Abstract
Peptides are molecules composed of two or more amino acids linked by peptide bonds, and they play essential biological roles. In recent decades, peptides have become pivotal bioactive ingredients in pharmaceuticals and cosmetics due to their unique features. Originally developed for therapeutic purposes, peptides have gained popularity in the cosmetic field, providing solutions for anti-aging, whitening, moisturizing, and skin repair. Moreover, innovations such as artificial intelligence-assisted peptide design, efficient delivery systems, and the integration of multifunctional ingredients have significantly contributed to the industry’s rapid evolution. This review explores the historical milestones of peptides in medicine and cosmetics, delves into cutting-edge synthesis technologies, and dissects the molecular mechanisms behind their cosmetic properties. Research in medicinal peptides has promoted the development of cosmetic peptides. Despite their potential, challenges such as stability, bioavailability, and cost-effective production remain barriers to widespread adoption. Future studies should focus on enhancing peptide stability, developing synergistic formulations, and conducting large-scale clinical trials to validate long-term efficacy. With continuous innovation, peptides are poised to redefine the cosmetic industry, bridging the gap between pharmaceuticals and skincare for safer and more effective solutions.
1. Introduction
Peptides have emerged as a cornerstone in the pharmaceutical and cosmetic industries due to their high specificity, biocompatibility, and diverse biological activities. As short chains of amino acids, peptides have been widely reported to function in critical physiological processes, including signaling, repair, and regulation at the molecular level [1]. Their unique properties have made them indispensable in addressing challenges ranging from chronic disease management to enhancing skin health.
Since the 1990s when the first peptide was introduced into cosmetics [2], various natural or synthetic peptides have been utilized as active ingredients for anti-aging, whitening, and anti-inflammation, et al. [3,4,5,6]. Advances in chemical and biochemical synthesis techniques largely facilitate the industrial-scale production of peptide-based drugs and promote the integration of peptides into cosmetics [7,8,9,10,11].
As the global market for peptide-based products, including therapeutic peptides, cosmetic peptides, and nutraceuticals, continues to experience significant growth, the cosmetic peptide manufacturing market alone is projected to grow at a compound annual growth rate (CAGR) of 10.3% through 2034, with its value expected to increase from USD 3770.00 million in 2024 to USD 8259.34 million by 2032 [12]. As the peptide market for peptide-based products continues to grow, understanding their historical evolution, technological breakthroughs, and underlying biological mechanisms is crucial for advancing both research and commercial applications in this rapidly expanding field.
This review aims to explore the multifaceted world of peptides by examining their historical development, the technologies that have shaped their production and application, and the mechanisms through which they achieve their effects. By analyzing these aspects, this article provides a comprehensive understanding of peptides’ roles and potential in advancing both pharmaceuticals and cosmetics.
2. Historical Discoveries: The Exploration of Peptides in Pharmaceuticals and Cosmetics
The evolution of peptide science spans more than a century, including both pharmaceutical and cosmetic innovations. A timeline of key milestones is illustrated in Figure 1.
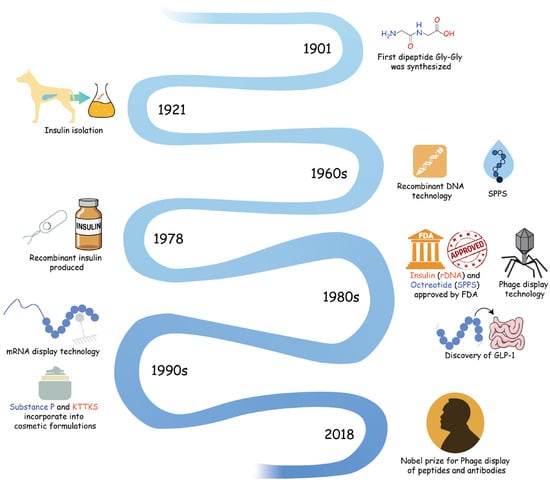
Figure 1.
Historical timeline of major breakthroughs in peptide research and development. The timeline illustrates the evolution of peptide science from the first dipeptide synthesis in 1901 to recombinant insulin production, the advent of SPPS, phage/mRNA display technologies, and recent advances in cosmetic applications.
2.1. Early Discoveries and Milestones
Peptide research can be traced back to the early 20th century [13,14,15]. In 1901, the dipeptide Gly-Gly was first synthesized by Fischer’s group [14], initiating peptide synthesis chemistry. Insulin was isolated from dog pancreas in 1921, setting the milestone of the therapeutic application of peptides in treating metabolic diseases such as diabetes [16]. After that, several peptides were identified as bioactive molecules, such as substance P, which is involved in blood pressure regulation and the induction of corticotropin in endocrine signaling [17,18]. It is worth mentioning that not until 1951 was insulin fully sequenced by Frederick Sanger [19], and bovine insulin was successfully chemically synthesized in the mid-1960s [16,20,21]. This represents the progress of peptide synthesis, thereby enabling the massive production of peptide-based drugs, shifted from natural extracts.
2.2. Advancements in Synthesis Techniques
The advent of solid-phase peptide synthesis (SPPS) in 1963 revolutionized peptide production, marking a transformative milestone in synthetic chemistry [7,8,11]. This groundbreaking technique streamlined the assembly of peptide chains through its innovative stepwise approach on an insoluble polymer support, dramatically enhancing both synthetic efficiency and cost-effectiveness. The clinical application of SPPS-synthesized peptides reached a significant milestone in the late 1980s [7,11], as evidenced by the FDA approval of octreotide in 1988 [22]. This landmark achievement not only validated SPPS as a robust platform for pharmaceutical production but also demonstrated its unparalleled capacity for the large-scale synthesis of structurally complex, biologically active molecules with precise sequence control.
The emergence of recombinant DNA technology in the 1960s marked a new era for peptide research. A pivotal advancement occurred in 1978 with the successful production of recombinant insulin by David Goeddel and his colleagues [16,23], which demonstrated the feasibility of industrial-scale peptide manufacturing through genetic engineering techniques. This breakthrough culminated in the 1982 approval of recombinant insulin, marking the first commercially available genetically engineered peptide drug and establishing a new paradigm for therapeutic development [23,24]. The subsequent discovery of glucagon-like peptide-1 (GLP-1) in 1986 by Mojsov and colleagues further expanded peptides’ therapeutic applications, particularly in metabolic disease management [25]. Recently, the GLP-1 market has experienced rapid expansion, fueled by its applications in type 2 diabetes, obesity, cardiovascular diseases, and beyond. With global leaders like Novo Nordisk and Eli Lilly dominating the market, along with emerging players from China driving innovation in oral formulations and multi-target agonists, the GLP-1 sector is on track to become a multi-billion-dollar industry. These milestones collectively transformed peptide science from laboratory curiosities to mainstream therapeutic agents, laying the foundation for the modern biopharmaceutical industry.
Display technologies, particularly phage display, which was awarded the Nobel Prize in 2018 [26], have greatly enhanced the efficiency and simplicity of peptide screening. Phage display is a technology based on the presentation of functional exogenous peptides, enabling the selection and amplification of specific peptides in vitro, like natural selection. Through multiple rounds of screening under selective pressure, peptides with a high affinity for target proteins can be enriched and amplified, while those with weak binding are eliminated.
Both the anti-tumor necrosis factor α (anti-TNF-α) antibody Adalimumab and the anti-B lymphocyte stimulator antibody Belimumab were discovered using phage display technology. Meanwhile, the anti-interleukin-13 (anti-IL-13) antibody Tralokinumab was discovered using RNA display technology. These drugs have all been approved for clinical use. Display technology has made significant advancements in drug discovery, with several drugs developed through this technology entering clinical trials [27].
Many peptide drug companies have secured a significant position in the peptide drug research field by leveraging their proprietary screening technologies. For example, Bicycle Therapeutics plc, a biopharmaceutical company, focuses on the development of “Bicycle® molecules”—a new class of therapeutics composed of small bicyclic peptides. The company’s unique bicyclic peptide design and synthesis technology is its most representative asset. Through an innovative combination of phage display and chemical cyclization at three cysteine sites, Bicycle Therapeutics has established a structurally stable and highly efficient bicyclic peptide library, forming a distinctive drug discovery platform in the field of targeted therapies [28].
