Natural Mineral Water–Plant Extract Combinations as Potential Anti-Aging Ingredients: An In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Extracts, Hydrolates, and Natural Mineral Waters Understudy
2.1.1. Plant and Agro-Industrial Residue Extracts
2.1.2. Natural Mineral Waters
2.2. In Vitro Safety Assessment Through MTT Assay: Cell Biocompatibility in L929 and RAW 264.7 Cell Lines
2.3. Efficacy Testing
2.3.1. DPPH Assay for Antioxidant Activity
2.3.2. Senescence-Associated β-Galactosidase in L929 Cells
2.3.3. Cell Migration Assay in L929: Skin Regenerating Potential
2.3.4. Reactive Oxygen Species Quantification in RAW 264.7 Cells
2.3.5. Superoxide Dismutase (SOD) Activity in RAW 264.7 Cells
2.4. Data Analises: Statistical Approach
3. Results and Discussion
3.1. Assessing Biocompatibility in L929 and RAW 264.7 Cell Lines and Selection of Optimal Concentrations for Cellular Efficacy Testing
3.2. Assessing Antioxidant Activity
3.3. Overview of the Efficacy Results Using Cell Models
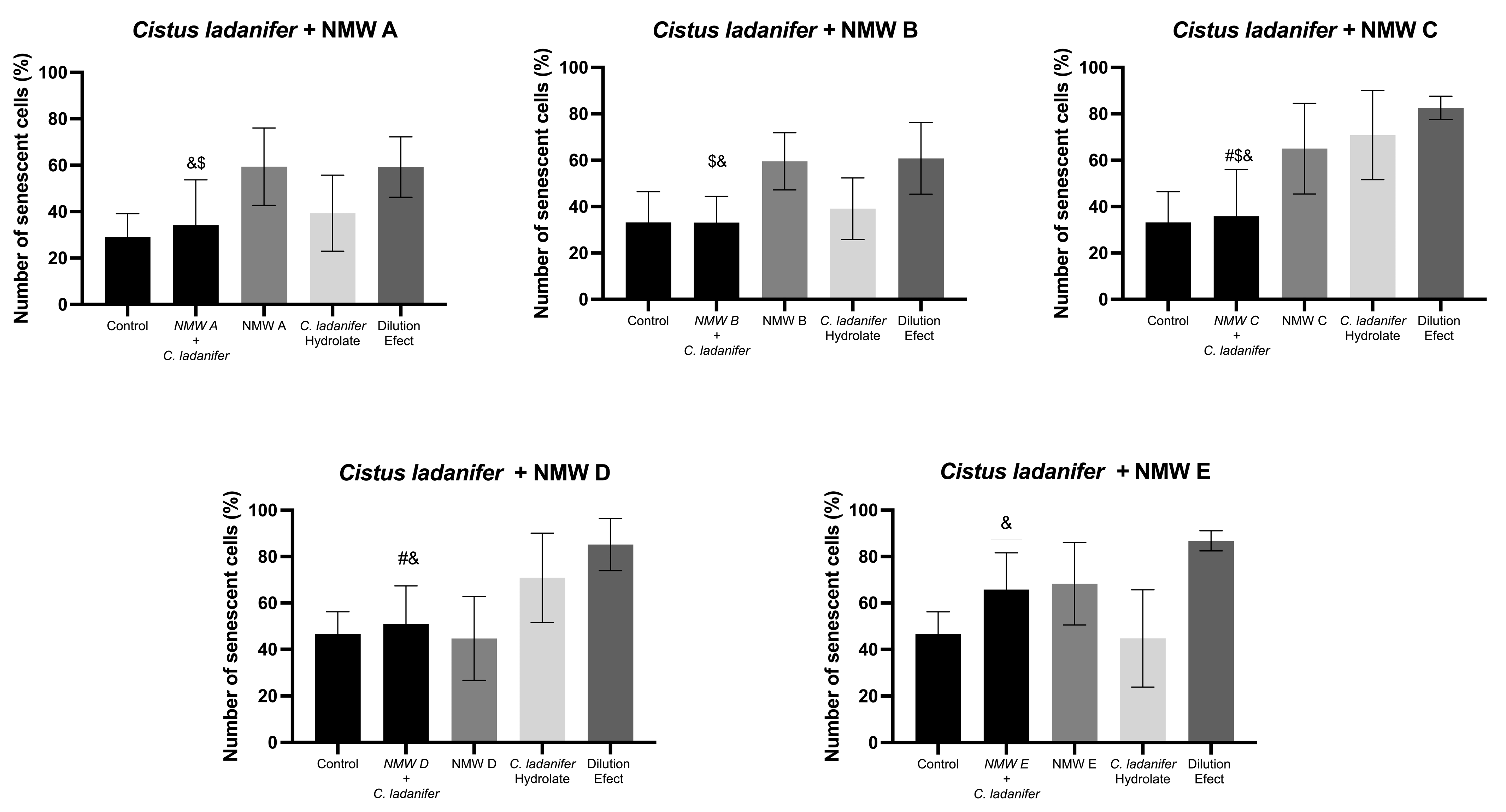
3.4. Senescence-Associated β-Galactosidase Senescence in L929 Cell Line
3.5. Skin Regenerating Potential of the Mixtures
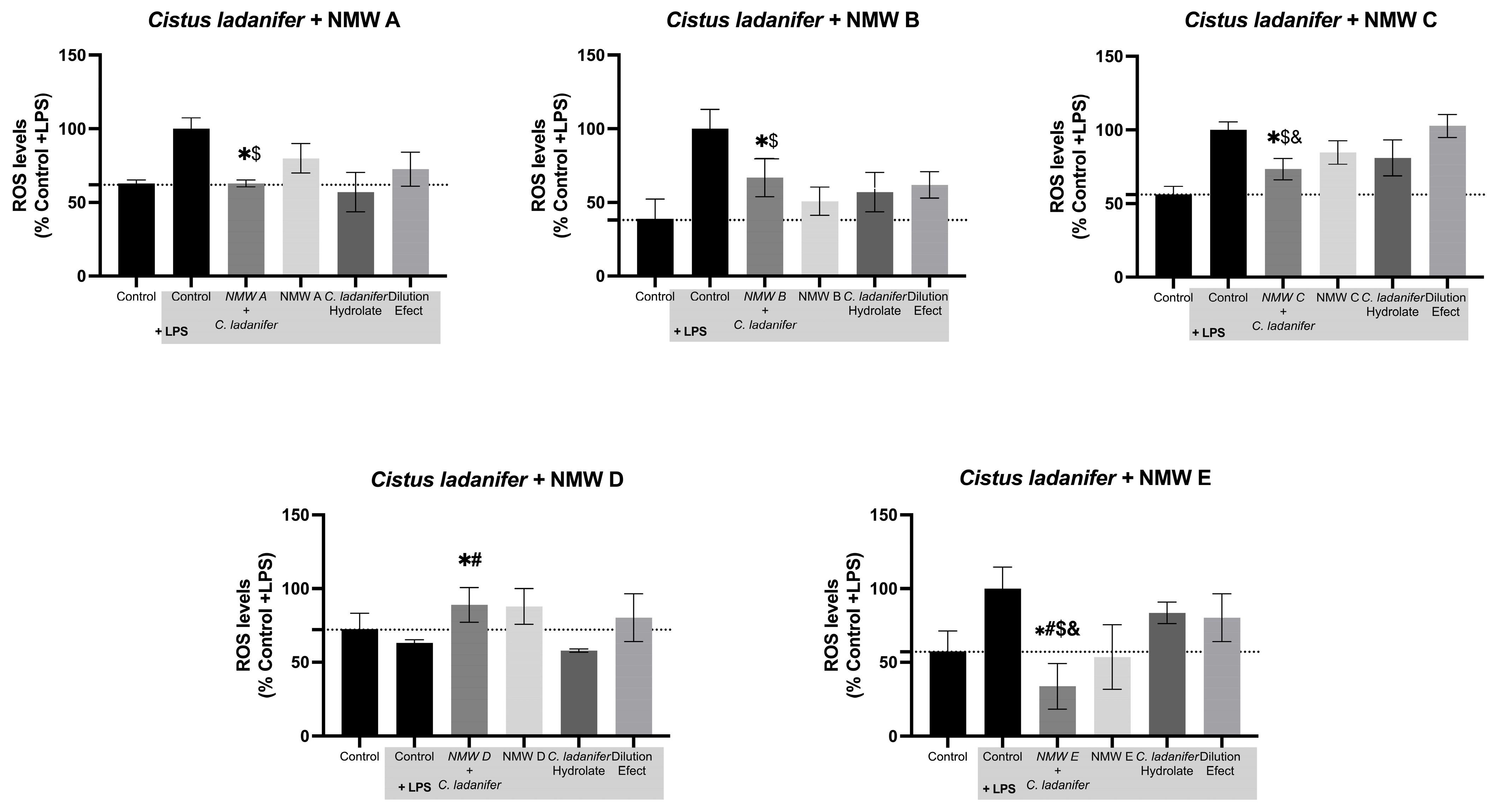
3.6. Reactive Oxygen Species Quantification
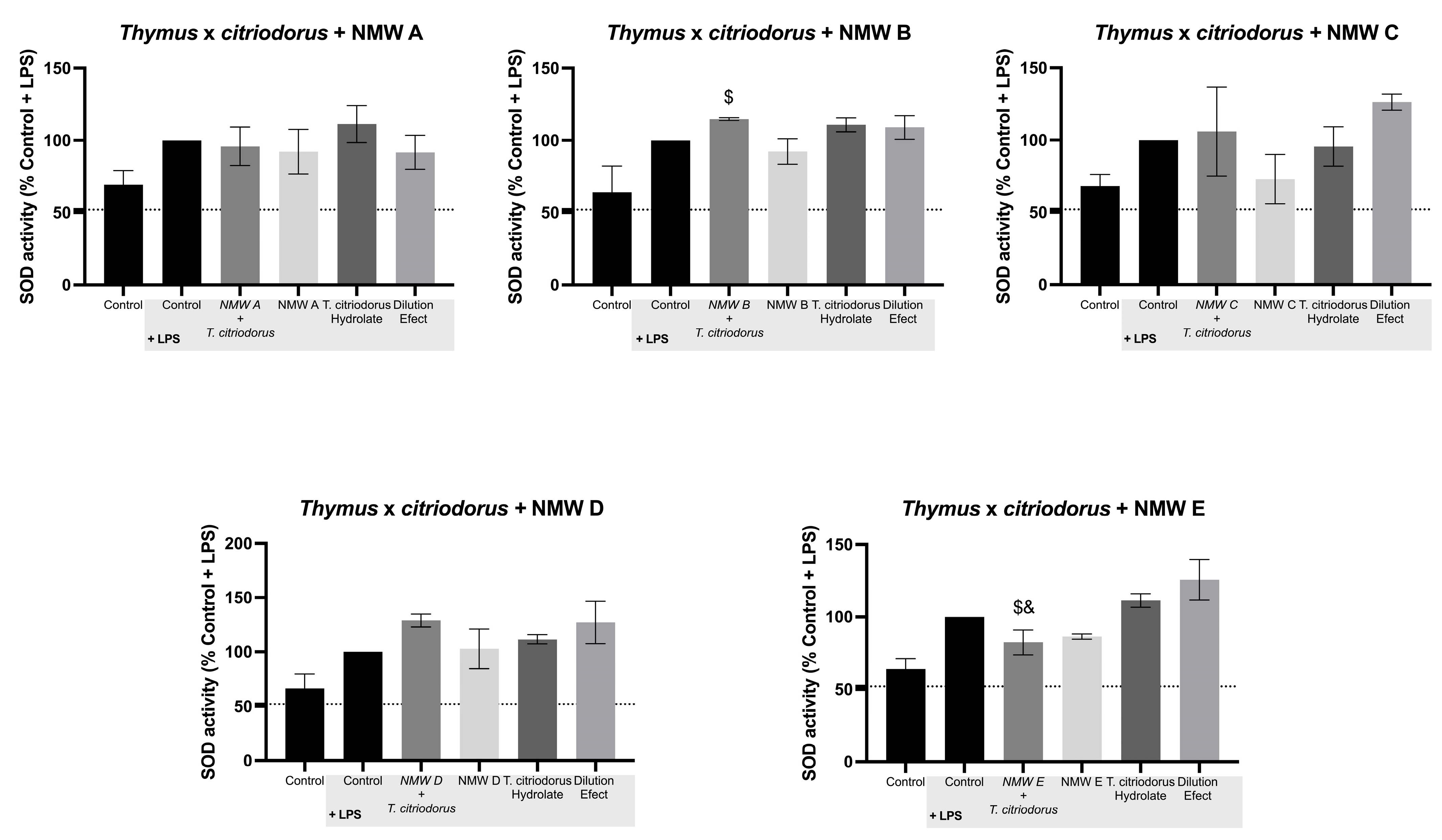
3.7. Effect on Superoxide Dismutase (SOD) Activity
3.8. Integrated Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Corrêa, M.A.; Chorilli, M. Sustainability, natural and organic cosmetics: Consumer, products, efficacy, toxicological and regulatory considerations. Braz. J. Pharm. Sci. 2015, 51, 17–26. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.S.H. Natural anti-aging skincare: Role and potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chermahini, S.H.; Adibah, F.; Majid, A.; Sarmidi, M.R. Cosmeceutical Value of Herbal Extracts as Natural Ingredients and Novel Technologies in Anti-Aging. J. Med. Plants Res. 2011, 5, 3074–3077. [Google Scholar]
- Martins, I.; Almeida, D.; Yoon, I.-S.; Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent Advances in Herbal-Derived Products with Skin Anti-Aging Properties and Cosmetic Applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef]
- Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Roccia, M.G.; Fioranelli, M.; Gianfaldoni, R.; Lotti, T. History of the Baths and Thermal Medicine. Open Access Maced. J. Med. Sci. 2017, 5, 566. [Google Scholar] [CrossRef]
