Skin Anti-Aging Properties of the Glycopeptide- and Glycoprotein-Enriched Fraction from a Cosmetic Variation of the Longevity Medicine, Gongjin-Dan
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals
2.3. Preparation of the Mixed Extract (ME)
2.4. Preparation of Glycopeptide- and Glycoprotein-Enriched Fraction (GEF)
2.5. Chemical Analysis of GEF
2.5.1. Identification of Glycopeptides and Glycoproteins
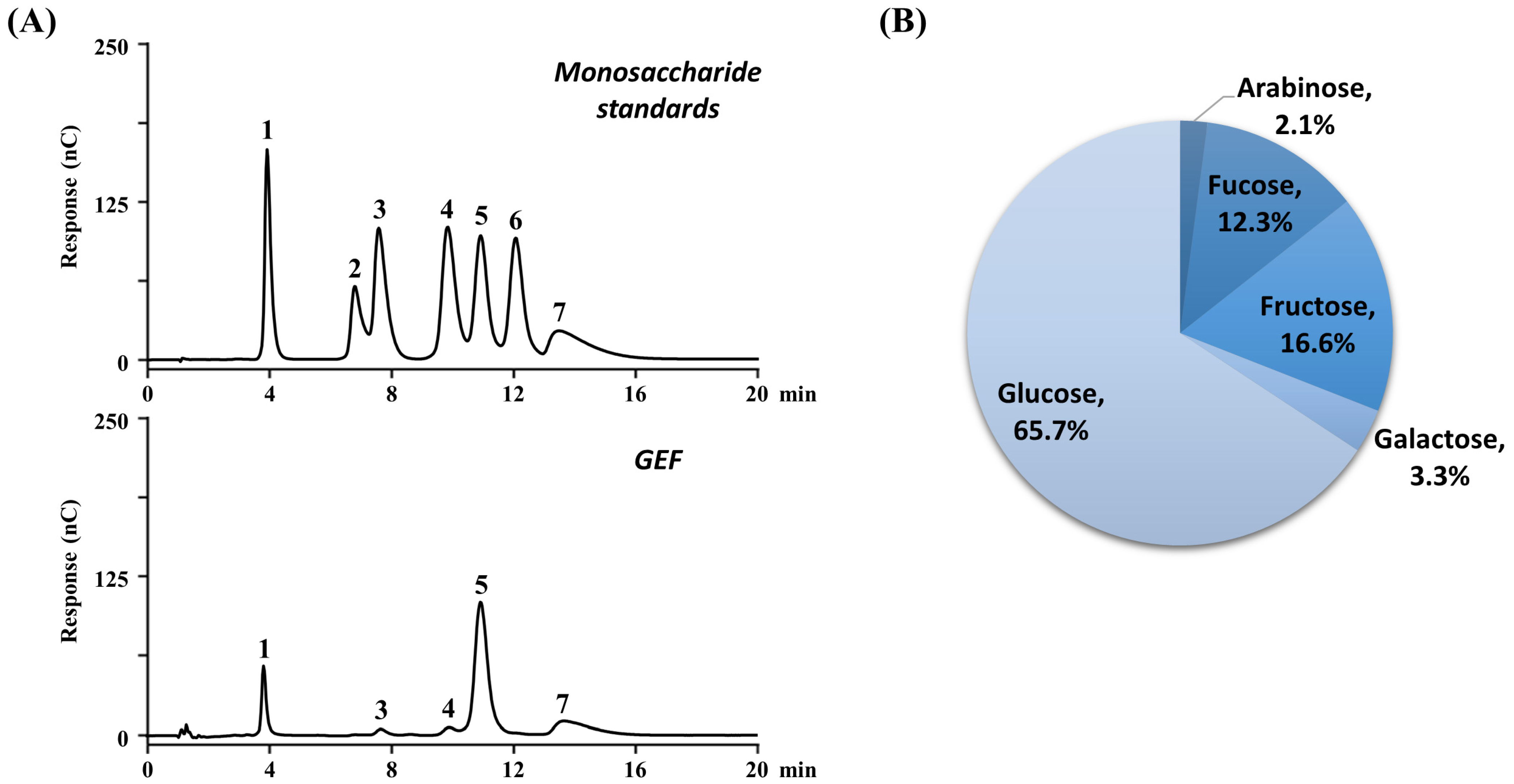
2.5.2. Carbohydrate Analysis
2.5.3. Protein Quantification and Amino Acid Composition
2.6. In Vitro Skin Anti-Aging Properties of GEF
2.6.1. Cell Culture
2.6.2. Immunocytochemical Analysis
2.6.3. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.6.4. Statistical Analysis
3. Results and Discussions
3.1. Extraction and Identification of GEF
3.2. Chemical Characterization of GEF
3.3. Inhibitory Effects on the Breakdown of Elements in Skin Layers Against Photoaging
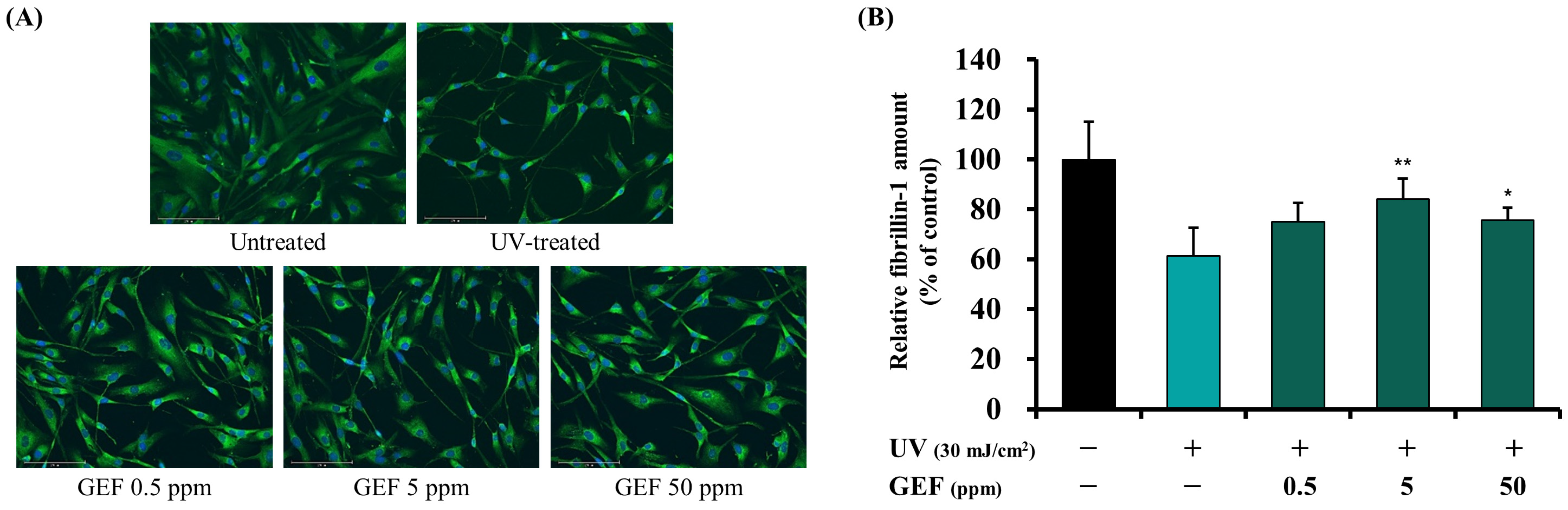
3.3.1. Effect of GEF on Fibrillin-1 Synthesis
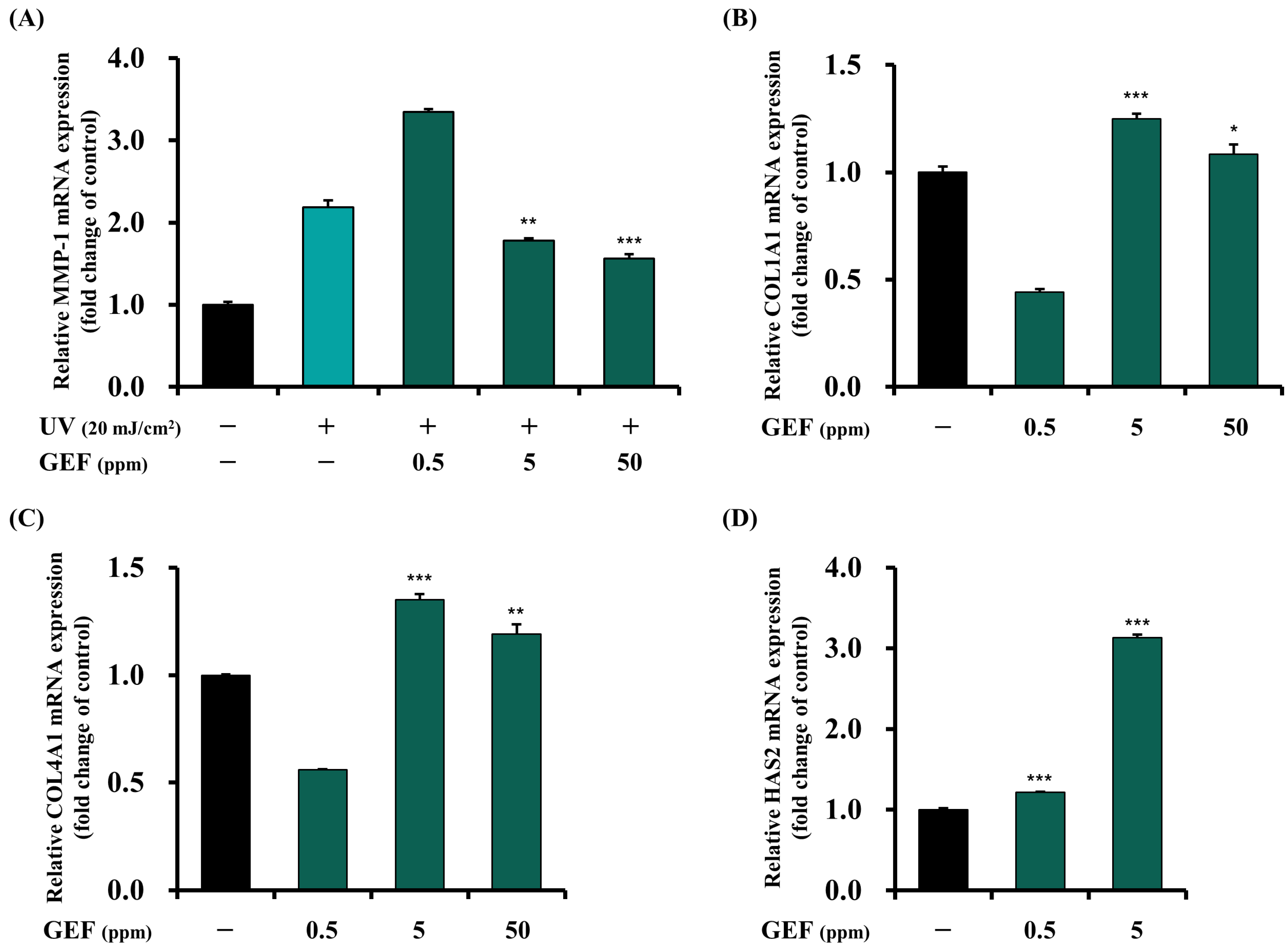
3.3.2. Inhibitory Effect of GEF on MMP-1 Gene Expression
3.4. Facilitating Effects on the Synthesis of Elements in Skin Layers
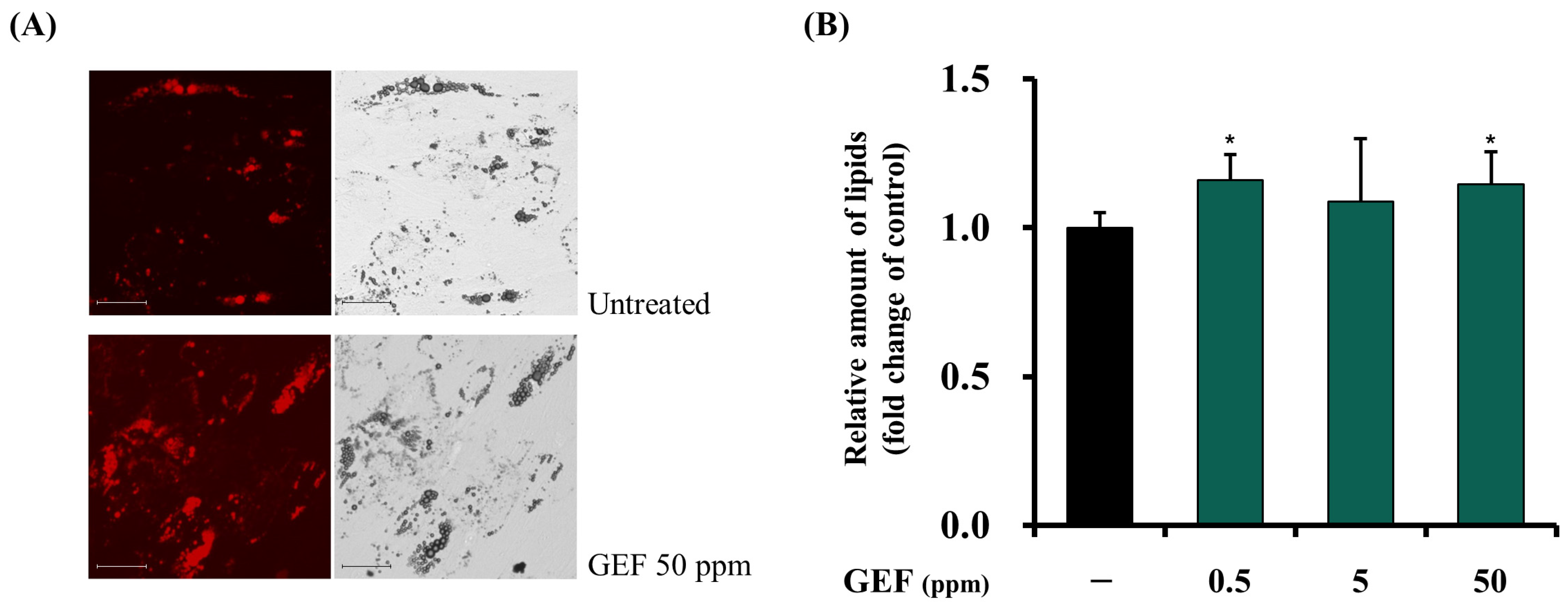
3.4.1. Facilitating Effects of GEF on Adipogenesis
3.4.2. Facilitating Effects of GEF on COL1A1, COL4A1, and HAS2 Gene Expressions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Prunel, A.; Keufer, B.; Abric, A.; Wang, Y.; Reni, A.; Cassier, M.; Delaunay, C. Changes in Facial Signs Due to Age and Their Respective Weights on the Perception of Age and Skin Plumpness among Differently Aged Korean Women. Ski. Res. Technol. 