Peptides in Cosmetics: From Pharmaceutical Breakthroughs to Skincare Innovations
Abstract
1. Introduction
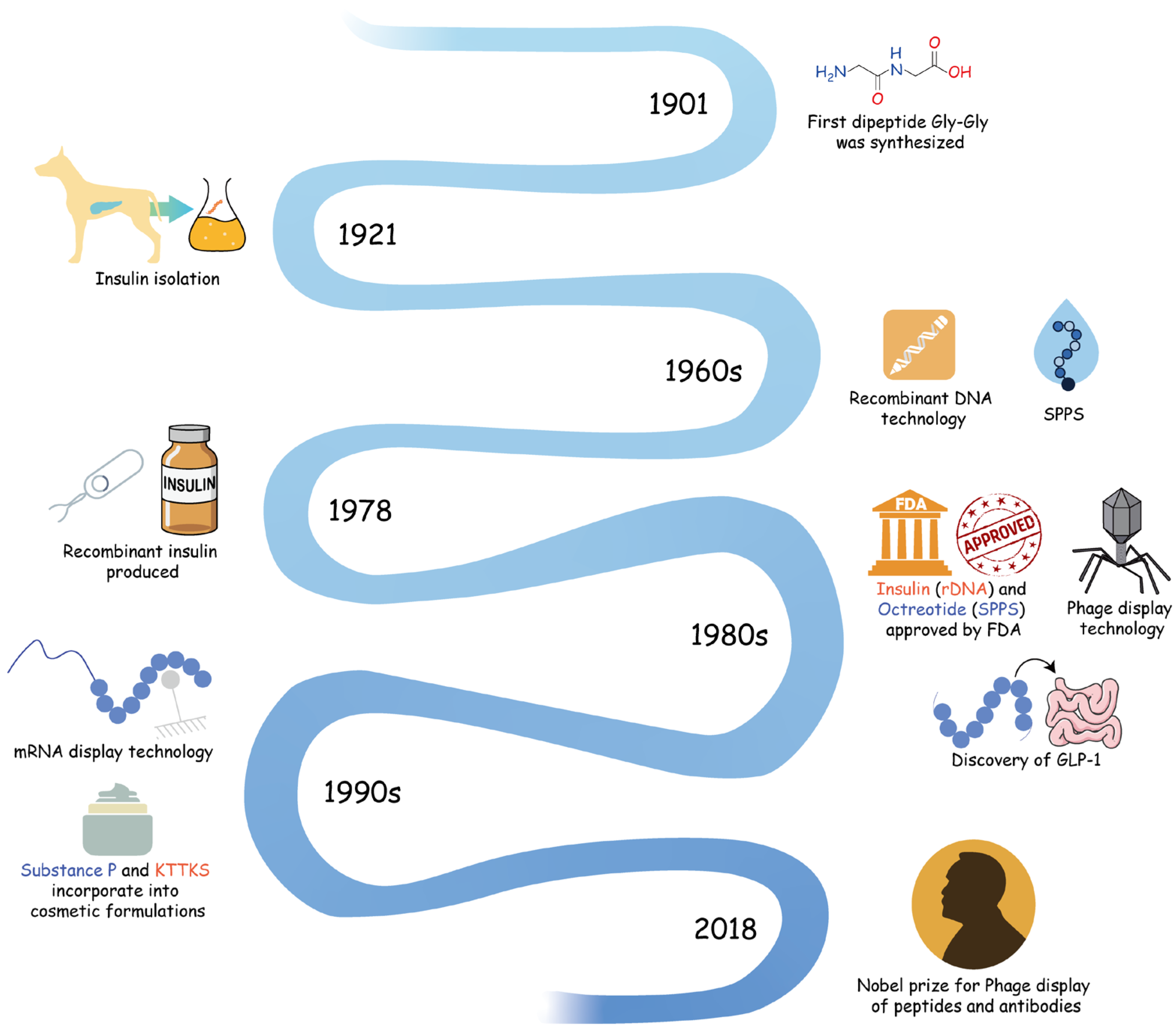
2. Historical Discoveries: The Exploration of Peptides in Pharmaceuticals and Cosmetics
2.1. Early Discoveries and Milestones
2.2. Advancements in Synthesis Techniques
2.3. Transition into Cosmetics
3. Peptide Synthesis Technologies
3.1. Chemical Synthesis of Peptides
3.2. Other Methods of Peptide Synthesis
3.3. Peptide Modifications
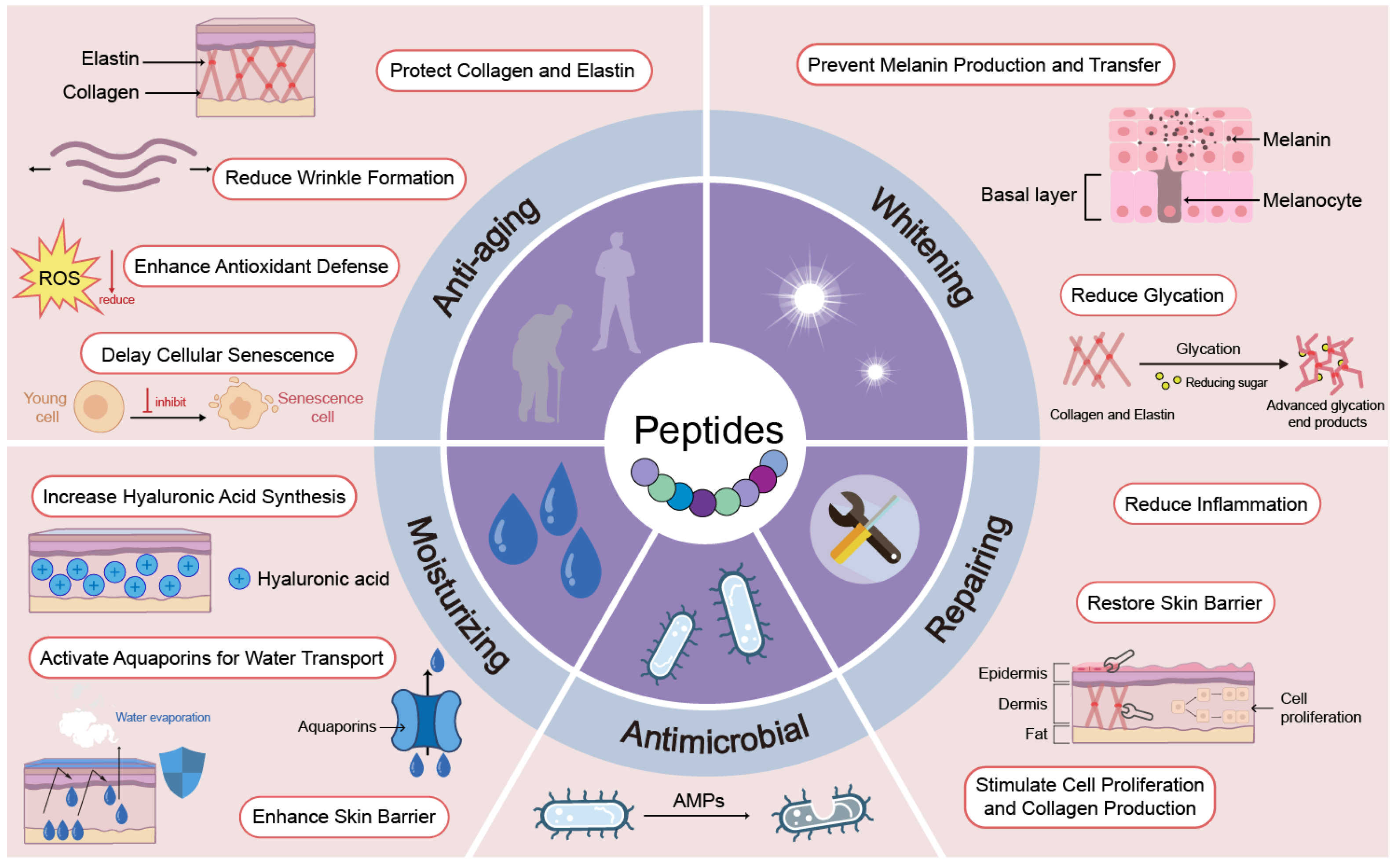
4. Targets and Mechanisms of Action
4.1. Anti-Aging Peptides
4.1.1. Protect Collagen and Elastin
4.1.2. Reduce Wrinkle Formation
4.1.3. Enhance Antioxidant Defense
4.1.4. Delay Cellular Senescence
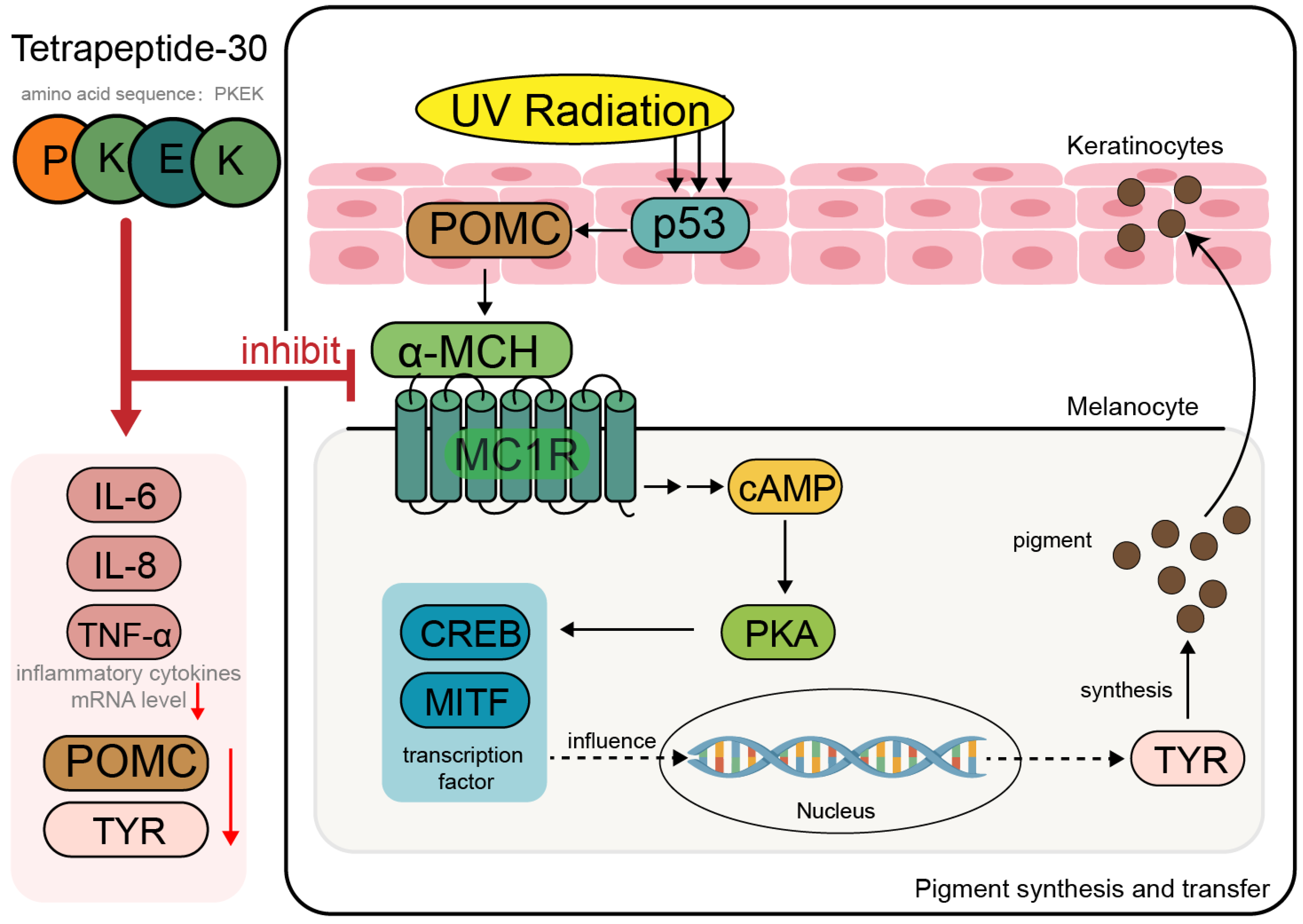
4.2. Whitening Peptides
4.3. Moisturizing Peptides
4.4. Repair Peptides
4.5. Antimicrobial Peptides
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ahx | Aminocaproic acid |
| Aib | 2-Aminoisobutyric acid |
| AMPs | Antimicrobial peptides |
| anti-TNF-α | Anti-tumor necrosis factor α |
| anti-IL-13 | Anti-interleukin-1 |
| CAGR | Compound annual growth rate |
| CSPS | Classical solution peptide synthesis |
| EM-1 | Endomorphin-1 |
| FIT | Flexible in vitro translation system |
| FTDR | Fluorine-thiol displacement reaction |
| GHK | Glycine-histidine-lysine |
| Gly | Glycine |
| GLP-1 | Glucagon-like peptide-1 |
| GSH | Glutathione |
| HA | Hyaluronic acid |
