1. Introduction
Microbial contamination of cosmetics represents a serious risk to public health, arising both from inherent contamination during production and from user-induced contamination during use by consumers [
1]. This issue calls for a thorough evaluation of bacteriological standards and the efficacy of preservatives in cosmetic formulations. Implementing robust quality control measures, including stringent cleaning procedures, environmental monitoring, and microbiological testing, is crucial for manufacturers to prevent contamination and ensure product safety [
2].
Cosmetic products, particularly those with high water content, are notably susceptible to contamination by a range of microorganisms, including
Escherichia coli,
Pseudomonas aeruginosa,
Staphylococcus aureus,
Aspergillus brasiliensis, and
Candida albicans [
3,
4].
E. coli, typically associated with fecal contamination, suggests poor manufacturing practices or the use of contaminated raw materials, posing serious health risks, especially when these products are applied near sensitive areas like the eyes or on damaged skin [
5].
P. aeruginosa, a resilient bacterium that thrives in aqueous environments, is a common contaminant in cosmetic products such as lotions and creams. It is a major concern for those with compromised immune systems and is notorious for its resistance to antibiotics, complicating treatment efforts [
6].
S. aureus, which is commonly found on the skin and in the nasal mucosa, can easily contaminate cosmetics during manufacture or use, leading to skin infections, particularly in areas where the skin barrier is compromised [
7].
A.
brasiliensis, a fungus, can degrade product quality and pose health risks, especially to immunocompromised individuals, through the production of harmful secondary metabolites [
8].
C. albicans, a polymorphic fungus, is typically innocuous but can induce infections varying from superficial skin conditions to severe systemic infections under certain conditions [
9]. This fungus acts as an opportunistic pathogen, particularly in immunocompromised individuals, causing a spectrum of infections including painful mucosal conditions [
10]. The pathogenic mechanisms of
C. albicans are multifaceted, involving adherence to and invasion of host cells, biofilm formation, and resistance to antifungal agents [
11]. Therefore, rigorous microbiological control of pathogens in cosmetic products is vital for safeguarding consumer health.
Traditional microbial detection methods, such as inoculation by plating on selective media, have been the industry standard in sectors including food, pharmaceuticals, and cosmetics. Microbial control in cosmetics, mandated by European Union legislation, is critical for ensuring that microbial limits are maintained throughout product development, production, storage, and usage [
12].
In addition to routine microbiological testing, one of the most widely used methods for assessing microbiological safety in industry is the challenge test, a standardized procedure that evaluates the efficacy of preservative systems over time by deliberately inoculating products with known microbial strains and monitoring their survival or reduction over a period of 28 days (ISO 11930:2019) [
4]. This assessment is typically carried out during formulation design and is essential to ensure the long-term safety and microbial stability of the final product.
While the challenge test is effective, it does not provide real-time information on the presence or identity of specific microorganisms. Additionally, the methodological design of ISO 11930:2012 [
4] introduces concerns regarding specificity and accuracy. The protocol employs a single, non-selective medium—Tryptic Soy Agar (TSA)—for the detection of three distinct bacterial species:
S. aureus,
P. aeruginosa, and
E. coli. The use of a universal medium without selective properties increases the risk of inaccurate enumeration due to interference by background microbiota or non-target organisms present in the cosmetic matrix. Since the standard does not require the use of selective media, this may result in false positives or underestimation of the target microorganisms, potentially compromising the reliability of the results.
In contrast, the FISH method developed in this study enables rapid and specific detection of microbial contaminants, such as C. albicans, at the single-cell level without the need for culture or prolonged incubation. As such, FISH represents a powerful complementary method for routine microbiological quality control and could significantly enhance the reliability and efficiency of the challenge test protocol.
Thus, while both approaches serve important roles in microbiological quality control, FISH offers a complementary, rapid diagnostic tool, particularly useful for early detection and source tracking in manufacturing environments.
FISH has emerged as a powerful tool in microbiological diagnostics due to its ability to detect and identify microorganisms directly in their native environments, without the need for culturing. Unlike traditional culture-based methods, which often require 24–72 h for organism growth and identification, FISH offers near real-time detection by targeting specific sequences of ribosomal RNA or DNA with fluorescently labeled probes [
13]. This allows for the rapid identification of microbial contaminants, even those that are viable but non-culturable (VBNC), which are commonly undetected by conventional plating techniques [
14].
In addition to its speed and accuracy, FISH provides high specificity by enabling the visualization of individual microbial cells within complex matrices, such as cosmetic formulations. This single-cell resolution is particularly advantageous in heterogeneous samples, where contaminants may be present in low abundance or masked by other components. Furthermore, when combined with flow cytometry (flow-FISH), the method becomes highly scalable and quantifiable, making it suitable for routine quality control in industrial settings [
15,
16]. These attributes make FISH a compelling alternative or complement to classical microbiological testing in the cosmetics industry.
