtered, and evaporated using a rotary evaporator. The P. bivalvis crude extracts were stored at −20 °C until further investigation.
2.3. Phytochemical Screening
The P. bivalvis extracts were screened for phytochemical constituents, including phenolics, flavonoids, anthocyanins, coumarins, saponins, tannins, terpenoids, steroids, and alkaloids. Each extract (10 mg/mL) was prepared by dissolving it in water for aqueous and acidic extracts and in 95% ethanol for the ethanolic extract. The qualitative secondary metabolites of the crude extracts were then determined by observing color changes in each assay.
2.3.1. Phenolics Test
The
P. bivalvis extract solutions were determined for phenolic compounds using the ferric chloride test. In each extract solution prepared in a test tube, 3–4 drops of 10%FeCl
3 was added and any color change was observed (blue, violet, or dark green colors indicating the presence of phenolic compounds) [
22,
23].
2.3.2. Flavonoids and Anthocyanins Tests
The crude extract solutions were tested for flavonoid and anthocyanin constituents using Shinoda’s test and an acid-base reaction, respectively [
24]. For Shinoda’s test, 2 pieces of magnesium ribbon were added to the test solution, followed by 2–3 drops of concentrated HCl. The presence of an orange-red color indicated the presence of flavonoids. For the anthocyanidin test, NH
3 T.S (3 drops) in aqueous solution was used as the basic condition, and 2N HCl was used as the acidic condition. The appearance of a blue-green color in the basic condition and a red-violet color in the acidic condition indicated the presence of anthocyanidins.
2.3.3. Coumarins Test
The crude extract solutions were assessed for coumarins using a fluorescence test. The crude extract solution tube was placed in a water bath covered with a filter membrane for 5 min. Then, 1–2 drops of 10% NaOH were added to the membrane filter and observed under 366 nm UV light. A greenish or yellowish light-blue fluorescence indicated the presence of coumarin compounds.
2.3.4. Tannins Test
The extract solutions were determined for tannin content using the ferric chloride test and the gelatin test [
24]. For the ferric chloride test, 2–3 drops of 10% FeCl
3 were added to the test solution, while 5 drops of 2% gelatin were used for the gelatin test. In the ferric chloride test, the presence of a blackish-blue color indicated gallic tannins, while a blackish-green color indicated catechol tannins. In the gelatin test, the formation of a white precipitate confirmed the presence of tannin compounds.
2.3.5. Terpenoids and Steroids Tests
Each extract was tested for terpenoid and steroid constituents using the Liebermann–Burchard test. The reaction between acetic anhydride, concentrated sulfuric acid, and terpenoids or steroids results in a color change. In this test, 2–3 drops of acetic anhydride were added to the crude extract, followed by 3–4 drops of concentrated sulfuric acid. The presence of a greenish-blue color indicates steroid content, while a reddish-purple or reddish-brown color suggests the presence of terpenoids [
24,
25].
2.3.6. Saponins Test
Each extract was tested for saponins content using the froth test in a 50 mL test tube. A total of 10 mL of water were added to the crude extract sample, shaken vigorously for about 5 min, and left to stand for 30 min. The presence of a honeycomb-like froth indicated the presence of saponins [
24].
2.3.7. Alkaloids Test
In this test, Dragendorff’s, Mayer’s, and tannic acid reagents were used for screening alkaloid content in each extract solution. For the Dragendorff test, 2 mL of Dragendorff reagent was added to the test solution, and the formation of an orange, orange-red, or reddish-brown precipitate indicated the presence of alkaloid compounds [
22,
24,
26]. For Mayer’s test, 1 mL of Mayer reagent was added to the test solution, and a white-yellow precipitate indicated the presence of alkaloid compounds. Similarly, the addition of 1 mL of tannic acid resulted in a white precipitate, confirming the presence of alkaloids.
2.4. Total Phenolic and Flavonoids Contents
2.4.1. Total Phenolic Contents (TP)
The TP of
P. bivalvis extracts were assessed using the Folin–Ciocalteu colorimetric method following previous description [
27,
28]. A 1 mg/mL solution of each crude extract was prepared and mixed in a 96-well plate with 10% Folin–Ciocalteu reagent. Then, 10% Na
2CO
3 was added, mixed well, and left to react for 30 min. The absorbance was then measured using a microplate reader (Synergy H1, BioTek Instruments, Winooski, VT, USA) at 756 nm. Gallic acid was used to create a standard curve with final concentrations ranging from 0.1 to 18 µg/mL.
2.4.2. Total Flavonoids Contents (TF)
The TF of
P. bivalvis extracts was determined using the aluminum chloride method, following a previously reported protocol [
27,
28]. A 1 mg/mL solution of each crude extract was prepared, mixed with 2% AlCl
3 in a 96-well plate, and left to react for 60 min. The absorbance was then measured at 420 nm (BioTek Instruments), and quercetin (with final concentrations ranging from 0.63 to 50 µg/mL) was used to create a standard calibration curve.
2.5. Antioxidant Activity
The P. bivalvis extracts were investigated for their ability to exhibit antioxidant activities using DPPH, ABTS, and FRAP assays.
2.5.1. DPPH Assay
The antioxidant activity of
P. bivalvis extracts via the hydrogen atom transfer mechanism was evaluated using the DPPH assay, following the method described earlier [
27]. Briefly, various concentrations of
P. bivalvis extracts were prepared. Water and acidic crude extracts (at working concentrations of 150–3000 µg/mL) were prepared in 30% Tween 80, while ethanolic extracts (at working concentrations of 18–1200 µg/mL) were dissolved in 95% ethanol. The extract solutions were then mixed with a 0.2 mM DPPH solution in a 96-well plate and incubated in the dark for 30 min. Subsequently, the absorbance of the test samples was measured at 515 nm (BioTek Instruments). Trolox was used as the positive control, and the antioxidant activity was expressed as IC
50 value.
2.5.2. ABTS Assay
The antioxidant activity of
P. bivalvis extracts via the scavenging of radical cations was performed using the ABTS assay, following a previously reported method [
25]. Water
P. bivalvis extracts (at a working concentration of 9–600 µg/mL) and acidic and ethanolic
P. bivalvis extracts (at a working concentration of 30–3000 µg/mL) were prepared and mixed with an incubated (12 h) mixture of 0.7 mM ABTS reagent solution and 2.45 mM potassium persulfate. The test plate was then left for 6 min and read using a microplate reader at 734 nm (BioTek Instruments). Trolox (at a working concentration of 1.5–180 µg/mL) was used as the positive control, and the cationic scavenging ability of the extracts was expressed as the IC
50 value.
2.5.3. FRAP Assay
The antioxidant activity of
P. bivalvis extracts via the electron transfer mechanism was evaluated using the FRAP assay, performed according to a previously described method [
27]. A 2 mg/mL solution of crude extracts was prepared and assayed with the FRAP reagent solution, mixed in a 1:1:10 ratio of 20 mM FeCl
3, 10 mM TPTZ dissolved in 40 mM HCl, and 300 mM acetate buffer (pH 3.6). The test plate was then left in the dark for 30 min and measured at 595 nm (BioTek Instruments). Ferrous sulfate (at a working concentration of 0.01–0.5 mM) was used to generate the standard curve, and the reduction capacity was expressed as mmol Fe
2+/g extract.