In Japan, PeptiDream Inc. specializes in mRNA display technology, with its core platform RaPID (Random non-standard Peptides Integrated Discovery) system that is used for the high-throughput screening of high-affinity, structurally diverse macrocyclic peptides. This system covalently links each peptide to its encoding mRNA, enabling the precise identification of binding sequences. Combined with PeptiDream’s proprietary FIT (Flexible In Vitro Translation system), RaPID enables the incorporation of a wide variety of non-standard amino acids during peptide synthesis, allowing the construction of peptide libraries with a scale of up to 10¹² unique sequences [29,30]. Due to its high throughput, chemical diversity, and adaptability to various targets, the RaPID system is the core competitive advantage of PeptiDream in the global peptide drug discovery field.
Display technology also holds tremendous potential in the cosmetics field, particularly with the growing trend of peptide-based cosmetics. For instance, in 2018, Malcolm A. Leissring’s team discovered a novel IDE peptide inhibitor, P12-3A, using phage display technology. This cyclic dodecapeptide has been shown to enhance several insulin-induced processes, including the transcription, translation, and secretion of type I collagen in primary mouse dermal fibroblasts, as well as keratinocyte migration in scratch wound assays. With these properties, P12-3A exhibits great therapeutic and cosmetic potential for topical applications [31].
2.3. Transition into Cosmetics
The application of peptides has extended beyond pharmaceuticals into the cosmetic industry. In 1993, the pentapeptide KTTKS, derived from type I procollagen, was identified for its ability to stimulate extracellular matrix production, leading to its adoption in anti-aging skincare products [32]. Similarly, in 1994, substance P antagonists were incorporated into cosmetic formulations, demonstrating their efficacy in improving skin health and appearance. These applications exemplify how pharmaceutical peptides have been repurposed to address cosmetic challenges.
3. Peptide Synthesis Technologies
3.1. Chemical Synthesis of Peptides
Peptide synthesis has advanced through three distinct methodologies—Classical Solution Peptide Synthesis (CSPS), Solid-Phase Peptide Synthesis (SPPS), and Liquid-Phase Peptide Synthesis (LPPS) [8]. Each represents a significant leap in addressing the challenges of purity, scalability, and sustainability. This evolution reflects the growing demand for efficient peptide production, particularly in pharmaceutical and industrial applications, while aligning with the principles of green chemistry.
CSPS, the earliest method of peptide synthesis, relies on stepwise reactions carried out in solution. The concept dates back to the pioneering work of Fischer and Fourneau in 1901 [14]. A major advancement in peptide synthesis came in 1932 when Bergmann and Zervas developed the first reversible Nα-protecting group, the carbobenzoxy group, which enabled stepwise chain elongation with precise protection and deprotection strategies [33]. CSPS ensures precise control of reaction conditions and allows for extensive purification of intermediates at each stage, yielding products with exceptional purity. However, the process is labor-intensive, requiring significant manual effort for intermediate isolation and characterization and making it inefficient for long peptides or large-scale production [14]. Despite these limitations, CSPS remains valuable for specific applications, such as the preparation of fragments for convergent synthesis, e.g., oxytocin [34].
The introduction of SPPS by Bruce Merrifield marked a paradigm shift in peptide synthesis [35]. By immobilizing the growing peptide chain on a solid resin, SPPS streamlined the process, eliminating the need for intermediate isolation. The synthesis process begins with the attachment of the first amino acid, corresponding to the C-terminal residue, to the solid-phase resin. Following the successful coupling of the amino acid to the resin, the resin is thoroughly washed to remove by-products and excess reagents. Subsequently, the N-terminal protecting group of the immobilized amino acid is removed, typically through deprotection reactions, and the resin is washed again to ensure the removal of residual deprotection agents. The next amino acid is then activated in the presence of a coupling reagent and conjugated to the growing peptide chain on the resin. This cycle of coupling, washing, and deprotection is repeated iteratively until the desired peptide sequence is fully assembled. Finally, all side-chain protecting groups are removed, the resin is washed to eliminate any remaining reagents, and the peptide is cleaved from the resin under appropriate cleavage conditions to yield the final product [35]. SPPS significantly shortened synthesis times compared to CSPS, with large-scale facilities achieving multi-kilogram production. SPPS has been instrumental in the synthesis of therapeutic peptides, such as insulin analogs and GLP-1 receptor agonists like liraglutide and semaglutide. Furthermore, GHK was synthesized by SPPS before LPPS came out.
SPPS also allowed for automation, reducing manual labor and enabling the production of peptides at a scale sufficient for clinical and commercial use [36]. Other innovations, including microwave-assisted SPPS, have further enhanced its efficiency by reducing reaction times and solvent usage [37]. However, the widespread use of excess coupling reagents and solvents, particularly for washing steps, presented environmental concerns. Moreover, the crude products from SPPS often required extensive downstream purification to achieve high purity, particularly for complex sequences. Recent advancements in SPPS have introduced a wash-free process that addresses the significant waste and time limitations of traditional methods [38]. This innovation eliminates the need for solvent-intensive washing steps between each cycle by employing bulk evaporation of the deprotection base under elevated temperatures and directed headspace nitrogen flushing to prevent condensation. Key features include the use of pyrrolidine as a deprotection base, which enhances evaporation efficiency and reduces reagent consumption. The wash-free methodology maintains high peptide purity and is scalable for both research and production, achieving up to a 95% reduction in waste while preserving product quality. Applications to challenging peptides and long sequences, including therapeutic peptides like liraglutide, demonstrate its robustness and potential for sustainable peptide manufacturing.
With the advent of LPPS, more sustainable and scalable methods became available, offering an alternative to SPPS for producing GHK and similar peptides with reduced environmental impact. LPPS combines the strengths of CSPS and SPPS while mitigating their limitations. In this method, the growing peptide chain is anchored to a soluble polymer or tag, such as polyethylene glycol (PEG) [39]. This soluble support enables reactions to occur in solution, preserving the homogeneous reaction kinetics of CSPS while facilitating purification through simple precipitation or filtration. LPPS has demonstrated its potential for industrial-scale production, achieving high yields with significantly reduced solvent consumption compared to SPPS. For example, PEG-tagged LPPS has been effectively employed to synthesize cyclic peptides like eptifibatide [40], utilizing precipitation-based purification to minimize waste. Further advancements in LPPS include the integration of fluorous tags [41,42,43] and simplified purification processes for small peptides through liquid–liquid extractions, while membrane-enhanced peptide synthesis has introduced nanofiltration to optimize reagent use and reduce environmental impact [44]. These innovations underscore LPPS’s capability to align peptide synthesis with sustainability goals, making it an attractive choice for large-scale applications [8].
One of the most renowned cosmetic peptides, GHK-Cu (glycyl-L-histidyl-L-lysine copper complex), exemplifies the application of LPPS in the industry [45]. The synthesis of GHK tripeptide involves a stepwise process beginning with the acetylation of lysine (H-Lys(Ac)-OH) using acetic acid p-nitrophenyl acetate in aqueous sodium hydroxide and tetrahydrofuran (THF). Protected intermediates such as butyloxycarbony (Boc)-Gly-COOSu and Boc-Gly-His(Boc)-OH are prepared via reactions involving Boc-Gly-OH, N-maloyl imine, and DCC, followed by coupling with histidine. Boc-Gly-His(Boc)-COOSu is then reacted with H-Lys (Ac)-OH in an aqueous THF solution to form Boc-Gly-His(Boc)-Lys(Ac)-OH. Finally, the deprotection of Boc groups using trifluoroacetic acid yields the GHK tripeptide with high specificity and purity, highlighting a meticulous sequence of activation, coupling, and deprotection steps.
