Abstract
2-aza-8-oxohypoxanthine (AOH) has been shown to improve skin barrier function according to DNA microarray studies of normal human epidermal keratinocytes. This study aimed to evaluate the cosmetic efficacy of AOH in wrinkle improvement. A 12-week clinical trial involved 23 women (mean age 44 ± 8 years), who applied 0.01% or 0.05% AOH lotion to each half of their face twice daily. Skin assessments were conducted before and after application at 4, 8, and 12 weeks by measuring stratum corneum water content using a Corneometer and evaluating wrinkles through image analysis of replicas. Application of the lotion enhanced stratum corneum water content. Use of 0.01% AOH reduced the wrinkle area percentage and maximum wrinkle depth at 8 and 12 weeks post-application, respectively. The 0.05% lotion showed a significant reduction in the wrinkle area percentage and mean maximum wrinkle depth at 12 weeks and a significant decrease in maximum wrinkle depth at 4 weeks. A between-group comparison revealed that the 0.05% AOH lotion significantly reduced mean maximum wrinkle depth compared to the 0.01% AOH lotion at 8 and 12 weeks, indicating a concentration-dependent effect of AOH. Overall, AOH improves wrinkles in a concentration-dependent manner, confirming its efficacy as a cosmetic ingredient for wrinkle improvement.
1. Introduction
The “fairy rings” phenomenon occurs when turfgrass growth is either accelerated or inhibited in a ring pattern, with mushrooms often emerging within this formation. According to western legends, these rings were believed to be spots where fairies danced, hence the term “fairy rings” [1,2,3]. 2-azahypoxanthine (AHX), imidazole-4-carboxamide (ICA), and 2-aza-8-oxohypoxanthine (AOH) have been identified as compounds responsible for the formation of fairy rings (Figure 1) [4,5,6]. After the title of a Nature article introducing our work, we refer to these compounds (AHX, ICA, and AOH) as fairy chemicals (FCs) collectively [7].
Figure 1.
Chemical structures of fairy chemicals. (a) 2-azahypoxanthine (AHX), (b) imidazole-4-carboxamide (ICA), and (c) 2-aza-8-oxohypoxanthine (AOH).
Preliminary studies with FCs showed that only AOH had a cytostatic effect on normal human epidermal keratinocytes (NHEKs). Therefore, we have focused on AOH among the three types of FCs to verify its usefulness and safety on skin. Safety evaluation studies of AOH have shown no adverse reactions in any tests, which supports its use in cosmetics [8,9]. Additionally, AOH exists endogenously in various plants, including major crops such as rice, wheat, and potatoes [10,11]. Previous studies have highlighted the potential of AOH among FCs as a functional cosmetic ingredient [12,13].
The efficacy of AOH was further substantiated by the finding that it did not exert any adverse effects on the viability of NHEKs [12]. Microarray-based expression profiling of genes related to skin aging and diseases in NHEKs revealed that AOH treatment generally upregulates genes involved in skin barrier function [12]. A clinical trial confirmed the effectiveness of AOH in enhancing skin barrier function, evidenced by increased stratum corneum water content and reduced trans-epidermal water loss (TEWL) [13]. Overall, the results of cellular-level experiments and clinical trials suggest that AOH plays a vital role in the regulation of skin barrier function. Another clinical trial focused on changes in L* values, an index of skin lightness, after applying a lotion containing 0.1% AOH for 8 weeks. After 8 weeks, L* values increased significantly, demonstrating the skin lightening effect of AOH [14].
Given that decreased stratum corneum water content from compromised skin barrier function can lead to wrinkle formation [15,16,17], potency in wrinkle improvement by AOH was suggested. Therefore, the present clinical study was conducted to prove the effect of AOH by assessing changes in stratum corneum water content and wrinkle parameters before and after prolonged AOH application.