- Araujo, A.R.T.S.; Sarraguça, M.C.; Ribeiro, M.P.; Coutinho, P. Physicochemical fingerprinting of thermal waters of Beira Interior region of Portugal. Env. Geochem. Health 2017, 39, 483–496. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Vaz, C.V.; Silva, A.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.; Cruz, M.T.; et al. In vitro evaluation of potential benefits of a silica-rich thermal water (Monfortinho Thermal Water) in hyperkeratotic skin conditions. Int. J. Biometeorol. 2020, 64, 1957–1968. [Google Scholar] [CrossRef]
- Silva, A.; Oliveira, A.S.; Vaz, C.V.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.M.F.; Palmeira-de-Oliveira, A.; et al. Anti-inflammatory potential of Portuguese thermal waters. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Vaz, C.V.; Oliveira, A.S.; Silva, A.; Cortes, L.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.F.; et al. Protective role of Portuguese natural mineral waters on skin aging: In Vitro evaluation of anti-senescence and anti-oxidant properties. Int. J. Biometeorol. 2022, 66, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Nissen, H.P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int. J. Dermatol. 2005, 44, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Rodrigues, M.; Mourelle, M.L.; Araujo, A.R.T.S. Thermal Spring Waters as an Active Ingredient in Cosmetic Formulations. Cosmetics 2023, 10, 27. [Google Scholar] [CrossRef]
- Yasin, Z.A.M.; Ibrahim, F.; Rashid, N.N.; Razif, M.F.M.; Yusof, R. The Importance of Some Plant Extracts as Skin Anti-aging Resources: A Review. Curr. Pharm. Biotechnol. 2017, 18, 864–876. [Google Scholar] [CrossRef]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Martins, C.V.B.; Fariña, L.O.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Enciso-Martínez, Y.; Zuñiga-Martínez, B.S.; Ayala-Zavala, J.F.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Viuda-Martos, M. Agro-Industrial By-Products of Plant Origin: Therapeutic Uses as well as Antimicrobial and Antioxidant Activity. Biomolecules 2024, 14, 762. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- Filho, A.V.; Avila, L.B.; Lacorte, D.H.; Martiny, T.R.; Rosseto, V.; Moraes, C.C.; Dotto, G.L.; Carreno, N.L.V.; da Rosa, G.S. Brazilian Agroindustrial Wastes as a Potential Resource of Bioative Compounds and Their Antimicrobial and Antioxidant Activities. Molecules 2022, 27, 6876. [Google Scholar] [CrossRef]