In addition to the advancements in LPPS, novel peptide synthesis strategies have emerged to further enhance efficiency, minimize by-product formation, and optimize the construction of long and complex peptides. One such approach is the ynamide coupling strategy, which has demonstrated significant potential in both C → N and N → C peptide synthesis [46]. Traditional C → N peptide synthesis often requires precise protection and deprotection steps to maintain stereochemical integrity. However, ynamide reagents, such as MYTsA and MYMsA, enable efficient peptide bond formation under mild conditions, preserving chirality and eliminating the need for additional catalysts or additives. These reagents facilitate a highly selective two-step, one-pot reaction that integrates hydroacyloxylation and aminolysis. More importantly, ynamide chemistry also supports N → C peptide synthesis, which overcomes solubility and nucleophilicity challenges associated with zwitterionic amino acids. This strategy employs transiently protected amino acids to streamline synthesis while reducing operational complexity through a single-pot reaction. Its broad substrate compatibility, including non-natural amino acids and complex peptide active pharmaceutical ingredients, makes it a promising approach for advanced peptide manufacturing [47]. Moreover, this method is compatible with green solvents, significantly reducing solvent consumption compared to traditional SPPS and LPPS methods and thus contributing to a more sustainable and scalable peptide synthesis process.
In summary, SPPS remains the dominant method for therapeutic peptide synthesis, while LPPS offers a more sustainable and scalable alternative for industrial applications. Simultaneously, the introduction of ynamide-based strategies has further refined C → N and N → C peptide synthesis, providing a new solution for constructing complex and long peptides. Moving forward, the integration of these methods may drive the development of more efficient and environmentally friendly peptide synthesis technologies. The comparative strengths and limitations of these methods are summarized in Table 1.

Table 1.
Comparison of CSPS, SPPS, and LPPS.
3.2. Other Methods of Peptide Synthesis
Enzymatic synthesis represents a sustainable alternative, using proteolytic enzymes to catalyze peptide bond formation under moderate conditions. Immobilized enzyme systems further enhance this process by allowing enzyme recovery and reuse, which reduces production costs and minimizes waste. Enzymatic methods are particularly effective for producing short peptide chains with high specificity. Moreover, these methods avoid harsh conditions, preserving the bioactivity of sensitive peptides. Despite these advantages, enzymatic synthesis is often limited by the availability and cost of specific proteases.
The development of enzymatic synthesis in the field of peptide production represents a pivotal shift toward greener and more selective methodologies. By employing enzymes such as trypsin [48,49], proteases [49,50], and lipases [51,52], peptide bonds can be formed under mild conditions that are less harmful to sensitive functional groups. This method circumvents the need for extensive protection and deprotection steps, a common requirement in conventional chemical synthesis, thereby reducing process complexity and environmental impact.
The underlying mechanisms of enzymatic peptide synthesis can be broadly categorized into equilibrium-controlled and kinetically controlled strategies [53,54]. Equilibrium-controlled methods leverage reaction conditions like pH and solvent composition to shift the balance toward peptide bond formation, whereas kinetically controlled approaches often involve activated acyl donors to drive rapid and efficient synthesis. These methods have been further optimized through the introduction of innovative reaction media, including ionic liquids and alternative organic solvents, which enhance enzyme performance and expand the range of compatible substrates.
Enzymatic peptide synthesis is widely used in the pharmaceutical field. The chemoenzymatic synthesis of Endomorphin-1 (EM-1) combines enzymatic and chemical methods for efficient production [55]. Solvent-stable proteases, such as WQ9-2 and PT121, were used to synthesize key intermediates with high yields, minimizing the need for purification and side-chain protection. The final product, EM-1, was obtained with over 99.8% purity through deprotection and high-speed countercurrent chromatography.
In cosmetics, the synthesis of L-carnosine (β-alanine-L-histidine) [56], an antioxidant to protect skin cells from oxidative stress and environmental damage, has employed β-peptidases as whole-cell biocatalysts. Recombinant yeast and bacterial strains overexpressing β-aminopeptidases have been increasingly utilized for the efficient synthesis of β-peptides, which exhibit enhanced stability and longer half-lives compared to α-peptides. By optimizing reaction conditions, high yields of up to 71% of L-carnosine were achieved with E. coli, which also demonstrated stability over five repeated batches. In a fed-batch process, L-carnosine accumulated to 3.7 g/L, highlighting the scalability and effectiveness of this enzymatic method for peptide production.
Recombinant DNA technology enables the synthesis of peptides with precise amino acid sequences through gene cloning and overexpression in microbial systems. This approach is particularly valuable for the large-scale production of peptides that are difficult to synthesize chemically. Microorganisms such as Escherichia coli and Pichia pastoris serve as common hosts for recombinant peptide production. Recombinant techniques also allow for the incorporation of post-translational modifications, expanding the functional repertoire of peptides for cosmetic applications. Recombinant collagen offers enhanced stability, biocompatibility, and tunable properties, making it a sustainable and versatile alternative to animal-derived collagen for applications in regenerative medicine, tissue engineering, and cosmetics. Recombinant DNA technology has enabled the production of human collagen type I α-1 and its 45 kDa fragment using transgenic barley seeds as an expression platform. By employing seed-specific (GluB1), constitutive (Ubi-I), and germination-specific (α-amylase) promoters, researchers optimized expression levels and protein stability, with the GluB1 promoter achieving the highest yield of up to 45 mg/kg in dry seeds [57]. Wang’s study investigates recombinant human collagen (RHC) synthesized through recombinant DNA technology, emphasizing its superior protective effects on UVA-damaged skin fibroblasts compared to animal-derived collagens [58]. The RHC was expressed in Pichia pastoris and subjected to simulated gastrointestinal digestion to produce digestates. The study analyzed the molecular characteristics of RHC digestates, including higher Pro content and larger average molecular weight, which contributed to enhanced biological activity. Experimental results demonstrated that RHC digestates significantly improved fibroblast proliferation, migration, and extracellular matrix expression while reducing oxidative stress by increasing antioxidant enzyme activity. These findings highlight the potential of RHC as a safe, effective, and sustainable alternative for anti-skin-aging applications in both cosmetics and functional foods [58].
The use of DNA recombinant technology has also significantly advanced the production of GHK. Recombinant expression systems, including E. coli and yeast, have been employed to produce high yields of GHK, offering a sustainable alternative to traditional extraction methods [59]. The recombinant GHK demonstrated enhanced stability, bioavailability, and activity in promoting collagen synthesis and tissue regeneration. These findings highlight the potential of recombinant DNA technology to optimize peptide production for therapeutic and cosmetic applications, providing a scalable and cost-effective platform for GHK and similar peptides.
The development of efficient synthetic strategies for cyclic peptides has significantly advanced in recent years, driven by their structural rigidity and promising therapeutic applications. Modern approaches focus on overcoming challenges associated with macrocyclization and the incorporation of complex functional motifs. Techniques such as SPPS integrated with CSPS have proven particularly effective for constructing large and structurally constrained cyclic peptides [60].
Various condensation and substitution reactions, such as azide-alkyne cycloaddition [61] and S(N)Ar substitution [60,62], have been employed for peptide macrocyclization. Traditional methods like olefin ring-closing metathesis [63] are also effective but often require additional catalysts and rely on interactions with native amino acids like cysteine, which play critical roles in protein stability and function. A novel fluorine-thiol displacement reaction (FTDR) addresses these limitations by enabling the selective functionalization of fluoroacetamide side chains in unprotected peptides [64]. Using benzenedimethanethiol linkers, FTDR stabilizes α-helical structures, enhances cellular uptake up to ninefold, and improves protease resistance and cancer cell inhibition, providing a chemoselective, catalyst-free, and efficient alternative to conventional stapling methods. These advancements in macrocyclization techniques have greatly expanded the toolbox for constructing complex cyclic peptides, enabling precise control over their structural and functional properties. Recent applications of these strategies have successfully facilitated the synthesis of biologically significant cyclic peptides, demonstrating their potential in medicinal chemistry.
For instance, α-amanitin was successfully synthesized by employing an iridium-catalyzed approach to introduce the challenging 6-hydroxy-tryptathionine unit, followed by acid-mediated cyclization and oxidation [65]. Similarly, scytonemide A [66] utilized an imine-driven macrocyclization strategy, demonstrating the efficiency of simple solid-phase synthesis combined with post-assembly modifications. Another notable example is callyaerin A [67], where formylglycine was used as a precursor for spontaneous imine formation, providing a streamlined pathway for generating endiamine-containing rings. Despite these advances, significant challenges remain in enhancing the efficiency and yield of these methods, particularly for synthesizing peptides with demanding structures like thioenamides or multi-bridged macrocycles. Continued innovation in cyclic peptide synthesis is critical for expanding their therapeutic potential and enabling their transition into clinical applications. While these case studies highlight the versatility of modern synthetic methodologies, significant challenges remain, particularly in improving reaction efficiency and yields for highly constrained structures such as thioenamides or multi-bridged macrocycles. Continued innovation in cyclic peptide synthesis, including catalyst-free and selective transformation strategies, will be essential to further expand their therapeutic potential and facilitate their clinical translation.