2021, 27, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Sherratt, M.J.; Griffiths, C.E.M.; Watson, R.E.B. A New Wrinkle on Old Skin: The Role of Elastic Fibres in Skin Ageing. Int. J. Cosmet. Sci. 2010, 32, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.B.; Griffiths, C.E.M.; Craven, N.M.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-Rich Microfibrils Are Reduced in Photoaged Skin. Distribution at the Dermal–Epidermal Junction. J. Investig. Dermatol. 1999, 112, 782–787. [Google Scholar] [CrossRef]
- Loo, Y.C.; Hu, H.-C.; Yu, S.-Y.; Tsai, Y.-H.; Korinek, M.; Wu, Y.-C.; Chang, F.-R.; Chen, Y.-J. Development on Potential Skin Anti-Aging Agents of Cosmos Caudatus Kunth via Inhibition of Collagenase, MMP-1 and MMP-3 Activities. Phytomedicine 2023, 110, 154643. [Google Scholar] [CrossRef]
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Kim, H.-W.; Jung, Y.-A.; Yun, J.-M.; Kim, Y.; Kim, S.-A.; Suh, S.-I.; Ryoo, Y.-W. Effects of Poly-L-Lactic Acid on Adipogenesis and Collagen Gene Expression in Cultured Adipocytes Irradiated with Ultraviolet B Rays. Ann Dermatol 2023, 35, 424–431. [Google Scholar] [CrossRef]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef]
- Park, K.M.; Yoo, Y.J.; Ryu, S.; Lee, S.H. Nelumbo nucifera Leaf Protects against UVB-Induced Wrinkle Formation and Loss of Subcutaneous Fat through Suppression of MCP3, IL-6 and IL-8 Expression. J. Photochem. Photobiol. B Biol. 2016, 161, 211–216. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Derm. -Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Shang, L.; Li, M.; Xu, A.; Zhuo, F. Recent Applications and Molecular Mechanisms of Hyaluronic Acid in Skin Aging and Wound Healing. Med. Nov. Technol. Devices 2024, 23, 100320. [Google Scholar] [CrossRef]
