Abstract
Introduction: Skin aging is influenced both by intrinsic factors and environmental exposures, such as UV radiation, which accelerate structural changes within the skin’s extracellular matrix (ECM). Understanding these changes is crucial for developing effective anti-aging treatments. Materials and Methods: This pilot cross-sectional study examined skin biopsy samples from three Caucasian male subjects with different levels of UV exposure, aiming to evaluate the effectiveness of two-photon microscopy (2PM) and Second Harmonic Generation (SHG) in visualizing and quantifying structural changes associated with skin aging. The samples were analyzed using 2PM to assess the structure and density of collagen and elastin fibers within the ECM. Integrated optical density (IOD) and the SHG-to-Autofluorescence Aging Index of the Dermis (SAAID) were used for quantitative analysis. Results: This study revealed a significant decrease in collagen density and increased disorganization in the ECM with age. Photo-exposed skin showed a more pronounced degradation of collagen and a higher increase in elastin content compared to non-photo-exposed skin. The average IOD for collagen was notably lower in elderly subjects compared to younger subjects, with a marked decrease in chronically photo-exposed skin. Discussion: The SAAID values indicated a substantial impact of photoaging, with lower scores in photo-exposed elderly skin compared to non-exposed skin. Conclusions: In conclusion, 2PM and SHG microscopy were effective in visualizing and quantifying age- and UV-induced skin remodeling, providing valuable insights into the distinct mechanisms driving intrinsic and extrinsic aging.
1. Introduction
Two-photon microscopy (2PM) is a sophisticated nonlinear optical technique utilized for the investigation of the structure and dynamics of biological systems, both in vitro and in vivo. This method enables a detailed characterization of tissue architecture, effectively highlighting pathological alterations [1,2,3,4,5]. The use of near-infrared light in two-photon microscopy allows deep tissue penetration, enabling detailed observations of both intra- and extracellular structures within the epidermis and dermis, up to several hundred microns deep. The images obtained are characterized by high clarity, contrast, and resolution, allowing for clear visualization of various tissues in living organisms [6,7,8].
In 2PM, two photons engage in a simultaneous interaction with a molecule, resulting in the promotion of the molecule to an excited state that subsequently emits fluorescence, as shown in Figure 1. Furthermore, near-infrared light facilitates the generation of optical harmonics through nonlinear photon interactions. Second Harmonic Generation (SHG) occurs when two photons are scattered simultaneously, leading to the production of a single photon that possesses twice the energy and half the wavelength of the original photons. SHG is particularly beneficial for examining ordered structural protein assemblies [8]. This technique relies on the principles of nonlinear photon absorption and scattering, which are influenced by the molecular properties of the tissue under investigation [8].
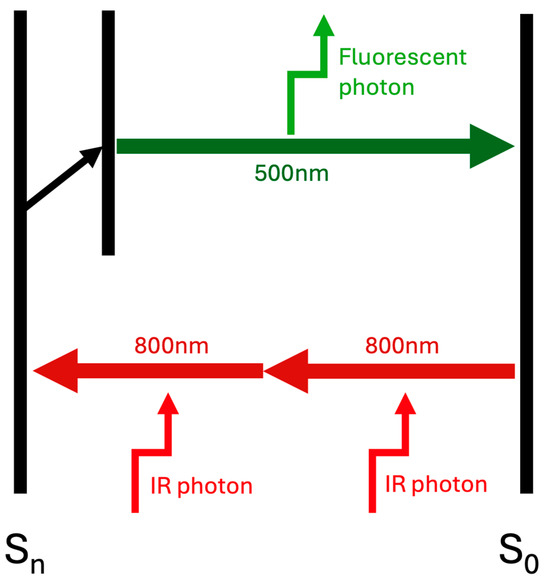
Figure 1.
Simplified diagram illustrating the principle of two-photon excitation. Two infrared photons (800 nm each) are absorbed simultaneously by a fluorophore, promoting it from the ground state (S0) to an excited electronic state (Sn). A single fluorescent photon (500 nm) is then emitted upon relaxation.
The skin is compound of various endogenous molecules with inherent fluorescence and ordered structural characteristics, including elastin, keratin, melanin, and collagen fibers. Harmonic generation does not necessitate actual absorption but is enhanced when close to resonance, although it may incur some degree of parasitic absorption from the analyzed tissue. Notably, SHG does not generate a fluorescence signal; instead, it produces visible light that can be detected using optical filters. This capability facilitates detailed imaging of molecular structure and orientation, particularly for ordered assemblies such as collagen fibers.