FISH, a culture-independent technique, enables the specific identification of microbial species at the single-cell level through the use of fluorescently labeled probes that bind to complementary RNA or DNA sequences within target cells [
13,
14,
17,
18]. This technique has extensive applications in microbial ecology, allowing for the examination of microbial community distribution and behavior in natural environments such as soil, water, wine, and within living organisms [
19,
20]. Clinically, FISH is invaluable for the rapid detection of pathogens in patient samples, significantly enhancing the diagnosis and treatment of infectious diseases [
21,
22,
23].
Kempf et al. [
23] developed a DNA-FISH probe specifically for detecting
C. albicans in blood samples, which is effective only in the presence of 20% (
v/
v) formamide. However, the use of formamide raises safety concerns due to its carcinogenic properties [
24]
Therefore, the present study aims to develop a novel DNA-FISH probe requiring 0% formamide for the specific identification of C. albicans in cosmetic products, while also significantly enhancing the speed and accuracy of microbial detection through the application of a flow cytometry-coupled FISH protocol (flow-FISH).
2. Materials and Methods
2.1. Design and In Silico Evaluation of a C. albicans Specific Probe
To design a species-specific DNA-FISH probe for
C. albicans, several 26S ribosomal RNA (rRNA) sequences from the target group were retrieved from the National Center for Biotechnology Information (NCBI) website (
https://www.ncbi.nlm.nih.gov, accessed on 1 April 2025) [
25] and aligned using the BioEdit program [
26]. These sequences were then submitted to the Design Probes web tool within the DECIPHER program [
27] to generate species-specific DNA-FISH probes for
C. albicans. From the results, the top ten probes were selected based on their specificity scores and efficiencies.
For in silico evaluation, the following steps were conducted: (a) To confirm probe specificity, a BLASTN search was performed on the NCBI website [
25]; (b) the probes properties, such as molecular weight, melting temperature, GC content, self-complementarity, and hairpin loop formation, were calculated using the Oligo Calc: Oligonucleotide Properties Calculator [
28]; and (c) in silico simulations of FISH performance, including hybridization efficiency, probe affinity, FA melting point ([FA]m), and dissociation profiles, were carried out using the mathFISH program [
29]. Finally, the probe with the highest specificity and optimal FISH performance was selected for experimental testing.
2.2. Analyses of the Performance and Specificity of the DNA-FISH Probe
2.2.1. Microorganisms: Strains and Growth Conditions
In this work, the following strains were used: Escherichia coli ATCC 8739 (American Type Collection, Manassas, VA, United States of America), Candida albicans ATCC 18804 (American Type Collection, Manassas, United States of America), Pichia kudriavzevii ISA 2290 (Instituto Superior de Agronomia, Universidade de Lisboa, Lisboa, Portugal), Staphylococcus aureus ATCC 25992 (American Type Collection, Manassas, United States of America), Saccharomyces cerevisiae ISA 1028 (Instituto Superior de Agronomia, Universidade de Lisboa, Portugal), and Wickerhamomyces anomalus NCYC 434 (National Collection of Yeast Cultures, Norwich, United Kingdom).
E. coli was maintained in Luria–Bertani (LB) agar medium (BD Difco, Franklin Lakes, NJ, USA) and Staphylococcus aureus ATCC 25992 in Brain Heart Infusion (BHI) agar medium (BD Difco, NJ, USA), C. albicans ATCC 18804, Pichia kudriavzevii ISA 2290, S. cerevisiae ISA 1028, and W. anomalus NCYC 434 were maintained on YEPD agar (containing 20 g/L glucose, 20 g/L peptone, 10 g/L yeast extract, and 20 g/L agar, pH 6) and stored at 4 °C. Inoculums of E. coli and S. aureus were obtained by transferring biomass of each species (pre-grown at 37 °C for 24 h) into 100 mL of LB broth medium (BD Difco, NJ, USA) and to BHI broth medium (BD Difco, NJ, USA), respectively. Inoculums of yeasts were obtained by transferring biomass of each species (pre-grown at 30 °C for 24–48 h) into 100 mL of YEPD broth medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose, pH 6). Bacterial cultures were incubated at 37 °C for 4 h without agitation, and yeasts were incubated at 30 °C for 8 h without agitation.
2.2.2. DNA-FISH Probes
The DNA-FISH probes used in this study included the following: EUK516, a universal rRNA probe for eukaryotes, serving as a positive control for yeasts; EUB338, a universal rRNA probe for eubacteria, used as a yeast-negative and bacteria-positive control; and Cad202, a species-specific probe targeting C. albicans. All the probes were labeled with ATTO 647N at the 5′ end.
