Abstract
The porosity of hair fibers can be modified by chemical and physical damage, influencing their response to cosmetic treatments. To investigate the effect of commonly applied hair care protocols on textured hair, virgin and once-bleached tresses were subjected to multiple cycles of washing, blow-drying while combing, and styling with a hot flat iron, simulating a consumer routine spanning one to six months. Porosity-related properties were evaluated using swelling test, fluorescence, atomic force, and scanning electron microscopies, high-pressure differential scanning calorimetry, and tensile testing. Both chemical and physical processes induced significant changes in the hair’s water permeability, surface topography, and appearance, alongside a reduction in mechanical and thermal properties, indicating substantial structural alterations compared to virgin hair. Increased porosity compared to virgin hair possibly reduced the heat conductivity of bleached hair, leading to less pronounced effects of heat exposure. These findings underscore the damaging potential of routine practices for textured hair and emphasize the need for targeted cosmetic solutions to protect and repair these fibers as part of consumers’ hair care regimens.
1. Introduction
Hair fibers are keratinous materials. The distinct compositions and arrangements of hair structures determine their properties and behavior. The fibrous cortex, consisting of alpha-keratin fibrils connected to a matrix of keratin-associated proteins (KAPs), is primarily responsible for the fiber’s mechanical properties. The surrounding cuticle comprises several layers of overlapping flat rigid cells externally linked to a lipid coating. The cuticle has the main function of protecting the fiber against external stressors and is associated with hair’s sensory properties. These layers and structures are bonded by a lipo-protein component known as the cell membrane complex (CMC), which plays a crucial role in molecules’ diffusion into the hair fiber [1,2].
Like all keratinous fibers, hair is naturally porous, denoting its ability to absorb fluids due to the presence of voids or empty spaces at nano- to macroscale levels [3,4,5,6]. Exposure to chemicals (e.g., dyes, bleaches) and physical/environmental factors (e.g., solar radiation, combing, elevated temperatures) is known to induce structural damage, resulting in the formation of cracks, holes, and split ends, in addition to loss of protein and lipid material, decreased shine and smoothness, and increased breakage [7,8,9,10,11,12,13]. By altering hair fiber’s external and internal structure, damage processes affect hair porosity, impacting their void fraction and surface area, permeability to fluids, and mechanical and thermal properties [6,14,15,16,17]. Beyond damaging processes, porosity levels are influenced by factors such as hair texture and aging [18,19,20,21]. Alongside physicochemical factors, porosity plays a vital role in material diffusion into hair fibers, significantly impacting the effectiveness of cosmetic products. It influences hair permeability to external agents, such as water and active ingredients, thereby affecting their retention and efficacy within the hair fibers [1,3,22,23]. It is, therefore, essential to comprehend the impact of hair porosity when evaluating real-world hair care practices, which, although potentially milder, are frequently employed by consumers.
While the impact of chemical and physical treatments, such as bleaching, perming, UV exposure, and heat application, on straight to loosely wavy Caucasian hair fibers has been extensively investigated [1,7,8,9,10,11,12,13], equivalent research on textured hair (including curly, coily, and kinky hair types) remains limited. Textured hair has unique structural characteristics—such as a helical shape and a flattened elliptical cross section—that make it more prone to mechanical damage than straight hair [1,19]. Grooming practices commonly employed to “easily manage” textured hair, including heat styling, chemical relaxers, and tight braiding, have been associated with long-term damage to the scalp and hair shaft, and in some cases, broader health implications [1,19]. Furthermore, the combined effects of chemical and physical damage, common in consumer practices, on textured hair porosity have not been explored. Thus, prioritizing the study of textured hair represents a critical step toward promoting equity and inclusivity in scientific research and clinical practice.
This study employed well-established instrumental techniques, including hair swelling and tensile tests, alongside various microscopy-based assessments (fluorescence microscopy, scanning electron microscopy [SEM], and atomic force microscopy [AFM]), and high-pressure differential scanning calorimetry (HPDSC) to examine hair properties and condition. Through this approach, the study aims to assess the correlation between a standardized hair care routine, incorporating commonly applied chemical and physical stressors, and the porosity level of textured hair.
Elucidating the impact of prevalent hair care protocols on the health of textured hair constitutes a critical area of inquiry as it addresses existing knowledge gaps and enhances consumer awareness, thereby enabling more informed decision-making regarding hair care practices. This topic holds considerable significance for dermatologists, supporting appropriate medical recommendations, and cosmetic scientists, who advocate for the development of specialized cosmetic formulations tailored to diverse hair types and needs, designed to protect and/or repair hair fibers as a fundamental aspect of consumers’ hair care practices.
2. Materials and Methods
2.1. Hair Samples
Virgin textured hair (Curl V—IHIP/17 cm long) was obtained from International Hair Importers & Products, Inc. (New York, NY, USA). Fifty tresses with 2.5 g of hair each were prepared. The tresses were washed with a sodium lauryl ether sulfate (SLES) 10% solution, using a ratio of 0.1 mL/g of hair. Each tress was wet, massaged with the surfactant solution for 30 s, rinsed for 30 s under warm tap water (4 L/min at 33 ± 3 °C), and left to dry under controlled temperature (22 ± 2 °C) and humidity (50 ± 5% RH) conditions.
2.2. Damaging Protocols
2.2.1. Chemical Damage
Half of the previously prepared hair tresses were subjected to a bleaching process using a 1:2 mixture of commercially available bleaching powder (Yamá Cosméticos, Cotia, Brazil) and 12% hydrogen peroxide emulsion (Oxycreme, Yamá Cosméticos, Cotia, Brazil) in a ratio of 5 g/g of hair. The mixture, prepared according to the manufacturer’s instructions, was spread along the entire length of the tresses, individually wrapped in aluminum foil, and placed in an oven at 45 °C for 30 min. The hair tresses were thoroughly rinsed under running water (4 L/min at 33 ± 3 °C), washed three times with a 10% SLES solution as described above, and then left to dry under controlled conditions (22 ± 2 °C and 50 ± 5% RH).