The skin is a complex organ composed of epidermal cells, fibroblasts, keratinocytes, and vascular and neural structures embedded within a dense collagen-rich extracellular matrix (ECM). The stability of collagen fibers is maintained through interactions with elastin and fibulin. Aging or exposure to sunlight prompts remodeling of the ECM. In younger individuals, collagen fibers typically intersect at approximately 90 degrees; however, in older individuals, these structures tend to become disorganized, resulting in a decrease in the density of collagen and other ECM components, which creates gaps between the fibers. Such alterations can consequently impact the mechanical properties of the tissue.
In this study, we employed 2PM to characterize ECM remodeling associated with skin aging or induced by photo-exposure. We integrated classical histochemical analysis with SHG imaging to achieve a more comprehensive evaluation of collagen organization and structural changes within the ECM. The SHG analysis was conducted directly on unstained samples, allowing for a faster and more cost-effective assessment compared to traditional histological or immunofluorescence techniques. The absence of the need for exogenous labeling in SHG minimizes sample preparation time, reduces potential artifacts introduced by staining procedures, and preserves tissue integrity. This advantage renders it particularly valuable for real-time imaging in biomedical research, clinical diagnostics, and regenerative medicine, where understanding structural changes in tissues, notably collagen organization, is essential for elucidating disease progression, aging, and treatment responses.
2. Materials and Methods
2.1. Sample Collection and Histopathological Analysis
This study involved the participation of three Caucasian male subjects with skin phototype III, who consented verbally and in writing. The ages of the participants were 20, 79, and 80 years, respectively. Written informed consent was obtained in accordance with the protocol approved by the Research Review Board and the Ethics Committee of ASST Spedali Civili, Brescia, Italy (study reference number: 5552). Each participant underwent surgical excision of melanocytic lesions, necessitating removal through rhomboid excision, aligned with Italian guidelines. Histopathological examination confirmed that the lesions were compound nevi without cytological atypia. From the excised specimens, a 4 mm punch biopsy of healthy skin was taken from one pole of each rhomboid. For the young subject and one of the elderly subjects, samples were obtained from a non-photoexposed area of the buttocks, while a chronically photoexposed area (neck) was biopsied from the other elderly subject. The samples were fixed in 10% formaldehyde and processed using standard protocols involving alcohol dehydration, xylene immersion, and paraffin embedding. Sections of 2–5 μm thickness were prepared for histological and immunohistochemical analysis. These sections underwent deparaffinization and rehydration, followed by initial staining with hematoxylin–eosin (H&E). Subsequently, immunohistochemical reactions were performed using specific antibodies against collagen IV (clone PHM-12, 1:100) and elastin (clone BA-4, 1:200) (Novocastra Antibodies, Leica, Milan, Italy).
2.2. Mutiphoton-Induced Autofluorescence and SHG
Three sections from each sample were thoroughly examined at the Imaging Platform Laboratory within the Department of Molecular and Translational Medicine at the University of Brescia. Cellular nuclei were labeled with To-Pro 3 iodide, a carbocyanine monomer nucleic acid stain (Thermo Fisher, Milan, Italy). Collagen fibers were visualized using SHG with LSM880 point scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) equipped with a Chameleon multiphoton (2P) tunable laser (Coherent Inc., Santa Clara, CA, USA) (excitation wavelength: 860 nm; emission wavelength: 430/10 nm). Images were acquired with a Plan-Neofluar 40X/0.75 objective (Carl Zeiss, Oberkochen, Germany). Additionally, elastin was visualized through autofluorescence, utilizing excitation at 860 nm and capturing emission within the range of 508–535 nm [9,10].
Quantitative analyses of collagen and elastin fibers were conducted by measuring the integrated optical density (IOD) of each using their respective optical frequencies: autofluorescence for elastin and SHG for collagen [11,12,13]. The non-invasive and objective parameter for evaluating skin aging, referred to as the SHG-to-Autofluorescence Aging Index of the Dermis (SAAID), was calculated using the following equation: SAAID = (SHG − AF)/(SHG + AF) [12].
Both IOD and SAAID measurements were obtained from three non-overlapping, anatomically consistent regions of interest (ROIs) per section, selected across three serial sections from each skin sample, resulting in nine ROIs per condition. Each ROI measured 100 × 50 μm and was positioned within the upper dermis, avoiding areas with artifacts, adnexal structures, or blood vessels. All images were acquired under fixed laser power, detector gain, and pinhole settings. Quantitative analysis was performed using ImageJ software (version 1.53c, National Institutes of Health, Bethesda, MD, USA; https://imagej.net/ij/download.html, accessed on 15 March 2021),and maximum, minimum, and average intensity values were calculated. ROI selection and data analysis followed predefined anatomical and morphological criteria applied uniformly across all samples to ensure methodological consistency and reproducibility. All image analyses were conducted under blinded conditions. The operator performing ROI selection and quantitative measurements was unaware of the sample origin or group assignment during data acquisition and processing.
