The Epidemiology of Acne in the Current Era: Trends and Clinical Implications
Abstract
1. Introduction
2. Relevant Sections
2.1. Epidemiology
2.2. Pathophysiology
| Pathophysiologic Mechanism | Details | Future Perspectives |
|---|---|---|
| Sebum overproduction | Increased sebaceous gland activity under the influence of androgen hormones | Development of molecules that directly inhibit sebum production or target specific androgen receptors |
| Follicular hyperkeratinization | Blockage of pilosebaceous follicles due to keratinocyte obstruction | Use of more selective topical retinoids or gene therapies to regulate keratinocyte differentiation |
| Cutibacterium acnes colonization | Overgrowth of C. acnes bacteria, exacerbating inflammation | Development of bacteriophages or microbiome therapies to restore bacterial balance in the skin |
| Inflammation | Local inflammatory response with formation of pustules, papules, and nodules | Introduction of anti-inflammatory biologic therapies (IL-1, IL-6, IL-17 inhibitors) for severe inflammatory acne |
| Role of the skin microbiome | Imbalance of the skin microbiome can contribute to the development and worsening of skin lesions | Research on topical probiotics and prebiotics to optimize microbiome health |
| Genetic factors | Genetic polymorphisms influence acne sensitivity and susceptibility | Gene editing technologies (CRISPR-Cas9) to correct mutations involved in acne predisposition |
| Dietary and metabolic influence | Diet high in simple carbohydrates and dairy may exacerbate acne | Development of personalized dietary guidelines and use of metabolic biomarkers for prevention and treatment |
2.3. Microbiome
- “Seborrheic” areas (forehead, scalp, chest, back);
- “Wet” areas (armpit, cubital fossa, groin, inguinal fold, popliteal fossa, umbilicus (navel), buttock fold, soles, inner finger spaces);
2.4. Genetic Predisposition
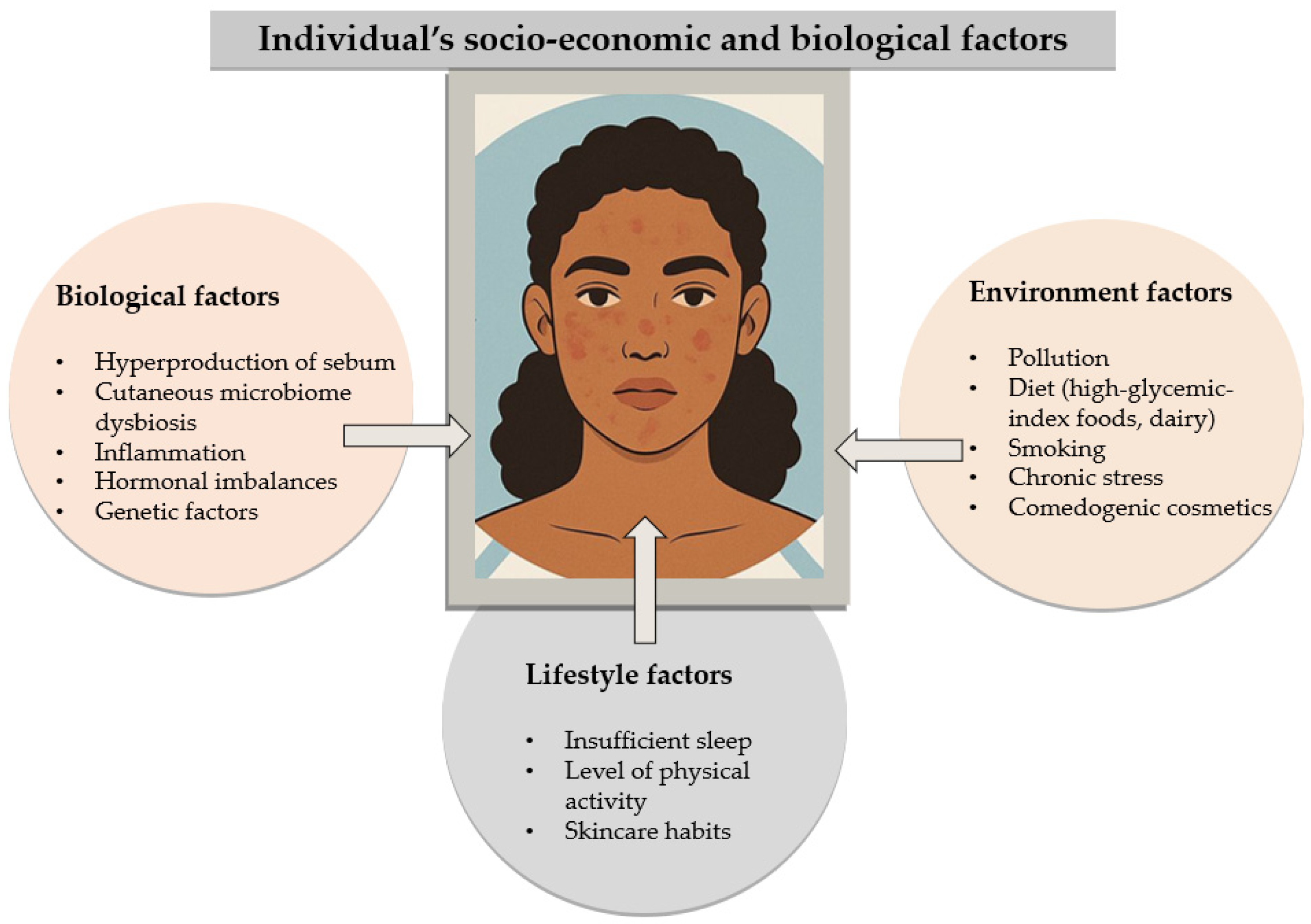
2.5. Economic Factors
2.6. Diet
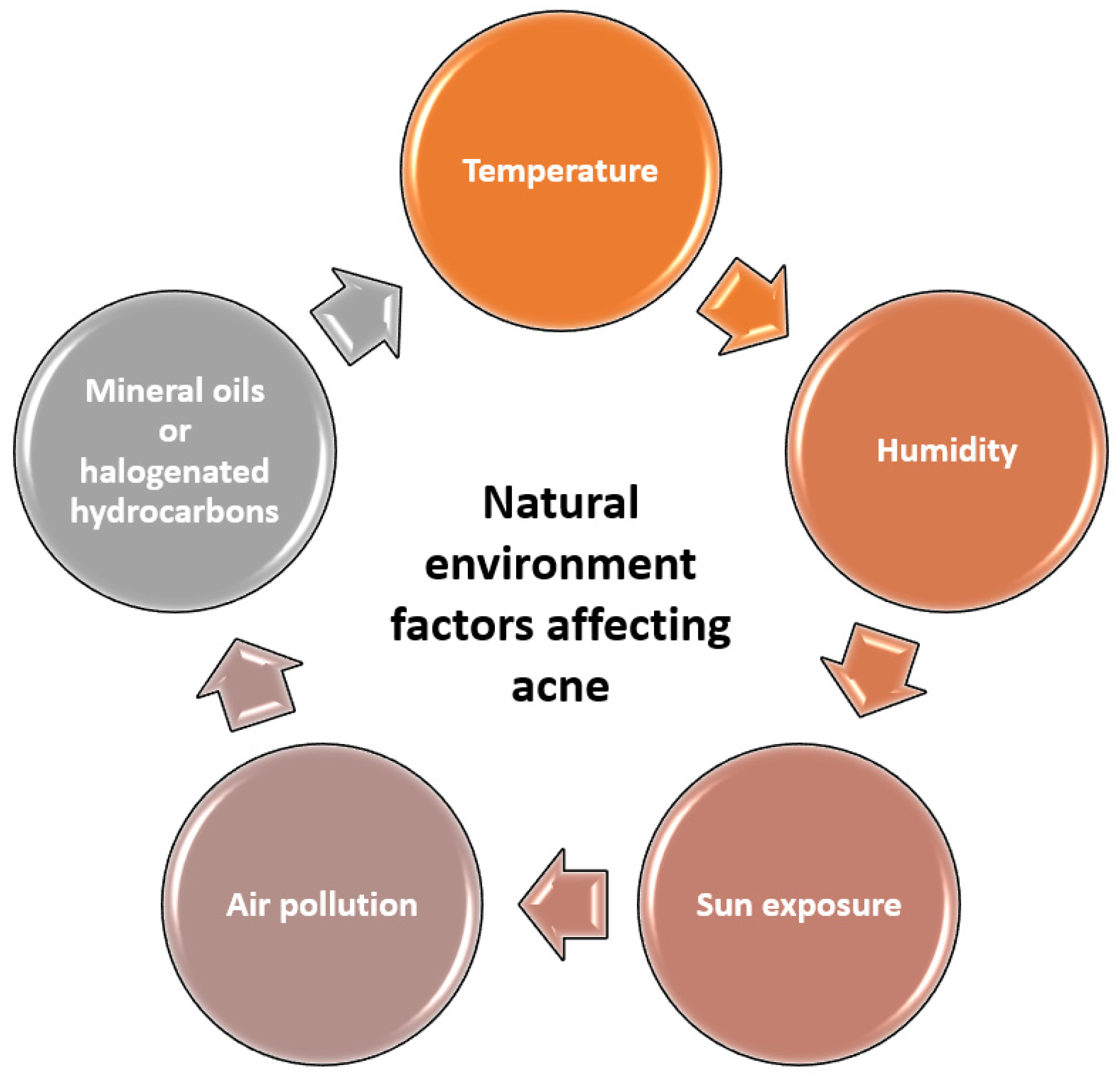
2.7. Environmental Factors
2.8. Cytokines Involved in Acne Pathogenesis
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfeifer, J.H.; Berkman, E.T. The development of self and identity in adolescence: Neural evidence and implications for a value-based choice perspective on motivated behavior. Child Dev. Perspect. 2018, 12, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Alexis, A.; Tan, J.; Rocha, M.; Kerob, D.; Demessant, A.; Ly, F.; Wu, Y.; Sachdev, M.; Kurokawa, I. Is Acne the Same Around the World? J. Clin. Aesthetic Dermatol. 2024, 17, 16–22. [Google Scholar]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Dermatology: How to manage acne vulgaris. Drugs Context 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, M.J.; Yu, Y.F.; Fan, X.Y.; Chen, J.; Lai, Y.F.; Liu, Y.; Ye, H.Y.; Zhang, Z.Y.; Zhao, Y.; et al. Acromegaly presented with acne vulgaris: A retrospective study with 123 cases. J. Endocrinol. Investig. 2024, 47, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Jena, A.; Panda, M.; Debasmita, B.; Ipsita, D. Randomized prospective study of low-dose isotretinoin alone and combination with salicylic acid and mandelic peel against acne tarda. J. Cosmet. Dermatol. 2022, 21, 4398–4404. [Google Scholar] [CrossRef]