2.2.2. Physical Damage
Prepared virgin (VG) and bleached (BL) hair was divided into five-tress groups and subjected to 0 (VG and BL controls), 4 (VG4C and BL4C), 8 (VG8C and BL8C), 12 (VG12C and BL12C), or 24 (VG24C and BL24C) cycles of physical damage. Each cycle comprised three steps: I. Washing with 10% SLES solution (as described in the pre-cleaning section); II. Blow-drying for 1 min using hair dryers (Taiff Vulcan 2500W—Taiff—São Paulo, Brazil) at maximum speed and temperature while brushing with heat-resistant brushes (7511T—Marco Boni—Cotia, Brazil) using an automated combing machine (BLPA 300—Bioluz—Campinas, Brazil—at speed 8 = 200 combings/min); III. Heat straightening using a hot flat iron (BaByLiss Pro Titanium—Conair—Stamford, CT, USA)—3 passes of 10 s each at 232 °C (450 °F).
2.3. Damage Assessment
2.3.1. Fiber Swelling Assessment
Fifty hair fibers (10 per tress) from each group were prepared for the swelling test at an automated system with a dynamic swelling module (ALS1500/DSM770—Dia-Stron—Andover, UK). Each fiber was crimped with plastic tabs at both ends, leaving a 3 cm space between them. The fibers were positioned in the sample preparation press in a manner that the central part of the fiber was considered for later analysis. The diameter of each one of the fibers was measured for 180 s at the DSM770, using a laser scan micrometer (LSM6200—Mitutoyo—Kanagawa, Japan). The swelling module comprised an acrylic cell where the crimped individual fibers were positioned for diameter measurements. After the fiber was well placed, a first measurement was made, and the cell was automatically filled with water. Continuous measurements of the fiber’s diameter were made while submerging it in water for a set period of 180 s.
2.3.2. Fluorescence Microscopy Analysis
Small portions of hair (containing approximately 50 fibers) were taken from each tress (5 portions per group). The central portion of the fibers was cut to approximately 7 cm in length, and the hair tufts were tied at both ends using sewing thread. These tufts were incubated for 1 h with a 10 µg/mL Rhodamine B (Sigma-Aldrich—St. Louis, MO, USA) solution, then rinsed under running water (4 L/min at 33 ± 3 °C) for 30 s and placed to dry in an oven at 45 °C for 15 min. Samples were embedded in histological resin (Technovit® 7100—Kulzer—Hanau, Germany) following the manufacturer’s instructions. Samples’ cross sections (10 µm thick) were obtained using a rotary microtome (CUT6062—SLEE medical—Nieder-Olm, Germany), opened in water, and placed on microscope slides. Three cuts were prepared per sample. The sections were left to dry for at least 2 h under controlled conditions (22 ± 2 °C and 50 ± 5% RH—protected from light). Images were acquired in a fluorescence microscope (Olympus BX53F, Camera DP73, and U-RFL-T source—Olympus—Tokyo, Japan) using a GWI filter, 10× magnification objective, an exposure time of 3.03 ms, ISO 400, and medium contrast. The average fluorescence intensity in the obtained images was measured using FluoSeg 1.1.1 software (QIMA Newtone—Lyon, France).
2.3.3. Atomic Force Microscopy (AFM)
Two fibers from each group were analyzed, and three images were recorded from the central portion (1 cm cut) of each fiber. The images were obtained using a MultiMode® 8 SPM (Bruker—Billerica, MA, USA), in contact mode, with a silicon tip attached to a cantilever in V (DNPS—Bruker—Billerica, USA). The setup was adjusted for aspect ratio 4, 128 lines, and 512 points per line at a 1 Hz scan rate. The scan window for all images was 90 μm × 22.5 μm. The images were pre-processed using a third-order Plane Fit XY using the Nanoscope Analysis software v1.5. The step height and roughness (RMS) parameters were calculated using a specific algorithm (LFF-USP, São Paulo, Brazil).
2.3.4. Scanning Electron Microscopy (SEM)
Three fibers from each tested group were mounted on conductive carbon tape. The samples were coated with Au using the Denton Vacuum DESK II (Denton Vacuum—Moorestown, NJ, USA) sputtering for 180 s, with a current of 10 mA. Since the deposition rate is approximately 0.065 nm/s, around 12 nm were deposited. The images were acquired using a Jeol 6460 LV SEM (Joel—Tokyo, Japan), with an accelerating voltage of 15 kV.
2.3.5. Mechanical Properties Evaluation
Forty-five hair fibers (9 per tress) from each group were prepared for the tensile test. Each fiber was crimped at both ends, leaving a 3 cm space between them (central portion of the fiber). The cross-sectional area of each fiber was automatically measured at 5 points with a laser scan micrometer (LSM6200—Mitutoyo—Kanagawa, Japan) using the Automated Loading System—ALS1500 and the Fibre Dimensional Analysis System—FDAS770 (Dia-Stron—Andover, UK). The fibers were then transferred to the Miniature Tensile Tester—MTT680 (Dia-Stron—Andover, UK) and analyzed using a constant strain speed of 15 mm/min. For the tensile dry, the experiment was performed at a relative humidity of 55 ± 5% and 22 ± 2 °C, while for the tensile wet, the fibers were soaked in water for 1 h before the test by filling in the cannelure of the equipment’s carrousel. The parameters of interest were extracted using UvWin 4.2.4.2 software (Dia-Stron—Andover, UK).