3. Results
Paraffin-embedded skin samples from both young individuals and elderly subjects, categorized into non-photo-exposed and chronically photo-exposed groups, were analyzed using laser scanning microscopy. The imaging analysis indicated a reduction in epidermal thickness and a flattening of the dermo-epidermal junction in the skin of elderly subjects, irrespective of photo-exposure, when compared to that of young individuals.
The images acquired through 2PM in the three analyzed samples are represented in Figure 2. The cell nuclei, marked with To-Pro 3 iodide, are highlighted in blue; collagen fibers, captured through SHG, appear white; elastin fibers, visualized by AF, are colored green. Comparing the images qualitatively, there is a flattening of the dermo-epidermal junction in the skin of elderly subjects (Figure 2B,C), both in non-photoexposed and chronically photoexposed sites, compared to the young subject (Figure 2A). A progressive decrease in collagen fibers is observed from young skin to the non-photoexposed skin of the elderly subject (Figure 2B), with further reduction in the chronically photoexposed skin of the elderly subject (Figure 2C). Conversely, elastin fibers show a progressive increase across the three samples, coming to occupy most of the dermis in the chronically photoexposed elderly skin (Figure 2C). In the third sample, a morphological alteration in the elastin fibers can also be observed, which are irregularly and heterogeneously arranged, variably thickened, sometimes forming a vortex-like pattern (Figure 2C).
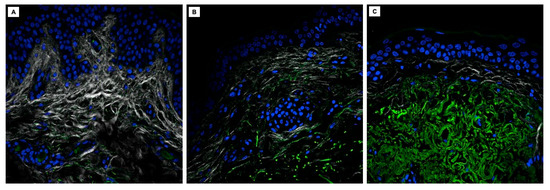
Figure 2.
2PM images of skin samples from a young subject in a non-photoexposed site (A), an elderly subject in a non-photoexposed site (B), and an elderly subject in a chronically photoexposed site (C).
Histological analysis in hematoxylin and eosin (Figure 3) was performed on the sections under study; here, too, a flattening of the dermo-epidermal junction in elderly skin is evident (Figure 3B,C), regardless of the degree of photodamage. Parallel to what was observed in 2PM, the morphological evaluation of the dermis in hematoxylin–eosin shows an accumulation of amorphous, dense, and tangled material in the superficial and middle dermis of photodamaged skin (Figure 3C), without, however, the ability to highlight the molecular nature of the fibers and thus distinguish elastin from collagen. Therefore, it is not possible in hematoxylin–eosin to detect the reduction in dermal collagen highlighted with the method under study (Figure 3B,C).
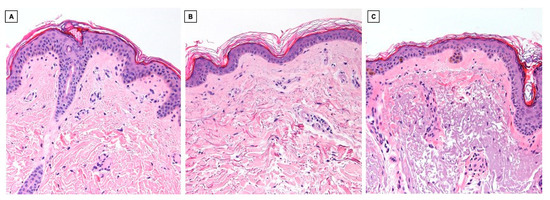
Figure 3.
Hematoxylin-eosin histological images (10×) of skin samples from a young subject in a non-photoexposed site (A), an elderly subject in a non-photoexposed site (B), and an elderly subject in a chronically photoexposed site (C).
Immunohistochemistry for collagen IV (Figure 4) highlights its presence at the level of the basal membranes and capillary wall, which qualitatively appears similar in all three samples.
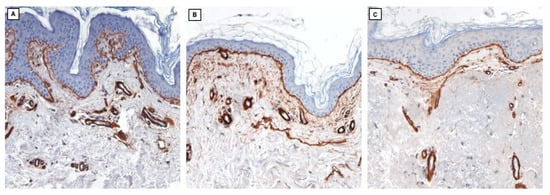
Figure 4.
Collagen IV (10×; immunohistochemistry clone PHM-12, Leica) in skin samples from a young subject in a non-photoexposed site (A), an elderly subject in a non-photoexposed site (B), and an elderly subject in a chronically photoexposed site (C).
In immunohistochemical staining, elastin fibers, minimally represented in young skin (Figure 5A), show a progressive increase in the elderly subject in the non-photoexposed site (Figure 5B) and are immensely expressed and qualitatively abnormal in the chronically photodamaged skin of sample 3 (Figure 5C), consistently with observations made through 2PM. While immunohistochemical staining allows precise localization of specific proteins such as elastin and collagen IV through antibody–antigen interactions, 2PM provides a label-free approach to visualize the native architecture of these fibers based on their intrinsic optical signals. The contrast offered by immunohistochemistry is superior in terms of specificity and clarity, but 2PM enables structural imaging of the extracellular matrix in its unaltered, unstained condition. As such, these two modalities offer complementary information: molecular precision on one side, and unmodified structural context on the other.