D-peptides have gained considerable attention in both pharmaceutical and cosmetic applications due to their unique biochemical properties. Unlike L-peptides, which are susceptible to enzymatic degradation, D-peptides exhibit remarkable proteolytic resistance, allowing for prolonged biological activity. Their low immunogenicity further enhances their potential as therapeutic agents, reducing the risk of immune responses commonly associated with peptide-based drugs [68]. In cosmetics, D-peptides have been explored for their stability and bioactivity in formulations aimed at skin rejuvenation, anti-aging, and enhanced wound healing. These advantages make D-peptides highly attractive for drug development, bioactive skincare formulations, and novel peptide-based therapies.
The synthesis and screening of D-peptides, derived entirely from D-amino acids, have advanced significantly, driven by their proteolytic resistance and low immunogenicity. Chemical synthesis, particularly high-efficiency SPPS [69], remains the cornerstone for producing D-peptides due to the inability of recombinant systems to incorporate D-amino acids. Techniques like native chemical ligation (NCL) [70,71] enable the efficient assembly of large peptides, overcoming size constraints inherent to SPPS. NCL is a reaction in which peptide fragment 1, with a C-terminal thioester, is coupled to peptide fragment 2, with an N-terminal cysteine residue. The reaction proceeds as follows: under the action of a catalyst, the thiol group of peptide fragment 2 performs a nucleophilic attack on the thioester group of peptide fragment 1, resulting in a thiol–thioester exchange reaction at room temperature to form a new thioester intermediate. This intermediate subsequently undergoes an intramolecular S, N-acyl shift, yielding a new ligated peptide chain. In NCL, thiols or imidazoles can serve as catalysts to accelerate the rate-limiting step of the thiol–thioester exchange reaction [72].
For screening, mirror-image phage display (MIPD) has emerged as a pivotal technology. The process begins with the chemical synthesis of the mirror image (D-enantiomer) of a target protein, a necessary step because natural recombinant methods cannot produce D-proteins. Using techniques like native chemical ligation, synthetic D-proteins are created, mirroring the structure of their natural L-protein counterparts. These mirror-image targets are then used to screen L-peptide libraries displayed on phages. During the screening process, phages displaying L-peptides are incubated with the D-protein target. High-affinity binders are enriched through iterative rounds of binding, washing, and amplification. Because of the reciprocal nature of molecular chirality [73], the identified L-peptides that bind D-proteins can be converted into their D-enantiomers, which will specifically bind to the original L-protein target. MIPD has been successfully applied to discover D-peptides targeting key disease-related proteins such as PD-L1 [74], with several candidates demonstrating potent activity and advancing to clinical trials.
The development of D-protein VEGF-A antagonists highlights advances in chemical synthesis with potential applications beyond therapeutics, including cosmetics. Using NCL, mirror-image VEGF-A (D-VEGF-A) was synthesized and employed as a target for phage display to identify high-affinity binders from a GB1 protein scaffold. The optimized D-protein ligand, D-RFX001, demonstrated chiral specificity, binding exclusively to L-VEGF-A and effectively blocking its interaction with VEGFR1 [75]. This activity suggests its potential in anti-angiogenic applications, such as reducing vascularization and inflammation, which are key in cosmetic formulations for anti-aging and skin-calming products. Resistant to proteolysis and minimally immunogenic, D-RFX001 offers a promising alternative to traditional peptides for both medical and cosmetic innovations. Notably, these approaches leverage the reciprocal chirality of D-peptides and L-proteins, ensuring specificity and robustness in therapeutic applications.
3.3. Peptide Modifications
The natural properties of peptides, such as their specificity and potency, make them ideal candidates for therapeutic and cosmetic applications. However, their clinical and cosmetic efficacy is often limited by factors like instability, poor membrane permeability, and rapid degradation. To overcome these challenges, various peptide modifications have been developed to enhance their stability, bioactivity, and delivery [1,76]. These modifications enable peptides to better meet the demands of different applications, from skin care to targeted therapeutics.
Native GLP-1 demonstrates multifaceted metabolic benefits, including β-cell proliferation, suppression of glucagon secretion, and appetite regulation through central nervous system modulation [77]. However, its therapeutic potential is inherently limited by two physiological vulnerabilities: rapid enzymatic degradation by dipeptidyl peptidase-4 and rapid renal clearance (degraded in less than 1 min in the bloodstream), thereby necessitating continuous intravenous infusion to maintain sustained efficacy [77,78]. To overcome these limitations and enhance the clinical utility of GLP-1-based therapies, various structural modifications have been explored.
For example, liraglutide, a GLP-1 analog, incorporates an amino acid substitution at position 34 (K34 → R34) and has an elimination half-life of approximately 13 h in phase III clinical trials [79,80]. Another GLP-1 analog, semaglutide, was designed for once-weekly administration through the introduction of several key modifications. These include the addition of a fatty acid moiety, two amino acid substitutions (Aib(8) and Arg(34)), and C18 fatty diacid conjugated at Lys(26), which together enhance albumin binding and improve stability. As a result, semaglutide exhibits a significantly extended plasma half-life of 46.1 h and a mean residence time of 63.6 h in mini-pigs, compared to liraglutide’s shorter duration. These modifications ensure prolonged therapeutic action, and semaglutide is currently undergoing phase III clinical trials [81].
Peptide modifications can be categorized into backbone modifications, side-chain modifications, lipidation, polymer conjugation, and structural refinements. Backbone modifications enhance stability and membrane permeability through strategies like cyclization and the incorporation of D-amino acids. Side-chain modifications improve structural integrity by introducing non-natural amino acids or stable disulfide bond mimetics. Lipidation and polymer conjugation enhance solubility, pharmacokinetics, and biocompatibility, while structural refinements, such as truncation and helix stabilization, optimize receptor binding and resistance to degradation. These strategies collectively improve the functionality, stability, and therapeutic potential of peptides.
Molecular modifications to peptides play a crucial role in enhancing their delivery and bioactivity for skin care applications. By introducing aminocaproic acid (Ahx) at the N-terminus of KTTKS, the lipophilicity and skin permeability were significantly increased. The peptide was purified to over 97% purity using preparative HPLC and characterized by mass spectrometry [82]. Functional studies showed that Ac-WAhx-KTTKS is non-toxic at effective concentrations and enhances collagen production in fibroblasts by up to 80%, underscoring its potential as an advanced anti-aging cosmetic ingredient.
The synthesis and modification of the cosmetic peptide Eyeseryl (Ac-βAHSH-OH, B1) to enhance its copper(II) ion-binding properties is critical for skin regeneration. Using chemical synthesis, the peptide sequence was modified to create a derivative (Ac-HAEH-OH, B2) by altering the arrangement of amino acids, introducing glutamic acid, and optimizing the spacing between histidine residues [83]. These modifications were confirmed through potentiometric titration, isothermal titration calorimetry, and mass spectrometry, which demonstrated higher thermodynamic stability of the modified peptide–metal complexes. The enhanced binding affinity of the modified peptide increases its potential to support collagen synthesis and tissue repair, highlighting the importance of precise sequence design in developing advanced cosmetic peptides.
Lim’s study investigated analogs of Argireline (Arg0), a hexapeptide mimetic of Botox, by introducing esterification and functional group alterations to increase lipophilicity and reduce the formation of zwitterions [84]. These structural modifications improved skin permeation, with Arg2 and Arg3 showing significantly higher permeation through human cadaver skin compared to the parent compound. Additionally, in vitro tests on neuronal cell cultures demonstrated enhanced inhibition of glutamate release, indicating improved efficacy for wrinkle reduction. This approach highlights the potential of molecular modifications to optimize peptide delivery and functionality in cosmetic applications.