- Kirsten, N.; Mohr, N.; Augustin, M. Prevalence and cutaneous comorbidity of acne vulgaris in the working population. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1393–1400. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33, Erratum in J. Am. Acad. Dermatol. 2020, 82, 1576. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172 (Suppl. S1), 3–12. [Google Scholar] [CrossRef]
- Gollnick, H.; Abanmi, A.; Al-Enezi, M.; Al Hammadi, A.; Galadari, I.; Kibbi, A.; Zimmo, S. Managing acne in the Middle East: Consensus recommendations. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. S7), 4–35. [Google Scholar] [CrossRef]
- Li, Q.; Patrick, M.T.; Sreeskandarajan, S.; Kang, J.; Kahlenberg, J.M.; Gudjonsson, J.E.; He, Z.; Tsoi, L.C. Large-scale epidemiological analysis of common skin diseases to identify shared and unique comorbidities and demographic factors. Front. Immunol. 2024, 14, 1309549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahto, A. Acne vulgaris. Medicine 2017, 45, 386–389. [Google Scholar] [CrossRef]
- Szepietowska, M.; Bień, B.; Krajewski, P.K.; Stefaniak, A.A.; Matusiak, Ł. Prevalence, Intensity and Psychosocial Burden of Acne Itch: Two Different Cohorts Study. J. Clin. Med. 2023, 12, 3997. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.H.; Badaruddin, N.S.F.; Foo, S.Y.; Bujang, M.A.; Muniandy, P. Prevalence and psychosocial impact of acne vulgaris among high school and university students in Sarawak, Malaysia. Med. J. Malays. 2022, 77, 446–453. [Google Scholar]
- Huet, F.; Taieb, C.; Corgibet, F.; Brenaut, E.; Richard, M.A.; Misery, L. Pruritus, Pain, and Depression Associated with the Most Common Skin Diseases: Data from the French Study “Objectifs Peau”. Dermatology 2022, 238, 448–453. [Google Scholar] [CrossRef]
- Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.E.; Misery, L.; Szabo, C.; Linder, D.; Sampogna, F.; et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Investig. Dermatol. 2015, 135, 984–991. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, X.; Luo, Z.; Liu, M.; Zhang, H.; Zheng, H.; Li, J. Global, regional and national burdens of acne vulgaris in adolescents and young adults aged 10–24 years from 1990 to 2021: A trend analysis. Br. J. Dermatol. 2025, 192, 228–237. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, S.; Ren, J.; Zhang, Y. Analysis of the epidemiological burden of acne vulgaris in China based on the data of global burden of disease 2019. Front. Med. 2022, 9, 939584. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Alsulaimani, H.; Kokandi, A.; Khawandanh, S.; Hamad, R. Severity of Acne Vulgaris: Comparison of Two Assessment Methods. Clin. Cosmet. Investig. Dermatol. 2020, 13, 711–716. [Google Scholar] [CrossRef]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Gold, L.F.S.; Tan, J.K.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef]