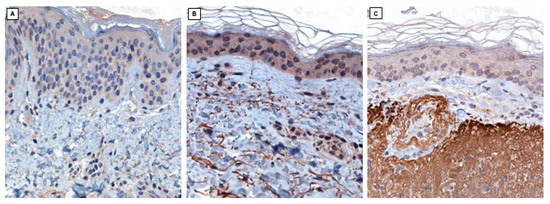
Figure 5.
Elastin (40×; immunohistochemistry clone BA-4, Novocastra) in skin samples from a young subject in a non-photoexposed site (A), an elderly subject in a non-photoexposed site (B), and an elderly subject in a chronically photoexposed site (C).
Quantification of collagen and elastin was performed using 2PM images, with IOD values calculated to assess ECM alterations in both chronological aging and photoaging among elderly subjects (Table 1).

Table 1.
Average integrated optical density (IOD) values for collagen and elastin, along with the SAAID for non-exposed (NE) and chronically exposed (CE) skin from young (Y) and elderly (E) subjects.
The collagen IOD values exhibited a strong correlation with chronological aging but not with chronic sun exposure. The average IOD values recorded were 65.3 for non-photo-exposed skin of the young subject, 21.9 for non-photoexposed skin of the elderly subject, and 19.8 for chronically photoexposed skin of another elderly subject. When compared to the young subject, the percentage of the average IOD values indicated a decrease of 66.5% in non-photoexposed aged skin and 69.7% in chronically photo-exposed aged skin.
Furthermore, the average IOD value for the auto-fluorescent signal intensity attributable to elastin was also calculated (Table 1). The analysis indicated that the total elastin content increases with age, with values of 0.10 IOD in young non-photoexposed skin compared to 19.9 IOD in chronically photoexposed aged skin, and this increase was strongly influenced by chronic sun exposure (0.61 IOD in non-photoexposed aged skin). The density of elastin in the chronically photoexposed skin of elderly individuals increases approximately 200-fold. In contrast, the increase in elastin density in non-photoexposed elderly skin is limited to about fivefold. Lastly, the SHG and SAAID were calculated for each sample within the same defined regions [12]. The SAAID index was notably lowest (−0.0025) in chronically photoexposed skin from elderly subjects, in contrast to values approaching 1 for non-photoexposed skin from both elderly and young subjects (0.946 and 0.997, respectively). Average IOD values for collagen and elastin are graphically represented in Figure 6, while the corresponding variation in SAAID values is reported in Figure 7.
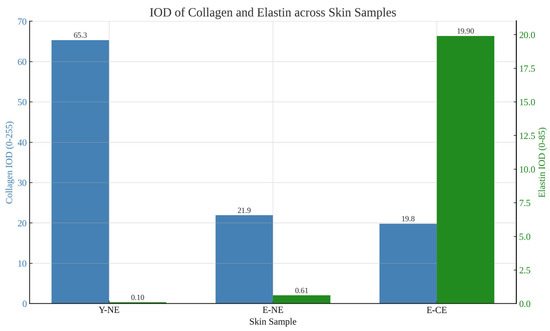
Figure 6.
Bar plot representing average IOD values for collagen and elastin in non-photoexposed young skin (Y-NE), non-photoexposed elderly skin (E-NE), and chronically photoexposed elderly skin (E-CE). Data highlight a marked decrease in collagen with aging and a significant elastin increase associated with chronic photoexposure.
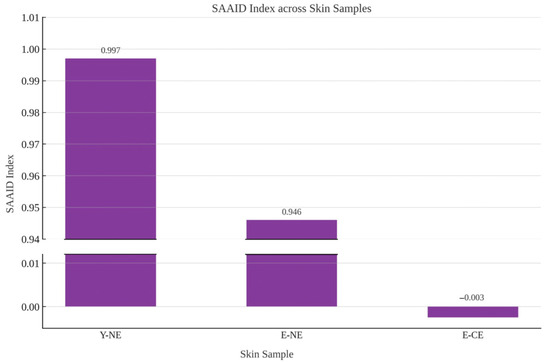
Figure 7.
Graphical representation of the SAAID values across the three skin samples displaying the sharp contrast between high SAAID values in non-photoexposed skin and the drastic reduction observed in chronically photoexposed elderly skin (E-CE).