2.3.6. Dynamic Scanning Calorimetry (DSC)
The central portion of the hair fibers was cut to approximately 1 mm fragments, and 6–7 mg of this hair was placed in high-capacity stainless steel capsules (PerkinElmer—Waltham, MA, USA). Fifty microliters of deionized water (DI) were added to each capsule before they were sealed using an appropriate press. The samples were analyzed on a DSC4000 (PerkinElmer—Waltham, USA) in the temperature range of 70 °C to 180 °C, with a heating rate of 5 °C/min and a nitrogen flow of 20 L/min. A capsule without hair was used as a reference for the analysis. The temperature and enthalpy values of the keratin denaturation peak were obtained using the Pyris 13.4 software (PerkinElmer—Waltham, USA). Five samples from each group (one per hair tress) were prepared and analyzed.
2.4. Statistical Analysis
Comparisons among groups were made using Student’s t-test for means or one-way ANOVA followed by Tukey HSD post hoc test, considering a 95% confidence interval. Tests were conducted using the XLStat 19.02 extension for Excel (Lumivero—Denver, CO, USA).
3. Results
3.1. Porosity Evaluation: Water Intake and Cationic Dye Adsorption
3.1.1. Fiber Swelling
The chemical damage imparted by the bleaching process increased the values of diameter variation of the textured hair fibers due to water intake by more than 2x compared to virgin hair. On the other hand, the effect of physical damaging processes on this parameter was softer, with some increases in the water absorption being observed after 4 to 8 cycles, followed by a reduction in the diameter variation with additional rounds of damage (Figure 1).
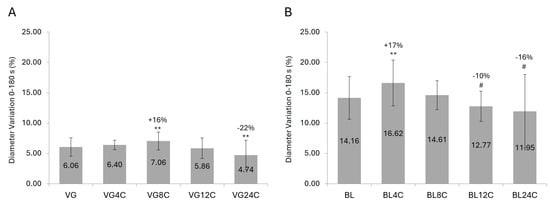
Figure 1.
Diameter variation % (mean ± SD) of textured hair fibers after 180 s submerged in water. (A) Virgin hair. (B) Bleached hair. VG = virgin hair. BL = bleached hair. 4C–24C = number of cycles of physical damage. # p < 0.1 and ** p < 0.01 versus VG or BL controls.
3.1.2. Intake/Adsorption of Rhodamine B
A notable increase in the fluorescence intensity of bleached textured hair was exhibited (Figure 2). The values rose by 57% following 4 cycles of damage and by 86% after 8 cycles, relative to control hair. However, a subsequent decline in the intake of the fluorescent dye (Rhodamine B) was evident after 12 and 24 cycles of physical damage compared to the levels observed after 8 cycles.
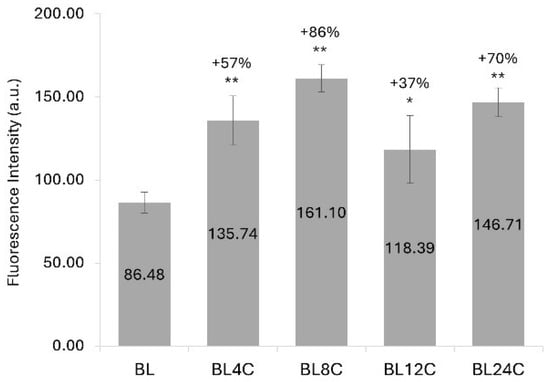
Figure 2.
Fluorescence intensity (mean ± SD) of bleached textured hair samples incubated with Rhodamine B. BL = bleached hair. 4C–24C = number of cycles of physical damage. * p < 0.05 and ** p < 0.01 versus BL control.
3.2. Surface Evaluation—AFM and SEM Analysis
3.2.1. Cuticle Conditions and Surface Roughness—AFM
An increase in the step height values was observed when comparing bleached to virgin hair fibers (Table 1). In contrast, the cuticles’ step height measured in the AFM for the hair fibers after 24 cycles of mechanical and heat damage was, respectively, 17% and 22% lower than those of virgin and bleached fibers that were not subjected to any cycles.

Table 1.
Step height and RMS values obtained from AFM analysis (mean). The percent reduction in both parameters was calculated versus VG and BL (controls). VG = virgin hair. BL = bleached hair. 4C–24C = number of cycles of physical damage. ** p < 0.01 and *** p < 0.001 versus BL or VG control.
3.2.2. Visual Analysis of Hair Surface—SEM
Representative images of the textured hair fibers from different damage groups obtained on SEM analysis are illustrated in Figure 3.
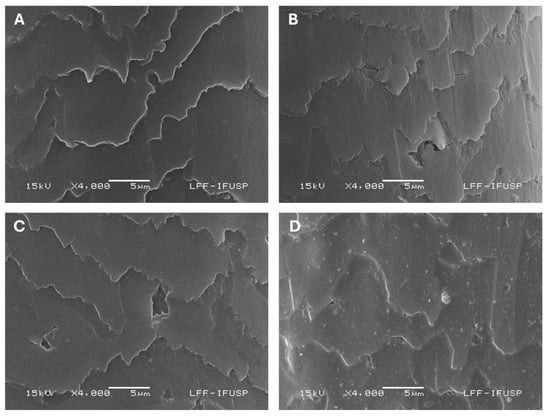
Figure 3.
Scanning electron microscopy images of textured hair samples—4000x magnification. (A) Virgin hair—control. (B) Bleached hair—control. (C) Virgin hair—after 24 cycles of hair styling routine simulation. (D) Bleached hair—after 24 cycles of hair styling routine simulation.