2.2.3. RNA-FISH Procedure
After incubation, the cultured cells were collected through centrifugation and washed with PBS. A total of 50.0 mL of PBS (Phosphate-Buffered Saline: 130.0 mM NaCl, 8.0 mM NaH
2PO
4, 2.7 mM KCl, 1.5 mM KH
2PO
4, pH 7.2) was prepared. The cells were then fixed in absolute ethanol and incubated for 1 h at room temperature to preserve cellular integrity while permeabilizing the membranes. The fixed cells were stored in a 50/50 ethanol/PBS mixture (
v/
v) at −20.0 °C for later use. Before further processing, the cells were washed with PBS, and a suspension containing 10
6 cells (yeasts) and 10
8 cells (bacteria) was transferred to 1.5 mL microtubes for centrifugation. For yeast strains, cell counts were determined using a Neubauer chamber, allowing direct quantification of yeast cells to obtain suspensions with approximately 10
6 cells/mL. For bacterial strains, cell density was estimated by measuring the optical density at 600 nm. Based on standard growth curves for the bacterial species used in this study (
E. coli and
S. aureus), an OD
600 of approximately 1.0 corresponds to ~10
8 cells/mL, which was used to prepare the bacterial suspensions [
30]. The resulting pellet was resuspended in 80.0 μL of hybridization buffer (HB), which contained 0.9 M NaCl, 20 mM Tris-HCl, 0.1% SDS aqueous solution, pH 7.2, with [FA]% concentrations ranging from 0 to 45 (
v/
v). Next, 1.0 μL of a DNA-FISH probe stock solution (120 ng/μL) was added to each assay. The FISH assays included (i) blank controls (to assess natural and FISH-induced autofluorescence) without the DNA-FISH probe, and (ii) positive controls using the EUK516-ATTO 647N probe (universal for eukaryotes, targeting yeast) and EUB338-ATTO 647N probe (universal for eubacteria, targeting bacteria).
All FISH assays were incubated in the dark in a water bath for 2 h at 46 °C with continuous shaking. After centrifugation, the cells were washed with 100 μL of Washing Buffer (WB) for 30 min in the same water bath under the conditions used for hybridization. The stringency of the WB was adjusted based on the formamide concentration [FA]% used during the hybridization step, following the protocol of [
29] with slight modifications. The composition of the WB for cells treated with hybridization buffer (HB) containing FA (0–45%) is detailed in
Table S1. For cells treated with HB with 0% FA, the WB used was the HB. After washing, the cells were pelleted by centrifugation, resuspended in 500 μL of PBS, and analyzed using flow cytometry (FC). The entire process was conducted under aseptic conditions, and centrifugation steps were performed for 5 min at 13,000 rpm and 4 °C.
2.3. Flow Cytometry (FC) Analysis
The flow cytometer (Cytoflex, Beckman, Brea, CA, USA) used in this work is equipped with a blue laser and five fluorescence detectors. All light scattering and fluorescence signals were plotted in logarithmic scale cytograms, using the CytExpert 2.4 software (Beckman Coulter, CA, USA). The equipment was daily calibrated using a suspension of fluorospheres around 3 µm in size with an emission range from 410 to 800 nm (Beckman Coulter, CytoFLEX Daily QC Fluorospheres, CA, USA).
The fluorescence intensity specifically conferred on the cells by the Cad202-ATTO 647N probe after FISH treatment was calculated by the following formula:
FI = Σ(FI after Cad202 probe hybridization) − Σ(FI in the corresponding negative control). The percentage of fluorescent cells obtained after RNA-FISH treatment was calculated according to the following formula: % fluorescent cells = [(Σ fluorescent cells in positive control or Cad202-treated sample) − (Σ fluorescent cells in blank control)] × 100/2000. Each sample was run in triplicate.
2.4. Cosmetic Formulation Tested
The cosmetic base formulation evaluated was a
Cucumis Sativus lotion supplied by Elisa Câmara
® Lda. (Lisbon, Portugal), with a pH of 5.2. The formulations studied were prepared in the Elisa Câmara Laboratory in accordance with Good Manufacturing Practices (ISO 22716:2007) [
31]. The ingredients comply with the International Nomenclature of Cosmetic Ingredients (INCI) and are as follows:
Aqua, PEG-7 Glyceryl Cocoate (SABODERM® HE MB-RNM; PRODUTOS QUÍMICOS, S.A., Porto, Portugal), PEG-40 Hydrogenated Castor Oil (EUMULGIN® HRE 40; Cognis, Monheim, Germany), Glycerin (Glycerine USP, PH. Eur.; Interfat-natural oils, Barcelona, Spain), Cucumis Sativus Fruit Extract (CUCUMBER EXTRACT H.GL., Provita; Barcelona, Espanha), Tocopheryl Acetate (Covi-ox® T 90 C, BASF®; Personal Care, Monheim, Germany), Allantoin (ALLANTOIN, AKEMA S.R.L., Fine chemicals; Rimini, Italia) Propylene Glycol (PROPILENGLICOL USP; INEOS, Köln, Germany), Parfum (FLORAL® No. 54.690.0225; Sensient Fragrances, S.A., Granada, Spain), Lactic Acid (Jungbunzlauer Lactic Acid 90®; Jungbunzlauer S.A, Marckolsheim, France), Tetrasodium EDTA (TETRASODIUM ETHYLENEDIAMINETETRAACETATE; Quimidroga S.A., Barcelona, Spain).