2. Materials and Methods
2.1. Preparation of the Test Formulation
AOH was synthesized from 5-aminoimidazole-4-carboxamide, as previously described [18,19]. Methyl p-hydroxybenzoate (final concentration: 0.4%, FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) was dissolved in 1,3-butanediol (final concentration: 5%, ITO Co., Ltd., Tokyo, Japan) by heating. Phenoxyethanol (final concentration: 0.3%; FUJIFILM Wako Pure Chemical Corporation), xanthan gum (KELCOL CG LAX-T, Sansho Co., Ltd., Osaka, Japan), and polyoxyethylene hydrogenated castor oil 60 (final 0.2%, Nikko Chemicals Co., Ltd., Tokyo, Japan) were then added to this 1.3-butanediol solution. Next, AOH (final concentration: 0.01% or 0.05%) in sodium phosphate buffer (pH 7.0, final 10 mM, FUJIFILM Wako Pure Chemical Corporation) and purified water (final 33.8%) were added to the solution. Prior to the clinical study, a human patch test was performed to evaluate the safety of the test formulation. The patch test was performed on the skin of 16 men and 6 women from Japan, aged 21–59 y (ave. 39 y). The test samples (0.01% or 0.05% AOH lotion) were placed on a Finn Chamber on Scanpor Tape and applied to each subject’s upper back for 24 h in an occlusive condition. Skin conditions were evaluated 1 and 24 h after the removal of the test sample. Skin reactions were evaluated according to the International Contact Dermatitis Research Group standards and the guidelines of the Japanese Dermatological Association [20].
2.2. Participant Selection for the Clinical Trial
The following selection criteria were applied: (1) women (yellow race) in their 30s to 50s at the time of consent; (2) participants who were concerned about dry skin and wrinkles at the corners of their eyes; (3) participants whose usual post-cleansing (or post-bathing) skin care routine consisted of only one or two products (i.e., lotion and emulsion); and (4) participants who received a full explanation of the study’s purpose and procedures, possessed the capacity to consent, and voluntarily agreed to participate by providing written informed consent. The exclusion criteria were as follows: (1) any skin condition at the evaluation site that may affect the study results, such as urticaria or inflammation; (2) any history or current history of atopic dermatitis or predisposition to atopy; (3) participants who had undergone cosmetic treatment on the evaluated area; (4) participants who had undergone skin care treatment at a beauty salon or esthetic clinic on the evaluated area within the past 4 weeks; (5) those who were deemed by the study investigator to be unsuitable for participation.
2.3. Clinical Trial Study
To target participants with dry skin, TEWL was measured in 40 subjects before the start of the clinical trial, and 24 subjects with high TEWL were selected. Twenty-four healthy Japanese women were enrolled in the study, which was conducted in Osaka from 19 January to 27 April 2023. This trial employed a double-blind, split-face, left–right randomized design. After washing their face twice in the morning and evening, participants applied a lotion containing 0.01% AOH to one side of their face and a lotion containing 0.05% AOH to the other side for twelve weeks. Approximately 0.3 g of each product was applied throughout the 12-week period, starting on day 1. After cleansing the face with water, the skin’s biophysical parameters were measured at baseline (the day before the first application) and again at 4, 8, and 12 weeks by the attending researcher. All participants completed a usability questionnaire at 4, 8, and 12 weeks after the start of the study. Skin evaluations were conducted following the “Anti-Wrinkle Evaluation Guidelines for Obtaining New Indications (Quasi-drugs)” [21].
2.4. Evaluation
All measurements were conducted under controlled environmental conditions (temperature, 21 ± 1 °C; relative humidity, 50 ± 10%), following at least a 30 min acclimatization period. Stratum corneum hydration was assessed by capacitance measurements using a Corneometer (Courage + Khazaka electronic GmbH, Cologne, Germany). Each measurement was taken at least three times, and the mean of three stable measurements was used. A replica of the wrinkle was taken using the replica agent SILFLO (Monaderm, Rue des Violettes, Monaco). A 4 cm × 4 cm area was sampled from the external canthus outward on both sides. Wrinkle data were collected using an ASA-03RXD reflective replica analysis system and reflective 3D skin analysis software Version 3.51 (Asch Japan Co., Ltd., Tokyo, Japan). The main analysis region was a 10 mm × 10 mm square located approximately 5 mm lateral to the external canthus. Parameters evaluated included wrinkle area percentage (%), maximum wrinkle depth (μm), mean maximum wrinkle depth (μm), and total mean wrinkle depth (μm).