As shown in Figure 3C, the bleaching process alone led to the appearance of holes in the hair cuticles. Indicatives of cuticle lixiviation, with exposure of endocuticle and/or CMC, are also visualized. Cuticle fusion caused by excessive heat after 24 cycles of washing and physical damage is identified in Figure 3B,D. Additionally, Figure 3D reveals the presence of bubbles and small pores within the cuticle structure.
3.3. Thermal and Mechanical Properties of Hair Fibers
3.3.1. Hair Fiber Structural Arrangement—DSC
The influence of the chemical and physical damage in the temperature (Td) associated with the degradation of proteins within the hair fibers is shown in Figure 4.
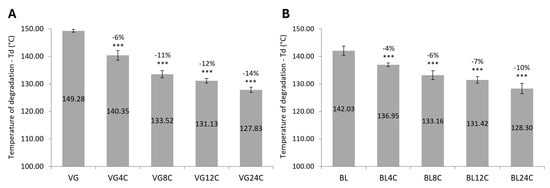
Figure 4.
Temperature of keratin denaturation (mean ± SD) of textured hair samples. (A) Virgin hair. (B) Bleached hair. VG = virgin hair. BL = bleached hair. 4C–24C = number of cycles of physical damage. *** p < 0.001 versus VG or BL controls.
The bleaching process caused a 7 °C reduction in the textured hair degradation temperature.
After only 4 cycles of physical damage, significant reductions in the temperature of keratin degradation were observed for virgin and bleached hair. The reduction became more pronounced with the increase in the number of cycles.
The virgin hair presented larger reductions in temperature of keratin degradation compared to bleached hair (10% vs. 14% after 24 cycles compared to the respective control).
3.3.2. Hair Mechanical Properties—Tensile Assessment
The elastic modulus results obtained on both dry (55% RH) and wet conditions are shown in Figure 5. After bleaching, textured hair fibers became 15% stiffer in dry conditions and 38% more flexible in wet conditions than those evaluated in the virgin state. A depletion of both hair’s elastic modulus and resistance to strain was observed even after the first 4 cycles of physical damage. The subsequent rise in the damage cycles was accompanied by increasingly large impacts on these parameters for both virgin and bleached hair. However, given the percentage reductions calculated regarding the respective controls, virgin hair was more heavily affected by the damage cycles than bleached hair.
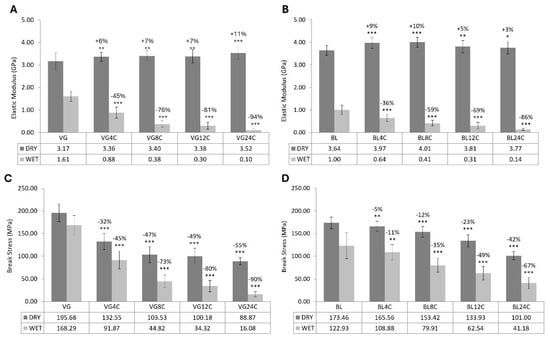
Figure 5.
Elastic modulus and break stress (mean ± SD) of virgin (A,C) and bleached (B,D) textured hair fibers. VG = virgin hair. BL = bleached hair. 4C–24C = number of cycles of physical damage. * p < 0.05, ** p < 0.01, and *** p < 0.001 versus BL or VG control in the same humidity condition.
The number of fibers that broke before 15% of strain in the dry condition escalated with the cycles’ progress, reaching 96% and 80% of the total number of virgin and bleached fibers, respectively, after 24 cycles.
The results obtained from tensile wet experiments were significantly lower than those from tensile dry. This reduction in the values of tensile parameters became even greater with the increase in the number of cycles (Table 2).

Table 2.
The percent reduction in elastic modulus and break stress values of virgin and bleached hair under wet conditions versus dry conditions. VG = virgin hair. BL = bleached hair. 4C–24C = number of cycles of physical damage.
4. Discussion
In this study, textured hair samples underwent hair care procedures, including chemical and/or physical treatments designed to simulate real-life damage conditions. Consequently, the bleaching process was conducted using commercially available products, according to the manufacturer’s instructions. Employing a realistic protocol, the bleaching process itself was responsible for causing significant damage to the hair. Hair bleaching involves a severe oxidative reaction that targets melanin degradation to lighten hair color [1,24]. However, this process is not melanin-specific and leads to cleavage of other chemical bonds, resulting in increased polarity or hydrophilicity, intense protein loss, and the appearance of holes and fractures on the surface of the strands. In extreme cases, cuticle loss with cortex exposure may be observed [9,10,11,25,26].
The observed substantial increase in the permeability of textured hair fibers to water following bleaching, as indicated by the swelling test results, suggests an alteration in the level of hair porosity [5,27]. As hair fibers undergo anisotropic swelling when hydrated, meaning that water absorption substantially increases their diameter but not their length [1,28,29], the diameter variation measured in the swelling test represents a suitable parameter for hair porosity evaluation. Although porosity is an inherent characteristic of hair fibers originating from various structural components [3], the exacerbation of this trait is a recognized consequence of chemical damage [6,15].
The physical damage cycles focused on heat damage but also included cleansing with non-conditioning shampoo and brushing while blow-drying. The temperatures utilized in this protocol are commonly employed by individuals with textured hair as it is significantly more challenging to straighten or align this type of hair at lower temperatures. Assuming a washing frequency of once or twice per week, four cycles of this simulation may correspond to approximately one month or fifteen days of a real-life routine, while twenty-four cycles can simulate a six-month hair care regimen.