4. Discussion
Aging and chronic solar exposure profoundly impact the composition, organization, and structural integrity of the extracellular matrix in human skin. This study demonstrates that the complementary use of SHG and 2PM facilitates a highly detailed and quantitative assessment of these complex ECM alterations, presenting notable advantages over traditional histological techniques. Previous investigations employing 2PM have allowed for precise real-time evaluations of cellular and stromal modifications in skin tumors, thereby enhancing our understanding of the underlying mechanisms of carcinogenesis and the structural changes in collagen associated with specific genetic mutations, such as those found in Ehlers–Danlos syndrome [11,14,15,16]. The ability of these advanced imaging modalities to provide in-depth and high-resolution visualization of the skin’s ECM components, particularly collagen and elastin, offers insights into the intricate pathophysiology of both chronological aging and photoaging.
In our findings, the extracellular matrix remodeling observed in the skin samples was characterized by a significant reduction in collagen fibers, coupled with the accumulation of amorphous and dense materials in the photodamaged skin. This observation aligns with earlier studies indicating that prolonged ultraviolet exposure not only inhibits the synthesis of collagen but also contributes to its degradation through the increased activity of matrix metalloproteinases [17,18]. Recent studies employing qPCR, Western blotting, and gelatin zymography have demonstrated that chronic UV irradiation activates MAPK and AP-1 pathways, leading to the upregulation and enzymatic activation of MMP-1, MMP-3, and MMP-9 in skin cells, key mediators of collagen degradation and photoaging [19,20,21,22,23]. Furthermore, the accumulation of elastin observed in photoaged skin is now recognized as the result of an imbalanced remodeling process. UV-induced oxidative stress alters fibroblast gene expression, promoting aberrant elastin synthesis while downregulating elastolytic activity, thereby contributing to the formation of solar elastosis [24]. These dysregulated molecular mechanisms underline the progressive loss of extracellular matrix homeostasis in photoaged skin and provide promising therapeutic targets.
Quantitative analysis of 2PM images provided deeper insights into the relative contributions of chronological aging versus chronic sun exposure on collagen density within the skin’s ECM. The data indicated that the significant decline in collagen integrated optical density values observed in both aged and photoaged skin samples suggests that intrinsic, age-related factors are the primary drivers of ECM degradation. This finding implies that the natural aging process, rather than chronic photoexposure, is the dominant factor underlying the reduction in dermal collagen content. Conversely, the variations in dermal collagen density imply that prolonged ultraviolet radiation exposure does not substantially amplify the collagen loss associated with the natural aging process. To further quantify the extent of dermal changes, the study employed the SHG and SAAID. In contrast to previous studies, the SAAID values for non-photoexposed skin were not significantly influenced by the age of the subjects involved [11]. In contrast, chronic solar exposure was shown to decrease these values, indicating an inverse correlation with the degree of photoaging. This finding suggests that the SAAID index may serve as a useful tool for differentiating between the effects of chronological aging and photoaging on the skin’s extracellular matrix, particularly in terms of the relative impacts on collagen organization and structure.
The elastin quantification performed in our study revealed a progressive increase in elastin content with age and a particularly marked accumulation in chronically photoexposed skin, where levels were approximately 200-fold higher compared to young non-photoexposed skin. This disproportionate increase reflects a profound disruption in the physiological balance between elastin synthesis and degradation, ultimately leading to the formation of solar elastosis [25]. The altered elastin architecture, together with its excessive deposition, is a hallmark of photoinduced extracellular matrix remodeling and contributes to the loss of skin elasticity and resilience observed in advanced photoaging [25].
Additionally, our analysis revealed that the elastic fiber network in photoaged skin is markedly disrupted, with excessive and disorganized elastin deposition contributing to the progressive loss of skin elasticity, resilience, and mechanical properties typically observed in advanced photodamage. The disruption of the elastic fiber architecture impairs the skin’s ability to effectively respond to mechanical stresses and deformations, leading to the sagging and loss of skin firmness associated with chronic sun exposure. These comprehensive findings emphasize the necessity of implementing preventive strategies against photoaging and suggest that targeting ECM homeostasis may represent a promising avenue for future therapeutic interventions aimed at mitigating the effects of both chronological and photodamage-induced skin aging. To further investigate the underlying mechanisms of ECM remodeling, future studies will integrate 2PM/SHG analysis with molecular approaches such as quantitative PCR, Western blotting, and gelatin zymography. These techniques will allow for the quantification of MMP expression and activity, as well as elastin-related regulatory markers, providing validation of the structural alterations observed through optical imaging.