2.5. Assessment of Physicochemical and Microbiological Analysis in Cosmetic Formulations
To determine the physicochemical analysis of the
Cucumis sativus lotion formulations, pH was assessed by the potentiometric method (ISO 4316:1977) [
32] (Metrohm; 692 pH/Ion Meter 09187, Herisau, Sweden) and physical stability by centrifugation for 1 h at 5000 rpm (ISO/TR 18811:2018) [
33] (P. Selecta; Centronic 3ZZ102, Barcelona, Spain). In addition, microbiological analyses were performed according to standard methods, testing for enumeration and detection of mesophilic bacteria (ISO 21149:2017) [
34],
S. aureus (ISO 22718:2015) [
35],
E. coli (ISO 21150:2015) [
36],
P. aeruginosa (ISO 22717:2015) [
37],
C. albicans (ISO 18416:2015) [
38], and total yeast and mold (ISO 16212:2017) [
39] to ensure that the formulation is not contaminated. Confirming both the microbiological and physicochemical quality of formulations is critical before initiating further testing to ensure the safety and efficacy of the product.
2.6. FISH Procedure in the Cosmetic Formulation
The tonic formulation was artificially contaminated with C. albicans to assess the applicability of the DNA-FISH probe in a cosmetic matrix. C. albicans was inoculated into the tonic at a cell density of 108 cells/mL and incubated for 2 h to allow microbial interaction with the formulation components. Following incubation, the FISH procedure described in 2.2.3 was applied to evaluate probe hybridization efficiency.
4. Discussion
Microbiological quality control in cosmetics is essential for ensuring consumer safety and product integrity. Contamination by bacteria or fungi can not only degrade product performance and shelf life but also pose serious health risks, particularly for immunocompromised individuals or when applied to sensitive areas such as the eyes or damaged skin [
3,
7]. Given the increasing use of natural and water-rich formulations—more prone to microbial growth—cosmetic products must be routinely tested to meet strict microbiological safety standards. Regulatory frameworks such as ISO 16212:2017 and ISO 21149:2017 [
34,
39] provide guidelines on acceptable microbial limits and testing protocols. Effective quality control strategies, including both preservative efficacy testing (e.g., challenge tests) and rapid routine microbial detection methods, are critical to prevent contamination and ensure compliance with safety regulations [
4,
12]. Although challenge tests are essential for regulatory compliance and preservative validation, this method is time-consuming, taking up to four weeks to complete, and does not identify the microbial species present [
4]. It also lacks sensitivity in detecting viable but non-culturable organisms, which may still pose safety risks [
42].
The RNA-FISH method described in this study represents a significant advancement in microbiological surveillance by enabling specific, rapid, and culture-independent identification of C. albicans within hours. This technique can be particularly valuable in early-phase contamination screening, during manufacturing or stability testing, where rapid microbial detection is critical for decision-making. Rather than replacing the challenge test, FISH provides a complementary strategy that strengthens the overall microbiological control framework by addressing its main limitations—namely, speed and specificity.
4.1. In Silico Evaluation of C. albicans DNA FISH Probes
The development of a DNA-FISH probe for the specific detection of C. albicans in cosmetic products represents a valuable advancement in microbiological quality control.
The selection of the Cad202 DNA-FISH probe was based on a comprehensive evaluation of specificity score, hybridization efficiency, and sequence match identity, as outlined in
Table 1. Among the tested probes, Cad202 demonstrated superior performance across these critical parameters, establishing it as the optimal choice for
C. albicans detection.
The specificity score of Cad202 was among the highest (lowest negative value) when compared to other probes, indicating its strong discriminatory ability between C. albicans and non-target species. Although other probes exhibited acceptable specificity values, Cad202 consistently outperformed them by minimizing potential cross-hybridization with non-target sequences. This enhanced specificity is particularly relevant for applications requiring high diagnostic accuracy, such as microbial contamination assessment in cosmetic formulations.