2.6. Antiglycation Activity
The antiglycation activity of
P. bivalvis extracts was assessed using the non-enzymatic BSA–fructose assay, following a previous study [
29]. Briefly, various concentrations of crude extracts (at working concentrations of 75, 150, and 300 µg/mL) were prepared and mixed with 30 mg/mL BSA and 300 mM D-fructose in a 96-well plate. The test plate was then covered with aluminum foil, incubated at 37 ± 2 °C for 60 h, and measured for intrinsic fluorescence at excitation and emission wavelengths of 340 and 435 nm (BioTek Instruments), respectively. Aminoguanidine HCl was used as the positive control.
2.7. Anti-Lipid Peroxidative Activity
The anti-lipid peroxidative activity of
P. bivalvis extracts was investigated using the thiobarbituric acid reactive substances (TBARS) assay, as previously reported [
28]. In this assay, crude extracts (at a working concentration of 500–2000 µg/mL) were mixed with the lipid source reagent in a microcentrifuge tube and incubated at 37 °C for 30 min. After that, 2 mM ascorbic acid and 4 mM Fe
2SO
4 were added, mixed well, and further incubated for 60 min. TBARS reagent was then added, mixed well, and incubated at 90 °C for 1 h. Finally, the test solution was centrifuged at 10,000 rpm for 5 min, and the supernatant was measured at 530 nm (BioTek Instruments). Trolox was used as the positive control.
2.8. Cell Viability Assay
2.8.1. Cell Culture and Conditions
Human skin fibroblast cells (WS-1; ATCC CRL-1502) were cultured in high-glucose DMEM supplemented with 10%FBS and maintained in a 5% CO2 humidified atmosphere at 37 °C.
2.8.2. Determination of Cell Viability
The effects of
P. bivalvis extracts, obtained using different extraction solvents, on fibroblast cell viability were assessed using the MTT assay following the method described previously [
25]. Briefly, WS-1 cells (8.0 × 10
3 cells/well) were cultured in 96-well plates and incubated overnight at 37 °C in a 5% CO
2 atmosphere. The cells were then treated with varying concentrations of each
P. bivalvis extract (1–100 µg/mL) and incubated overnight under the same conditions. After incubation, MTT reagent (5 mg/mL) was added into each well and incubated with 5% CO
2 at 37 °C for 4 h. Finally, the formazan crystals formed were dissolved with DMSO, and the absorbance was measured at 570 nm using a microplate reader (Enspire, Perkin Elmer, Waltham, MA, USA). Control cells were treated with 0.1% DMSO in complete medium. The percentage of cell viability was calculated using the following equation:
2.9. Development of Water-in-Oil (W/O) Nanoemulsions
2.9.1. Preparation of Unloaded W/O Nanoemulsions
The W/O nanoemulsions were prepared using a high-energy emulsification method. The composition of the unloaded W/O nanoemulsions is presented in
Table 1. The F1, F2, F3, and F4 formulations contain varying ratios of the surfactant mixture composed of PEG-30 dipolyhydroxystearate and polyglyceryl-3 diisostearate at 1:1, 2:1, 3:1, and 4:1, respectively. Briefly, the aqueous phase (phase A) and the oil phase (phase B) were separately heated to 70–75 °C using a digital heating plate with constant stirring until homogeneous. The nanoemulsion was then formed by mixing the oil and aqueous phases using a T 25 digital ULTRA-TURRAX
® homogenizer (IKA Works (Thailand) Co., Ltd., Bangkok, Thailand) at 10,000 rpm for 10 min. At 45 °C, antioxidants (phase C) and preservatives (phase D) were added to the nanoemulsion and homogenized using a homogenizer at 10,000 rpm for 5 min. All formulations were allowed to rest for 24 h before undergoing nanoemulsions characterization. The formulation that exhibited an optimal physical appearance and good stability for 21 days was selected for the incorporation of the
P. bivalvis extract.
2.9.2. Preparation of W/O Nanoemulsions Loaded P. bivalvis Leaf Crude Extract
The P. bivalvis extracts with excellent biological activities, and non-toxicity on human fibroblast were selected to prepare nanoemulsions. The composition of nanoemulsion is the same as unloaded nanoemulsions. P. bivalvis extracts were dissolved in deionized water and then added to aqueous phase. Subsequently, the nanoemulsion was prepared following the previously described method, followed by characterization and stability assessment.
2.9.3. Characterization and Stability Assessment of W/O Nanoemulsions
All W/O nanoemulsions were characterized based on their physical appearance through visual observation. The pH value was measured using pH meter (SevenDirect SD20., Mettler-Toledo International Inc., Columbus, OH, USA) and viscosity was measured by the DVNext Cone/Plate viscometer (AMETEK Brookfield, Middleborough, MA, USA). Particle size and the polydispersity index (PDI) were measured using a Zetasizer Nano-ZS90 (Malvern Panalytical Ltd., Malvern, Worcestershire, UK).
The stability of unloaded W/O nanoemulsions were evaluated by observing changes in their physical appearance after samples were stored at 4 °C, room temperature, and 45 °C for 21 days, as well as the samples to a heating/cooling cycle for 6 cycles (heating/cooling 1 cycle: 24 h at 4 °C followed by 24 h at 45 °C), following the previous method [
10,
25] with some modifications. The storage stability of nanoemulsions loaded with
P. bivalvis extracts was assessed by monitoring changes in terms of particle size, PDI, pH values, and viscosity under room temperature after 21 days and a heating/cooling cycle for 6 cycles.
2.9.4. Determination of Entrapment Efficiency (EE)
The encapsulation efficiency of W/O nanoemulsions loaded
P. bivalvis extract was calculated by measuring the TP in the nanoemulsions and comparing it to the free TP in the filtrate concentrate. Briefly, W/O nanoemulsions were diluted with 95% ethanol (1:10 ratio), and 0.5 mL of this sample solution was added to an Ultra-0.5 Centrifugal Filter Devices (Merck Millipore Ltd., Carrigtwohill, Co., Cork, Ireland) with a molecular weight cut-off of 50 kDa (pore size 10 to 100 nm). The samples were then centrifuged at 10,000 rpm (MPW-352R, MPW Med. Instruments, Warsaw, Poland) at 25 °C for 15 min. The filtrate part was collected, and the free TP was measured. The percentage of entrapment efficiency (%EE) was determined using the following equation:
where C1 is the TP in nanoemulsions, and C2 is the free TP in the filtration part after centrifugation.
2.10. Statistical Analysis
All data were expressed as mean ± standard deviation (SD) based on independent experiments with triplicate samples (n = 3). Parametric data comparison of more than two groups was analyzed using one-way ANOVA, followed by Tukey’s HSD test comparisons. The significance level of p < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 24 for Windows (SPSS, Chicago, IL, USA).
3. Results and Discussion
3.1. Extraction Yield and Phytochemical Screening of P. bivalvis Extracts
The P. bivalvis leaves were extracted using different solvents, DI water, 95% ethanol, and 1% HCl in 50% ethanol, through the maceration process to obtain P. bivalvis leaf crude extracts of WE, EE, and AE, respectively. The results of the extraction procedures showed that using the acidic solvent (1% HCl in 50% ethanol; AE) resulted in the highest extraction yield of the crude extract at 20.23%, while the ethanolic (EE) and water extractions (WE) yielded lower amounts, at 9.88% and 10.14%, respectively. These results indicate that the acidic extraction method was more effective in breaking down plant tissues compared to the other techniques.