- Rayon, C.; Lerouge, P.; Faye, L. The Protein N-Glycosylation in Plants. J. Exp. Bot. 1998, 49, 1463–1472. [Google Scholar] [CrossRef]
- Baik, K.K.; Song, W.-Y.; Song, D.K.; Yun, J.; Jang, J.H.; Oh, J.Y.; Lee, M.-J.; Go, E.; Lee, K.J.; Roh, E.; et al. Protective Effects of Sesame Glycoproteins on Ultraviolet-Induced Skin Aging: In Vitro and In Vivo Studies. Pharmaceuticals 2024, 17, 1306. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Lee, Y.-M.; Park, J.-P.; Lim, K.-T.; Lee, S.-J. Phytoglycoprotein Isolated from Dioscorea Batatas Decne Promotes Intestinal Epithelial Wound Healing. Chin. J. Nat. Med. 2020, 18, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, S.Y.; Hong, S.-M.; Kwon, E.-H.; Lee, T.-K. Skin Whitening and Anti-Corrugation Activities of Glycoprotein Fractions from Liquid Extracts of Boiled Sea Cucumber. Asian Pac. J. Trop. Med. 2016, 9, 1002–1006. [Google Scholar] [CrossRef]
- Hong, S.-S.; Lee, J.; Lee, J.-S.; Lee, H.-W.; Kim, H.-G.; Lee, S.-K.; Park, B.-K.; Son, C.-G. The Traditional Drug Gongjin-Dan Ameliorates Chronic Fatigue in a Forced-Stress Mouse Exercise Model. J. Ethnopharmacol. 2015, 168, 268–278. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Choi, B.; Kwon, O.; Seo, C.-S.; Kim, A.-R.; Shin, H.; Kim, K. Efficacy and Safety of Herbal Medicine Gongjin-Dan and Ssanghwa-Tang in Patients with Chronic Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Integr. Med. Res. 2024, 13, 101025. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, W.; Hong, J.; Lee, J.; Yeo, C.; Lee, Y.; Baek, S.; Ha, I. Gongjin-Dan Enhances Neurite Outgrowth of Cortical Neuron by Ameliorating H2O2-Induced Oxidative Damage via Sirtuin1 Signaling Pathway. Nutrients 2021, 13, 4290. [Google Scholar] [CrossRef]