Cad202 achieved a hybridization efficiency of 99.99%, the highest among the tested probes, ensuring robust and reliable binding to target sequences under 0% formamide conditions. The high hybridization efficiency of this probe was a key factor in its selection, as several alternative probes demonstrated lower efficiencies, which could compromise detection sensitivity.
Unlike traditional probes that require formamide to achieve high stringency, Cad202 was specifically designed and validated to function effectively under formamide-free conditions. The ability to operate under 0% formamide not only eliminates the need for a toxic reagent but also simplifies the hybridization protocol, reducing health and environmental risks associated with formamide exposure. This feature is particularly useful for applications in the cosmetics industry, where safety regulations require the exclusion of hazardous substances.
Furthermore, BLAST analysis confirmed that Cad202 exhibited a 100% sequence match identity with 494 C. albicans sequences, underscoring its precise targeting capability. While other probes also demonstrated high sequence match identities, the combination of superior specificity and hybridization efficiency rendered Cad202 the most effective option. The integration of this probe into microbiological quality control workflows has the potential to enhance the accuracy and reliability of C. albicans detection in cosmetic products, thereby contributing to improved product safety and compliance with regulatory standards.
Our study builds upon and advances previous research on the use of DNA-FISH probes for the detection of
C. albicans. Notably, Kempf et al. [