Phytochemical constituent screening of
P. bivalvis extracts, including phenolics, flavonoids, coumarins, tannins, terpenoids, steroids, saponins, and alkaloids, was performed, and the results are shown in
Table 2. The results showed that all crude extracts contained phenolics, flavonoids, anthocyanins, coumarins, and terpenoids, while tannins and saponins were not detected in any of the extracts. However, steroids and alkaloids were only detected in the EE sample. The presence of steroids and alkaloids in EE may be due to the semi-polarity of the 95% ethanol solvent, which can extract non-polar compounds like steroids and alkaloids. The ability of ethanol to extract these compounds has been previously reported [
30,
31,
32]. The phytochemical constituents, including phenolics, flavonoids, anthocyanidins, coumarins, terpenoids, and alkaloids, in
P. bivalvis leaf crude extracts have been correlated and identified in previous studies [
13,
20].
3.2. TP and TF of P. bivalvis Extracts
Secondary metabolites of plant species are synthesized after the photosynthesis process for specific functions, such as defense against insects or other pests, protection from harmful environmental factors, and attraction for pollination [
33]. Phenolic and flavonoid compounds are considered a large group of secondary metabolites in plants, playing an important role in these specific functions. These compounds also exhibit various bioactivities including skin health promoting effects, such as antioxidant, anti-inflammatory, and anti-aging effects, etc. Determining the phenolic and flavonoid compounds in plant crude extracts can be used for quality control and to predict some bioactivities of the plant extracts. In this experiment, different types of
P. bivalvis extracts were analyzed for TP and TF, and the results are shown in
Table 3. The results showed that all crude extracts contained phenolic and flavonoid compounds, with the highest TP and TF observed in EE, at 146.72 ± 4.74 mg GAE/g extract and 510.74 ± 7.41 mg QE/g extract, respectively. WE exhibited lower levels of TP and TF, with 110.91 ± 3.28 mg GAE/g extract and 305.80 ± 5.66 mg QE/g extract, while AE showed the lowest content, with 103.36 ± 7.16 mg GAE/g extract and 82.35 ± 7.71 mg QE/g extract. These results indicated that different types of solvent extraction affect phytochemical contents, and the highest contents in EE may stem from the non-polarity of ethanol, as described in the previous section. In addition, the phenolic and flavonoid compounds of the
P. bivalvis extracts were predicted to include anthocyanidin (pelargonidin) and anthocyanins (such as alezelechin (4-8) pelargonidyl glucoside, cyanidin, and cyanidin-3-
O-
β-
D-glucoside), as noted in previous studies [
13,
20].
3.3. Antioxidant Activity of P. bivalvis Extracts Using DPPH, ABTS, and FRAP Assays
The oxidation process in biological systems generates harmful reactive oxygen species (ROS), reactive nitrogen species (RNS), and other free radical molecules. The excessive production of these free radicals leads to cells, tissue, and organ injury and damage. Determining the antioxidant activity of substances could help mitigate and address these problems. In this study,
P. bivalvis extracts were assessed for antioxidant activities through various methods, including DPPH, ABTS, and FRAP assays. The results of these antioxidant activities are shown in
Table 3. In the DPPH free radical scavenging assay, EE exhibited the highest free radical scavenging activity, with an IC
50 value of 136.10 ± 2.69 µg/mL, which was significantly different from that of WE (IC
50 = 397.78 ± 4.22 µg/mL) and AE (IC
50 = 478.44 ± 7.03 µg/mL). Trolox, the positive control, had an IC
50 value of 8.22 ± 0.07 µg/mL. This activity was similar to the FRAP assay, where EE exhibited the highest antioxidant capacity, with a reducing power of 41.48 ± 0.81 µmol Fe
2+/g extract, whereas WE and AE showed reducing power values of 36.74 ± 0.85 and 33.09 ± 1.30 µmol Fe
2+/g extract, respectively. In addition, the antioxidant capacity of the positive control, Trolox, was 86.62 ± 3.30 µmol Fe
2+/g Trolox. In contrast, in the free radical scavenging cationic assay via ABTS, the crude extracts showed that WE had the strongest free radical cation scavenging activity (IC
50 = 87.36 ± 2.52 µg/mL), which was significantly different from EE and AE, with IC
50 values of 215.85 ± 0.78 µg/mL and 170.72 ± 1.78 µg/mL, respectively. Trolox, the positive control, exhibited activity with an IC
50 value of 11.09 ± 0.07 µg/mL.
These results elucidated that
P. bivalvis extracts possess antioxidant properties across all types of solvent extracts (WE, EE, and AE), with EE showing the greatest overall antioxidant potential in all test assays. The antioxidant activities of
P. bivalvis extracts have been previously reported [
16]. These activities might stem from the presence of secondary metabolites previously identified in the plant, such as flavonoids [anthocyanidins (pelargonidin and cyanidin)], anthocyanins (cyanidin-3-O-β-D-glucoside), terpenoids (carotenoids), and coumarins. In addition, the strong antioxidant properties of pelargonidin [
34,
35], cyanidin [
36,
37], cyanidin-3-O-β-D-glucoside [
37], carotenoids [
38,
39], and coumarins [
40] have been confirmed and previously reported.
3.4. Anti-Glycation Activity of P. bivalvis Extracts Using a Non-Enzymatic BSA–Fructose Assay
Aging is an inevitable biological phenomenon that alters the morphology of skin layers, resulting from the synergistic effects of both extrinsic and intrinsic factors. The general mechanism of enzymatic reactions, such as those involving elastase, collagenase, and hyaluronidase, plays a critical role in damaging or destroying skin structure. However, non-enzymatic reactions, such as the glycation or the Maillard reaction, are also harmful to tissue skin. The interaction between reducing sugars and amino acids, peptides, or proteins in the glycation process spontaneously generates Amadori and Advanced Glycation End Products (AGEs), which are harmful and contribute to inflammation and skin aging [
41,
42]. Therefore, inhibiting the glycation process can help prevent aging and other forms of skin tissue damage.