- Kang, J.-W.; Cho, H.-E.; Choi, H.-M.; Lee, I.-C. Anti-Wrinkle Properties of Angelica Gigas Nakai Root Extracts Using Mineral-Rich Water. J. Cosmet. Dermatol. 2023, 22, 328–334. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, H.; Liu, M.; Wang, N.; Ye, L.; Guo, C.; Zheng, B. Cornus Officinalis Extract Enriched with Ursolic Acid Ameliorates UVB-Induced Photoaging in Caenorhabditis elegans. Molecules 2024, 29, 2718. [Google Scholar] [CrossRef]
- Cuong, V.T.; Chen, W.; Shi, J.; Zhang, M.; Yang, H.; Wang, N.; Yang, S.; Li, J.; Yang, P.; Fei, J. The Anti-Oxidation and Anti-Aging Effects of Ganoderma lucidum in Caenorhabditis elegans. Exp. Gerontol. 2019, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Yu, L.-X.; Yan, X.; Guo, C.; Xiong, Y. Effects of Root and Stem Extracts of Asparagus Cochinchinensis on Biochemical Indicators Related to Aging in the Brain and Liver of Mice. Am. J. Chin. Med. 2011, 39, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Jeong, N.-H.; Jang, B.-S. Antioxidative Activity and Antiaging Effect of Carrot Glycoprotein. J. Ind. Eng. Chem. 2015, 25, 216–221. [Google Scholar] [CrossRef]
- Jang, B.-S.; Lee, M.-J.; Jeong, N.-H. Preparation and Availability Analysis of Glycoprotein from Canola Meal. J Food Sci Technol 2021, 58, 377–382. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Li, L.; Yang, X. Purification, Characterization, and Functional Properties of a Novel Glycoprotein from Tartary Buckwheat (Fagopyrum tartaricum) Seed. Food Chem. 2020, 309, 125671. [Google Scholar] [CrossRef]