For the analyses, measurements taken at baseline and 4, 8, and 12 weeks after application were compared among participants between the two groups, i.e., 0.01% AOH and 0.05% AOH lotion applications (within-county comparison). Additionally, changes from baseline to the subsequent time points (at 4, 8, and 12 weeks) were also compared between groups (between-county comparisons).
2.5. Statistical Analyses
All statistical analyses were conducted using IBM® SPSS® Statistics Version 23 (IBM Corp, New York, NY, USA). Two-tailed tests were used, and p-values were calculated using the corresponding t-tests. A Bonferroni correction was applied to account for multiple comparisons, setting the overall significance level at 5%.
3. Results
3.1. Evaluation of Stratum Corneum Water Content
Human patch tests showed no serious skin reactions in any of the subjects at 1 h and 24 h after removal of the test sample. In addition, 0.01% AOH lotion and 0.05% AOH lotion had a skin irritation index of 2.3 and 0.0, respectively. Based on these results, it was concluded that both lotions were safe products.
A total of 22 participants (16 males and 6 females; aged 21–59 years, mean: 39 years) underwent human patch testing with the test formulations. As a result, the skin irritation indices for the 0.01% and 0.05% AOH lotions were 2.3 and 0.0, respectively, indicating that both formulations were safe for use. Of the 24 participants initially enrolled, 23 participants (mean age 44 ± 8 years, range: 32–58 years) completed the clinical trial. They applied the test samples twice daily to their faces for 12 weeks. No participants reported adverse reactions on the usability questionnaire, and no adverse events related to the test product were observed.
Figure 2 shows the time course of the amount of change in stratum corneum water content compared to baseline (0 week). In the 0.01% AOH lotion group, an increasing trend in stratum corneum water content was observed, although the within-county comparison did not show statistical significance. Conversely, the within-county comparison for the 0.05% AOH lotion group revealed a statistically significant increase in stratum corneum water content at 8 weeks (p = 0.014) and 12 weeks (p = 0.004) compared to baseline. Furthermore, the between-county comparison showed that the 0.05% AOH lotion group had a statistically significant increase in stratum corneum water content at 4 weeks (p = 0.025), 8 weeks (p = 0.002), and 12 weeks (p = 0.012) compared to the 0.01% AOH lotion group, demonstrating a concentration-dependent effect.
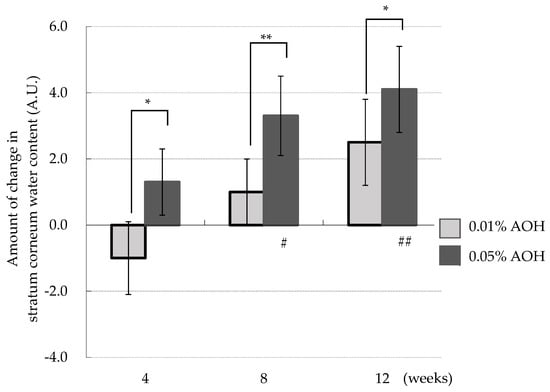
Figure 2.
Change in stratum corneum water content compared with 0 weeks. Means ± SD, n = 23. * p < 0.05, ** p < 0.01 (0.01% AOH vs. 0.05% AOH), # p < 0.05, ## p < 0.01 (baseline vs. after 4, 8, 12 weeks).
In the current study, while the 0.01% AOH lotion group did not show a significant change, there was a trend toward increasing stratum corneum water content over time. Notably, the 0.05% AOH lotion group exhibited increases in stratum corneum water content of 2.7%, 7.3%, and 9.4% at 4, 8, and 12 weeks, respectively, compared to baseline. These findings underscore that continuous application of AOH lotion enhances stratum corneum water content in a concentration-dependent manner.
3.2. Image Analysis of Wrinkles Using Skin Replicas
Figure 3 illustrates the time course of the change in wrinkle area percentage from baseline. At the site where 0.01% AOH was applied, the wrinkle area percentage significantly decreased at 8 weeks (p = 0.015) and 12 weeks (p = 0.043). At the 0.05% AOH-applied site, there was a significant reduction at 12 weeks (p = 0.014).