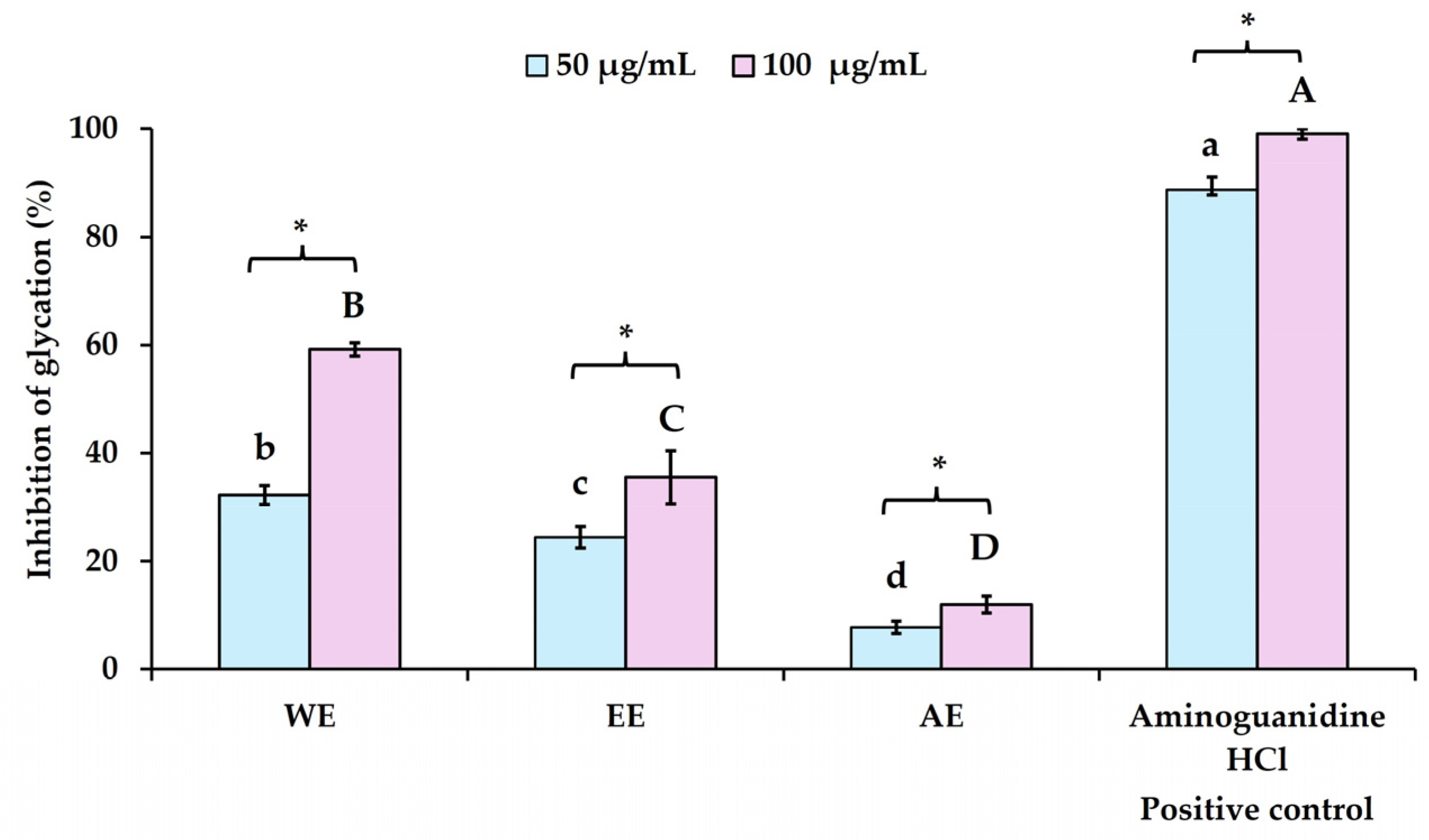
The results of the anti-glycation activity of
P. bivalvis extracts indicated that WE exhibited significantly higher activity (
p < 0.05) compared to the other extracts, with an inhibition of 59.23 ± 1.26% at 100 µg/mL (see
Figure 2). At the same concentration, EE and AE showed inhibition rates of 35.48 ± 4.90% and 11.94 ± 1.59%, respectively. A similar inhibition pattern was observed at a lower extract dosage (50 µg/mL), where WE, EE, and AE showed inhibition rates of 32.19 ± 1.80%, 24.48 ± 1.98%, and 7.72 ± 1.15%, respectively. Aminoguanidine HCl, used as a positive control at concentrations of 50 and 100 µg/mL, exhibited high inhibition of the glycation process, with rates of 88.75 ± 2.38% and 99.03 ± 0.94%, respectively, and an IC
50 value of 9.09 ± 0.81 µg/mL. The anti-glycation activity of the crude extracts was related to their TP and TF contents, as well as their antioxidant capacity. Both WE and EE, which had higher TP, TF, and antioxidant levels, exhibited greater activity than AE. This is because the extraction technique using strong HCl for maceration over three days led to the degradation of bioactive compounds, such as anthocyanins and other phenolic compounds [
43,
44]. Phenolic and flavonoid compounds inhibit glycation by reducing reactive oxygen species (ROS), which act as a glycation inducer in its early stages, as previously reported [
42]. Interestingly, polyphenolic substances—such as phenolic acids, flavonoids, stilbenes, and lignans—found in natural extracts are well known for their anti-glycation activity [
42]. Additionally, anthocyanins, reported as major constituents of
P. bivalvis leaf extracts, have been noted not only for their inhibitory effects at the intermediate stage but also for playing a critical role in the entire non-enzymatic glycation process [
45].
3.5. Anti-Lipid Peroxidative Activity of P. bivalvis Extracts Using TBARS Assay
The lipid peroxidation process plays a critical role in various pathologies, including cells, tissue and organ injury, inflammation, and skin aging. This phenomenon occurs spontaneously in the human body as a result of free radicals attacking lipids, leading to lipid degradation and the formation of harmful lipid peroxyl radicals and hydroperoxides. Inhibiting or reducing the level of this process is primarily considered a key strategy for preventing undesired skin damage and aging.
The results of this study are presented in
Figure 3. At the lowest concentration tested (25 µg/mL), WE, EE, and AE exhibited anti-lipid peroxidation activity of 6.89 ± 1.80%, 17.48 ± 1.46%, and 3.78 ± 0.55%, respectively. At 50 µg/mL, all crude extracts significantly increased their inhibitory activity, with WE, EE, and AE demonstrating 19.21 ± 0.65%, 32.75 ± 1.29%, and 18.12 ± 1.32%, respectively. At the highest concentration tested (100 µg/mL), moderate anti-lipid peroxidation activity was observed, with WE, EE, and AE inhibiting lipid peroxidation by 35.51 ± 3.16%, 46.74 ± 1.61%, and 32.23 ± 1.37%, respectively. Among the extracts, EE exhibited the highest activity compared to other extracts. These results demonstrate that all extracts showed concentration-dependent inhibitory effects in lipid peroxidation. Trolox, used as a positive control, showed strong anti-lipid peroxidation activity, with inhibition ranging from 83.16 ± 1.43% to 95.42 ± 0.91% at concentrations of 25 µg/mL to 100 µg/mL.
The anti-lipid peroxidative activities of the
P. bivalvis extracts have been reported [
16]. This inhibitory activity might stem from antioxidant properties, which involve both free radical scavenging and electron transfer mechanisms (see
Table 3) of the plant crude extracts. EE showed a higher tendency for TP, TF, and antioxidant activity than the others, even though lower antioxidant activity was observed in the ABTS assay, leading to higher overall activity in this experiment. The higher activity of EE might also stem from other lipophilic compounds, such as terpenoids and steroids, detected in some crude extracts (see
Table 2), which could be extracted in higher yields using ethanol solvents compared to water and acidic extractions of WE and AE. Lipophilic compounds, such as α-tocopherol [
46,
47], terpenoids, and steroids [
48], have also been noted for their anti-lipid peroxidative inhibitory activity. In addition, the ethanolic extract exhibited a higher potential for anti-lipid peroxidative activity compared to the water extract, which was consistent with previous studies [
16].