The cycles of physical damage exerted a comparable effect on the water intake profile of both the virgin and bleached textured hair fibers. An initial increase in permeability was observed after a few cycles (4–8); however, this trend was subsequently reversed, with a reduction in fiber diameter variation noted in groups subjected to 12 or more cycles. An increase in porosity was anticipated as all three steps involved in the hair routine simulation could potentially lead to increased polarity or hydrophilicity due to lipid extraction or degradation, cuticle damage and lixiviation, protein loss, and the formation of cracks, fractures, and pores [13,17,30,31,32,33]. However, the decrease in water permeability after 12 and 24 cycles is possibly a consequence of a different phenomenon. Repeated exposure of hair to high temperatures that reach 232 °C when using a hot flat iron possibly leads to fusion of the cuticle layers, thereby making the diffusion process more challenging [32,34,35].
Analyses utilizing atomic force microscopy (AFM) and scanning electron microscopy (SEM) facilitated the assessment of the topography of hair fibers [7,36,37]. The results obtained corroborated the porosity findings derived from the swelling test. The process of bleaching resulted in a rougher hair surface, as evidenced by increased step height values in AFM analysis when compared to the undamaged control. In contrast, the cuticle step height measured for hair fibers after 24 cycles of mechanical and heat damage was 17% and 22% lower than that of virgin and bleached fibers not subjected to any cycles. A cuticle morphology consistent with these findings was also observed in SEM images of textured hair fibers after 24 cycles, confirming the cuticle fusion hypothesis, which aligns with previous studies employing extreme heat damage [35,38]. Additionally, other morphological changes observed in the SEM images, such as the presence of bubbles, fractures, and holes, indicated the significant damage caused by the proposed protocol [31,32,34,38,39]. These changes may be associated with consumer perceptions of dull, rough, and weak hair [40].
The use of Rhodamine B for fluorescence microscopy analysis allowed the evaluation of not only the hair permeability to the dye aqueous solution but also the assessment of the hair damage level [41,42]. Rhodamine B, a cationic dye, binds to the negative sites formed during hair bleaching. As virgin hair exhibits few negatively charged sites on its structure and more hydrophobic behavior, the fluorescence generated using this methodology is minimal, and bleached hair is a more appropriate substrate for analysis [41,43]. The increase in the fluorescent dye intake by the bleached textured hair fibers after four and eight cycles compared to the control group, which was only bleached, can again be related to increased porosity as hair permeability was augmented. Although a decrease in fluorescence intensity in the 8-cycle group was observed after 12 and 24 cycles, the fluorescence intensity for the hair subjected to 24 cycles of routine hair care was 70% higher than that of the control. This outcome likely results from the balance between hair permeability and affinity for the cationic dye. Despite the occurrence of cuticle fusion after 12 or 24 cycles, hardening the water entrance into the fiber, Rhodamine B can still bind to the overly damaged surface of these cuticles, generating a greater fluorescence intensity compared to hair that was only bleached.
In addition to the altered absorption of fluids, the depletion of thermal and mechanical properties of hair fibers serves as another indication of increased hair porosity. Wet high-pressure differential scanning calorimetry (HPDSC) was utilized to examine the effects of chemical and physical damage on the structural integrity of textured hair fibers [44,45,46]. The denaturation temperature was correlated with the viscosity of the cortical matrix. This viscosity is associated with both the quantity and nature of the cross-links found in the matrix. Consequently, a less viscous matrix may be related to more porous hair [44,46,47,48]. This understanding correlates well with the intense decrease in the temperature of keratin denaturation observed following hair bleaching and exposure to routine hair styling cycles. This reduction in temperature values can be attributed to a less compact matrix that corresponds to increased porosity compared to hair that has not undergone the damage process. After 24 simulation cycles, the keratin denaturation peak was barely distinguishable, with temperature values decreasing over 21 °C for virgin hair and 13 °C for bleached hair, reflecting extreme damage to the hair structure.
Considering that water functions as a plasticizer of the internal structure of hair fibers [1,49], a stress–strain test was conducted to evaluate the mechanical properties of hair under two distinct relative humidity (RH) conditions: 50% (dry hair) and 100% (wet hair). When comparing the elastic modulus and break stress values of virgin and bleached hair without additional physical damage under dry conditions, it was found that bleached hair exhibited increased stiffness (+15%) and decreased resistance (−11%) compared to virgin hair. These findings are in line with previous studies [1,50,51,52]. An increase in the elastic modulus suggests that the structure of the fiber is more rigid, potentially due to the formation of weak chemical bonds between the oxidized amino acid residues resulting from the damage process [10,14,53]. Significant increases in this parameter can be interpreted as indicative of more brittle hair, which is usually associated with intensified premature hair breakage [1,53]. This behavior was mainly observed in virgin hair, where a breakage rate before 15% stretching of 96% was noted after 24 cycles of thermal damage and brushing, compared to 80% obtained for bleached hair under the same conditions.
In contrast to what occurs under dry conditions, wet bleached hair is expected to exhibit greater elasticity (reduced rigidity) than wet virgin hair [1,53]. As previously discussed, water possesses the capacity to disrupt weaker bonds, such as van der Waals interactions, salt bridges, and hydrogen interactions, which are abundant within the internal structure of the hair fiber. These bonds are mainly responsible for the elastic behavior of hair fibers; once disrupted in the presence of water, the hair becomes more easily extensible. Given that some covalent bonds are broken during the bleaching process, resulting in new interactions between the formed residues, bleached fibers present a greater number of these weak bonds compared to virgin hair. Consequently, bleached hair is more intensely affected by the presence of water than virgin hair [1,53]. Furthermore, bleached hair is characterized by increased polarity and porosity, leading to greater water absorption than virgin hair [15,16,54], as demonstrated by the swelling test.