- Durairaj, A.; Elumalai, K.; Shanmugam, A. Cystic acne treatment: A comprehensive review. Med. Adv. 2023, 1, 318–329. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Dong, G.; Wang, X.; Liu, T. Acne treatment: Research progress and new perspectives. Front. Med. 2024, 11, 1425675. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. A Comprehensive Review of Acne Vulgaris. J. Clin. Pharm. 2019, 1, 17–45. [Google Scholar] [CrossRef]
- Heath, C.R.; Usatine, R.P. Acne vulgaris. Cutis 2021, 108, 167. [Google Scholar] [CrossRef]
- Haenssle, H.; Fink, C.; Toberer, F.; Winkler, J.; Stolz, W.; Hofmann-Wellenhof, R.; Buhl, T.; Zutt, M.; Abassi, M.; Thomas, L.; et al. Man against machine reloaded: Performance of a market-approved convolutional neural network in classifying a broad spectrum of skin lesions in comparison with 96 dermatologists working under less artificial conditions. Ann. Oncol. 2020, 31, 137–143. [Google Scholar] [CrossRef]
- Cecilia, M.S.; Satapathy, S.; Ramam, M.; Ramam, M. Assessment of body image disturbance, self esteem and quality of life among adolescents and young adults with acne in a tertiary care facility of India. Indian J. Dermatol. 2022, 67, 93. [Google Scholar] [CrossRef]
- Haenssle, H.; Fink, C.; Toberer, F.; Winkler, J.; Stolz, W.; Hofmann-Wellenhof, R.; Buhl, T.; Zutt, M.; Abassi, M.; Thomas, L.; et al. Understanding the impact of acne vulgaris and associated psychological distress on self-esteem and quality of life via regression modeling with CADI, DLQI, and WHOQoL. Sci. Rep. 2023, 13, 21084. [Google Scholar] [CrossRef]
- Agrawal, D.A.; Khunger, N.; Morphological, A. Study of acne scarring and its relationship between severity and treatment of active acne. J. Cutan. Aesthetic Surg. 2020, 13, 210–216. [Google Scholar] [CrossRef]
- Reszke, R.; Szepietowski, J.C. Itch and Psyche: Bilateral Associations. Acta Derm. Venereol. 2020, 100, 27–35. [Google Scholar] [CrossRef]
- Tan, J.; Beissert, S.; Cook-Bolden, F.; Chavda, R.; Harper, J.; Hebert, A.; Lain, E.; Layton, A.; Rocha, M.; Weiss, J.; et al. Impact of facial and truncal acne on quality of life: A multi-country population-based survey. JAAD Int. 2021, 3, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wolkenstein, P.; Machovcova, A.; Szepietowski, J.C.; Tennstedt, D.; Veraldi, S.; Delarue, A. Acne prevalence and associations with lifestyle: A cross-sectional online survey of adolescents/young adults in 7 European countries. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, Y.T.; Ali, F.M.; Salek, M.S.; Finlay, A.Y. Clinical experience and psychometric properties of the Cardiff Acne Disability Index (CADI). Br. J. Dermatol. 2021, 185, 711–724. [Google Scholar] [CrossRef]
- Szepietowski, J.; Salomon, J.; Finlay, A.Y.; Klepacki, A.; Chodynicka, B.; Marionneau, N.; Taieb, C.; Myon, E. Dermatology Life Quality Index (DLQI): Polish version. Dermatol Klin 2004, 6, 63–70. [Google Scholar]