4. Targets and Mechanisms of Action
Peptides used in cosmetics exert their effects through multiple biological pathways, targeting distinct cellular and molecular processes within the skin. As summarized in Figure 2, these peptides can be broadly categorized based on their primary functions—anti-aging, whitening, repairing, moisturizing, and antimicrobial—each involving specific mechanisms such as enhancing collagen synthesis, inhibiting melanin transfer, restoring the skin barrier, or modulating immune responses.
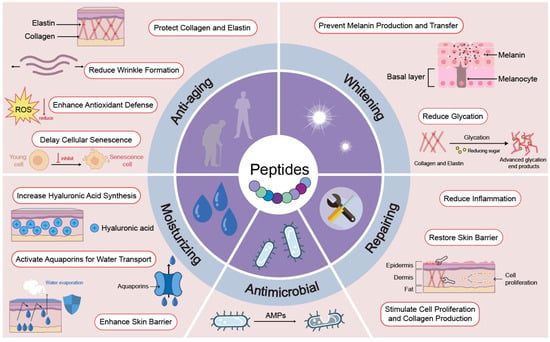
Figure 2.
Biological functions and mechanisms of action of cosmetic peptides. Peptides exert multifaceted effects on skin health through distinct mechanisms across five primary functional categories: anti-aging, whitening, repairing, moisturizing, and antimicrobial.
4.1. Anti-Aging Peptides
4.1.1. Protect Collagen and Elastin
With aging, the synthesis of collagen and elastin in the skin gradually declines, while the activity of enzymes responsible for their degradation increases. This imbalance leads to a reduction in the levels of collagen and elastin, contributing to skin laxity and the formation of wrinkles [85]. Anti-aging peptides effectively combat these processes by regulating the synthesis and degradation of collagen and elastin. Some peptides have been found to stimulate the production of these essential proteins [86].
Palmitoyl Pentapeptide-4 is a bioactive peptide widely used in anti-wrinkle skincare products. Introduced in 2000, it effectively promotes the synthesis of collagen and elastin in the dermis. Its core peptide sequence is KTTKS, a fragment derived from the propeptide of type I collagen [87].
To enhance skin penetration, palmitic acid is conjugated to the N-terminal lysine, forming Pal-KTTKS (Figure 3). This lipidation (palmitoylation) increases the hydrophobicity of the molecule, improving its stability, reducing enzymatic degradation, and enhancing membrane permeability [88].
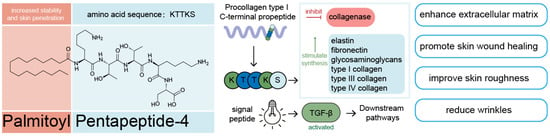
Figure 3.
Mechanism of action of Palmitoyl Pentapeptide-4 (Pal-KTTKS). Pal-KTTKS is a collagen-derived peptide with enhanced skin penetration due to its palmitoyl group. It promotes extracellular matrix synthesis, inhibits collagenase, activates TGF-β signaling, and contributes to skin repair by improving texture, reducing wrinkles, and supporting wound healing.
In 1993, researchers at the University of Tennessee identified KTTKS as a potent stimulator of collagen synthesis in human lung fibroblasts during a screening of overlapping peptides derived from the COOH-terminal propeptide of type I procollagen. Subsequent studies showed that KTTKS can stimulate the synthesis of type I, III, and IV collagens, elastin, fibronectin, and glycosaminoglycans, while also inhibiting collagenase activity, thereby enhancing the extracellular matrix and promoting wound healing [89,90].
In a 4-month double-blind clinical study involving 49 women, Pal-KTTKS significantly increased the density of elastic fibers, improved the expression of type IV collagen at the dermal–epidermal junction, and reduced skin roughness, wrinkle volume, and depth [91].
Mechanistically, KTTKS functions as a signal peptide that enhances type I collagen expression through both transcriptional and post-transcriptional regulation, partly by upregulating TGF-β, a key upstream regulator of collagen gene expression that drives tissue regeneration and skin repair [92].
Hexapeptide-9, a synthetic peptide that also mimics a portion of the collagen sequence (GPQGPQ), stimulates fibroblasts to enhance collagen production, thereby improving skin firmness and elasticity [93].
Furthermore, certain cyclic peptides, such as those that inhibit matrix metalloproteinase-2 (MMP2) activity at low concentrations, can reduce the degradation of collagen and elastin by interfering with the protein–protein interaction between proMMP2 and tissue inhibitor of metalloproteinases-2 [94].
4.1.2. Reduce Wrinkle Formation
During the aging process, the thickness and elasticity of the skin are compromised, leading to skin atrophy, sagging, and wrinkles. Excessive muscle activity can contribute to the folding and creasing of the skin. Certain peptides mimic neurotransmitter activity to alleviate facial muscle contractions, thereby reducing the appearance of dynamic wrinkles.
For example, acetyl hexapeptide-8 and pentapeptide-18 modulate the release of acetylcholine at the neuromuscular junction. These peptides can relax facial muscles, diminishing the formation of dynamic wrinkles such as crow’s feet and frown lines by inhibiting acetylcholine release. This neuro-modulatory effect provides a non-invasive alternative to botulinum toxin, offering consumers a safer and more effective approach to minimizing facial expression lines.
Acetyl hexapeptide-8 is a synthetic anti-wrinkle cosmetics ingredient. It is a peptide that is a fragment of SNAP25, a substrate of botulinum toxin (Botox). Acetyl hexapeptide-8 is marketed as Argireline® peptide by Lubrizol Corporation (Wickliffe, OH, USA). By mimicking a fragment of the SNAP25 protein, the stability of the SNARE complex is disrupted, thereby interfering with the release of neurotransmitters (Figure 4). This small peptide exhibits the great advantage of its insignificant acute toxicity as compared with BoNT. Furthermore, the hexapeptide does not exhibit primary skin irritation in an intracutaneous test nor genotoxicity as determined by the AMES test, thus making its use highly safe and physician-independent [95].
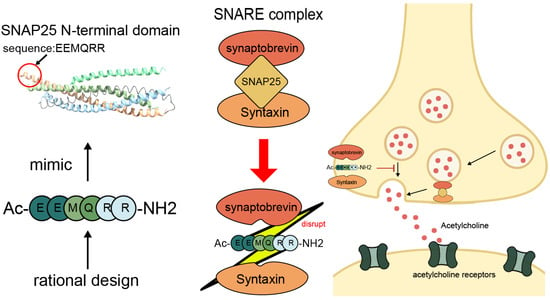
Figure 4.
Mechanism of action of a SNAP25-derived peptide inhibitor. A rationally designed peptide (Ac-EEMQRR-NH2), mimicking the N-terminal domain of SNAP25, disrupts the assembly of the SNARE complex by interfering with the interaction between synaptobrevin, SNAP25, and syntaxin. This inhibition reduces acetylcholine release at the neuromuscular junction, thereby reducing muscle contraction and softening expression lines.
Pentapeptide-18 (Leuphasyl®) is a less-studied neurotransmitter inhibitor peptide with promising properties. It decreases neuronal activity and neurotransmitter release due to a similar mechanism of action with endogenous enkephalins [96].
4.1.3. Enhance Antioxidant Defense
Reactive oxygen species (ROS) are a class of free radical species produced mainly by the mitochondrial respiratory chain and are involved in oxidative stress signaling in normal cells. However, if the accumulation of ROS exceeds the capacity of the cellular free radical scavenging system, these reactive species trigger uncontrolled reactions with non-target biomolecules (lipids, proteins, and DNA) and cells, and mediate the subsequent activation of pro-inflammatory or pro-apoptotic pathways. UV irradiation especially leads to the genesis of ROS that are, in turn, the main contributors to the aging of skin [97]. Antioxidant peptides can protect the skin from oxidative stress by neutralizing free radicals and reducing oxidative damage, thereby delaying the appearance of aging.
Glutathione (GSH), a potent antioxidant, helps maintain cellular health by eliminating reactive oxygen species and serving as a cofactor for glutathione peroxidase in reducing peroxides to water [98]. GSH plays a key role in protecting skin cells from oxidative damage. Carnosine, also a naturally occurring dipeptide, has been shown to protect the skin from UV-induced oxidative damage and photoaging. Carnosine works by scavenging reactive carbonyl species, such as acrolein and hydroxynonenal, that are produced during UV exposure, preventing the modification of key skin proteins like elastin and collagen and thus preserving skin structure and function [97]. Soybean peptides are bioactive substances derived from soybean proteins. Some special soybean peptides exhibit strong antioxidant properties, improving skin elasticity and hydration [99]. These peptides help reduce the damage caused by free radicals and contribute to maintaining a youthful appearance by enhancing skin resilience and strength.