| KTTKS | Lysine-threonine- threonine-lysine-serine |
| MAPK | Mitogen-activated protein kinase |
| MC1R | Melanocortin 1 receptor |
| MIPD | Mirror-image phage display (MIPD) |
| MMP2 | Matrix metalloproteinase-2 |
| MoCRA | Modernization of Cosmetics Regulation Act |
| NCL | Native chemical ligation |
| PEG | Polyethylene glycol |
| RaPID | Random non-standard peptides integrated discovery |
| RHC | Recombinant human collagen |
| ROS | Reactive oxygen species |
| SPPS | Solid-phase peptide synthesis |
| THF | Tetrahydrofuran |
| TYR | Tyrosinase |
| LPPS | Liquid-phase peptide synthesis |
References
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide Chemistry Toolbox—Transforming Natural Peptides into Peptide Therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K. Composition Cosmetiques ou Dermopharmaceutiques Contenant le Tripeptide n-n-biotinyl-gly-his-lys pour Prevenir, Reduire ou Supprimer la Chute des Cheveux Ainsi que Pour Favoriser Leur Repousse. Patent WO2000058347A1, 5 October 2000. [Google Scholar]
- Lupo, M.P.; Cole, A.L. Cosmeceutical Peptides. Dermatol. Ther. 2007, 20, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.V.; Bhandari, P.; Shukla, P. Topical Peptides as Cosmeceuticals. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 9–18. [Google Scholar] [CrossRef]