- Gonçalves, S.; Caramelo, A. The Role of Elderberry Hydrolate as a Therapeutic Agent in Palliative Care. Antioxidants 2025, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Cruciani, S.; Garroni, G.; Sarais, G.; Kavak, F.F.; Satta, R.; Montesu, M.A.; Floris, M.; Ventura, C.; Maioli, M. Effect of Helichrysum italicum in Promoting Collagen Deposition and Skin Regeneration in a New Dynamic Model of Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 4736. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.V.; Palmeira-de-Oliveira, R.; Guiomar, L.; Vaz, C.V.; Rolo, J.; Gaspar, C.; Oliveira, A.S.; Caramelo, D.; Breitenfeld, L.; Gonçalves, J.C.; et al. Humulus lupulus aqueous extract and hydrolate as a potential ingredient for cosmetics: Chemical characterization and in vitro antimicrobial, cytotoxicity, antioxidant and anti-inflammatory assessment. Fitoterapia 2024, 175, 105861. [Google Scholar] [CrossRef] [PubMed]
- Ayu, K.; Budiasih, S.; Gede, I.; Handrean, E. The Anti-Aging Properties of Panax Ginseng: A Narrative Review of Mechanisms and Clinical Evidence. Int. J. Health Pharm. (IJHP) 2025, 5, 157–164. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Y.; Fu, J.; He, L.; Guo, X.; Ye, H.; Yin, C.; Li, H.; Jiang, H. Beneficial Effects of Epigallocatechin Gallate in Preventing Skin Photoaging: A Review. Molecules 2024, 29, 5226. [Google Scholar] [CrossRef]
- Nobile, V.; Dudonné, S.; Kern, C.; Roveda, G.; Garcia, C. Antiaging, Brightening, and Antioxidant Efficacy of Fermented Bilberry Extract (Vaccinium myrtillus): A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 2203. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Ramos, L.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Thymus mastichina (L.) L. and Cistus ladanifer L. for skin application: Chemical characterization and in vitro bioactivity assessment. J. Ethnopharmacol. 2023, 302, 115830. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Ramos, L.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Chemical characterization and bioactive potential of Thymus × citriodorus (Pers.) Schreb. preparations for anti-acne applications: Antimicrobial, anti-biofilm, anti-inflammatory and safety profiles. J. Ethnopharmacol. 2022, 287, 114935. [Google Scholar] [CrossRef]