Following four cycles of washing and physical damage, a notable decrease in the elastic modulus of wet fibers was observed in comparison to the virgin and bleached controls, indicating an enhancement in hair elasticity. This behavior was reinforced as the cycles progressed and was accompanied by an escalated reduction in tensile strength. After 24 cycles, decreases in tensile strength of 90% and 67% were observed for virgin and bleached hair, respectively, with a statistical difference between the two hair types.
The less pronounced decline in thermal and mechanical parameters observed in bleached hair compared to virgin hair after the styling routine cycles may be attributed to the differing heat conductivities of the two substrates. Heat diffusion is influenced by the porosity of the materials [4], so it manifests differently in virgin and bleached hair [55]. The increased presence of air and/or water within the structure of bleached hair may account for its lower thermal conductivity compared to the more compact structure of virgin hair, thereby resulting in reduced heat damage.
5. Conclusions
The employed analytical methods were effective in demonstrating that chemical and physical damage imparted by common consumer hair care practices significantly affected the porosity of textured hair. Besides influencing hair fibers’ capacity to absorb water and cationic dye, both studied stressors negatively affected their thermal and mechanical properties. Along with washing and repeated grooming, the elevated temperatures used in consumers’ routines, particularly those from heat styling tools, led to permanent damage of cuticle and cortical structures. The augmented porosity of bleached hair altered its thermal conductivity, unexpectedly leading to overall less severe reductions in hair’s thermal and mechanical properties compared to virgin hair. Understanding the interplay between hair care practices and hair porosity is crucial for developing effective strategies to mitigate damage and maintain hair health. Further research in this area, including different types of hair, such as textured ones, is warranted to elucidate precise mechanisms and inform the development of targeted hair care interventions.
Author Contributions
Conceptualization, R.M.G. and G.R.L.; methodology, R.M.G.; formal analysis, R.M.G.; investigation, R.M.G.; writing—original draft preparation, R.M.G.; writing—review and editing, C.B.L. and G.R.L.; supervision, G.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Symrise and Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Finance Code 001.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors would like to acknowledge the “Laboratório de Filmes Finos do IFUSP” for conducting AFM and SEM analysis at the SPM facility (FAPESP Proc.#95/5651-0) and the HCC team for supporting sample preparation and instrumental testing.
Conflicts of Interest
Rebeca Mantuan Gasparin and Carolina Botelho Lourenço are employed by Symrise. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that the company Symrise provided part of the funding. They were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| CMC | Cell Membrane Complex |
| AFM | Atomic Force Microscopy |
| SEM | Scanning Electron Microscopy |
References
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-25611-0. [Google Scholar]
- Feughelman, M. Morphology and Properties of Hair. In Hair and Hair Care; Routledge: London, UK, 2018; pp. 1–12. [Google Scholar]
- Müllner, A.R.M.; Pahl, R.; Brandhuber, D.; Peterlik, H. Porosity at Different Structural Levels in Human and Yak Belly Hair and Its Effect on Hair Dyeing. Molecules 2020, 25, 2143. [Google Scholar] [CrossRef] [PubMed]
- Křemenáková, D.; Militký, J.; Venkataraman, M.; Mishra, R. Thermal Insulation and Porosity—From Macro-to Nanoscale. In Thermal Physics and Thermal Analysis. Hot Topics in Thermal Analysis and Calorimetry; Šesták, J., Hubík, P., Mareš, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 11, pp. 425–448. [Google Scholar]
- Espinal, L. Porosity and Its Measurement. In Characterization of Materials; Wiley: Hoboken, NJ, USA, 2012; pp. 1–10. [Google Scholar]
- Habe, T.; Inoue, S.; Breakspear, S.; Noecker, B.; Popescu, C. Thermoporometry Measurements of Human Hair via Differential Scanning Calorimetry. Int. J. Cosmet. Sci. 2024, 47, 234–239. [Google Scholar] [CrossRef]
- Monteiro, V.F.; Pinheiro, A.S.; Leite, E.R.; Agnelli, J.A.M.; Pereira-da-silva, M.A.; Longo, E. UV Radiation: Aggressive Agent to the Hair-AFM, a New Methodology of Evaluation. J. Cosmet. Sci. 2003, 54, 271–281. [Google Scholar] [PubMed]