- Fu, T.-K.; Kuo, P.-H.; Lu, Y.-C.; Lin, H.-N.; Wang, L.H.-C.; Lin, Y.-C.; Kao, Y.-C.; Lai, H.-M.; Chang, M.D.-T. Cell Penetrating Peptide as a High Safety Anti-Inflammation Ingredient for Cosmetic Applications. Biomolecules 2020, 10, 101. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Insights into Bioactive Peptides in Cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Palomo, J.M. Solid-Phase Peptide Synthesis: An Overview Focused on the Preparation of Biologically Relevant Peptides. RSC Adv. 2014, 4, 32658–32672. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; de la Torre, B.G.; Albericio, F. Liquid-Phase Peptide Synthesis (LPPS): A Third Wave for the Preparation of Peptides. Chem. Rev. 2022, 122, 13516–13546. [Google Scholar] [CrossRef]
- Chow, H.Y.; Zhang, Y.; Matheson, E.; Li, X. Ligation Technologies for the Synthesis of Cyclic Peptides. Chem. Rev. 2019, 119, 9971–10001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, C.; Chen, K.; Sun, S.; Zhang, D.; Meng, X. Screening Technology of Cyclic Peptide Library Based on Gene Encoding. Med. Drug Discov. 2022, 16, 100145. [Google Scholar] [CrossRef]
- Guzmán, F.; Aróstica, M.; Román, T.; Beltrán, D.; Gauna, A.; Albericio, F.; Cárdenas, C. Peptides, Solid-Phase Synthesis and Characterization: Tailor-Made Methodologies. Electron. J. Biotechnol. 2023, 64, 27–33. [Google Scholar] [CrossRef]
- Cosmetic Peptide Manufacturing Market Read. Available online: https://www.credenceresearch.com/report/cosmetic-peptide-manufacturing-market (accessed on 20 March 2025).