- Plasencia, P.; Heleno, S.A.; Finimundy, T.; Carocho, M.; Calhelha, R.C.; Añibarro-Ortega, M.; Alves, M.J.; Oludemi, T.; Quidiongo, N.; Barreiro, F.; et al. Recovery of High Valuable Bioactive Molecules from Vaccinium myrtillus L. Bioresidues. Waste Biomass Valorization 2023, 14, 2873–2884. [Google Scholar] [CrossRef]
- Shiraishi, C.S.H.; Zbiss, Y.; Roriz, C.L.; Dias, M.I.; Prieto, M.A.; Calhelha, R.C.; Alves, M.J.; Heleno, S.A.; V., d.C.M.; Carocho, M.; et al. Fig Leaves (Ficus carica L.): Source of Bioactive Ingredients for Industrial Valorization. Processes 2023, 11, 1179. [Google Scholar] [CrossRef]
- Plasencia, P.; Finimundy, T.C.; Carocho, M.; Calhelha, R.C.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Barreiro, F.; Garcia, P.A.; Barros, L.; Heleno, S.A.; et al. Extraction of Bioactive Compounds from Rubus Idaeus Bioresidues: A Full Screening on Phenolic Composition and Bioactive Potential. Waste Biomass Valorization 2025, 16, 737–747. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S.; Bridgewater, L. (Eds.) Standard Methods for the Examination of Water and Wastewater; American Public Health Association American Water Works Association Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Hidrogenoma. Available online: https://hidrogenoma.dgeg.gov.pt/ (accessed on 15 February 2025).
- Oliveira, A.S.; Vaz, C.V.; Silva, A.; Ferreira, S.S.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.; et al. Chemical signature and antimicrobial activity of Central Portuguese Natural Mineral Waters against selected skin pathogens. Environ. Geochem. Health 2020, 42, 2039–2057. [Google Scholar] [CrossRef]
- Da Silveira, T.F.F.; Echegaray, N.; Guimarães, R.; Lemos, A.; Pires, T.C.S.P.; Ferreira, I.C.F.R.; Alves, M.J.; Barros, L. Unveiling the impact of thermal water in German chamomile infusions: Effects on phenolic compounds, antimicrobial and antioxidant properties. Food Chem. 2025, 463, 141481. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO 10993-5:2009. Available online: https://www.iso.org/standard/36406.html (accessed on 10 May 2023).