- Wingfield, P. Protein Precipitation Using Ammonium Sulfate. Curr. Protoc. Protein Sci. 1998, 13, A.3F.1–A.3F.8. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Deepachandi, B.; Weerasinghe, S.; Andrahennadi, T.P.; Karunaweera, N.D.; Wickramarachchi, N.; Soysa, P.; Siriwardana, Y. Quantification of Soluble or Insoluble Fractions of Leishmania Parasite Proteins in Microvolume Applications: A Simplification to Standard Lowry Assay. Int. J. Anal. Chem. 2020, 2020, 6129132. [Google Scholar] [CrossRef]
- Wallis, D.D.; Tan, F.K.; Kielty, C.M.; Kimball, M.D.; Arnett, F.C.; Milewicz, D.M. Abnormalities in Fibrillin 1–Containing Microfibrils in Dermal Fibroblast Cultures from Patients with Systemic Sclerosis (Scleroderma). Arthritis Rheum. 2001, 44, 1855–1864. [Google Scholar] [CrossRef]
- Manetti, M.; Romano, E.; Rosa, I.; Fioretto, B.S.; Praino, E.; Guiducci, S.; Iannone, F.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Systemic Sclerosis Serum Steers the Differentiation of Adipose-Derived Stem Cells Toward Profibrotic Myofibroblasts: Pathophysiologic Implications. J. Clin. Med. 2019, 8, 1256. [Google Scholar] [CrossRef]
- Schmid, K.; Kaufmann, H.; Isemura, S.; Bauer, F.; Emura, J.; Motoyama, T.; Ishiguro, M.; Nanno, S. Structure of A1-Acid Glycoprotein. The Complete Amino Acid Sequence, Multiple Amino Acid Substitutions, and Homology with the Immunoglobulins. Biochemistry 1973, 12, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Medjoubi-N, N.; Porquet, D. Alpha-1-Acid Glycoprotein. Biochim. Et Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2000, 1482, 157–171. [Google Scholar] [CrossRef]
- Thalacker-Mercer, A.; Riddle, E.; Barre, L. Protein and Amino Acids for Skeletal Muscle Health in Aging. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 29–64. [Google Scholar]