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Fourneau, E. Über Einige Derivate Des Glykocolls. Berichte Dtsch. Chem. Ges. 1901, 34, 2868–2877. [Google Scholar] [CrossRef]
- Fischer, E. Ueber Einige Derivate Des Glykocolls, Alanins Und Leucins. Berichte Dtsch. Chem. Ges. 1902, 35, 1095–1106. [Google Scholar] [CrossRef]
- Sims, E.K.; Carr, A.L.J.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 Years of Insulin: Celebrating the Past, Present and Future of Diabetes Therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef]
- Faulhaber, H.D.; Oehme, P.; Baumann, R.; Enderlein, J.; Rathsack, R.; Rostock, G.; Naumann, E. Substance P in Human Essential Hypertension. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. 12), S172–S176. [Google Scholar] [CrossRef]
- Asadi, S.; Alysandratos, K.-D.; Angelidou, A.; Miniati, A.; Sismanopoulos, N.; Vasiadi, M.; Zhang, B.; Kalogeromitros, D.; Theoharides, T.C. Substance P (SP) Induces Expression of Functional Corticotropin-Releasing Hormone Receptor-1 (CRHR-1) in Human Mast Cells. J. Investig. Dermatol. 2012, 132, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Tuppy, H. The Amino-Acid Sequence in the Phenylalanyl Chain of Insulin. 1. The Identification of Lower Peptides from Partial Hydrolysates. Biochem. J. 1951, 49, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Marglin, A.; Merrifield, R.B. The Synthesis of Bovine Insulin by the Solid Phase Method. J. Am. Chem. Soc. 1966, 88, 5051–5052. [Google Scholar] [CrossRef]
- Kung, Y.T.; Du, Y.C.; Huang, W.T.; Chen, C.C.; Ke, L.T. Total Synthesis of Crystalline Bovine Insulin. Sci. Sin. 1965, 14, 1710–1716. [Google Scholar]
- Lamberts, S.W.J.; Hofland, L.J. ANNIVERSARY REVIEW: Octreotide, 40 Years Later. Eur. J. Endocrinol. 2019, 181, R173–R183. [Google Scholar] [CrossRef]
- Quianzon, C.C.; Cheikh, I. History of Insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 18701. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.S. Human Insulin from Recombinant DNA Technology. Science 1983, 219, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Mojsov, S.; Heinrich, G.; Wilson, I.B.; Ravazzola, M.; Orci, L.; Habener, J.F. Preproglucagon Gene Expression in Pancreas and Intestine Diversifies at the Level of Post-Translational Processing. J. Biol. Chem. 1986, 261, 11880–11889. [Google Scholar] [CrossRef]
- Jaroszewicz, W.; Morcinek-Orłowska, J.; Pierzynowska, K.; Gaffke, L.; Węgrzyn, G. Phage Display and Other Peptide Display Technologies. FEMS Microbiol. Rev. 2022, 46, fuab052. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, R.; Zhang, X.; Wang, Z.L.; Xiong, F.; Zhang, S.; Li, G.; Wang, Y.; Zhang, Z.; Zhang, Q.-W.; et al. Encoding and Display Technologies for Combinatorial Libraries in Drug Discovery: The Coming of Age from Biology to Therapy. Acta Pharm. Sin. B 2024, 14, 3362–3384. [Google Scholar] [CrossRef]
- Heinis, C.; Rutherford, T.; Freund, S.; Winter, G. Phage-Encoded Combinatorial Chemical Libraries Based on Bicyclic Peptides. Nat. Chem. Biol. 2009, 5, 502–507. [Google Scholar] [CrossRef]
- Goto, Y.; Suga, H. The RaPID Platform for the Discovery of Pseudo-Natural Macrocyclic Peptides. Acc. Chem. Res. 2021, 54, 3604–3617. [Google Scholar] [CrossRef] [PubMed]
- Reid, P.C.; Goto, Y.; Katoh, T.; Suga, H. Charging of tRNAs Using Ribozymes and Selection of Cyclic Peptides Containing Thioethers. Methods Mol. Biol. 2012, 805, 335–348. [Google Scholar] [CrossRef]
- Suire, C.N.; Nainar, S.; Fazio, M.; Kreutzer, A.G.; Paymozd-Yazdi, T.; Topper, C.L.; Thompson, C.R.; Leissring, M.A. Peptidic Inhibitors of Insulin-Degrading Enzyme with Potential for Dermatological Applications Discovered via Phage Display. PLoS ONE 2018, 13, e0193101. [Google Scholar] [CrossRef]
- Katayama, K.; Armendariz-Borunda, J.; Raghow, R.; Kang, A.H.; Seyer, J.M. A Pentapeptide from Type I Procollagen Promotes Extracellular Matrix Production. J. Biol. Chem. 1993, 268, 9941–9944. [Google Scholar] [CrossRef]
- Bergmann, M.; Zervas, L. Über Ein Allgemeines Verfahren Der Peptid-Synthese. Berichte Dtsch. Chem. Ges. B Ser. 1932, 65, 1192–1201. [Google Scholar] [CrossRef]