- Molyneux, P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- JP-DM Protocol and Undefined 2012 Procedure: Preparation of DPPH Radical, and Antioxidant Scavenging Assay. Researchgate.netJM PrietoDPPH Microplate Protocol, Researchgate.Net. 2012. Available online: https://www.researchgate.net/profile/Jose-Prieto-10/post/Can_anyone_explain_the_DPPH_method_for_antioxidant_activity_in_details/attachment/59d61efac49f478072e9772d/AS%3A271744332435456%401441800305338/download/Prieto+DPPH+protocol.pdf (accessed on 10 May 2025).
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Pascoal, A.S.P.; Moura, M.J.; Ferreira, P.C.N.; Ramos, M.L.C.; Salavessa, M.A.N.; Duarte, B.P.M. A Conceptual Engineering Approach to Developing a Bio-Based Hair Mask. Cosmetics 2025, 12, 45. [Google Scholar] [CrossRef]
- Mac-Mary, S.; Creidi, P.; Marsaut, D.; Courderot-Masuyer, C.; Cochet, V.; Gharbi, T.; Guidicelli-Arranz, D.; Tondu, F.; Humbert, P. Assessment of effects of an additional dietary natural mineral water uptake on skin hydration in healthy subjects by dynamic barrier function measurements and clinic scoring. Ski. Res. Technol. 2006, 12, 199–205. [Google Scholar] [CrossRef]
- Lestari, U.; Muhaimin, M.; Chaerunisaa, A.Y.; Sujarwo, W. Anti-Aging Potential of Plants of the Anak Dalam Tribe, Jambi, Indonesia. Pharmaceuticals 2023, 16, 1300. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Gour, H.; Sagar, V.; Kumar, R. Medicinal Plants as Source of Anti-Ageing Agents: A Review. World J. Pharm. Pharm. Sci. 2023, 12, 946–957. [Google Scholar] [CrossRef]
- Papaccio, F.; D’arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Duarte, D.; Diallo, M.; Santos, J.; Ribeiro, E.; Vale, N.; Vilas-Boas, M. Improvement of the In Vitro Cytotoxic Effect on HT-29 Colon Cancer Cells by Combining 5-Fluorouacil and Fluphenazine with Green, Red or Brown Propolis. Molecules 2023, 28, 3393. [Google Scholar] [CrossRef]
- Tun, J.O.; Salvador-Reyes, L.A.; Velarde, M.C.; Saito, N.; Suwanborirux, K.; Concepcion, G.P. Synergistic Cytotoxicity of Renieramycin M and Doxorubicin in MCF-7 Breast Cancer Cells. Mar. Drugs 2019, 17, 536. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Pavlović, D.; Grigorov, M.; Martinović, M.; Tasić-Kostov, M. Hydrolates: From Waste Products to Potential Cosmetic Actives. Arch. Pharm. 2021, 71, S86. [Google Scholar]