- Zhou, C.; Vempati, A.; Tam, C.; Khong, J.; Vasilev, R.; Tam, K.; Hazany, S.; Hazany, S. Beyond the Surface: A Deeper Look at the Psychosocial Impacts of Acne Scarring. Clin. Cosmet. Investig. Dermatol. 2023, 16, 731–738. [Google Scholar] [CrossRef]
- Tan, J.; Chavda, R.; Leclerc, M.; Dréno, B. Projective personification approach to the experience of people with acne and acne scarring-expressing the unspoken. JAMA Dermatol. 2022, 158, 1005–1012. [Google Scholar] [CrossRef]
- Tam, C.; Khong, J.; Tam, K.; Vasilev, R.; Wu, W.; Hazany, S. A comprehensive review of non-energy-based treatments for atrophic acne scarring. Clin. Cosmet. Investig. Dermatol. 2022, 2022, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, Z.H.; Thiboutot, D.; Homsi, H.A.; Perez-Chada, L.M.; Barbieri, J.S. Patient-reported outcome measures for health-related quality of life in patients with acne vulgaris: A systematic review of measure development and measurement properties. JAMA Dermatol. 2022, 158, 900. [Google Scholar] [CrossRef]
- Chauhan, P.N.; Sharma, A.; Rasheed, H.; Mathur, H.; Sharma, P. Treatment opportunities and technological progress prospective for acne vulgaris. Curr. Drug Deliv. 2023, 20, 1037–1048. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Hazarika, N. Acne vulgaris: New evidence in pathogenesis and future modalities of treatment. J. Dermatol. Treat. 2021, 32, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Maddiboyina, B.; Vanamamalai, H.K.; Roy, H.; Ramaiah; Gandhi, S.; Kavisri, M. Moovendhan, Food and drug industry applications of microalgae Spirulina platensis: A review. J. Basic Microbiol. 2023, 63, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.-L.; Zheng, Y.; Bu, J. Physiological and psychological effects of isotretinoin in the treatment of patients with acne: A narrative review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amuzescu, A.; Tampa, M.; Matei, C.; Georgescu, S.R. Adult Female Acne: Recent Advances in Pathophysiology and Therapeutic Approaches. Cosmetics 2024, 11, 74. [Google Scholar] [CrossRef]
- Jin, Z.; Song, Y.; He, L. A review of skin immune processes in acne. Front. Immunol. 2023, 14, 1324930. [Google Scholar] [CrossRef]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Saez-de-Ocariz, M. The human skin microbiome in selected cutaneous diseases. Front. Cell. Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef]
- Robert, C.; Cascella, F.; Mellai, M.; Barizzone, N.; Mignone, F.; Massa, N.; Nobile, V.; Bona, E. Influence of sex on the microbiota of the human face. Microorganisms 2022, 10, 2470. [Google Scholar] [CrossRef]
- O’Neill, A.M.; Nakatsuji, T.; Hayachi, A.; Williams, M.R.; Mills, R.H.; Gonzalez, D.J.; Gallo, R.L. Identification of a human skin commensal bacterium that selectively kills Cutibacterium acnes. J. Investig. Dermatol. 2020, 140, 1619–1628.e2. [Google Scholar] [CrossRef]