- Vigneaud, V.D.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The Synthesis of an Octapeptide Amide with the Hormonal Activity of Oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Mäde, V.; Els-Heindl, S.; Beck-Sickinger, A.G. Automated Solid-Phase Peptide Synthesis to Obtain Therapeutic Peptides. Beilstein J. Org. Chem. 2014, 10, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, C.; Assunção, N.A.; Gerhardt, J.; Miranda, M.T.M. Microwave-Assisted Solid-Phase Peptide Synthesis at 60 Degrees C: Alternative Conditions with Low Enantiomerization. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 808–817. [Google Scholar] [CrossRef]
- Collins, J.M.; Singh, S.K.; White, T.A.; Cesta, D.J.; Simpson, C.L.; Tubb, L.J.; Houser, C.L. Total Wash Elimination for Solid Phase Peptide Synthesis. Nat. Commun. 2023, 14, 8168. [Google Scholar] [CrossRef]
- Benaglia, M.; Danelli, T.; Fabris, F.; Sperandio, D.; Pozzi, G. Poly(Ethylene Glycol)-Supported Tetrahydroxyphenyl Porphyrin: A Convenient, Recyclable Catalyst for Photooxidation Reactions. Org. Lett. 2002, 4, 4229–4232. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, X.; Ren, X.; Wang, P. Liquid Phase Synthesis Method of. Eptifibatide. Patent CN102924569A, 13 February 2013. [Google Scholar]
- Curran, D.P.; Hadida, S. Tris(2-(Perfluorohexyl)Ethyl)Tin Hydride: A New Fluorous Reagent for Use in Traditional Organic Synthesis and Liquid Phase Combinatorial Synthesis. J. Am. Chem. Soc. 1996, 118, 2531–2532. [Google Scholar] [CrossRef]
- Development of an Efficient Liquid-Phase Peptide Synthesis Protocol Using a Novel Fluorene-Derived Anchor Support Compound with Fmoc Chemistry; AJIPHASE®. Tetrahedron Lett. 2012, 53, 1936–1939. [CrossRef]
- Luo, Z.; Williams, J.; Read, R.W.; Curran, D.P. Fluorous Boc ((F)Boc) Carbamates: New Amine Protecting Groups for Use in Fluorous Synthesis. J. Org. Chem. 2001, 66, 4261–4266. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, T.; Song, W.; Liu, X.; Zhang, Z.; Yang, X.; Zhao, J. Synthesis Method for GHK. Tripeptide. Patent CN103665102A, 26 March 2014. [Google Scholar]
- Hu, L.; Xu, S.; Zhao, Z.; Yang, Y.; Peng, Z.; Yang, M.; Wang, C.; Zhao, J. Ynamides as Racemization-Free Coupling Reagents for Amide and Peptide Synthesis. J. Am. Chem. Soc. 2016, 138, 13135–13138. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, Z.; Lai, M.; Hu, L.; Zhao, J. Inverse Peptide Synthesis Using Transient Protected Amino Acids. J. Am. Chem. Soc. 2024, 146, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Aso, K.; Kodaka, H.; Fukushi, H.; Lee, H.-H. Trypsin-Catalyzed Synthesis of the Arginyl-Arginine Dipeptide Froml-Arginine Ethyl Ester. Biotechnol. Lett. 1992, 14, 451–454. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Hou, R.-Z.; Yang, Y.; Li, G.; Huang, Y.-B.; Wang, H.; Liu, Y.-J.; Xu, L.; Zhang, X.-Z. Synthesis of a Precursor Dipeptide of RGDS (Arg-Gly-Asp-Ser) Catalysed by the Industrial Protease Alcalase. Biotechnol. Appl. Biochem. 2006, 44, 73–80. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Xu, L.; Yang, X.-C.; Wu, X.-X.; Zhang, X.-Z. Lipase-Catalyzed Synthesis of Precursor Dipeptides of RGD in Aqueous Water-Miscible Organic Solvents. Prep. Biochem. Biotechnol. 2003, 33, 1–12. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Cai, Y.; Yang, S.; Wang, H.; Hou, R.-Z.; Xu, L.; Xiao-Xia, W.; Zhang, X.-Z. Synthesis of Tetrapeptide Bz-RGDS-NH2 by a Combination of Chemical and Enzymatic Methods. J. Biotechnol. 2006, 125, 311–318. [Google Scholar] [CrossRef]
- Yılmaz, F. Polymer Science; IntechOpen: London, UK, 2013; ISBN 978-953-51-0941-9. [Google Scholar]
- Yazawa, K.; Numata, K. Recent Advances in Chemoenzymatic Peptide Syntheses. Molecules 2014, 19, 13755–13774. [Google Scholar] [CrossRef]
- Sun, H.; He, B.-F.; Xu, J.; Wu, B.; Ouyang, P. Efficient Chemo-Enzymatic Synthesis of Endomorphin-1 Using Organic Solvent Stable Proteases to Green the Synthesis of the Peptide. Green Chem. 2011, 13, 1680–1685. [Google Scholar] [CrossRef]
- Heyland, J.; Antweiler, N.; Lutz, J.; Heck, T.; Geueke, B.; Kohler, H.-P.E.; Blank, L.M.; Schmid, A. Simple Enzymatic Procedure for L-Carnosine Synthesis: Whole-Cell Biocatalysis and Efficient Biocatalyst Recycling. Microb. Biotechnol. 2010, 3, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, K.; Ritala, A.; Suntio, T.; Blumer, S.; Holkeri, H.; Wahlström, E.H.; Baez, J.; Mäkinen, K.; Maria, N.A. Production of a Recombinant Full-Length Collagen Type I Alpha-1 and of a 45-kDa Collagen Type I Alpha-1 Fragment in Barley Seeds. Plant Biotechnol. J. 2009, 7, 657–672. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, Y.; Zhu, Z.; Gao, R. Recombinant Human Collagen Digestates Exhibit Higher Protective Effect on UVA-Damaged Skin Fibroblasts than Animal-Derived Collagens. J. Funct. Foods 2024, 113, 106035. [Google Scholar] [CrossRef]