- Oğuz, İ.; Oğuz, H.İ.; Attar, Ş.H.; Sönmez, D.A.; Çelik, H.; Yaşa Kafkas, N.E. Preferable Berry Fruits for Tolerance to Global Climate Change and Dry Conditions; IntechOpen Limited: London, UK, 2023. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and Antimicrobial Activity of Mexican Oregano (Poliomintha longiflora) Essential Oil, Hydrosol and Extracts from Waste Solid Residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components antimicrobial antioxidant properties of the essential oil hydrosol extracts of Citrus aurantium L. flowers. J. Infect. Public. Health 2020, 13, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Gonçalves, M.J.; Silva, A.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical Profile Anti-Microbial Anti-Inflammaging Activities of Santolina rosmarinifolia L. Essential Oil from Portugal. Antibiotics 2023, 12, 179. [Google Scholar] [CrossRef]
- Warman, D.J.; Jia, H.; Kato, H. Effects of Thyme (Thymus vulgaris L.) Essential Oil on Aging-Induced Brain Inflammation and Blood Telomere Attrition in Chronologically Aged C57BL/6J Mice. Antioxidants 2023, 12, 1178. [Google Scholar] [CrossRef]
- Faria, A.; Oliveira, J.; Neves, P.; Gameiro, P.; Santos-Buelga, C.; de Freitas, V.; Mateus, N. Antioxidant Properties of Prepared Blueberry (Vaccinium myrtillus) Extracts. J. Agric. Food Chem. 2005, 53, 6896–6902. [Google Scholar] [CrossRef]
- Tsakiroglou, P.; Vandenakker, N.E.; Del Bo’, C.; Riso, P.; Klimis-Zacas, D. Role of Berry Anthocyanins and Phenolic Acids on Cell Migration and Angiogenesis: An Updated Overview. Nutrients 2019, 11, 1075. [Google Scholar] [CrossRef]
- Costa, G.; Francisco, V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Intracellular signaling pathways modulated by phenolic compounds: Application for new anti-inflammatory drugs discovery. Curr. Med. Chem. 2012, 19, 2876–2900. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.Y.; Sun, J.E.; Lin, W.H.; Zhou, R.X. ILK–PI3K/AKT pathway participates in cutaneous wound contraction by regulating fibroblast migration and differentiation to myofibroblast. Lab. Investig. 2016, 96, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Dugué, C.; Baiyasi, M.; Jackson, S.; Tolliver, S.; Daveluy, S. Baking Soda and the Skin: A Review of Baking Soda in Dermatology. J. Integr. Dermatology. 2024. Available online: https://www.jintegrativederm.org/article/122501-baking-soda-and-the-skin-a-review-of-baking-soda-in-dermatology (accessed on 16 May 2025).