3.6. Cytotoxicity of P. bivalvis Extracts Using MTT Assay
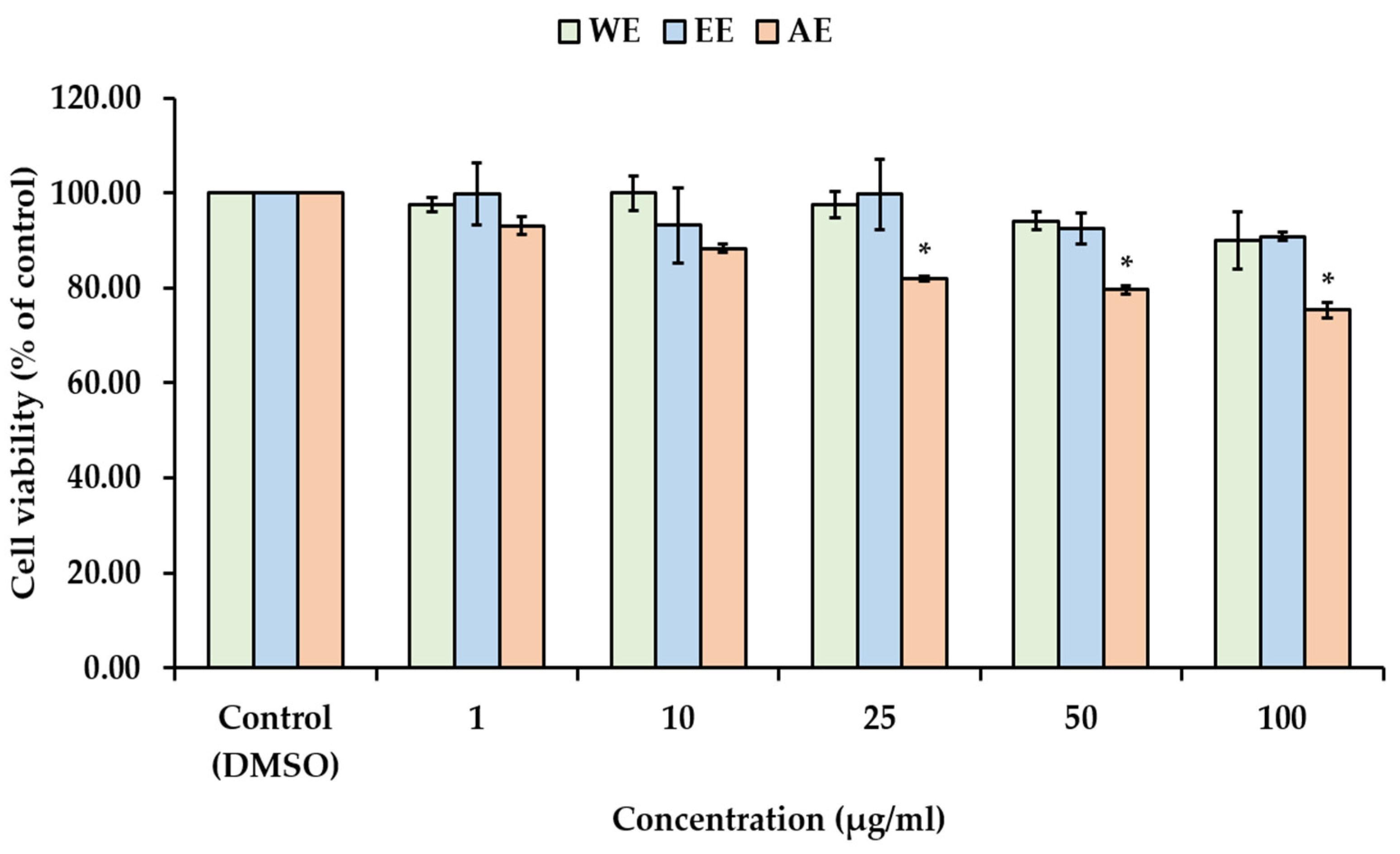
The depletion of dermal fibroblasts leads to reduced production of the main dermal components, such as collagen, elastic fibers, and hyaluronic acid, which contribute to skin aging. In general, the decline in both the viability and number of fibroblasts is a primary factor in the skin aging process [
49]. Cytotoxicity testing is a common and widely used method for assessing the potential of chemical compounds or natural plant-derived compounds to cause cell damage or inhibit cell growth. Determining the appropriate concentration or non-toxic doses is essential for the development of cosmetic and cosmeceutical products. In this study, the cytotoxic effects of
P. bivalvis extracts were investigated on human skin fibroblast cells (WS-1) using the MTT assay. The WS-1 cell viability were treated with various concentrations (1–100 µg/mL) of
P. bivalvis extracts (WE, EE, AE) as represented in
Figure 4. WE and EE exhibited non-cytotoxic doses at concentrations ranging from 1 to 100 µg/mL, whereas the
P. bivalvis extracts obtained from AE reduced cell viability in a dose-dependent manner (
p < 0.05). Despite AE exhibiting the highest % extraction yield, the solvent containing 1% HCl may affect cell growth. These findings imply that
P. bivalvis extracts obtained from deionized water and 95% ethanol are safer and can be considered suitable for incorporation into nanoemulsions.
Based on our results of phytochemical constituents, biological properties, antioxidants, anti-glycation, and anti-lipid peroxidative activities, as well as the lower fibroblast cytotoxicity of P. bivalvis extracts, WE and EE were selected for further investigation as natural active ingredient-loaded nanoemulsions and assessed for formulation stability testing.
3.7. Development and Characterization of Unloaded Water-in-Oil (W/O) Nanoemulsions
The novel nanoemulsions were developed to encapsulate phenolic and flavonoid compounds, namely anthocyanidin, which are the main compounds found in
P. bivalvis leaves. Anthocyanidins are colored, water-soluble pigments that are influenced by pH. However, they exhibit high stability under acidic conditions compared with basic conditions, and degradation occurs at higher pH levels [
50]. Hence, in the present study, water-in-oil nanoemulsions were formulated to encapsulate natural active ingredients using high-speed homogenization at 10,000 rpm for 15 min. The components of the nanoemulsions are as follows: the aqueous phase (internal phase) consists of humectants (propylene glycol and propanediol), an emulsion stabilizer (magnesium sulfate), and a preservative (diazolidinyl urea (and) iodopropynyl butylcarbamate (and) propylene glycol), while the oil phase (continuous phase) consists of a W/O emulsifier (PEG-30 dipolyhydroxystearate and polyglyceryl-3 diisostearate), emollients (CCT, squalane, and jojoba oil), and an antioxidant (tocopheryl acetate). These nanoemulsions were formulated with different ratios of surfactant mixtures (Smix) between PEG-30 dipolyhydroxystearate (HLB 5.5) and polyglyceryl-3 diisostearate (HLB 4) (see
Table 1), which are hydrophobic emulsifiers used to coat small droplets of water dispersed in an oil phase. In this study, the ratio of the aqueous phase, oil phase, and Smix was 78:15:7, which is classified as a high internal phase emulsion (HIPE) due to the internal phase volume exceeding 74.05% [
51]. Typically, the optimal water phase to oil phase ratio for stable emulsions ranges from 30:70 to 50:50 [
52,
53,
54]. However, the addition of magnesium sulfate to the W/O emulsion improved emulsion stability by increasing the resistance of the water droplets to coalescence and sedimentation [
51,
52,
55].