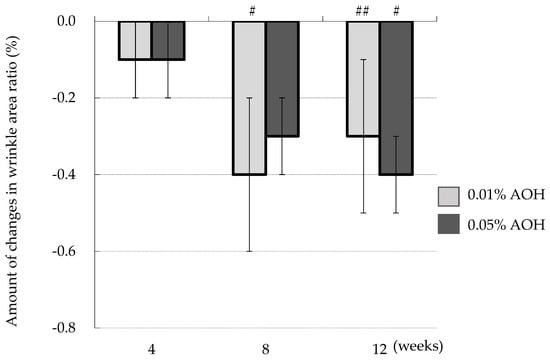
Figure 3.
Change in the wrinkle area ratio from baseline, as measured by image analysis of skin replicas. Mean ± SD, n = 23, # p < 0.05, ## p < 0.01 (0 weeks vs. 4, 8, or 12 weeks).
Figure 4 presents the time course of the reduction in maximum wrinkle depth at the 0.01% AOH application site from baseline. The maximum wrinkle depth at the 0.01% AOH site was significantly reduced at 12 weeks (p = 0.042). In contrast, maximum wrinkle depth at the 0.05% AOH application site was significantly reduced at 4 weeks (p = 0.002), 8 weeks (p < 0.001), and 12 weeks (p < 0.001). Additionally, between-group comparisons indicated a statistically significant greater reduction in maximum wrinkle depth in the 0.05% AOH lotion group at 4, 8 and 12 weeks compared to the 0.01% group, demonstrating a concentration-dependent effect.
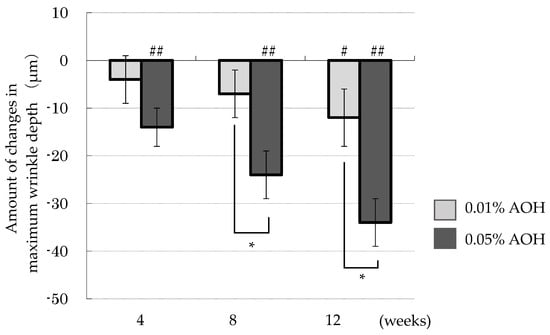
Figure 4.
Changes in maximum wrinkle depth from baseline. Means ± SD, n = 23. * p < 0.05 (0.01% AOH vs. 0.05% AOH), # p < 0.05, ## p < 0.01 (0 weeks vs. after 4, 8, 12 weeks).
Figure 5 displays the time course of the change in mean maximum wrinkle depth relative to baseline. The mean maximum wrinkle depth at the 0.01% AOH-applied site displayed a decreasing trend, but no statistical significance was observed. In contrast, the mean maximum wrinkle depth at the 0.05% AOH application site was significantly reduced at 12 weeks (p = 0.005). Total average wrinkle depth did not change in either the 0.01% or the 0.05% AOH application site until 12 weeks.
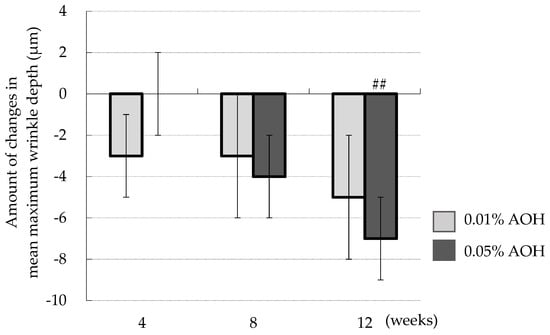
Figure 5.
Changes in mean maximum wrinkle depth from baseline. Means ± SD, n = 23. ## p < 0.01 (0 weeks vs. after 12 weeks).
Skin images of the representative participants taken by a Canon EOS 5D (Tokyo, Japan, Canon Inc.) are shown in Figure 6. In all the participants, wrinkles at the corners of the eyes were improved at 12 weeks, with greater enhancement in areas where the 0.05% AOH lotion was applied compared to the 0.01% AOH lotion.