- Dario, M.F.; Baby, A.R.; Velasco, M.V.R. Effects of Solar Radiation on Hair and Photoprotection. J. Photochem. Photobiol. B 2015, 153, 240–246. [Google Scholar] [CrossRef]
- Gillece, T.; Senak, L.; McMullen, R.L. Characterization of Bleached Hair Vibrational Spectroscopy, Thermal Analysis, and Determination of Equivalent Damage Factor. J. Cosmet. Sci. 2021, 72, 519–546. [Google Scholar]
- Nogueira, A.; Nakano, A.; Joekes, I. Impairment of Hair Mechanical Properties by Sun Exposure and Bleaching Treatments. J. Cosmet. Sci. 2004, 55, 533–537. [Google Scholar]
- Dyer, J.M.; Bell, F.; Koehn, H.; Vernon, J.A.; Cornellison, C.D.; Clerens, S.; Harland, D.P. Redox Proteomic Evaluation of Bleaching and Alkali Damage in Human Hair. Int. J. Cosmet. Sci. 2013, 35, 555–561. [Google Scholar] [CrossRef]
- Aparecida França-Stefoni, S.; Ferrera Dario, M.; Cristina Sá-Dias, T.; Bedin, V.; Jos de Almeida, A.; Rolim Baby, A.; Val eria Velasco, M.R. Protein Loss in Human Hair from Combination Straightening and Coloring Treatments. J. Cosmet. Dermatol. 2015, 14, 204–208. [Google Scholar] [CrossRef]
- Lima, C.R.R.D.C.; Couto, R.A.A.D.; Freire, T.B.; Goshiyama, A.M.; Baby, A.R.; Velasco, M.V.R.; Constantino, V.R.L.; Matos, J.D.R. Heat-damaged Evaluation of Virgin Hair. J. Cosmet. Dermatol. 2019, 18, 1885–1892. [Google Scholar] [CrossRef]
- Syed, A.N.; Ayoub, H. Correlating Porosity and Tensile Strength of Chemically Modified Hair. Cosmet. Toilet. 2002, 117, 57–64. [Google Scholar]
- Hessefort, Y.Z.; Holland, B.T.; Cloud, R.W. True Porosity Measurement of Hair: A New Way to Study Hair Damage Mechanisms. J. Cosmet. Sci. 2008, 59, 303. [Google Scholar] [PubMed]
- Barba, C.; Martí, M.; Manich, A.M.; Carilla, J.; Parra, J.L.; Coderch, L. Water Absorption/Desorption of Human Hair and Nails. Thermochim Acta 2010, 503–504, 33–39. [Google Scholar] [CrossRef]
- Nagase, S.; Shibuichi, S.; Ando, K.; Kariya, E.; Satoh, N. Influence of Internal Structures of Hair Fiber on Hair Appearance. I. Light Scattering from the Porous Structure of the Medulla of Human Hair. J. Cosmet. Sci. 2002, 53, 89–100. [Google Scholar] [PubMed]
- Mcmullen, R.L.; Gillece, T.; Schiess, T. Physicochemical Properties of Textured Hair. J. Cosmet. Sci. 2021, 72, 711–731. [Google Scholar]
- Franbourg, A.; Hallegot, P.; Baltenneck, F.; Toutain, C.; Leroy, F. Current Research on Ethnic Hair. J. Am. Acad. Dermatol. 2003, 48, S115–S119. [Google Scholar] [CrossRef]
- O’Sullivan, J.D.B.; Nicu, C.; Picard, M.; Chéret, J.; Bedogni, B.; Tobin, D.J.; Paus, R. The Biology of Human Hair Greying. Biol. Rev. 2021, 96, 107–128. [Google Scholar] [CrossRef]
- Duvel, L.; Herbal, A.; Daniels, L.; Kong, R.; Hillebrand, G.G. Age, Lifestyle and Self-perceptions of Hair: Is There an Association with Hair Diameter and Tensile Properties? Int. J. Cosmet. Sci. 2019, 41, 509–515. [Google Scholar] [CrossRef]
- Hill, V.; Loni, E.; Cairns, T.; Sommer, J.; Schaffer, M. Identification and Analysis of Damaged or Porous Hair. Drug Test. Anal. 2014, 6, 42–54. [Google Scholar] [CrossRef]
- Gasparin, R.M.; Thomaz, F.M.; Lourenço, C.B.; Nakano, A.K.; Marsaioli, A.J. Influence of Ethnicity and Damage Levels on Fragrance Substantivity on Hair. J. Cosmet. Sci. 2021, 72, 741–752. [Google Scholar]
- Alessandrini, A.; Piraccini, B. Essential of Hair Care Cosmetics. Cosmetics 2016, 3, 34. [Google Scholar] [CrossRef]
- Ryu, S.R.; Jang, W.; Yu, S.-I.; Lee, B.-H.; Kwon, O.-S.; Shin, K. FT-IR Microspectroscopic Imaging of Cross-Sectioned Human Hair during a Bleaching Process. J. Cosmet. Dermatol. Sci. Appl. 2016, 06, 181–190. [Google Scholar] [CrossRef]
- Grosvenor, A.J.; Deb-Choudhury, S.; Middlewood, P.G.; Thomas, A.; Lee, E.; Vernon, J.A.; Woods, J.L.; Taylor, C.; Bell, F.I.; Clerens, S. The Physical and Chemical Disruption of Human Hair after Bleaching—Studies by Transmission Electron Microscopy and Redox Proteomics. Int. J. Cosmet. Sci. 2018, 40, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Hook, J.R. An Introduction to Porosity. Petrophysics—SPWLA J. Form. Eval. Reserv. Descr. 2003, 44. [Google Scholar]
- Gouws, X.A.; Mastnak, A.; Kreplak, L.; Rutenberg, A.D. Anisotropic Swelling Due to Hydration Constrains Anisotropic Elasticity in Biomaterial Fibers. J. Mech. Behav. Biomed. Mater. 2024, 160, 106749. [Google Scholar] [CrossRef]
- Breakspear, S.; Noecker, B.; Popescu, C. Hair Mechanical Anisotropy-What Does It Tell Us? J. Cosmet. Sci. 2018, 69, 305–313. [Google Scholar] [PubMed]
- Song, S.-H.; Park, H.-S.; Jeon, J.; Son, S.K.; Kang, N.-G. Hair Pores Caused by Surfactants via the Cell Membrane Complex and a Prevention Strategy through the Use of Cuticle Sealing. Cosmetics 2023, 10, 161. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.D.; Hyun, H.J.; Pi, L.Q.; Jin, X.; Lee, W.S. Hair Shaft Damage from Heat and Drying Time of Hair Dryer. Ann. Dermatol. 2011, 23, 455–462. [Google Scholar] [CrossRef]