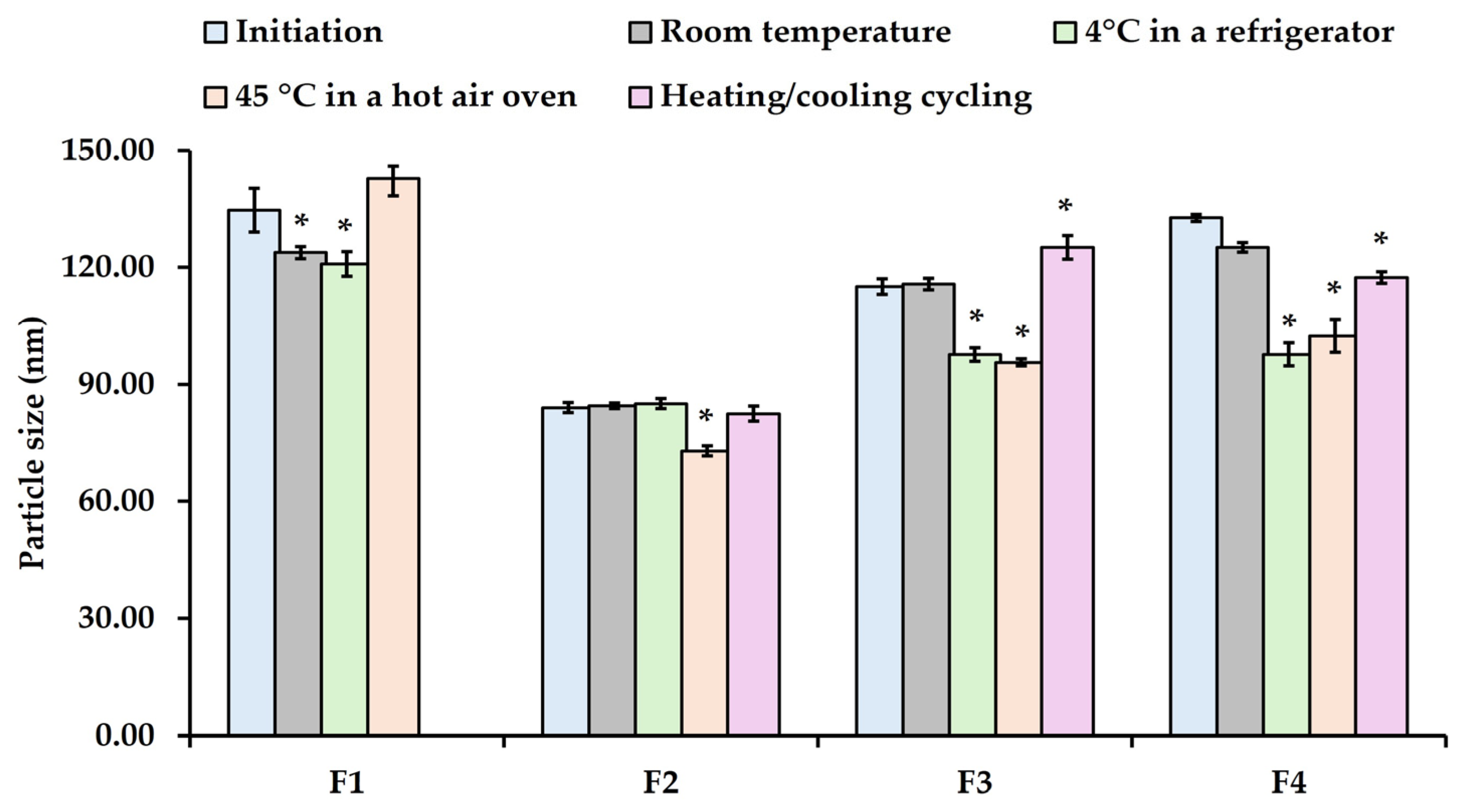
Initial nanoemulsions were characterized as shown in
Figure 5 and
Figure 6. As shown in
Figure 5, the increasing Smix ratio from 2:1 (F2) to 4:1 (F4) resulted in a slight increase in the average particle size from 84 to 133 nm. Despite this, at a 1:1 ratio, the average particle size was significantly larger than that of the other ratio due to the balance between PEG-30 dipolyhydroxystearate and polyglyceryl-3 diisostearate, which may not effectively reduce the interfacial tension between the oil and water phases. This ratio could lead to larger particle size due to aggregation, resulting in less stable nanoemulsions (phase-separated during the third cycle). However, a higher concentration of PEG-30 dipolyhydroxystearate can reduce the interfacial tension of the W/O system and stabilize the nanoemulsion [
56]. These results indicated that the Smix ratio (1:1, 2:1, 3:1, and 4:1) demonstrates a significant role in the formation of nanoemulsions. Interestingly, all unloaded nanoemulsions (F1-F4) are nano-sized droplets, with a narrow PDI (less than 0.4), indicating that the high-shear homogenization method can break down macroemulsion into nano-sized droplets. Additionally, the particle size of nanoemulsions depends on the composition of the oil–surfactant–water ratio, the type of homogenizer, and the operating conditions, including time and temperature [
3,
4].
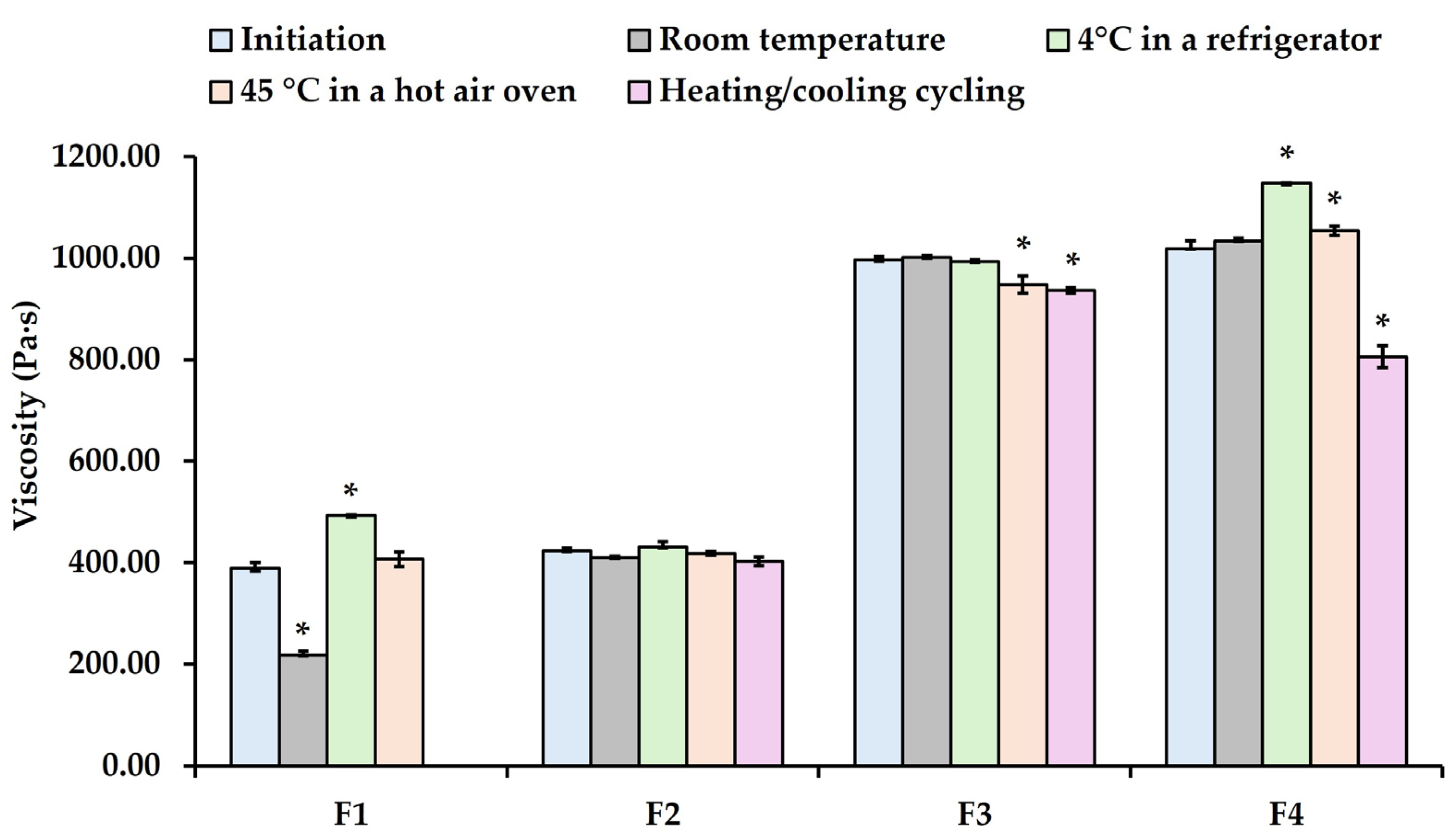
Furthermore, the Smix ratio affects the viscosity of nanoemulsions. Increasing the Smix ratio from 1:1 to 4:1 significantly increased the viscosity (
p < 0.05). Among all nanoemulsions, the apparent viscosity increased from 324.24 ± 11.28 Pa·s at the Smix ratio of 1:1 to 423.34 ± 4.89, 989.03 ± 6.85, and 1017.75 ± 10.89 Pa·s at the Smix ratios of 2:1, 3:1, and 4:1, respectively (
Figure 6). These results found that the viscosity of these nanoemulsions depends on the amount of PEG-30 dipolyhydroxystearate, which is a non-ionic surfactant that acts as polymeric emulsifier and has the appearance of brown wax. Likewise, previous studies reported that the emulsifier concentration affected emulsion viscosity [
52,
57,
58]. Additionally, the internal phase volume, continuous volume, internal phase viscosity, continuous phase viscosity, and continuous phase components affected the viscosity of the W/O nanoemulsions [
52,
58].
The appearance of unloaded nanoemulsions (F1–F4) was white viscous cream (see
Figure 7A,B), without sedimentation and phase separation after 24 h (initial formulation). All formulations exhibited a pH value range of 5.53 to 5.72.
3.8. Stability Assessment of Unloaded Water-in-Oil (W/O) Nanoemulsions
To identify the most stable formulation, the study initially conducted a screening process. Thermodynamic stability is a crucial characteristic for determining the shelf life of nanoemulsions, as the systems are susceptible to destabilization phenomena such as coalescence, creaming, Ostwald ripening, and phase separation. The F1 to F4 formulations were stored at various temperatures for 21 days, including room temperature, 4 °C (refrigerator), and 45 °C (hot air oven), in addition to undergoing a six-cycle heating/cooling stability test. During the stability study, the appearance, particle size, PDI, viscosity, and pH were monitored.
All nanoemulsions (F1–F4) maintained a white, creamy, semi-solid appearance without phase separation, except for the F1 formulation under a six-cycle heating/cooling stability test. The pH values of the nanoemulsions remained stable over time across all storage conditions, ranging from 5.53 to 5.73. After the incubation period, it was observed that the particle size of F1 formulation at room temperature and 4 °C decreased significantly (see