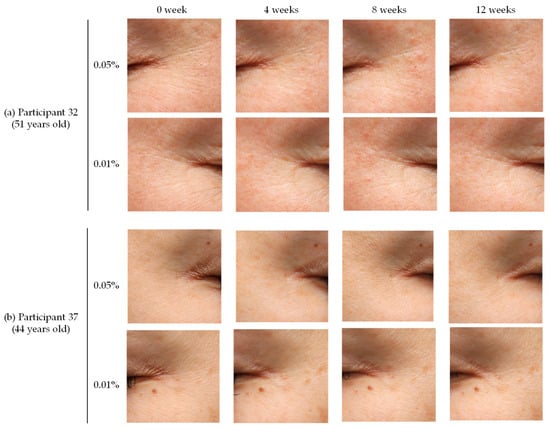
Figure 6.
Examples of wrinkle improvement assessed photographically in the crow’s feet area of participants (a) 32 and (b) 37 using VISIA imaging, showing results from before application (0 weeks) and after 4, 8, and 12 weeks.
Furthermore, in the analysis of wrinkle improvement by skin replicas, indices such as the wrinkle area ratio, maximum wrinkle depth, average wrinkle depth, and total average wrinkle depth were evaluated. In the 0.01% AOH lotion group, maximum wrinkle depth reductions were observed to be −4 µm, −7 µm, and −12 µm at 4, 8, and 12 weeks, respectively, compared to baseline. In the 0.05% AOH lotion group, the reductions were more pronounced, with maximum wrinkle depths decreasing to −14 µm, −24 µm, and −36 µm at the respective time points. This pattern further supports the concentration-dependent efficacy of AOH in wrinkle reduction.
4. Discussion
We have continuously explored the effects of AOH on skin at both cellular and clinical levels. This study presents the first report on the concentration-dependent effects of AOH on both increasing stratum corneum water content and wrinkle reduction.
It is recognized that decreases in stratum corneum water content and increases in TEWL are factors in wrinkle formation [15,16,17]. Hyaluronic acid plays a crucial role in maintaining or regulating the water content of the stratum corneum and epidermis. Research indicates that the concentration of hyaluronic acid in the epidermis diminishes with age, and this age-related dryness may also contribute to wrinkle development [22,23]. DNA microarray analysis of NHEK has shown increased expression of hyaluronic acid synthase 3 (HAS3) in the epidermis [12]. Additionally, AOH boosted hyaluronic acid production in in vitro experiments with human fibroblasts (unpublished data), suggesting that AOH application may enhance hyaluronic acid levels in the skin, thereby aiding in wrinkle reduction.
Another factor contributing to decreased stratum corneum water content is the increased water evaporation associated with impaired skin barrier function. Tight junctions (TJs), essential for skin barrier integrity to prevent water loss, are intercellular connections in the granular layer, and they are crucial not only for preventing water evaporation but also for the formation of mature stratum corneum and intercellular lipids [24,25]. Furthermore, the protein components of TJs diminish with aging and exposure to UV light [26]. Previous DNA microarray analysis using NHEK demonstrated that AOH upregulates a set of genes that enhance skin barrier function [12]. The results of this DNA microarray and our clinical study suggested that AOH may stimulate hyaluronic acid production and improve skin barrier function, resulting in increasing stratum corneum water content and a subsequent reduction in wrinkles in humans.
5. Conclusions
In this study, a long-term continuous-use study was conducted using 0.01% and 0.05% AOH lotion to verify the concentration-dependent effect of AOH on wrinkle improvement. The results showed that water content increased in a concentration-dependent manner, and the wrinkle area ratio, maximum wrinkle depth, and mean maximum wrinkle depth improved.
Considering that no adverse reactions or events were observed in this study, we concluded that AOH is a safe cosmetic ingredient with the potential to reduce the appearance of fine lines and wrinkles caused by dryness of the skin.