- Hsiao, C.-D.; Wu, H.-H.; Malhotra, N.; Liu, Y.-C.; Wu, Y.-H.; Lin, Y.-N.; Saputra, F.; Santoso, F.; Chen, K.H.-C. Expression and Purification of Recombinant GHK Tripeptides Are Able to Protect against Acute Cardiotoxicity from Exposure to Waterborne-Copper in Zebrafish. Biomolecules 2020, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- De Leon Rodriguez, L.M.; Williams, E.T.; Brimble, M.A. Chemical Synthesis of Bioactive Naturally Derived Cyclic Peptides Containing Ene-Like Rigidifying Motifs. Chem. Eur. J. 2018, 24, 17869–17880. [Google Scholar] [CrossRef]
- Jo, H.; Meinhardt, N.; Wu, Y.; Kulkarni, S.; Hu, X.; Low, K.E.; Davies, P.L.; DeGrado, W.F.; Greenbaum, D.C. Development of α-Helical Calpain Probes by Mimicking a Natural Protein-Protein Interaction. J. Am. Chem. Soc. 2012, 134, 17704–17713. [Google Scholar] [CrossRef]
- West, C.W.; Rich, D.H. Novel Cyclic Tripeptides and Substituted Aromatic Amino Acids via Ruthenium-Activated S(N)Ar Reactions. Org. Lett. 1999, 1, 1819–1822. [Google Scholar] [CrossRef]
- Blackwell, H.E.; Grubbs, R.H. Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis. Angew. Chem. Int. Ed. Engl. 1998, 37, 3281–3284. [Google Scholar] [CrossRef]
- Islam, M.S.; Junod, S.L.; Zhang, S.; Buuh, Z.Y.; Guan, Y.; Zhao, M.; Kaneria, K.H.; Kafley, P.; Cohen, C.; Maloney, R.; et al. Unprotected Peptide Macrocyclization and Stapling via a Fluorine-Thiol Displacement Reaction. Nat. Commun. 2022, 13, 350. [Google Scholar] [CrossRef]
- Wieland, T.; Faulstich, H. Fifty Years of Amanitin. Experientia 1991, 47, 1186–1193. [Google Scholar] [CrossRef]
- Krunic, A.; Vallat, A.; Mo, S.; Lantvit, D.D.; Swanson, S.M.; Orjala, J. Scytonemides A and B, Cyclic Peptides with 20S Proteasome Inhibitory Activity from the Cultured Cyanobacterium Scytonema Hofmanii. J. Nat. Prod. 2010, 73, 1927–1932. [Google Scholar] [CrossRef]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the Marine Sponge Callyspongia Aerizusa: Cyclic Peptides with Antitubercular Activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.; Xie, Z.; Xie, C.; Zhan, C.; Hu, X.; Shen, Q.; Wei, X.; Su, B.; Wang, J.; et al. D-Peptides as Recognition Molecules and Therapeutic Agents. Chem. Rec. 2016, 16, 1772–1786. [Google Scholar] [CrossRef]
- Collins, J.M.; Porter, K.A.; Singh, S.K.; Vanier, G.S. High-Efficiency Solid Phase Peptide Synthesis (HE-SPPS). Org. Lett. 2014, 16, 940–943. [Google Scholar] [CrossRef]
- Giesler, R.J.; Erickson, P.W.; Kay, M.S. Enhancing Native Chemical Ligation for Challenging Chemical Protein Syntheses. Curr. Opin. Chem. Biol. 2020, 58, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cistrone, P.A.; Bird, M.J.; Flood, D.T.; Silvestri, A.P.; Hintzen, J.C.J.; Thompson, D.A.; Dawson, P.E. Native Chemical Ligation of Peptides and Proteins. Curr. Protoc. Chem. Biol. 2019, 11, e61. [Google Scholar] [CrossRef]
- Agouridas, V.; El Mahdi, O.; Diemer, V.; Cargoët, M.; Monbaliu, J.-C.M.; Melnyk, O. Native Chemical Ligation and Extended Methods: Mechanisms, Catalysis, Scope, and Limitations. Chem. Rev. 2019, 119, 7328–7443. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.C.; Milton, S.C.; Kent, S.B. Total Chemical Synthesis of a D-Enzyme: The Enantiomers of HIV-1 Protease Show Reciprocal Chiral Substrate Specificity. Science 1992, 256, 1445–1448. [Google Scholar] [CrossRef]
- Chang, H.-N.; Liu, B.-Y.; Qi, Y.-K.; Zhou, Y.; Chen, Y.-P.; Pan, K.-M.; Li, W.-W.; Zhou, X.-M.; Ma, W.-W.; Fu, C.-Y.; et al. Blocking of the PD-1/PD-L1 Interaction by a D-Peptide Antagonist for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2015, 54, 11760–11764. [Google Scholar] [CrossRef]