23] successfully employed a DNA-FISH assay for identifying
C. albicans in blood cultures, demonstrating strong specificity. However, their method required the use of 20% formamide during hybridization, a compound known for its toxicity and environmental concerns. In contrast, the Cad202 probe developed in this work maintains high hybridization efficiency and specificity under formamide-free conditions, offering a safer and more sustainable alternative without compromising diagnostic accuracy. This improvement is particularly valuable for implementation in industrial contexts such as cosmetics manufacturing, where minimizing hazardous reagent use is essential for both operator safety and environmental compliance.
Our findings also expand upon the work of Branco et al. [
16], who applied an RNA-FISH method to detect
Dekkera bruxellensis in wine environments. Similarly to that study, our method combines FISH with flow cytometry, enabling single-cell resolution and quantitative analysis. However, applying this technique to detect
C. albicans in complex, formulated cosmetic products represents a novel and significant advancement. The high specificity, rapid detection time, and effective performance in a formulated product highlight the potential for broader application of FISH in quality control across multiple industries. Together, these results validate the robustness of FISH-based detection while demonstrating significant advancements in probe design and hybridization conditions.
4.2. Evaluation of Cad202-ATTO 647N DNA-FISH Probe Performance and Specificity
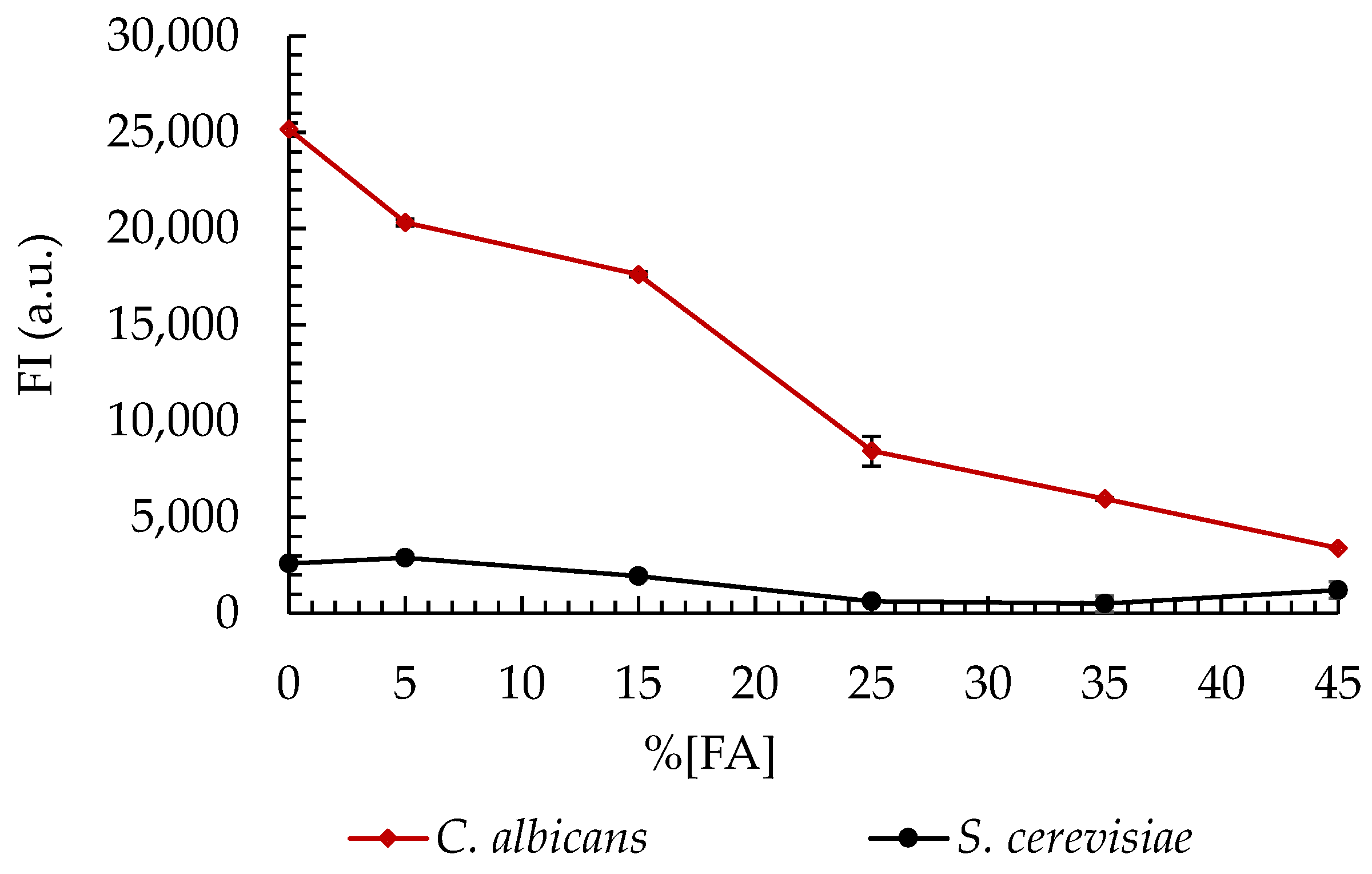
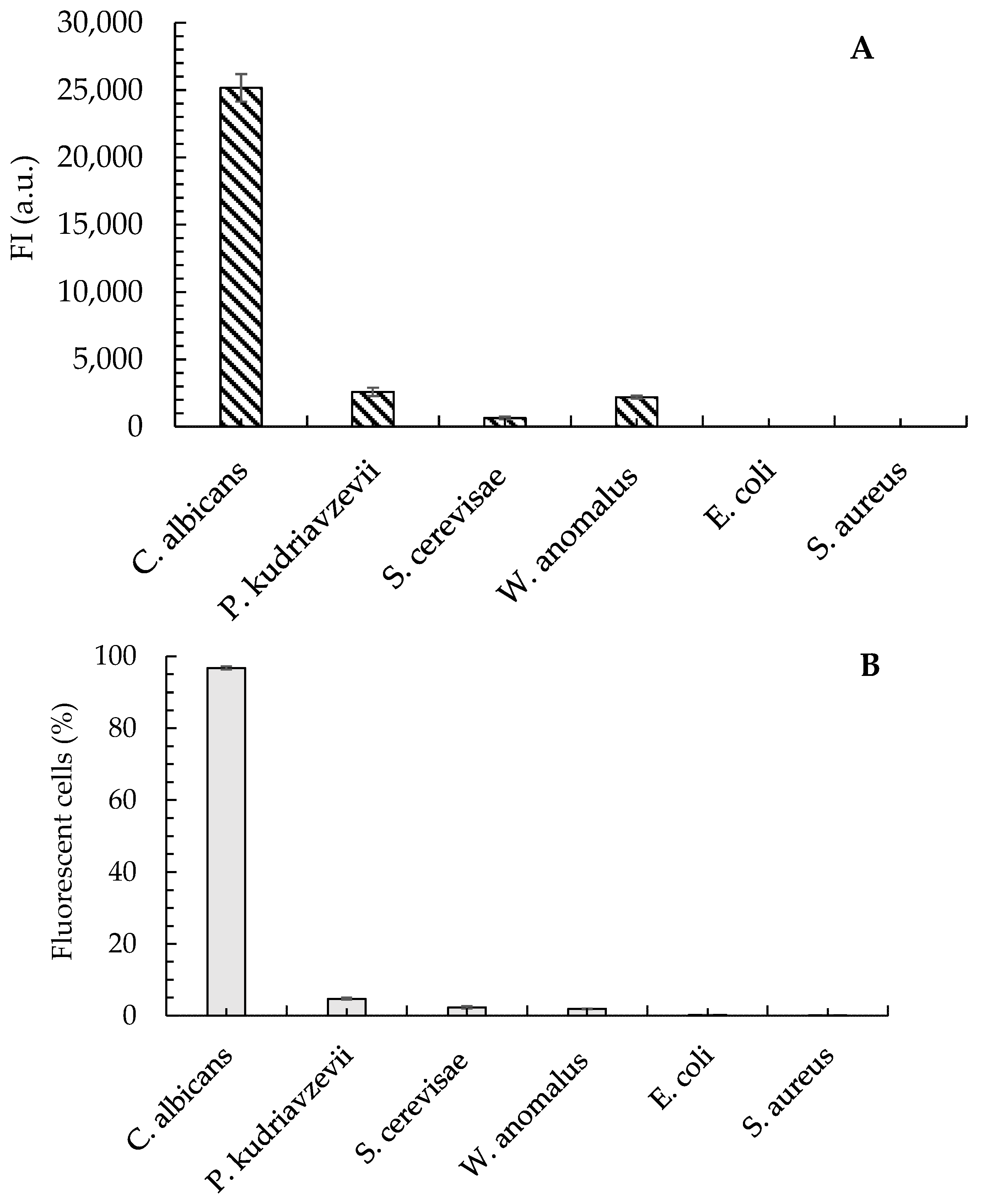