- Conwill, A.; Kuan, A.C.; Damerla, R.; Poret, A.J.; Baker, J.S.; Tripp, A.D.; Alm, E.J.; Lieberman, T.D. Anatomy promotes neutral coexistence of strains in the human skin microbiome. Cell Host Microbe 2022, 30, 171–182.e7. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A. The Microbiome and Acne: Perspectives for Treatment. Dermatol. Ther. 2024, 14, 31–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Chen, Z.; Qiao, S.; Zhu, Q.; Zuo, Z.; Guo, B. Analysis of Alterations of the Gut Microbiota in Moderate to Severe Psoriasis Patients Using 16S rRNA Gene Sequencing. Indian J. Dermatol. 2022, 67, 495–503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tse, H.; Yuen, K.-Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, W.; Wang, M.; Wang, X.; Wang, S. Causal roles of skin and gut microbiota in skin appendage disorders suggested by genetic study. Front. Immunol. 2024, 15, 1427276. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, Z.; Yang, X.; Li, K.; Xie, A.; Dong, F.; Wang, S.; Yan, J.; Liu, J. An overview of detecting gene-trait associations by integrating GWAS summary statistics and eQTLs. Sci. China Life Sci. 2024, 67, 1133–1154. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Garay, J.A.R.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The skin microbiome: A new actor in inflammatory acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef]
- Chilicka, K.; Dzieńdziora-Urbińska, I.; Szyguła, R.; Asanova, B.; Nowicka, D. Microbiome and Probiotics in Acne Vulgaris—A Narrative Review. Life 2022, 12, 422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.; Song, H.; Jin, J.S.; Lee, W.J.; Kim, J. Genomic and phenotypic characterization of Cutibacterium acnes bacteriophages isolated from acne patients. Antibiotics 2022, 11, 1041. [Google Scholar] [CrossRef]
- Farfan, J.; Gonzalez, J.M.; Vives, M. The immunomodulatory potential of phage therapy to treat acne:a review on bacterial lysis and immunomodulation. PeerJ 2022, 10, e13553. [Google Scholar] [CrossRef]
- Rimon, A.; Rakov, C.; Lerer, V.; Sheffer-Levi, S.; Oren, S.A.; Shlomov, T.; Shasha, L.; Lubin, R.; Zubeidat, K.; Jaber, N.; et al. Topical phage therapy in a mouse model of Cutibacterium acnes-induced acne-like lesions. Nat. Commun. 2023, 14, 1005. [Google Scholar] [CrossRef]
- Bataille, V.; Snieder, H.; MacGregor, A.J.; Spector, T.D. The influence of genetics and environmental factors in the pathogenesis of acne: A twin study of acne in women. J. Investig. Dermatol. 2002, 119, 1317–1322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, X.; Wu, W.; Peng, M.; Shen, Q.; Feng, J.; Lai, W.; Zhu, H.; Tu, C.; Quan, X.; Chen, Y.; et al. Identity-by-Descent Analysis Reveals Susceptibility Loci for Severe Acne in Chinese Han Cohort. J. Investig. Dermatol. 2019, 139, 2049–2051.e20. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Pang, Y.; Zhu, H.; Qu, L.; Xiao, T.; Wei, H.; Chen, H.; He, C. The epidemiology of adolescent acne in North East China. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 953–957. [Google Scholar] [CrossRef]