The current study has several limitations that should be acknowledged. The small sample size, particularly the inclusion of only one young subject, and the lack of intra-individual comparisons between photoexposed and non-photoexposed regions reduce the statistical power of the study and limit the generalizability of the findings, as well as the ability to control for genetic and lifestyle-related confounders. Although technical replicates were included by analyzing multiple sections and regions of interest per sample, other potential confounding factors, such as cumulative sun exposure, smoking habits, and genetic predisposition, could not be controlled in this observational setting. Furthermore, all participants were male, and no photoexposed skin samples were obtained from the younger individual. These factors limit the gender representativeness of the results and preclude a comprehensive understanding of the independent and combined effects of chronological aging and photoexposure on extracellular matrix remodeling. These constraints reflect the pilot and exploratory nature of the investigation and the limited availability of suitable clinical specimens. Future investigations will aim to overcome these limitations by enrolling a larger, gender-balanced cohort. The inclusion of female participants and younger individuals with photoexposed skin will enable the identification of sex- and age-related differences in ECM remodeling. In addition, anatomical standardization of biopsy sites and control for variables such as Fitzpatrick skin phototype, sun exposure history, and lifestyle habits will enhance the reproducibility and translational relevance of the findings.
Despite these limitations, our investigations into photoaging and chronoaging have demonstrated the significant advantages of laser scan microscopy technology in providing highly accurate imaging of collagen and elastin. This approach effectively overcomes the limitations of traditional histological and immunohistochemical methods, as it enables the visualization and quantification of various tissue structures in a single image without the need for exogenous markers. Notably, we conducted SHG analysis directly on unstained samples, resulting in faster and more cost-effective analyses compared to conventional histology or immunofluorescence techniques. The absence of exogenous labeling in SHG minimizes sample preparation time, reduces the potential for artifacts introduced during staining, and preserves the integrity of the tissue. This advantage positions SHG as particularly beneficial for real-time imaging in biomedical research, clinical diagnostics, and regenerative medicine, where assessing structural changes in tissues, such as collagen organization, is critical for understanding disease progression, aging, and treatment responses. Furthermore, in addition to collagen and elastin analysis, laser scan microscopy and SHG technologies can be utilized to examine the redox metabolic state of samples by quantifying factors like NADH and FAD, as well as for analyzing keratin, thereby enabling accurate diagnosis of pigmented cutaneous melanoma [26,27,28]. The broader application of 2PM in clinical and routine settings is currently limited due to the high cost of instrumentation, the requirement for specialized equipment, and the technical complexity of image acquisition and interpretation.
5. Conclusions
This study highlights the effectiveness of 2PM and SHG microscopy in providing a comprehensive and quantitative assessment of the structural and compositional changes that occur in human skin as a result of both intrinsic aging and chronic sun exposure. These advanced imaging techniques enable detailed visualization and precise measurement of the key extracellular matrix components, collagen and elastin, which are profoundly impacted by these aging processes.
The study’s findings reveal distinct patterns of ECM remodeling, with collagen density primarily influenced by chronological aging, while chronic photoexposure primarily drives the excessive accumulation of elastin, a hallmark of solar elastosis. The ability to differentiate between collagen loss and elastin accumulation using 2PM and SHG microscopy provides valuable mechanistic insights into the divergent pathways underlying intrinsic and extrinsic skin aging. This knowledge is crucial for developing targeted interventions to address the detrimental effects of both chronological and photoaging on skin structure and function.
Furthermore, our study underscores the advantages of these imaging techniques over traditional histological methods, offering faster, more cost-effective, and artifact-free analysis of skin samples. Their non-invasive and label-free nature makes them particularly well-suited for applications beyond research, including clinical diagnostics and regenerative medicine, where precise assessment of tissue remodeling is essential for evaluating disease progression and treatment response.
In this context, the combined use of 2PM and SHG could represent a powerful tool to monitor anti-aging interventions, quantify treatment outcomes, and support the development of diagnostic parameters for photoaged skin, potentially enabling their integration into future clinical and personalized dermatological workflows. The incorporation of SHG-derived indices, such as the SAAID, alongside established clinical grading scales, such as the SCINEXA and Glogau scales, may improve the objectivity and reproducibility of photoaging assessments [29,30]. Moreover, these technologies could be applied longitudinally to evaluate the efficacy of anti-aging therapies, laser resurfacing protocols, or topical treatments in clinical trials, offering a non-invasive and quantitative readout of dermal remodeling over time.