The in silico analyses confirmed that the Cad202 probe exhibits high specificity, with minimal cross-reactivity with closely related yeast species, including C. krusei, S. cerevisiae, and W. anomalus. These findings were further validated through experimental evaluations, which demonstrated that only 2.3%, 1.9%, and 4.7% of the respective non-target cells exhibited hybridization with the Cad202 probe. Additionally, the fluorescence intensity (FI) of these non-target interactions was significantly lower than that observed for C. albicans, ensuring a clear distinction between target and non-target species.
The fluorescence intensity of C. albicans remained consistently high across a range of formamide (FA) concentrations, with the peak intensity observed under 0% FA conditions. This suggests strong hybridization efficiency in the absence of formamide, further supporting the robustness of the probe. In contrast, S. cerevisiae exhibited consistently low FI values across all FA concentrations tested, indicating minimal hybridization and reinforcing the probe’s specificity.
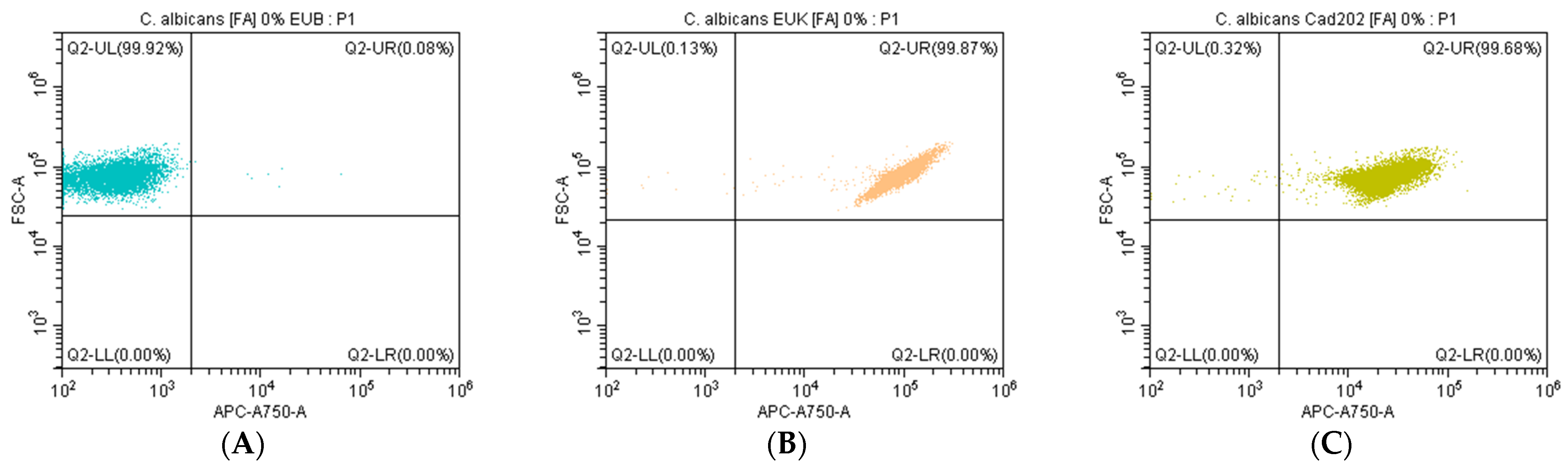
Dot plot analysis further demonstrated the ability of the Cad202-ATTO 647N probe to selectively detect C. albicans RNA under non-stringent conditions (0% FA). The negative control probe, EUB338, exhibited no significant fluorescence, confirming the absence of non-specific binding, while the positive control probe, EUK-ATTO 647N, effectively recognized eukaryotic RNA, serving as a validation reference. These results collectively establish Cad202-ATTO 647N as a highly reliable and specific tool for the detection of C. albicans under optimized hybridization conditions, highlighting its potential application in microbiological quality control.