- Stamu-O’Brien, C.; Jafferany, M.; Carniciu, S.; Abdelmaksoud, A. Psychodermatology of acne: Psychological aspects and effects of acne vulgaris. J. Cosmet. Dermatol. 2021, 20, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.S.; Mintem, G.C.; de Oliveira, I.O.; Horta, B.L.; Ramos, E.; Lopes, C.; Gigante, D.P. Consumption of ultra-processed foods and IL-6 in two cohorts from high-and middle-income countries. Br. J. Nutr. 2023, 129, 1552–1562. [Google Scholar] [CrossRef]
- Elhanboli, G.M. Role of InterLeukins in acne: A systematic review and meta-analysis. Fayoum Univ. Med. J. 2021, 9, 28–35. [Google Scholar]
- Kaminiów, K. Impact of Diet and Nutrition in Patients with Acne Vulgaris. Nutrients 2024, 16, 1476. [Google Scholar] [CrossRef]
- Meixiong, J.; Ricco, C.; Vasavda, C.; Ho, B.K. Diet and acne: A systematic review. JAAD Int. 2022, 7, 95–112. [Google Scholar] [CrossRef]
- Baldwin, H.; Tan, J. Effects of Diet on Acne and Its Response to Treatment. Am. J. Clin. Dermatol. 2020, 22, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Dall’Oglio, F.; Nasca, M.R.; Fiorentini, F.; Micali, G. Diet and acne: Review of the evidence from 2009 to 2020. Int. J. Dermatol. 2021, 60, 672–685. [Google Scholar] [CrossRef]
- Greywal, T.; Kusari, A.; Han, A.M.; Borok, J.; Proudfoot, J.A.; Ahluwalia, J.; Friedlander, S.F. Severe acne and its variants: Exploring its natural history and heritability. Pediatr. Dermatol. 2022, 39, 535–540. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34 (Suppl. S4), 4–25. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Xu, A.; He, L. A Review of Advancement on Influencing Factors of Acne: An Emphasis on Environment Characteristics. Front. Public Health 2020, 8, 450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daou, H.N. Exercise as an anti-inflammatory therapy for cancer cachexia: A focus on interleukin-6 regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R296–R310. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.C. Cytokine signaling in the modulation of post-acute and chronic systemic inflammation: A review of the influence of exercise and certain drugs. AIMS Allergy Immunol. 2020, 4, 100–116. [Google Scholar]
- Triatmakusuma, Y.; Praharsini, I.G.A.A.; Darmaputra, I.G.N.; Winaya, K.K.; Karna, N.L.P.R.V.; Puspawati, N.M.D. Serum Interleukin-6 Levels are Positively Correlated with the Severity of Acne Vulgaris. J. La Medihealtico 2024, 5, 158–166. [Google Scholar] [CrossRef]
- Heng, A.H.S.; Chew, F.T. Systematic review of the epidemiology of acne vulgaris. Sci. Rep. 2020, 10, 5754. [Google Scholar] [CrossRef]
- Al-tameemi, S.; Hameed, N.; Gomes, K.; Abid, H. Cigarette smoking increases plasma levels of IL-6 and TNF-α. Baghdad J. Biochem. Appl. Biol. Sci. 2022, 3, 60–68. [Google Scholar] [CrossRef]
- Stańkowska, A.; Bergler-Czop, B.; Brzezińska-Wcisło, L. Interleukins-6,-8 and-12p40 and C-reactive protein levels in patients with acne vulgaris with various severity of skin changes. Dermatol. Rev. Prz. Dermatol. 2020, 107, 308–322. [Google Scholar] [CrossRef]