4.1.4. Delay Cellular Senescence
Cellular senescence is a key factor in body aging. During this process, cells lose their ability to divide and function optimally. Cellular senescence occurs in response to endogenous and exogenous stresses, including telomere dysfunction, oncogene activation, and persistent DNA damage. Peptides designed to delay cellular senescence typically target these processes, improving cellular longevity and function.
Several peptides have been identified for their ability to rejuvenate cells by addressing key aging mechanisms such as mitochondrial dysfunction, DNA repair, and the activation of pro-survival pathways. For instance, Humanin and MOTSc are peptides derived from mitochondria [100]. They can promote cellular vitality by maintaining energy production and preventing oxidative damage within the mitochondria, which naturally decline with age.
4.2. Whitening Peptides
Traditional pharmacological whitening agents like hydroquinone, corticosteroids, and aminomercuric chloride have many side effects, including prickling sensation, contact dermatitis, irritation, and high toxicity and sensitivity. Whitening peptides offer a safer and milder way of avoiding the irritation and cytotoxicity commonly associated with harsher chemicals [101].
These peptides primarily act by targeting key steps in the melanogenesis pathway, including the inhibition of α-melanocyte-stimulating hormone (α-MSH) and tyrosinase (TYR) activity. UV radiation induces the binding of α-MSH to melanocortin 1 receptor (MC1R), activating the cAMP–PKA–CREB/MITF signaling pathway, which promotes the expression of TYR, thereby enhancing melanin synthesis and pigment transfer [102]. Tetrapeptide-30 and Hexapeptide-2 block the interaction between α-MSH and the MC1R on skin cells, preventing melanin synthesis and its transfer to surrounding cells. In vitro studies have demonstrated that Tetrapeptide-30 significantly suppresses the mRNA expression of inflammatory cytokines, including IL-6, IL-8, and TNF-α, in human keratinocytes exposed to UVB irradiation (Figure 5). It also markedly downregulates the expression of proopiomelanocortin, which encodes α-MSH, a key upstream regulator of melanin synthesis. Furthermore, the expression of TYR, a critical enzyme involved in melanogenesis, is also significantly reduced [103].
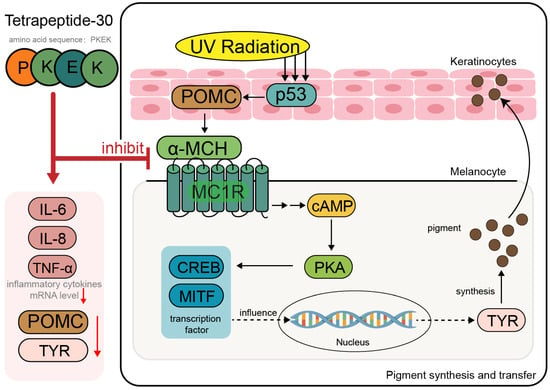
Figure 5.
Mechanism of action of Tetrapeptide-30 (PKEK) in pigmentation inhibition. Tetrapeptide-30 (PKEK) suppresses melanogenesis by downregulating inflammatory cytokines (IL-6, IL-8, TNF-α) and inhibiting the expression of POMC and TYR. It interferes with the UV-induced p53–POMC–α-MSH–MC1R signaling cascade, reducing cAMP production and downstream activation of the PKA–CREB/MITF pathway in melanocytes. This leads to decreased tyrosinase (TYR) expression and inhibition of pigment synthesis and transfer to keratinocytes. Red arrows in the figure indicate the inhibitory effects of Tetrapeptide-30 on the expression of inflammatory cytokines, POMC, and TYR.
In addition to directly inhibiting melanin production, certain whitening peptides, such as Glutathione and Carnosine, enhance skin brightening through antioxidant properties. By reducing oxidative stress and glycation [104], these peptides help to reduce pigment formation and support skin whitening [105,106].
A study conducted by Caregen R&D Center in 2021 showed that a novel peptide mixture effectively inhibited melanin production by modulating factors related to melanin formation, such as MITF (microphthalmia-associated transcription factor) and TYR. They also found that the peptide mixture reduced melanosome uptake in keratinocytes by inhibiting PAR-2 (protease-activated receptor-2) and promoted melanosome degradation by activating autophagy. In the skin model, the peptide mixture significantly reduced melanin content, showing its potential as an effective whitening agent for cosmetic applications [107].
4.3. Moisturizing Peptides
Hyaluronic acid (HA) is an important moisturizing component of the skin that helps to retain moisture and support skin elasticity. Peptides like Syn-Hycan can enhance skin hydration by promoting the synthesis of hyaluronic acid. HA not only helps maintain skin moisture but also improves skin elasticity and firmness [108].
Some peptides regulate cellular hydration by influencing water transport and retention in skin cells. A peptide of the general formula R1-(AA)n-X1-X2-X3-Pro-X4-X5-X7-(AA)P-R2 is reported to activate the synthesis of proteins of the aquaporin family [109], promoting water transport between skin cells and ensuring proper hydration across skin layers. This helps address dryness and dehydration issues.
Enhancing the skin’s barrier function is another key role of moisturizing peptides, as it helps prevent water loss. Collagen peptides have moisturizing properties because they can absorb water and form a moisturizing film on the skin’s surface, reducing water evaporation and keeping the skin hydrated.
4.4. Repair Peptides
Repair peptides promote skin repair and regeneration through various mechanisms to improve skin health. Repair peptides like Tripeptide-1 stimulate the proliferation of epidermal and fibroblast cells, which stimulates the production of collagen and elastin, accelerates skin regeneration, and improves skin elasticity and firmness [6].
Repair peptides also restore the integrity of the stratum corneum, reduce water loss, and enhance the barrier function of the skin. Tetrapeptide-7 reduces the release of inflammatory factors, alleviating skin inflammation caused by external stimuli or allergies. It can help soothe and repair the skin. With these effects, repair peptides become essential ingredients in soothing and restorative skincare products, especially for sensitive skin.
Repair peptides improve skin texture, smooth rough skin, and fade marks left by damage or inflammation, such as acne scars. Copper peptide (Cu-GHK) promotes the synthesis of collagen and elastin, thereby accelerating skin repair and regeneration and enhancing the self-repair ability of the skin. Copper peptide (GHK-Cu) regulates gene expression at the molecular level by facilitating the intracellular delivery of bioavailable copper ions. It upregulates a range of repair-associated genes, including those involved in the synthesis of collagen, elastin, integrins, and growth factors, while simultaneously activating the TGF-β signaling pathway to promote cell proliferation, angiogenesis, and nerve regeneration. Simultaneously, it downregulates pro-inflammatory cytokines (e.g., IL-6, TNF-α), blocks the activation of NFκB’s p65 and p38 MAPK (mitogen-activated protein kinase), enhances antioxidant defenses, and activates the ubiquitin–proteasome system, thereby stimulating tissue regeneration while suppressing harmful stress responses [110]. Through these actions, repair peptides not only improve overall skin health but also effectively address skin issues.
4.5. Antimicrobial Peptides
Antimicrobial peptides (AMPs) are a key part of the innate immune system in many animal species and have broad antimicrobial activity. They work by directly killing microbes, inhibiting their growth, or acting as immune modulators. In skincare, antimicrobial peptides are becoming a potential key ingredient in the cosmetics industry due to their effectiveness against skin pathogens. Since antimicrobial peptides target common skin microbes, particularly bacteria, fungi, and viruses, they have great potential in treating skin infections and preventing pathogen-related skin issues [111].
AMPs usually have a short chain of amino acid residues (10–50 aa), and most display a large number of hydrophobic and cationic residues [112]. Short-chain antimicrobial peptides, which are usually less than 20 amino acids, are ideal antimicrobial agents due to their high activity and ease of chemical synthesis. Unlike long-chain peptides, short-chain antimicrobial peptides lack disulfide bonds, making them easier to produce and stabilize on a large scale. They typically do not trigger immune responses, so they are considered safer for local application and well suited for skincare products [111].