- Mandal, K.; Uppalapati, M.; Ault-Riché, D.; Kenney, J.; Lowitz, J.; Sidhu, S.S.; Kent, S.B.H. Chemical Synthesis and X-ray Structure of a Heterochiral {D-Protein Antagonist plus Vascular Endothelial Growth Factor} Protein Complex by Racemic Crystallography. Proc. Natl. Acad. Sci. USA 2012, 109, 14779–14784. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like Peptide-1 Receptor: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of Glucose-Dependent Insulinotropic Polypeptide and Truncated Glucagon-like Peptide 1 In Vitro and In Vivo by Dipeptidyl Peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Shao, S.-C.; Kuo, S.; Yang, C.-Y.; Chen, H.-Y.; Chan, Y.-Y.; Ou, H.-T. Comparative Effectiveness of Dulaglutide versus Liraglutide in Asian Type 2 Diabetes Patients: A Multi-Institutional Cohort Study and Meta-Analysis. Cardiovasc. Diabetol. 2020, 19, 172. [Google Scholar] [CrossRef]
- Drucker, D.J.; Dritselis, A.; Kirkpatrick, P. Liraglutide. Nat. Rev. Drug Discov. 2010, 9, 267–268. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Guglielmi, D.A.S.; Martinelli, A.M.; Rissi, N.C.; Cilli, E.M.; Soares, C.P.; Chiavacci, L.A. Synthesis of the Peptide Ac-Wahx-KTTKS and Evaluation of the Ability to Induce In Vitro Collagen Synthesis. Protein Pept. Lett. 2016, 23, 544–547. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Kloska, A.; Zdrowowicz, M.; Wieczorek, R.; Makowska, J. Modification of Amino-Acid Sequence of Cosmetic Peptide Eyeseryl Enhances the Affinity towards Copper(II) Ion. Polyhedron 2022, 222, 115948. [Google Scholar] [CrossRef]
- Lim, S.H.; Sun, Y.; Thiruvallur Madanagopal, T.; Rosa, V.; Kang, L. Enhanced Skin Permeation of Anti-Wrinkle Peptides via Molecular Modification. Sci. Rep. 2018, 8, 1596. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef]
- Kunjulakshmi, R.; Kumar, A.; Vinod Kumar, K.; Sengupta, A.; Kundal, K.; Sharma, S.; Pawar, A.; Krishna, P.S.; Alfatah, M.; Ray, S.; et al. AagingBase: A Comprehensive Database of Anti-Aging Peptides. Database 2024, 2024, baae016. [Google Scholar] [CrossRef]
- Jones, R.R.; Castelletto, V.; Connon, C.J.; Hamley, I.W. Collagen Stimulating Effect of Peptide Amphiphile C16–KTTKS on Human Fibroblasts. Mol. Pharm. 2013, 10, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Abu Samah, N.H.; Heard, C.M. Topically Applied KTTKS: A Review. Int. J. Cosmet. Sci. 2011, 33, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Tałałaj, U.; Uścinowicz, P.; Bruzgo, I.; Surażyński, A.; Zaręba, I.; Markowska, A. The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity. Molecules 2019, 24, 3698. [Google Scholar] [CrossRef]
- Park, H.; An, E.; Cho Lee, A.-R. Effect of Palmitoyl-Pentapeptide (Pal-KTTKS) on Wound Contractile Process in Relation with Connective Tissue Growth Factor and α-Smooth Muscle Actin Expression. Tissue Eng. Regen. Med. 2017, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Hsu, C.-C.; Chung, C.-Y.; Lin, M.-S.; Li, S.-L.; Pang, J.-H.S. The Pentapeptide KTTKS Promoting the Expressions of Type I Collagen and Transforming Growth Factor-β of Tendon Cells. J. Orthop. Res. 2007, 25, 1629–1634. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Liu, T.; Zhao, Y.; Sun, X.; Lu, B.; Zhang, J.; Liu, Z.; Zhang, J. Pioneering Ionic Liquids in Neuro-Soothing: Enhanced Transdermal Delivery of Collagen Peptides and Their Synergistic Anti-Aging Functions. Mater. Today Bio 2025, 31, 101527. [Google Scholar] [CrossRef]
- Sarkar, P.; Li, Z.; Ren, W.; Wang, S.; Shao, S.; Sun, J.; Ren, X.; Perkins, N.G.; Guo, Z.; Chang, C.-E.A.; et al. Inhibiting Matrix Metalloproteinase-2 Activation by Perturbing Protein–Protein Interactions Using a Cyclic Peptide. J. Med. Chem. 2020, 63, 6979–6990. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Clemente, J.; Jodas, G.; Gil, A.; Fernández-Ballester, G.; Ponsati, B.; Gutierrez, L.; Pérez-Payá, E.; Ferrer-Montiel, A. A Synthetic Hexapeptide (Argireline) with Antiwrinkle Activity. Int. J. Cosmet. Sci. 2002, 24, 303–310. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.-A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
- Radrezza, S.; Carini, M.; Baron, G.; Aldini, G.; Negre-Salvayre, A.; D’Amato, A. Study of Carnosine’s Effect on Nude Mice Skin to Prevent UV-A Damage. Free Radic. Biol. Med. 2021, 173, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.B.; Scarlato, G.C.G.; Cavalini, D.F.; Shiguemoto, G.E. Humanin, MOTS-c and Physical Exercise: A New Perspective. Biomed. Res. Rev. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural Skin-Whitening Compounds for the Treatment of Melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef]