- Li, J.; Du, D.; Zhang, J.; Liu, W.; Wang, J.; Wei, X.; Xue, L.; Li, X.; Diao, P.; Zhang, L.; et al. Development and validation of an artificial intelligence-powered acne grading system incorporating lesion identification. Front. Med. 2023, 10, 1255704. [Google Scholar] [CrossRef]
- Franceschini, C.; Persechino, F.; Ardigò, M. In Vivo Reflectance Confocal Microscopy in General Dermatology: How to Choose the Right Indication. Dermatol. Pract. Concept. 2020, 10, e2020032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anaba, E.L.; Oaku, I.R. Adult female acne: A cross-sectional study of diet, family history, body mass index, and premenstrual flare as risk factors and contributors to severity. Int. J. Women’s Dermatol. 2021, 7, 265–269. [Google Scholar] [CrossRef]
- Akpinar Kara, Y.; Ozdemir, D. Evaluation of food consumption in patients with acne vulgaris and its relationship with acne severity. J. Cosmet. Dermatol. 2020, 19, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Penso, L.; Touvier, M.; Deschasaux, M.; Hercberg, S.; Ezzedine, K.; Sbidian, E. Association between adult acne and dietary behaviors: Findings from the NutriNet-Sante Prospective Cohort Study. JAMA Dermatol. 2020, 156, 854–862. [Google Scholar] [CrossRef]
- Dreno, B.; Shourick, J.; Kerob, D.; Bouloc, A.; Taieb, C. The role of exposome in acne: Results from an international patient survey. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1057–1064. [Google Scholar] [CrossRef]
- Podder, I.; Agarwal, K.; Anurag, A. Metabolic status, obesity, and quality of life in patients with acne vulgaris: A cross-sectional case-control study. Indian J. Dermatol. 2021, 66, 223. [Google Scholar] [CrossRef]
- Saha, B.; Mendiratta, V. Evaluating the body mass index, blood glucose, and serum insulin in adolescent acne. Indian J. Paediatr. Dermatol. 2023, 24, 19–23. [Google Scholar] [CrossRef]
- Kovács, D.; Fazekas, F.; Oláh, A.; Törőcsik, D. Adipokines in the Skin and in Dermatological Diseases. Int. J. Mol. Sci. 2020, 21, 9048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turchin, I.; Bourcier, M. The role of interleukins in the pathogenesis of dermatological immune-mediated diseases. Adv. Ther. 2022, 39, 4474–4508. [Google Scholar] [CrossRef] [PubMed]
- Mesas-Fernández, A.; Bodner, E.; Hilke, F.J.; Meier, K.; Ghoreschi, K.; Solimani, F. Interleukin-21 in autoimmune and inflammatory skin diseases. Eur. J. Immunol. 2023, 53, e2250075. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Fiocco, Z.; Satoh, T.K.; Peris, K.; French, L.E. Therapeutic potential of targeting interleukin-1 family cytokines in chronic inflammatory skin diseases. Br. J. Dermatol. 2022, 186, 925–941. [Google Scholar] [CrossRef]
- Borgia, F.; Custurone, P.; Li Pomi, F.; Cordiano, R.; Alessandrello, C.; Gangemi, S. IL-31: State of the art for an inflammation-oriented interleukin. Int. J. Mol. Sci. 2022, 23, 6507. [Google Scholar] [CrossRef]