Figure 5). In contrast, the particle sizes of F3 and F4 formulations at room temperature were similar to the initial size on day 1, whereas F2 formulation remained stable under all conditions, except at 45 °C. However, the particle sizes of F3 and F4 formulations at 4 °C and 45 °C decreased significantly compared to the initial size on day 1. Under heating and cooling conditions, the particle size of F3 and F4 formulations increased substantially compared to day 1. Additionally, phase separation was noted in F1 after the third heating/cooling cycle. Correspondingly, the viscosity of F1 decreased significantly, even when stored at room temperature. The viscosity of F4, however, increased significantly when stored at 4 °C and 45 °C, but dramatically decreased under the heating and cooling conditions (see
Figure 6).
At 4 °C, the near-freezing temperature reduced the movement of the emulsion droplets, effectively placing them in a near-frozen state and lowering their kinetic energy. Conversely, at 25 °C or 40 °C, higher temperatures facilitated an increase in the kinetic energy of the droplet particles due to enhanced Brownian motion. This heightened movement led to more frequent collisions, which promoted coalescence and an increase in particle size. These findings align with the results reported by Abdul Wahab et al. [
59] and Pongsumpun et al. [
60].
Among all the unloaded formulations, the F2 formulation, which contained the optimal Smix ratio of 2:1, demonstrated the smallest particle size of 84.05 ± 1.30 nm and a PDI of 0.24 ± 0.04 during the 21-day storage period, both at room temperature and under stress conditions. This formulation showed no changes in appearance, maintaining a homogeneous emulsion with no signs of precipitation or phase separation. Furthermore, the particle size and viscosity of F2 remained stable and did not significantly change under the stability test conditions. These results suggest that the F2 formulation exhibited greater thermodynamic stability compared to the other formulations.
3.9. Characterization and Stability Assessment of W/O Nanoemulsions Incorporating with P. bivalvis Extracts
After evaluating the phytochemical constituent screening, biological activities, and cytotoxicity of P. bivalvis extracts, it was found that the WE and EE exhibited the best performance for incorporation into nanoemulsions, based on high levels of TP, TF, antioxidant activities, anti-glycation, anti-lipid peroxidation, and non-toxicity. Thus, WE and EE at concentrations of 1% w/w were incorporated into F2 formulation (Smix ratio 2:1).
The
P. bivalvis ethanolic extract-loaded nanoemulsions (NE-EE) exhibited a bright reddish-orange color with the pH value of 4.52 ± 0.11 (see
Figure 7C), while
P. bivalvis water extract-loaded nanoemulsions (NE-WE) exhibited a light reddish-purple color with the pH value of 5.63 ± 0.29 (see
Figure 7D). This color difference is attributed to the influence of anthocyanin-based compounds, depending on the different pH values. Following preparation, both loaded formulations were within the nanoscale range, with average particle sizes and PDI values of 104.25 ± 0.78 nm and 0.28 ± 0.12 for NE-EE, and 139.67 ± 2.34 nm and 0.28 ± 0.12 for NE-WE, respectively. The average particle size of the
P. bivalvis extract-loaded formulations was larger than that of unloaded nanoemulsions. This finding is consistent with previous studies on jaboticaba extract-loaded nanoemulsions [
61] and
Phyllanthus niruri extract-loaded nanoemulsions [
62], which reported average particle sizes of less than 200 nm. The viscosity of NE-EE and NE-WE slightly decreased compared to unloaded nanoemulsions, possibly due to changes in the internal phase viscosity. The total phenolic content-loaded nanoemulsions exhibited moderate levels of phenolic content in both NE-EE (53.97 ± 5.23%) and NE-WE (56.09 ± 6.20%).