- Fujiwara, T.; Kanazawa, S.; Ichibori, R.; Tanigawa, T.; Magome, T.; Shingaki, K.; Miyata, S.; Tohyama, M.; Hosokawa, K. L-Arginine Stimulates Fibroblast Proliferation through the GPRC6A-ERK1/2 and PI3K/Akt Pathway. PLoS ONE 2014, 9, e92168. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.P.; de Andrade Berti, B.; Mendes, N.F.; Engel, D.F.; Zanesco, A.M.; Pereira de Souza, G.F.; de Medeiros Bezerra, R.; de Toledo Bagatin, J.; Maria-Engler, S.S.; Morari, J.; et al. Glutamic Acid Promotes Hair Growth in Mice. Sci Rep 2021, 11, 15453. [Google Scholar] [CrossRef]
- Kwon, K.C.; Won, J.G.; Seo, J.H.; Kwon, O.S.; Kim, E.H.; Kim, M.-S.; Park, S.-W. Effects of Arginine Glutamate (RE:Pair) on Wound Healing and Skin Elasticity Improvement after CO2 Laser Irradiation. J. Cosmet. Dermatol. 2022, 21, 5037–5048. [Google Scholar] [CrossRef]
- Strasser, R.; Seifert, G.; Doblin, M.S.; Johnson, K.L.; Ruprecht, C.; Pfrengle, F.; Bacic, A.; Estevez, J.M. Cracking the “Sugar Code”: A Snapshot of N- and O-Glycosylation Pathways and Functions in Plants Cells. Front. Plant Sci. 2021, 12, 640919. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Yamawaki, Y.; Sato, Y.; Muraoka, S.; Yoshida, M.; Okano, Y.; Masaki, H. Decreased Mitochondrial Function in UVA-Irradiated Dermal Fibroblasts Causes the Insufficient Formation of Type I Collagen and Fibrillin-1 Fibers. J. Dermatol. Sci. 2022, 108, 22–29. [Google Scholar] [CrossRef]
- Kähäri, V.-M.; Saarialho-Kere, U. Matrix Metalloproteinases in Skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef]
- Liu, M.; Lu, F.; Feng, J. Aging and Homeostasis of the Hypodermis in the Age-Related Deterioration of Skin Function. Cell Death Dis 2024, 15, 443. [Google Scholar] [CrossRef]