- Chilicka, K.; Rusztowicz, M.; Szyguła, R.; Nowicka, D. Methods for the improvement of acne scars used in dermatology and cosmetology: A review. J. Clin. Med. 2022, 11, 2744. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tan, K.; Hu, J.; Zhang, Z.; Dong, S. A review of fusion methods for omics and imaging data. IEEE ACM Trans. Comput. Biol. Bioinform. 2022, 20, 74–93. [Google Scholar] [CrossRef]
- Goodarzi, A.; Mozafarpoor, S.; Bodaghabadi, M.; Mohamadi, M. The potential of probiotics for treating acne vulgaris: A review of literature on acne and microbiota. Dermatol. Ther. 2020, 33, e13279. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jawade, S.; Madke, B.; Gupta, S. Recent Trends in the Management of Acne Vulgaris: A Review Focusing on Clinical Studies in the Last Decade. Cureus 2024, 16, e56596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Epidemiologic Aspect | Details |
|---|---|
| Prevalence | Over 85% of teenagers are affected by some form of acne (absolute differences) |
| Age distribution | Most common between 12 and 24 years of age; may persist in adults (absolute differences 15–20% of cases) |
| Gender distribution | More common in men in adolescence (due to androgen hormones); more common in women in adulthood |
| Geographical factors | More common in industrialized countries; lower incidence in non-Westernized populations (difficulty in access to dermatological care) |
| Genetic impact | Significant family predisposition (if one or both parents had severe acne) |
| Ethnic factors | Severity may vary: Caucasians tend to develop inflammatory forms more often. People of color may have hypertrophic or keloid scars more often |
| Hormonal factors | Acne is frequently associated with hormonal changes (puberty, pregnancy, polycystic ovary syndrome) |
| Severe forms | Acne conglobata and fulminans are rarer but predominantly affect men |
| Psychological impact | Affects quality of life and is associated with anxiety, depression, and low self-esteem |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guguluș, D.L.; Vâță, D.; Popescu, I.A.; Pătrașcu, A.I.; Halip, I.A.; Mocanu, M.; Solovăstru, L.G. The Epidemiology of Acne in the Current Era: Trends and Clinical Implications. Cosmetics 2025, 12, 106. https://doi.org/10.3390/cosmetics12030106
Guguluș DL, Vâță D, Popescu IA, Pătrașcu AI, Halip IA, Mocanu M, Solovăstru LG. The Epidemiology of Acne in the Current Era: Trends and Clinical Implications. Cosmetics. 2025; 12(3):106. https://doi.org/10.3390/cosmetics12030106
Chicago/Turabian StyleGuguluș, Dumitrița Lenuța, Dan Vâță, Ioana Adriana Popescu, Adriana Ionela Pătrașcu, Ioana Alina Halip, Mădălina Mocanu, and Laura Gheucă Solovăstru. 2025. "The Epidemiology of Acne in the Current Era: Trends and Clinical Implications" Cosmetics 12, no. 3: 106. https://doi.org/10.3390/cosmetics12030106
APA StyleGuguluș, D. L., Vâță, D., Popescu, I. A., Pătrașcu, A. I., Halip, I. A., Mocanu, M., & Solovăstru, L. G. (2025). The Epidemiology of Acne in the Current Era: Trends and Clinical Implications. Cosmetics, 12(3), 106. https://doi.org/10.3390/cosmetics12030106