Author Contributions
S.B.: Writing—original draft preparation, conceptualization; C.R. (Chiara Rovati): conceptualization, writing—original draft preparation; L.B.: writing—review and editing, validation; M.A.: validation, methodology; M.R.: validation, methodology; C.R. (Cosetta Ravelli): software, visualization, data curation, investigation; S.R.: writing—review and editing; M.V.: supervision, methodology; S.M.: writing—review and editing, funding acquisition, supervision, validation, investigation; P.C.-P.: project leadership, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Associazione Italiana Ricerca sul Cancro AIRC (AIRC grant IG17276 to S.M.).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of ASST Spedali Civili, Brescia, Italy (study reference number: 5552; Approval date: 5 November 2020).
Informed Consent Statement
Written informed consent has been obtained from the patients involved in this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors performed experiments at the Imaging Platform of the Department of Translational and Molecular Medicine of the University of Brescia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Masters, B.R.; So, P.T.; Gratton, E. Multiphoton excitation fluorescence microscopy and spectroscopy of in vivo human skin. Biophys. J. 1997, 72, 2405–2412. [Google Scholar] [CrossRef]
- Wang, H.; Shyr, T.; Fevola, M.J.; Cula, G.O.; Stamatas, G.N. Age-related morphological changes of the dermal matrix in human skin documented in vivo by multiphoton microscopy. J. Biomed. Opt. 2018, 23, 030501. [Google Scholar] [CrossRef]
- Chen, J.; Zhuo, S.; Jiang, X.; Zhu, X.; Zheng, L.; Xie, S.; Lin, B.; Zeng, H. Multiphoton microscopy study of the morphological and quantity changes of collagen and elastic fiber components in keloid disease. J. Biomed. Opt. 2011, 16, 051305. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Zhuo, S.; Xiong, S.; Zeng, H.; Jiang, X.; Chen, R.; Xie, S. Nonlinear spectral imaging of human hypertrophic scar based on two-photon excited fluorescence and second-harmonic generation. Br. J. Dermatol. 2009, 161, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.Z.; Tehrani, A.Y.; Jett, K.A.; Bernatchez, P.; van Breemen, C.; Esfandiarei, M. Quantification of aortic and cutaneous elastin and collagen morphology in Marfan syndrome by multiphoton microscopy. J. Struct. Biol. 2014, 187, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Koehler, M.J.; Preller, A.; Kindler, N.; Elsner, P.; König, K.; Bückle, R.; Kaatz, M. Intrinsic, solar and sunbed-induced skin aging measured in vivo by multiphoton laser tomography and biophysical methods. Skin Res. Technol. 2009, 15, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Decencière, E.; Tancrède-Bohin, E.; Dokládal, P.; Koudoro, S.; Pena, A.M.; Baldeweck, T. Automatic 3D segmentation of multiphoton images: A key step for the quantification of human skin. Skin Res. Technol. 2013, 19, 115–124. [Google Scholar] [CrossRef]
- Yasui, T.; Takahashi, Y.; Fukushima, S.; Ogura, Y.; Yamashita, T.; Kuwahara, T.; Hirao, T.; Araki, T. Observation of dermal collagen fiber in wrinkled skin using polarization-resolved second-harmonic-generation microscopy. Opt. Express. 2009, 17, 912–923. [Google Scholar] [CrossRef]
- Zoumi, A.; Yeh, A.; Tromberg, B.J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc. Natl. Acad. Sci. USA 2002, 99, 11014–11019. [Google Scholar] [CrossRef]
- Wang, B.G.; König, K.; Halbhuber, K.J. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J. Microsc. 2010, 238, 1–20. [Google Scholar] [CrossRef]
- Kiss, N.; Haluszka, D.; Lőrincz, K.; Kuroli, E.; Hársing, J.; Mayer, B.; Kárpáti, S.; Fekete, G.; Szipőcs, R.; Wikonkál, N.; et al. Ex vivo nonlinear microscopy imaging of Ehlers-Danlos syndrome-affected skin. Arch. Dermatol. Res. 2018, 310, 463–473. [Google Scholar] [CrossRef]
- Lin, S.-J.; Wu, R.-J.; Tan, H.-Y.; Lo, W.; Lin, W.-C.; Young, T.-H.; Hsu, C.-J.; Chen, J.-S.; Jee, S.-H.; Dong, C.-Y. Evaluating cutaneous photoaging by use of multiphoton fluorescence and second-harmonic generation microscopy. Opt. Lett. 2005, 30, 2275–2277. [Google Scholar] [CrossRef] [PubMed]