Antimicrobial peptides can serve as preventive skincare ingredients, helping to prevent pathogen infections and skin issues. For example, Tetrapeptide-7 and Tripeptide-2 have been shown to effectively inhibit the growth of pathogens like Staphylococcus aureus and Candida albicans. In addition, insect-derived antimicrobial peptides, such as lucifensin from the green bottle fly (Lucilia sericata), have shown strong antimicrobial activity against skin pathogens and can be easily synthesized for large-scale production [113].
To enhance antimicrobial effects and reduce hemolysis, the engineering design of antimicrobial peptides has become a key research focus. By modifying the amino acid composition of antimicrobial peptides, their specificity for skin pathogens can be increased while minimizing toxicity to healthy cells.
Additionally, the use of metal–organic derivatives has been shown to enhance the efficacy of antimicrobial peptides, providing more diverse mechanisms of action and helping to delay the development of resistance [114]. Antimicrobial peptides have broad prospects in cosmetics, particularly in preventing skin pathogen infections and promoting skin health. Further advances in design and production could make them a key ingredient in skincare.
5. Discussion
Peptides have emerged as promising functional ingredients in cosmetics, offering diverse biological activities to improve skin health. Despite their potential, challenges such as stability, bioavailability, and cost-effective production remain barriers to their widespread application.
In the United States, the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) has significantly expanded the FDA’s authority over cosmetic products. Under MoCRA, manufacturers are required to ensure the safety of their products, which includes substantiating the safety of ingredients like peptides. This encompasses assessments of stability, skin penetration efficiency, and potential allergenicity. Additionally, MoCRA mandates facility registration, product listing, and adherence to good manufacturing practices, aiming to enhance consumer safety and product transparency [115]. In the European Union, Regulation (EC) No 1223/2009 governs cosmetic products, requiring a comprehensive safety assessment before market placement. This includes evaluating the physical and chemical properties, microbiological quality, and toxicological profile of ingredients such as peptides. The regulation also mandates the maintenance of a Product Information File (PIF) and prohibits animal testing, promoting alternative methods for safety evaluation [116]. Safety considerations are paramount in the development of peptide-based cosmetics. Potential allergenicity is a concern, as peptides can elicit immune responses in some individuals. Therefore, thorough allergenicity assessments are essential during product development. Skin penetration efficiency is another critical factor, as peptides must effectively reach target layers to exert their intended effects. Advanced delivery systems, such as nanocarriers and cell-penetrating peptides, are being explored to enhance transdermal delivery. Moreover, the long-term stability of peptides in formulations is crucial to maintain efficacy and safety throughout the product’s shelf life. Stability testing under various conditions is necessary to ensure product integrity over time.
The convergence of peptide research in pharmacology and cosmetics has unveiled a synergistic pathway for innovation. Pharmacological advancements in peptide target identification (e.g., receptor-specific agonism/antagonism) and structural optimization (e.g., cyclization, PEGylation) have directly inspired cosmetic applications. For instance, anti-wrinkle peptides like Palmitoyl Pentapeptide-4 mimic collagen fragments to activate dermal fibroblasts, a concept borrowed from growth factor signaling pathways in drug development. Similarly, AI-driven peptide design, initially applied in drug discovery (e.g., Paxlovid’s protease inhibition), now accelerates the creation of high-affinity cosmetic peptides.
Technological cross-pollination is equally transformative. Drug delivery systems like cell-penetrating peptides and nanocarriers, developed for systemic drug delivery, enhance the transdermal efficacy of cosmetic peptides. Moreover, high-throughput screening methods from pharmacology enable the rapid identification of bioactive peptides for skin benefits, as seen in antimicrobial peptide Z8’s application against acne [117].
China has emerged as a pivotal player in the global cosmetic peptide industry, driven by its rapidly expanding market, technological innovations, and strategic investments in research and development. The Chinese cosmetic peptide market has witnessed remarkable growth, with a CAGR of 17.22% from 2017 to 2022, reaching a market size of 16.54 billion RMB (investigated by PUHUA POLICY). This growth is fueled by increasing consumer demand for high-quality, functional skincare products, particularly those targeting anti-aging, whitening, and hydration. China’s regulatory environment has also played a crucial role in fostering innovation. The implementation of the Cosmetic Supervision and Administration Regulation in 2021 has streamlined the approval process for new peptide-based ingredients, encouraging domestic companies to invest in R&D. As of January 2025, 22 out of 226 new cosmetic ingredients in China are peptide-based, reflecting the industry’s commitment to innovation (data acquired from National Medical Products Administration). Moreover, China’s focus on green and sustainable production aligns with global trends. Companies are increasingly adopting eco-friendly synthesis methods and biodegradable delivery systems, positioning Chinese cosmetic peptides as both effective and environmentally responsible.
Future directions should focus on integrating pharmacological precision (e.g., CRISPR-based target validation) with cosmetic needs while advancing eco-friendly synthesis methods. Collaborative efforts between pharmaceutical and cosmetic industries could unlock next-generation peptides with dual functionalities—therapeutic and aesthetic—ushering in an era of “cosmeceuticals” grounded in rigorous science.
Author Contributions
Conceptualization, L.Z., H.D. and X.L.; supervision, L.Z. and H.D.; writing—original draft, T.N. and Y.T.; writing—review and editing, X.L., H.D., T.N. and Y.T.; funding acquisition, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from Procter & Gamble Co.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All articles and data referenced in this review are publicly available online.
Acknowledgments
The authors would like to thank Hongxin Gao and Lu Zhang from Procter & Gamble Co. for their valuable support during this study.
Conflicts of Interest
Lu Zhang is employed by The Procter & Gamble Company. The other authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ahx | Aminocaproic acid |
| Aib | 2-Aminoisobutyric acid |
| AMPs | Antimicrobial peptides |
| anti-TNF-α | Anti-tumor necrosis factor α |
| anti-IL-13 | Anti-interleukin-1 |
| CAGR | Compound annual growth rate |
| CSPS | Classical solution peptide synthesis |
| EM-1 | Endomorphin-1 |
| FIT | Flexible in vitro translation system |
| FTDR | Fluorine-thiol displacement reaction |
| GHK | Glycine-histidine-lysine |
| Gly | Glycine |
| GLP-1 | Glucagon-like peptide-1 |
| GSH | Glutathione |
| HA | Hyaluronic acid |
| KTTKS | Lysine-threonine- threonine-lysine-serine |
| MAPK | Mitogen-activated protein kinase |
| MC1R | Melanocortin 1 receptor |
| MIPD | Mirror-image phage display (MIPD) |
| MMP2 | Matrix metalloproteinase-2 |
| MoCRA | Modernization of Cosmetics Regulation Act |
| NCL | Native chemical ligation |
| PEG | Polyethylene glycol |
| RaPID | Random non-standard peptides integrated discovery |
| RHC | Recombinant human collagen |
| ROS | Reactive oxygen species |
| SPPS | Solid-phase peptide synthesis |
| THF | Tetrahydrofuran |
| TYR | Tyrosinase |
| LPPS | Liquid-phase peptide synthesis |
References
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide Chemistry Toolbox—Transforming Natural Peptides into Peptide Therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K. Composition Cosmetiques ou Dermopharmaceutiques Contenant le Tripeptide n-n-biotinyl-gly-his-lys pour Prevenir, Reduire ou Supprimer la Chute des Cheveux Ainsi que Pour Favoriser Leur Repousse. Patent WO2000058347A1, 5 October 2000. [Google Scholar]
- Lupo, M.P.; Cole, A.L. Cosmeceutical Peptides. Dermatol. Ther. 2007, 20, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.V.; Bhandari, P.; Shukla, P. Topical Peptides as Cosmeceuticals. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 9–18. [Google Scholar] [CrossRef]
- Fu, T.-K.; Kuo, P.-H.; Lu, Y.-C.; Lin, H.-N.; Wang, L.H.-C.; Lin, Y.-C.; Kao, Y.-C.; Lai, H.-M.; Chang, M.D.-T. Cell Penetrating Peptide as a High Safety Anti-Inflammation Ingredient for Cosmetic Applications. Biomolecules 2020, 10, 101. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Insights into Bioactive Peptides in Cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Palomo, J.M. Solid-Phase Peptide Synthesis: An Overview Focused on the Preparation of Biologically Relevant Peptides. RSC Adv. 2014, 4, 32658–32672. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; de la Torre, B.G.; Albericio, F. Liquid-Phase Peptide Synthesis (LPPS): A Third Wave for the Preparation of Peptides. Chem. Rev. 2022, 122, 13516–13546. [Google Scholar] [CrossRef]
- Chow, H.Y.; Zhang, Y.; Matheson, E.; Li, X. Ligation Technologies for the Synthesis of Cyclic Peptides. Chem. Rev. 2019, 119, 9971–10001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, C.; Chen, K.; Sun, S.; Zhang, D.; Meng, X. Screening Technology of Cyclic Peptide Library Based on Gene Encoding. Med. Drug Discov. 2022, 16, 100145. [Google Scholar] [CrossRef]
- Guzmán, F.; Aróstica, M.; Román, T.; Beltrán, D.; Gauna, A.; Albericio, F.; Cárdenas, C. Peptides, Solid-Phase Synthesis and Characterization: Tailor-Made Methodologies. Electron. J. Biotechnol. 2023, 64, 27–33. [Google Scholar] [CrossRef]
- Cosmetic Peptide Manufacturing Market Read. Available online: https://www.credenceresearch.com/report/cosmetic-peptide-manufacturing-market (accessed on 20 March 2025).