Author Contributions
Conceptualization, H.A. and R.I.; methodology, H.A. and R.I.; investigation, H.A; data curation, H.A.; writing—original draft preparation, H.A; writing—review and editing, R.I. and H.K.; visualization, H.A.; supervision, H.K.; project administration, H.A.; funding acquisition, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that this study was funded with a research budget from the Vitamin C60 BioResearch Corporation (integrated into Mitsubishi Corporation Life Sciences Limited on 1 April 2024, Tokyo, Japan). This clinical trial was conducted by an external laboratory, and the funding agency, Vitamin C60 BioResearch Corporation, was not involved in the collection, analysis, or interpretation decisions of the data. This work was partially supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) for Specially Promoted Research “Science of Fairy Chemicals and Their Application Development” (grant number JP20H05620).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of DRC Co. Ltd. (IBR Number 16000189; date of approval: 13 January 2023).
Informed Consent Statement
Informed consent for participation was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this paper are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We wish to express appreciation to all the volunteers who kindly cooperated with the study processes. We also give special thanks to DRC Co., Ltd. (Osaka, Japan) for their assistance in subject recruitment, conducting the clinical trial, and data management.
Conflicts of Interest
Authors H.A. and R.I. were are employed by Vitamin C60 BioResearch Corporation. The remaining author (H.K) declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Vitamin C60 BioResearch Corporation and a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| AHX | 2-azahypoxanthine |
| AOH | 2-aza-8-oxohypoxanthine |
| FCs | Fairy chemicals |
| ICA | Imidazole-4-carboxamide |
| NHEK | Normal human epidermal keratinocytes |
| TEWL | Trans-epidermal water loss |
| TJs | Tight junctions |
References
- Ramsbottom, J. Rate of growth of fungus rings. Nature 1926, 117, 158–159. [Google Scholar] [CrossRef]
- Shantz, H.L.; Piemeisel, R.L. Fungus fairy rings in Eastern Colorado and their effect on vegetation. J. Agric. Res. 1917, 11, 191–245. [Google Scholar]
- Smith, J.D. Fungi and turf diseases. J. Sports Turf. Res. Inst. 1951, 8, 60–66. [Google Scholar]
- Choi, J.H.; Fushimi, K.; Abe, N.; Tanaka, H.; Maeda, S.; Morita, A.; Hara, M.; Motohashi, R.; Matsunaga, J.; Eguchi, Y.; et al. Disclosure of the “fairy” of fairy-ring-forming fungus Lepista sordida. Chem. Bio Chem. 2010, 11, 1373–1377. [Google Scholar] [CrossRef]
- Choi, J.-H.; Abe, N.; Tanaka, H.; Fushimi, K.; Nishina, Y.; Morita, A.; Kiriiwa, Y.; Motohashi, R.; Hashizume, D.; Koshino, H.; et al. Plant-growth regulator, imidazole-4-carboxamide, produced by the fairy ring forming fungus Lepista sordida. J. Agric. Food Chem. 2010, 58, 9956–9959. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ohnishi, T.; Yamakawa, Y.; Takeda, S.; Sekiguchi, S.; Maruyama, W.; Yamashita, K.; Suzuki, T.; Morita, A.; Ikka, T.; et al. The source of “fairy rings”: 2-azahypoxanthine and its metabolite found in a novel purine metabolic pathway in plants. Angew. Chem. Int. Ed. Engl. 2014, 53, 1552–1555. [Google Scholar] [CrossRef]
- Mitchinson, A. Fairy chemicals. Nature 2014, 505, 298–299. [Google Scholar] [CrossRef]
- Aoshima, H.; Hyodo, S.; Ibuki, R.; Wu, J.; Choi, J.-H.; Kawagishi, H. Safety evaluation of 2-aza-8-oxohypoxanthine based on in vitro and human patch tests. Fundam. Toxicol. Sci. 2020, 7, 207–214. [Google Scholar] [CrossRef]