- Skala, M.C.; Squirrell, J.M.; Vrotsos, K.M.; Eickhoff, J.C.; Gendron-Fitzpatrick, A.; Eliceiri, K.W.; Ramanujam, N. Multiphoton microscopy of endogenous fluorescence differentiates normal, precancerous, and cancerous squamous epithelial tissues. Cancer Res. 2005, 65, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K. Localization of collagen types in tissues. Int. Rev. Connect Tissue Res. 1981, 9, 265–324. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, R.; Massi, D.; Sestini, S.; Carli, P.; De Giorgi, V.; Lotti, T.; Pavone, F.S. Multidimensional non-linear laser imaging of Basal Cell Carcinoma. Opt. Express. 2007, 15, 10135–10148. [Google Scholar] [CrossRef]
- Entenberg, D.; Aranda, L.; Li, Y.; Toledo-Crow, R.; Schaer, D.; Li, Y. Multimodal microscopy of immune cells and melanoma for longitudinal studies. In Proceedings of the SPIE BIOS—SPIE, Multimodal Biomedical Imaging, San Jose, CA, USA, 21–26 January 2006; Volume 60810A. [Google Scholar] [CrossRef]
- Chen, B.; Li, R.; Yan, N.; Chen, G.; Qian, W.; Jiang, H.-L.; Ji, C.; Bi, Z.-G. Astragaloside IV controls collagen reduction in photoaging skin by improving transforming growth factor-β/Smad signaling suppression and inhibiting matrix metalloproteinase-1. Mol. Med. Rep. 2015, 11, 3344–3348. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Chen, L.Y.; Shih, M.F. Preventive effects of β-thujaplicin against UVB-induced MMP-1 and MMP-3 mRNA expressions in skin fibroblasts. Am. J. Chin. Med. 2012, 40, 387–398. [Google Scholar] [CrossRef]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix Metalloproteinases on Skin Photoaging. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef]
- Ivarsson, J.; Ferrara, F.; Vallese, A.; Guiotto, A.; Colella, S.; Pecorelli, A.; Valacchi, G. Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation. Int. J. Mol. Sci. 2023, 24, 16674. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, J.Y.; Kim, Y.; Kang, C.H. Latilactobacillus sakei Wikim0066 Protects Skin through MMP Regulation on UVB-Irradiated In Vitro and In Vivo Model. Nutrients 2023, 15, 726. [Google Scholar] [CrossRef]
- Lee, J.-S.; Min, J.-W.; Gye, S.-B.; Kim, Y.-W.; Kang, H.-C.; Choi, Y.-S.; Seo, W.-S.; Lee, B.-Y. Suppression of UVB-Induced MMP-1 Expression in Human Skin Fibroblasts Using Lysate of Lactobacillus iners Derived from Korean Women’s Skin in Their Twenties. Curr. Issues Mol. Biol. 2024, 46, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-Y.; Kim, G.-B.; Kim, J.-M.; Kang, S.Y.; Youn, H.-J.; Park, J.; Ro, S.Y.; Chung, E.-Y.; Park, K.-H.; Kim, J.-S. Fisetin Inhibits UVA-Induced Expression of MMP-1 and MMP-3 through the NOX/ROS/MAPK Pathway in Human Dermal Fibroblasts and Human Epidermal Keratinocytes. Int. J. Mol. Sci. 2023, 24, 17358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Mambwe, B.; Mellody, K.T.; Kiss, O.; O’Connor, C.; Bell, M.; Watson, R.E.B.; Langton, A.K. Cosmetic retinoid use in photoaged skin: A review of the compounds, their use and mechanisms of action. Int. J. Cosmet. Sci. 2025, 47, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, F.; Abramov, A.Y. Measurement of mitochondrial NADH and FAD autofluorescence in live cells. Methods Mol. Biol. 2015, 1264, 263–270. [Google Scholar] [CrossRef]
- Hu, L.; Wang, N.; Cardona, E.; Walsh, A.J. Fluorescence intensity and lifetime redox ratios detect metabolic perturbations in T cells. Biomed. Opt. Express. 2020, 11, 5674–5688. [Google Scholar] [CrossRef]
- Szyc, Ł.; Scharlach, C.; Haenssle, H.; Fink, C. In vivo two-photon-excited cellular fluorescence of melanin, NAD(P)H, and keratin enables an accurate differential diagnosis of seborrheic keratosis and pigmented cutaneous melanoma. J. Biomed. Opt. 2021, 26, 075002. [Google Scholar] [CrossRef]
- Vierkötter, A.; Ranft, U.; Krämer, U.; Sugiri, D.; Reimann, V.; Krutmann, J. The SCINEXA: A novel, validated score to simultaneously assess and differentiate between intrinsic and extrinsic skin ageing. J. Dermatol. Sci. 2009, 53, 207–211. [Google Scholar] [CrossRef]
- Glogau, R.G. Aesthetic and anatomic analysis of the aging skin. Semin. Cutan. Med. Surg. 1996, 15, 134–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).