4.3. Microbiologic Analyses of the Cosmetic Formulation
In order to carry out the test accurately, it is essential to ensure that the cosmetic formulations analyzed were prepared in accordance with the official quality control standards and the specifications established by Elisa Câmara, Lda for the product in question.
According to the results obtained, it was possible to conclude that all the parameters analyzed complied with all requirements, guaranteeing the quality of the formulation from an organoleptic, physicochemical, and microbiological standpoint. The presence of any microorganism, in particular
C. albicans, would be an uncontrolled interfering factor that could affect the validity of the test and the conclusions based on the controls established. The methodology used in the quality control followed the standardized classical methods for the quantification of microorganisms on agar plates, which allowed for verifying that the detection of pathogens by this approach has several inherent drawbacks [
41]. For example, the detection of
C. albicans is time consuming, requiring media preparation, inoculation, and an incubation period of up to 48 h. Although these methods are highly valid, their time consumption and susceptibility to cross-contamination can delay product release [
42]. Alternatively, the application of the FISH method [
22] with the Cad202 probe may provide a more rapid and efficient option. With its high specificity for
C. albicans, detection time is reduced to just a few hours, eliminating the need for complex growth and incubation steps, improving the accuracy and efficiency of the detection process. This technology simplifies the procedure and minimizes the risk of false positives and cross-contamination.
Future studies should investigate the application of the Cad202 DNA-FISH probe to different cosmetic formulations to assess its scalability and integration with automated detection systems, which could further optimize the speed and reliability of microbiological quality control in the cosmetics industry.
4.4. Applicability of the RNA-FISH Method for Detecting C. albicans in Cosmetic Formulations
The slightly lower percentage of hybridized C. albicans cells observed in the cosmetic tonic formulation (88.4%) compared to pure culture conditions (99.68%) suggests that certain components within the cosmetic matrix may interfere with the hybridization process. Factors such as pH variation, chemical composition, and the biphasic nature of the formulation are likely contributors to the observed reduction in hybridization efficiency.
FISH probe hybridization is highly sensitive to pH, with optimal efficiency typically achieved under neutral to slightly alkaline conditions. The tonic formulation used in this study has an estimated pH between 4.0 and 5.5, which is more acidic than standard hybridization buffers. These acidic conditions can destabilize nucleic acid interactions and reduce the binding affinity of the probe, ultimately compromising hybridization performance [
43].
In addition, specific ingredients commonly found in cosmetic products may further affect probe efficacy. For example, EDTA, frequently used as a chelating agent, can alter the ionic conditions required for effective probe-target binding. Previous studies have shown that EDTA may reduce non-specific probe adsorption in environmental FISH applications, but it may also unintentionally impact specific hybridization [
44].
The biphasic nature of the tonic, containing both aqueous and oil-based components, may also influence probe behavior. Lipid-rich environments are known to hinder probe diffusion and increase background fluorescence, as demonstrated in studies involving complex matrices [
44,
45]. The oil phase may act as a physical barrier, impeding probe access to target cells and leading to incomplete labeling.
Despite these challenges, the RNA-FISH method proved effective for C. albicans detection within a cosmetic product. However, adjustments to the hybridization protocol—such as modifying buffer composition, optimizing pH, or incorporating emulsifying agents—could further enhance detection performance in complex formulations. Future research should explore how specific formulation ingredients and pH conditions influence hybridization dynamics, with the goal of refining this method for routine microbiological quality control in the cosmetics industry.
5. Conclusions
The Cad202 DNA-FISH probe, characterized by its high specificity and optimized design, demonstrates significant potential for improving microbial surveillance in cosmetic products. Its ability to selectively detect Candida albicans while minimizing cross-reactivity underscores its utility in microbiological quality control. Future research should explore the probe’s scalability across diverse product matrices and assess its integration with automated detection systems to enhance efficiency in quality control workflows.
The selection of the Cad202 probe was justified by its superior performance across all in silico evaluated criteria, establishing it as a highly reliable and effective tool for C. albicans detection in cosmetic formulations. Notably, its ability to maintain high specificity and hybridization efficiency in the absence of formamide highlights its practical applicability and innovative contribution to microbiological quality control methodologies.
Furthermore, this study reinforces the potential of integrating flow cytometry with the FISH method (flow-FISH) as a robust approach for rapid and precise microbial detection. By enabling single-cell analysis, flow-FISH provides distinct advantages over conventional culture-based methods, including reduced detection time and enhanced sensitivity. Its implementation in microbiological quality control presents a valuable opportunity for real-time monitoring of microbial contaminants, ultimately improving the safety and compliance of cosmetic manufacturing processes.