- Aoshima, H.; Matsumoto, T.; Ibuki, R.; Kawagishi, H. Safety evaluation of 2-aza-8-oxohypoxanthine by in vitro skin sensitization and human tests. Fundam. Toxicol. Sci. 2021, 8, 123–133. [Google Scholar] [CrossRef]
- Kawagishi, H. Fairy chemicals—A candidate for a new family of plant hormones and possibility of practical use in agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 752–758. [Google Scholar] [CrossRef]
- Kawagishi, H. Are fairy chemicals a new family of plant hormones? Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, H.; Ito, M.; Ibuki, R.; Kawagishi, H. The potential of 2-aza-8-Oxohypoxanthine as a cosmetic ingredient. Cosmetics 2021, 8, 60. [Google Scholar] [CrossRef]
- Aoshima, H.; Ibuki, R.; Ito, M.; Kawagishi, H. Clinical evaluation of topical lotion containing 2-aza-8-oxohypoxanthine on skin barrier function against water loss. Cosmetics 2021, 8, 83. [Google Scholar] [CrossRef]
- Aoshima, H.; Ito, S.; Ibuki, R.; Kawagishi, H. Potential of fairy chemicals as functional cosmetic ingredients: Effect of 2-aza-8-oxohypoxanthine on skin lightness. Int. J. Med. Mushrooms 2022, 24, 41–47. [Google Scholar] [CrossRef]
- Horii, I.; Nakayama, Y.; Obata, M.; Tagami, H. Stratum corneum hydration and amino acid content in xerotic skin. Br. J. Dermatol. 1989, 121, 587–592. [Google Scholar] [CrossRef]
- Ghadially, R.; Brown, B.E.; Sequeira-Martin, S.M.; Feingold, K.R.; Elias, P.M. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J. Clin. Investig. 1995, 95, 2281–2290. [Google Scholar] [CrossRef]
- Lavker, R.M. Structural alterations in exposed and unexposed aged skin. J. Investig. Dermatol. 1979, 73, 59–66. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kikuchi, A.; Pumkaeo, P.; Hirai, H.; Tokuyama, S.; Kawagishi, H. Bioconversion of AHX to AOH by resting cells of Burkholderia contaminans CH-1. Biosci. Biotechnol. Biochem. 2016, 80, 2045–2050. [Google Scholar] [CrossRef]
- Ikeuchi, K.; Fujii, R.; Sugiyama, S.; Asakawa, T.; Inai, M.; Hamashima, Y.; Choi, J.-H.; Suzuki, T.; Kawagishi, H.; Kan, T. Practical synthesis of natural plant-growth regulator 2-azahypoxanthine, its derivatives, and biotin-labeled probes. Org. Biomol. Chem. 2014, 12, 3813–3815. [Google Scholar] [CrossRef]
- Japan Dermatological Association Contact Dermatitis Treatment Guidelines Committee. Contact Dermatitis Treatment Guidelines. Jpn. Dermatol. Assoc. J. 2009, 119, 1757–1793. [Google Scholar]
- Japanese Cosmetic Science Society. Guidelines for evaluation of antiwrinkle products (task force committee for evaluation of antiaging function). J. Jpn. Cosmet. Sci. Soc. 2006, 31, 411–431. [Google Scholar]
- Kurasawa, M.; Maeda, T.; Oba, A.; Yamamoto, T.; Sasaki, H. Tight junction regulates epidermal calcium ion gradient and differentiation. Biochem. Biophys. Res. Commun. 2011, 406, 506–511. [Google Scholar] [CrossRef]
- Kuroda, S.; Kurasawa, M.; Mizukoshi, K.; Maeda, T.; Yamamoto, T.; Oba, A.; Kishibe, M.; Ishida-Yamamoto, A. Perturbation of lamellar granule secretion by sodium caprate implicates epidermal tight junctions in lamellar granule function. J. Dermatol. Sci. 2010, 59, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-P.; Han, S.B.; Kim, Y.K.; Park, E.E.; Doh, E.J.; Kim, K.H.; Lee, D.H.; Chung, J.H. Changes in tight junction protein expression in intrinsic aging and photoaging in human skin in vivo. J. Dermatol. Sci. 2016, 84, 99–101. [Google Scholar] [CrossRef]
- Parrish, A.R. The impact of aging on epithelial barriers. Tissue Barriers 2017, 5, e1343172. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).