- Marini, A.; Farwick, M.; Grether-Beck, S.; Brenden, H.; Felsner, I.; Jaenicke, T.; Weber, M.; Schild, J.; Maczkiewitz, U.; Köhler, T.; et al. Modulation of Skin Pigmentation by the Tetrapeptide PKEK: In Vitro and in Vivo Evidence for Skin Whitening Effects. Exp. Dermatol. 2012, 21, 140–146. [Google Scholar] [CrossRef]
- Ghodsi, R.; Kheirouri, S. Carnosine and Advanced Glycation End Products: A Systematic Review. Amino Acids 2018, 50, 1177–1186. [Google Scholar] [CrossRef]
- del Marmol, V.; Solano, F.; Sels, A.; Huez, G.; Libert, A.; Lejeune, F.; Ghanem, G. Glutathione Depletion Increases Tyrosinase Activity in Human Melanoma Cells. J. Investig. Dermatol. 1993, 101, 871–874. [Google Scholar] [CrossRef]
- Sonthalia, S.; Jha, A.K.; Lallas, A.; Jain, G.; Jakhar, D. Glutathione for Skin Lightening: A Regnant Myth or Evidence-Based Verity? Dermatol. Pract. Concept. 2018, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Kim, J.; Jeong, M.K.; Lee, Y.M.; Chung, Y.J.; Kim, E.M. Whitening Effect of Novel Peptide Mixture by Regulating Melanosome Biogenesis, Transfer and Degradation. Korean J. Physiol. Pharmacol. 2021, 25, 15–26. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of Topical Hyaluronic Acid for Skin Quality and Signs of Skin Aging: From Literature Review to Clinical Evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef] [PubMed]
- Dal Farra, C.; Domloge, N.; Botto, J.-M. Peptide for Activating Aquaporin Synthesis. Patent WO2009112645A1, 17 September 2009. [Google Scholar]
- Pickart, L.; Margolina, A. Regenerative and Protective Actions of the GHK-Cu Peptide in the Light of the New Gene Data. Int. J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Vilcinskas, A. Short Antimicrobial Peptides as Cosmetic Ingredients to Deter Dermatological Pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 8847–8855. [Google Scholar] [CrossRef]
- Kamysz, W.; Okrój, M.; Łukasiak, J. Novel Properties of Antimicrobial Peptides. Acta Biochim. Pol. 2003, 50, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pöppel, A.-K.; Vogel, H.; Wiesner, J.; Vilcinskas, A. Antimicrobial Peptides Expressed in Medicinal Maggots of the Blow Fly Lucilia Sericata Show Combinatorial Activity against Bacteria. Antimicrob. Agents Chemother. 2015, 59, 2508–2514. [Google Scholar] [CrossRef]
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337. [Google Scholar] [CrossRef]
- Commissioner, O. of the Modernization of Cosmetics Regulation Act of 2022 (MoCRA). Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra (accessed on 17 April 2025).
- UNION, PEAN. Union Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union 2009, 342, 59–209. [Google Scholar]
- Zuo, Q.; Song, X.; Yan, J.; Bao, G.; Li, Y.; Shen, J.; He, Z.; Hu, K.; Sun, W.; Wang, R. Triazination/IEDDA Cascade Modular Strategy Installing Pyridines/Pyrimidines onto Tyrosine Enables Peptide Screening and Optimization. J. Am. Chem. Soc. 2025, 147, 9576–9589. [Google Scholar] [CrossRef]


| Aspect | CSPS | SPPS | LPPS |
|---|---|---|---|
| Scalability | Relatively low efficiency | Multi-kilogram production | Industrial-scale synthesis |
| Purity | High (stepwise) | Moderate (crude product) | High (efficient reactions) |
| Environmental Impact | High solvent use | High solvent/reagent waste | Lower solvent usage |
| Key Applications | Convergent synthesis, specialized peptides | Therapeutic peptides, analogs | Industrial and clinical peptides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Nie, T.; Zhang, L.; Liu, X.; Deng, H. Peptides in Cosmetics: From Pharmaceutical Breakthroughs to Skincare Innovations. Cosmetics 2025, 12, 107. https://doi.org/10.3390/cosmetics12030107
Tang Y, Nie T, Zhang L, Liu X, Deng H. Peptides in Cosmetics: From Pharmaceutical Breakthroughs to Skincare Innovations. Cosmetics. 2025; 12(3):107. https://doi.org/10.3390/cosmetics12030107
Chicago/Turabian StyleTang, Yuxiang, Tong Nie, Lu Zhang, Xiaohui Liu, and Haiteng Deng. 2025. "Peptides in Cosmetics: From Pharmaceutical Breakthroughs to Skincare Innovations" Cosmetics 12, no. 3: 107. https://doi.org/10.3390/cosmetics12030107
APA StyleTang, Y., Nie, T., Zhang, L., Liu, X., & Deng, H. (2025). Peptides in Cosmetics: From Pharmaceutical Breakthroughs to Skincare Innovations. Cosmetics, 12(3), 107. https://doi.org/10.3390/cosmetics12030107