- Hanyu, O.; Nakae, H.; Miida, T.; Higashi, Y.; Fuda, H.; Endo, M.; Kohjitani, A.; Sone, H.; Strott, C.A. Cholesterol sulfate induces expression of the skin barrier protein filaggrin in normal human epidermal keratinocytes through induction of RORα. Biochem. Biophys. Res. Commun. 2012, 428, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Nadanaka, S.; Kadomatsu, K.; Kitagawa, H. Chondroitin 6-sulfate represses keratinocyte proliferation in mouse skin, which is associated with psoriasis. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hołyńska-Iwan, I.; Szewczyk-Golec, K. Analysis of changes in sodium and chloride ion transport in the skin. Sci. Rep. 2020, 10, 18094. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Major selected monoterpenes α-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic Potentials of Superoxide Dismutase. Int. J. Health Sci. 2018, 12, 88. [Google Scholar]
- Božovic, M.; Pirolli, A.; Ragno, R. Mentha suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules 2015, 20, 8605–8633. [Google Scholar] [CrossRef]
- Costantino, M.; Giuberti, G.; Caraglia, M.; Lombardi, A.; Misso, G.; Abbruzzese, A.; Ciani, F.; Lampa, E. Possible antioxidant role of SPA therapy with chlorine-sulphur-bicarbonate mineral water. Amino Acids 2009, 36, 161–165. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Maraver, F. Sulphurous Mineral Waters: New Applications for Health. Evid.-Based Complement Altern. Med. 2017, 2017, 8034084. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Madeira, A.; Marto, J.; Graça, A.; Pinto, P.; Ribeiro, H. Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults. Cosmetics 2019, 6, 56. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef] [PubMed]

| Species | Common Name | Source | Major Compounds | Reference |
|---|---|---|---|---|
| Cistus ladanifer | Rockrose | Proentia® (Proença-a-Nova, Portugal) | E-pinocarveol (25.3%), borneol (14.0%), terpinene-4-ol (9.7%) | [29] |
| Ficus carica L. | Fig | CIMO-IPB (Bragança, Portugal) | Apigenin-C-hexoside-C-pentoside, Quercetin-O-deoxyhexosyl-hexoside | [32] |
| Rubus idaeus L. | Raspberry | CIMO-IPB (Bragança, Portugal) | Galloyl-HHDP-glucose, Procyanidin dimer, Procyanidin trimer, Methyl ellagic acid, hexoside | [33] |
| Thymus x citriodorus | Lemon thyme | Ervitas Catitas™ (Borba, Portugal) | 1,8-cineole (43.9%), α-terpineol (21.1%), borneol (11.5%) | [29] |
| Vaccinium myrtillus | Blueberry | CIMO-IPB (Bragança, Portugal) | 3-O-Cafeoylquinic acid, 5-O-Cafeoylquinic acid, Procyanidin trimer | [31] |
| Code | Main Composition | Secondary Composition | Major Compounds * | PH | Reference |
|---|---|---|---|---|---|
| A | Sodium Bicarbonate, Sulfuric | Carbonated, Fluoridated, Sulfhydrated | SiO2 (↑), Na+ (↑),HCO3− (↑) | 8.7 | [36] |
| B | Sodium Bicarbonate, Sulfuric | Carbonated, Fluoridated, Sulfhydrated | HCO3− (↑↑),Na+ (↑), SiO2 (↑) | 8.8 | [36] |
| C | Sulfate, Bicarbonate, Sodium | Sulfhydrated | HCO3− (↑↑), Na+ (↑), SiO2 (↓) | 8.3 | [36] |
| D | Silicate | Sodium bicarbonate | H3SiO4− (↓), SiO2 (↓),HCO3− (↓) | 5.6 | [36] |
| E | Sodium Bicarbonate, Gasocarbonate | Fluoridated | HCO3− (↑↑), Na+ (↑↑), SiO2 (↑) | 6.9 | [37] |
| Extract | Combination with NMW A | Combination with NMW B | Combination with NMW C | Combination with NMW D | Combination with NMW E |
|---|---|---|---|---|---|
| Cistus ladanifer | 25 (% v/v) NMW + 3.13 (% v/v) hyd | 12.5 (% v/v) NMW + 3.13 (% v/v) hyd | 25 (% v/v) NMW + 6.25 (% v/v) hyd | 12.5 (% v/v) NMW + 6.25 (% v/v) hyd | 25 (% v/v) NMW + 12.5 (% v/v) hyd |
| Ficus carica L. | 12.5 (% v/v) NMW + 2 mg/mL Ext | 12.5 (% v/v) NMW + 2 mg/mL Ext | 25 (% v/v) NMW + 1 mg/mL Ext | 12.5 (% v/v) NMW + 1 mg/mL Ext | 12.5 (% v/v) NMW + 2 mg/mL Ext |