- Khoo, N.K.H.; Fazzari, M.; Chartoumpekis, D.V.; Li, L.; Guimaraes, D.A.; Arteel, G.E.; Shiva, S.; Freeman, B.A. Electrophilic Nitro-Oleic Acid Reverses Obesity-Induced Hepatic Steatosis. Redox Biol. 2019, 22, 101132. [Google Scholar] [CrossRef]
- Kang, S.; Jun, S.-H.; Lee, J.; An, M.J.; Kang, N.-K. In Vitro Efficacies of Complex of Cedrol and Three Peptides, and Wrinkle Improvements and Lips Volumization effects of Applied Formulations. J. Soc. Cosmet. Sci. Korea 2023, 49, 365–373. [Google Scholar]
- Yan, Y.; Quan, H.; Guo, C.; Qin, Z.; Quan, T. Alterations of Matrisome Gene Expression in Naturally Aged and Photoaged Human Skin In Vivo. Biomolecules 2024, 14, 900. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, A.M.; van Loon, L.J.C. The Impact of Collagen Protein Ingestion on Musculoskeletal Connective Tissue Remodeling: A Narrative Review. Nutr Rev 2022, 80, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.C.; Puzzi, M.B. The Effect of Aging in Primary Human Dermal Fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Ikarashi, N.; Tabata, K.; Miyazawa, A.; Kon, R.; Sakai, H.; Hosoe, T. Expression Analysis of Genes Important for Maintaining Skin Function in a Senescence-Accelerated Mouse Prone Model. Geriatr. Gerontol. Int. 2023, 23, 951–957. [Google Scholar] [CrossRef]

| Target mRNA | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | CATGTTCGTCATGGGGTGAACCA | AGTGATGGCATGGACTGTGGTCAT |
| MMP-1 | CTGGCCACAACTGCCAAATG | CTGTCCCTGAACAGCCCAGTACTT |
| COL1A1 | GCTACTACCGGGCTGATGAT | ACCAGTCTCCATGTTGCAGA |
| COL4A1 | CTGCCTGGAGGAGTTTAGAAG | GAACATCTCGCTCCTCTCTATG |
| HAS2 | AATCCAGCTCTTCTACCGGG | CTTGGCGGGAAGTAAACTCG |
| Content b | GEF | ME |
|---|---|---|
| Total carbohydrate | 2219 (44.4%) | 1824 (36.5%) |