For accelerated physical stability studies, phase separation was induced by thermal treatment, as described by Yalcin et al. [
63]. The stability of the formulations was assessed based on the absence of phase separation. The rate of destabilization in the nanoemulsions was accelerated using heating and cooling cycle tests. At low temperatures, near the freezing point of the aqueous phase, droplet movement decreased. At higher temperatures, however, droplet migration increased, leading to faster movement and more frequent collisions, which promoted coalescence and accelerated phase separation. Moreover, the alternating heating and cooling cycles disrupted the structural integrity of the nanoemulsions, causing instability and initiating phase separation.
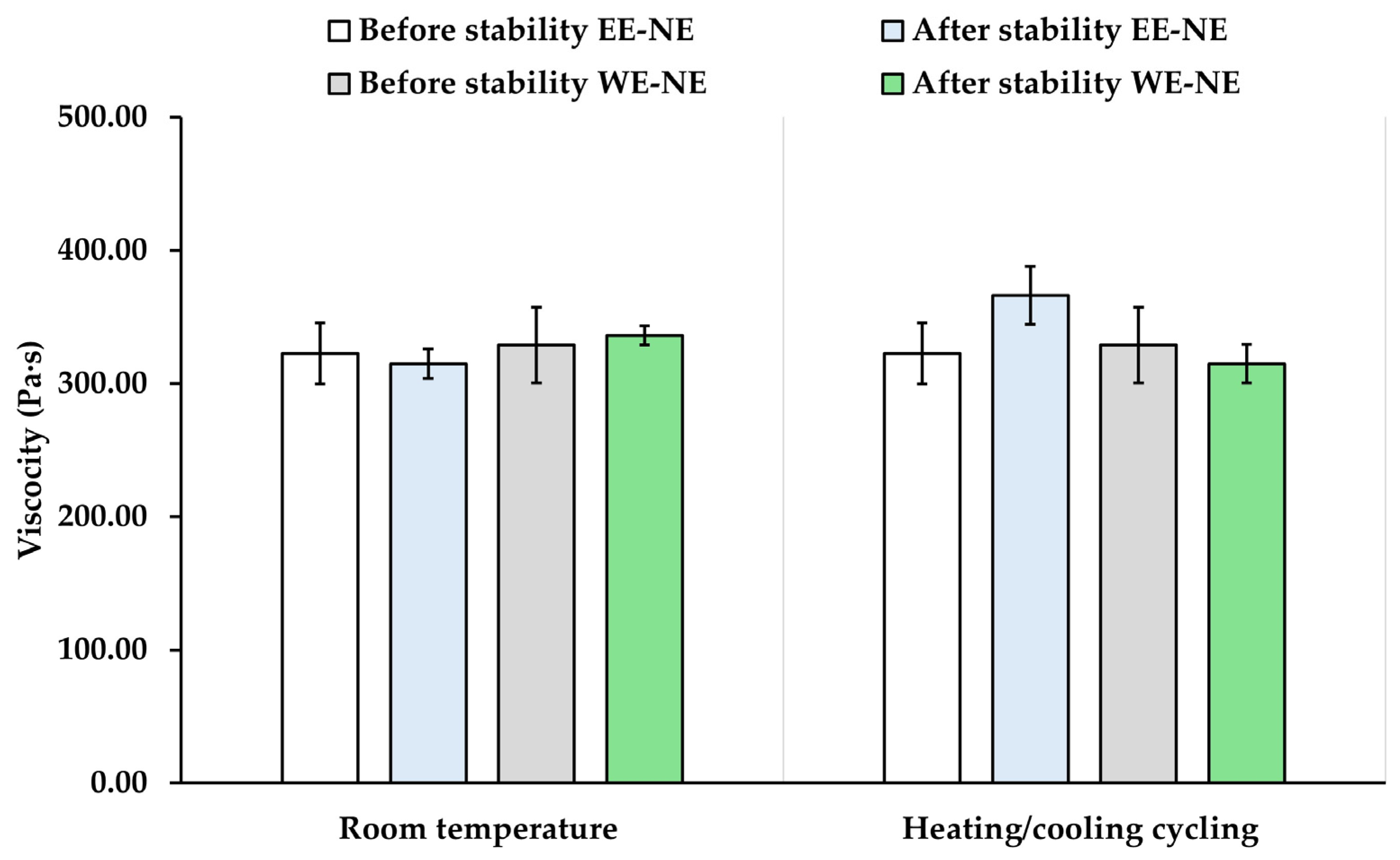
The characteristics of NE-EE and NE-WE were evaluated before and after stability assessment as shown in
Figure 8 and
Figure 9. The particle size and viscosity of both the NE-EE and NE-WE formulations did not change significantly after undergoing six cycles of heating and cooling and room temperature for 21 days (
p > 0.05). The
P. bivalvis extract-loaded nanoemulsions remained polydisperse and stable throughout the testing period, as evidenced by the narrow PDI values (less than 0.3). Both formulations were also homogeneous, creamy, and maintained unchanged color appearance and pH values. These results suggest that the emulsifier system used in this study effectively restricted droplet movement and prevented phase separation. On the other hand, at room temperature, the total phenolic content of NE-EE and NE-WE slightly decreased to 50.41 ± 1.92% and 55.71 ± 4.46%, respectively. However, under heating and cooling cycle tests, total phenolic content of NE-EE and NE-WE dramatically decreased to 31.20 ± 10.21% and 30.48 ± 11.49%, respectively. These results suggest that both nanoemulsions should be stored at low temperatures to prevent the degradation of active ingredients.
The limitation of this study is that the exact major constituents or bioactive compounds, which are essential for quality control, standardization, and permeation testing of P. bivalvis extract, have not yet been established or quantified. However, to provide stronger evidence of the nanoemulsion’s ability to act on the skin layer, further investigation is needed to determine the permeation profile of the P. bivalvis extract-loaded nanoemulsion.
4. Conclusions
The leaves of Peristrophe bivalvis (L.) Merr were extracted using the maceration method with different solvents (WE, EE, and AE). The highest percentage of extraction yield was obtained from AE (20.23%), followed by WE (10.14%) and EE (9.88%), respectively. The phytochemical constituents of P. bivalvis extracts found phenolics, flavonoids, anthocyanins, coumarins, and terpenoids in all extraction solvents, except for steroids and alkaloids, which were found only in EE samples. EE exhibited the highest TP and TF with excellent antioxidant activities, while AE exhibited the lowest TP, TF, and antioxidant activities compared to other solvents. However, WE exhibited moderate TP and TF with good antioxidant activity as measured by the ABTS assay. Additionally, all solvent extractions demonstrated anti-glycation and anti-lipid peroxidation in a dose-dependent manner (p < 0.05). EE and WE exhibited non-toxicity and the highest potential for biological activity in anti-glycation and anti-lipid peroxidation, respectively. These findings suggest that EE and WE act as natural active compounds with excellent biological activity, suitable for incorporation into nanoemulsions. The W/O nanoemulsions (F1–F4) were developed with different surfactant mixture ratios ranging from 1:1 to 4:1 using high-shear homogenization. The 2:1 ratio demonstrated that nanoemulsions remained stable under room temperatures for 21 days and accelerated conditions. The P. bivalvis ethanolic extract-loaded nanoemulsions (NE-EE) and P. bivalvis water extract-loaded nanoemulsions (NE-WE) exhibited small particle sizes with a narrow PDI and good stability under all stability conditions.