- Gamez-Garcia, M.; North, C. The Cracking of Human Hair Cuticles by Cyclical Thermal Stresses. J. Cosmet. Sci. 1998, 49, 141–153. [Google Scholar]
- Mcmullen, R.L.; Zhang, G.; Gillece, T. Quantifying Hair Shape and Hair Damage Induced during Reshaping of Hair. J. Cosmet. Sci. 2015, 66, 379–409. [Google Scholar]
- Mcmullen, R.; Jachowicz, J. Thermal Degradation of Hair. I. Effect of Curling Irons. J. Soc. Cosmet. Chem. 1998, 49, 223–244. [Google Scholar]
- Zhou, Y.; Rigoletto, R.; Koelmel, D.; Zhang, G.; Gillece, T.; Foltis, L.; Moore, D.J.; Sun, C. The Effect of Various Cosmetic Pre-Treatments on Protecting Hair from Thermal Damage by Hot Flat Ironing. J. Cosmet. Sci. 2011, 62, 265–282. [Google Scholar] [PubMed]
- Wei, G.; Bhushan, B.; Torgerson, P.M. Nanomechanical Characterization of Human Hair Using Nanoindentation and SEM. Ultramicroscopy 2005, 105, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Tomes, C.; Jones, J.T.; Carr, C.M.; Jones, D. Three-Dimensional Imaging and Analysis of the Surface of Hair Fibres Using Scanning Electron Microscopy. Int. J. Cosmet. Sci. 2007, 29, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ruetsch, S.B.; Kamath, Y.K. Effects of Thermal Treatments with a Curling Iron on Hair Fiber. J. Cosmet. Sci. 2004, 55, 13–27. [Google Scholar] [CrossRef]
- Harper, D.; Qi, C.; Kaplan, P. Thermal Styling: Efficacy, Convenience, Damage Tradeoffs. J. Cosmet. Sci. 2011, 62, 139–147. [Google Scholar]
- Sinclair, R.D. Healthy Hair: What Is It? J. Investig. Dermatol. Symp. Proc. 2007, 12, 2–5. [Google Scholar] [CrossRef]
- dos Santos Silva, A.L.; Joekes, I. Rhodamine B Diffusion in Hair as a Probe for Structural Integrity. Colloids Surf. B Biointerfaces 2005, 40, 19–24. [Google Scholar] [CrossRef]
- Ruetsch, S.B.; Yang, B.; Kamath, Y.K.; Princeton, T. Chemical and Photo-Oxidative Hair Damage Studied by Dye Diffusion and Electrophoresis. J. Cosmet. Sci. 2003, 54, 379–394. [Google Scholar]
- Longo, V.M.; Monteiro, V.F.; Pinheiro, A.S.; Terci, D.; Vasconcelos, J.S.; Paskocimas, C.A.; Leite, E.R.; Longo, E.; Varela, J.A. Charge Density Alterations in Human Hair Fibers: An Investigation Using Electrostatic Force Microscopy. Int. J. Cosmet. Sci. 2006, 28, 95–101. [Google Scholar] [CrossRef]
- Lima, C.R.R.D.C.; Machado, L.D.B.; Velasco, M.V.R.; Matos, J.D.R. DSC Measurements Applied to Hair Studies. J. Therm. Anal. Calorim. 2018, 132, 1429–1437. [Google Scholar] [CrossRef]
- Popescu, C.; Gummer, C. DSC of Human Hair: A Tool for Claim Support or Incorrect Data Analysis? Int. J. Cosmet. Sci. 2016, 38, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, F.-J.; Springob, C.; Sendelbach, G. Investigations of Cosmetically Treated Human Hair by Differential Scanning Calorimetry in Water. J. Cosmet. Sci. 2002, 53, 219–228. [Google Scholar] [PubMed]
- Marsh, J.M.; Clarke, C.J.; Meinert, K.; Dahlgren, R.M. Investigations of Cosmetic Treatments on High-Pressure Differential Scanning Calorimetry. J. Cosmet. Sci. 2007, 58, 319–327. [Google Scholar] [PubMed]
- Gummer, C.; Marsh, J.; Dangrew, R.M.; Meinhert, K. Understanding the Effect on Hair Fibers of Coloring and Bleaching Formulations Using High Pressure Differential Scanning Calorimetry (HPDSC). J. Cosmet. Sci. 2006, 58, 90–91. [Google Scholar]
- Evans, T. Measuring the water content of hair. Cosmet. Toilet. 2014, 129, 64–69. [Google Scholar]
- Yu, Y.; Yang, W.; Wang, B.; Meyers, M.A. Structure and Mechanical Behavior of Human Hair. Mater. Sci. Eng. C 2017, 73, 152–163. [Google Scholar] [CrossRef]
- Benzarti, M.; Pailler-Mattei, C.; Jamart, J.; Zahouani, H. The Effect of Hydration on the Mechanical Behaviour of Hair. Exp. Mech. 2014, 54, 1411–1419. [Google Scholar] [CrossRef]
- Evans, T. A Unifying Theory for Visualizing the Causes of Hair Breakage and Subsequent Strategies for Mitigation. J. Cosmet. Sci. 2017, 68, 137–140. [Google Scholar]
- Wortmann, F.J.; Quadflieg, J.M.; Wortmann, G. The Information Content of Tensile Tests of Human Hair (Wet) Is Limited: Variables Mainly Cluster in Just Two Principal Components. J. Mech. Behav. Biomed. Mater. 2022, 129, 105145. [Google Scholar] [CrossRef]
- Medice, A.; Lourenço, C.; Gasparin, R.; Nakano, A.; Marsaioli, A. Fragrance Retention in Virgin and Bleached Caucasian Hair. J. Cosmet. Sci. 2018, 69, 363–370. [Google Scholar]
- Hahn, J.; Felts, T.; Vatter, M.; Reid, T.; Marconnet, A. Measurement of Hair Thermal Diffusivity with Infrared Microscopy Enhanced Ångström’s Method. Materialia 2020, 12, 100733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).