| Rubus idaeus L. | 12.5 (% v/v) NMW + 1 mg/mL Ext | 25 (% v/v) NMW + 1 mg/mL Ext | 12.5 (% v/v) NMW + 0.25 mg/mL Ext | 12.5 (% v/v) NMW + 1 mg/mL Ext | 25 (% v/v) NMW + 1 mg/mL Ext |
| Thymus x citriodorus | 12.5 (% v/v) NMW + 3.13 (% v/v) hyd | 25 (% v/v) NMW + 3.13 (% v/v) hyd | 12.5 (% v/v) NMW + 6.25 (% v/v) hyd | 25 (% v/v) NMW + 12.5 (% v/v) hyd | 25 (% v/v) NMW + 12.5 (% v/v) hyd |
| Vaccinium myrtillus | 12.5 (% v/v) NMW + 0.25 mg/mL Ext | 25 (% v/v) NMW + 0.25 mg/mL Ext | 12.5 (% v/v) NMW + 0.25 mg/mL Ext | 12.5 (% v/v) NMW + 0.5 mg/mL Ext | 25 (% v/v) NMW + 0.25 mg/mL Ext |
| Sulfuric Bicarbonate Sodic | Silicated | Sodium, Bicarbonate, Gasocarbonic | ||||
|---|---|---|---|---|---|---|
| NMW A | NMW B | NMW C | NMW D | NMW E | W/o NMW (Extract IC50) | |
| Cistus ladanifer | 34.760 * + 34.760 * | 19.770 * + 19.770 * | 23.920 * + 23.920 * | 53.630 * + 53.630 * | 40.583 * + 40.583 * | 26.000 * |
| Ficus carica L. | 11.220 + 0.080 | 6.144 + 0.049 | 6.201 + 0.0448 | 6.566 + 0.0525 | 16.703 * + 0.133 | 0.041 |
| Rubus idaeus L. | 16.540 * + 0.010 | 8.137 + 0.006 | 10.85 + 0.008 | 11.470 + 0.009 | 20.932 * + 0.016 | 0.006 |
| Thymus x citriodorus | 37.770 * + 37.770 * | 24.230 * + 24.230 * | 31.050 * + 31.050 * | 40.820 * + 40.820 * | 51.846 * + 51.846 * | 36.980 * |
| Vaccinium myrtillus | 8.750 + 0.010 | 5.576 + 0.004 | 5.482 + 0.004 | 14.910 + 0.011 | 9.836 + 0.007 | 0.004 |
| W/o extract (NMW IC50) | 36.840 * | 25.260 * | 61.200 * | 67.540 * | 38.799 * | - |
| Senescence | Migration | ROS Production | SOD Activity | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract | A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | |
| NMW | |||||||||||||||||||||
| Cistus ladanifer | ↑↑ | ↑↑ | ↑↑ | ↑ | ↑ | ↓ | – | ↓ | – | ↓ | ↑↑ | – | ↑↑↑ | ↓ | ✓ | – | ↓ | ↓ | ↓ | ↓ | |
| Ficus carica L. | ↑ | ↑ | ↑↑↑ | ↓ | ↓ | ↑ | – | – | – | ↓ | ↑↑ | ↓ | ↓ | ↓ | ↑ | – | ↓ | – | – | – | |
| Rubus idaeus L. | – | ↓ | – | ↓ | – | ↑↑ | ↓ | ↑ | ↑↑↑ | ↑↑ | ↑ | – | ↑ | – | ↑ | ↓ | ↓ | ↑ | ↓ | – | |
| Thymus x citriodorus | ↓ | – | ↑↑ | ↑ | ↓ | – | ↓ | – | ↓ | ↓ | – | ↓ | ↑↑ | ↑ | ↑↑ | – | ↑ | – | – | ↓ | |
| Vaccinium myrtillus | ↓ | – | ↓ | – | ↓ | – | – | ↓ | – | ↑↑ | ↑↑ | ↑ | – | ↑ | ↓ | – | ↓ | ↑ | ↓ | ↓ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, C.P.; Oliveira, A.S.; Rolo, J.; da Silveira, T.F.F.; Palmeira de Oliveira, R.; Alves, M.J.; Plasencia, P.; Palmeira de Oliveira, A. Natural Mineral Water–Plant Extract Combinations as Potential Anti-Aging Ingredients: An In Vitro Evaluation. Cosmetics 2025, 12, 113. https://doi.org/10.3390/cosmetics12030113
Gomes CP, Oliveira AS, Rolo J, da Silveira TFF, Palmeira de Oliveira R, Alves MJ, Plasencia P, Palmeira de Oliveira A. Natural Mineral Water–Plant Extract Combinations as Potential Anti-Aging Ingredients: An In Vitro Evaluation. Cosmetics. 2025; 12(3):113. https://doi.org/10.3390/cosmetics12030113
Chicago/Turabian StyleGomes, Carolina P., Ana S. Oliveira, Joana Rolo, Tayse F. F. da Silveira, Rita Palmeira de Oliveira, Maria José Alves, Paula Plasencia, and Ana Palmeira de Oliveira. 2025. "Natural Mineral Water–Plant Extract Combinations as Potential Anti-Aging Ingredients: An In Vitro Evaluation" Cosmetics 12, no. 3: 113. https://doi.org/10.3390/cosmetics12030113
APA StyleGomes, C. P., Oliveira, A. S., Rolo, J., da Silveira, T. F. F., Palmeira de Oliveira, R., Alves, M. J., Plasencia, P., & Palmeira de Oliveira, A. (2025). Natural Mineral Water–Plant Extract Combinations as Potential Anti-Aging Ingredients: An In Vitro Evaluation. Cosmetics, 12(3), 113. https://doi.org/10.3390/cosmetics12030113