| Total protein | 683 (13.7%) | 398 (8.0%) |
| Protein | Concentration (mg/mL) | Absorbance (550 nm) |
|---|---|---|
| Blank | 0 | 0.296 |
| Lysozyme | 2.5 | 0.333 |
| Bovine serum albumin | 2.5 | 0.413 |
| Ovalbumin | 2.5 | 0.573 |
| apo-Transferrin | 2.5 | 0.798 |
| Fetuin | 2.5 | 5.593 |
| α1-Acid glycoprotein | 2.5 | 5.274 |
| GEF | 2.5 | 13.148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.J.; Park, J.; Jeon, H.J.; Kim, T.H.; Lee, H.; Kang, S.; Hwang, S.J.; Son, N.S.; Kang, N.-G. Skin Anti-Aging Properties of the Glycopeptide- and Glycoprotein-Enriched Fraction from a Cosmetic Variation of the Longevity Medicine, Gongjin-Dan. Cosmetics 2025, 12, 91. https://doi.org/10.3390/cosmetics12030091
Lee GJ, Park J, Jeon HJ, Kim TH, Lee H, Kang S, Hwang SJ, Son NS, Kang N-G. Skin Anti-Aging Properties of the Glycopeptide- and Glycoprotein-Enriched Fraction from a Cosmetic Variation of the Longevity Medicine, Gongjin-Dan. Cosmetics. 2025; 12(3):91. https://doi.org/10.3390/cosmetics12030091
Chicago/Turabian StyleLee, Gwang Jin, Jiwon Park, Hyeon Jun Jeon, Tae Heon Kim, Hyejin Lee, Seongsu Kang, Seung Jin Hwang, Nam Seo Son, and Nae-Gyu Kang. 2025. "Skin Anti-Aging Properties of the Glycopeptide- and Glycoprotein-Enriched Fraction from a Cosmetic Variation of the Longevity Medicine, Gongjin-Dan" Cosmetics 12, no. 3: 91. https://doi.org/10.3390/cosmetics12030091
APA StyleLee, G. J., Park, J., Jeon, H. J., Kim, T. H., Lee, H., Kang, S., Hwang, S. J., Son, N. S., & Kang, N.-G. (2025). Skin Anti-Aging Properties of the Glycopeptide- and Glycoprotein-Enriched Fraction from a Cosmetic Variation of the Longevity Medicine, Gongjin-Dan. Cosmetics, 12(3), 91. https://doi.org/10.3390/cosmetics12030091