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Fourneau, E. Über Einige Derivate Des Glykocolls. Berichte Dtsch. Chem. Ges. 1901, 34, 2868–2877. [Google Scholar] [CrossRef]
- Fischer, E. Ueber Einige Derivate Des Glykocolls, Alanins Und Leucins. Berichte Dtsch. Chem. Ges. 1902, 35, 1095–1106. [Google Scholar] [CrossRef]
- Sims, E.K.; Carr, A.L.J.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 Years of Insulin: Celebrating the Past, Present and Future of Diabetes Therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef]
- Faulhaber, H.D.; Oehme, P.; Baumann, R.; Enderlein, J.; Rathsack, R.; Rostock, G.; Naumann, E. Substance P in Human Essential Hypertension. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. 12), S172–S176. [Google Scholar] [CrossRef]
- Asadi, S.; Alysandratos, K.-D.; Angelidou, A.; Miniati, A.; Sismanopoulos, N.; Vasiadi, M.; Zhang, B.; Kalogeromitros, D.; Theoharides, T.C. Substance P (SP) Induces Expression of Functional Corticotropin-Releasing Hormone Receptor-1 (CRHR-1) in Human Mast Cells. J. Investig. Dermatol. 2012, 132, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Tuppy, H. The Amino-Acid Sequence in the Phenylalanyl Chain of Insulin. 1. The Identification of Lower Peptides from Partial Hydrolysates. Biochem. J. 1951, 49, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Marglin, A.; Merrifield, R.B. The Synthesis of Bovine Insulin by the Solid Phase Method. J. Am. Chem. Soc. 1966, 88, 5051–5052. [Google Scholar] [CrossRef]
- Kung, Y.T.; Du, Y.C.; Huang, W.T.; Chen, C.C.; Ke, L.T. Total Synthesis of Crystalline Bovine Insulin. Sci. Sin. 1965, 14, 1710–1716. [Google Scholar]
- Lamberts, S.W.J.; Hofland, L.J. ANNIVERSARY REVIEW: Octreotide, 40 Years Later. Eur. J. Endocrinol. 2019, 181, R173–R183. [Google Scholar] [CrossRef]
- Quianzon, C.C.; Cheikh, I. History of Insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 18701. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.S. Human Insulin from Recombinant DNA Technology. Science 1983, 219, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Mojsov, S.; Heinrich, G.; Wilson, I.B.; Ravazzola, M.; Orci, L.; Habener, J.F. Preproglucagon Gene Expression in Pancreas and Intestine Diversifies at the Level of Post-Translational Processing. J. Biol. Chem. 1986, 261, 11880–11889. [Google Scholar] [CrossRef]
- Jaroszewicz, W.; Morcinek-Orłowska, J.; Pierzynowska, K.; Gaffke, L.; Węgrzyn, G. Phage Display and Other Peptide Display Technologies. FEMS Microbiol. Rev. 2022, 46, fuab052. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, R.; Zhang, X.; Wang, Z.L.; Xiong, F.; Zhang, S.; Li, G.; Wang, Y.; Zhang, Z.; Zhang, Q.-W.; et al. Encoding and Display Technologies for Combinatorial Libraries in Drug Discovery: The Coming of Age from Biology to Therapy. Acta Pharm. Sin. B 2024, 14, 3362–3384. [Google Scholar] [CrossRef]
- Heinis, C.; Rutherford, T.; Freund, S.; Winter, G. Phage-Encoded Combinatorial Chemical Libraries Based on Bicyclic Peptides. Nat. Chem. Biol. 2009, 5, 502–507. [Google Scholar] [CrossRef]
- Goto, Y.; Suga, H. The RaPID Platform for the Discovery of Pseudo-Natural Macrocyclic Peptides. Acc. Chem. Res. 2021, 54, 3604–3617. [Google Scholar] [CrossRef] [PubMed]
- Reid, P.C.; Goto, Y.; Katoh, T.; Suga, H. Charging of tRNAs Using Ribozymes and Selection of Cyclic Peptides Containing Thioethers. Methods Mol. Biol. 2012, 805, 335–348. [Google Scholar] [CrossRef]
- Suire, C.N.; Nainar, S.; Fazio, M.; Kreutzer, A.G.; Paymozd-Yazdi, T.; Topper, C.L.; Thompson, C.R.; Leissring, M.A. Peptidic Inhibitors of Insulin-Degrading Enzyme with Potential for Dermatological Applications Discovered via Phage Display. PLoS ONE 2018, 13, e0193101. [Google Scholar] [CrossRef]
- Katayama, K.; Armendariz-Borunda, J.; Raghow, R.; Kang, A.H.; Seyer, J.M. A Pentapeptide from Type I Procollagen Promotes Extracellular Matrix Production. J. Biol. Chem. 1993, 268, 9941–9944. [Google Scholar] [CrossRef]
- Bergmann, M.; Zervas, L. Über Ein Allgemeines Verfahren Der Peptid-Synthese. Berichte Dtsch. Chem. Ges. B Ser. 1932, 65, 1192–1201. [Google Scholar] [CrossRef]
- Vigneaud, V.D.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The Synthesis of an Octapeptide Amide with the Hormonal Activity of Oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Mäde, V.; Els-Heindl, S.; Beck-Sickinger, A.G. Automated Solid-Phase Peptide Synthesis to Obtain Therapeutic Peptides. Beilstein J. Org. Chem. 2014, 10, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, C.; Assunção, N.A.; Gerhardt, J.; Miranda, M.T.M. Microwave-Assisted Solid-Phase Peptide Synthesis at 60 Degrees C: Alternative Conditions with Low Enantiomerization. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 808–817. [Google Scholar] [CrossRef]
- Collins, J.M.; Singh, S.K.; White, T.A.; Cesta, D.J.; Simpson, C.L.; Tubb, L.J.; Houser, C.L. Total Wash Elimination for Solid Phase Peptide Synthesis. Nat. Commun. 2023, 14, 8168. [Google Scholar] [CrossRef]
- Benaglia, M.; Danelli, T.; Fabris, F.; Sperandio, D.; Pozzi, G. Poly(Ethylene Glycol)-Supported Tetrahydroxyphenyl Porphyrin: A Convenient, Recyclable Catalyst for Photooxidation Reactions. Org. Lett. 2002, 4, 4229–4232. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, X.; Ren, X.; Wang, P. Liquid Phase Synthesis Method of. Eptifibatide. Patent CN102924569A, 13 February 2013. [Google Scholar]
- Curran, D.P.; Hadida, S. Tris(2-(Perfluorohexyl)Ethyl)Tin Hydride: A New Fluorous Reagent for Use in Traditional Organic Synthesis and Liquid Phase Combinatorial Synthesis. J. Am. Chem. Soc. 1996, 118, 2531–2532. [Google Scholar] [CrossRef]
- Development of an Efficient Liquid-Phase Peptide Synthesis Protocol Using a Novel Fluorene-Derived Anchor Support Compound with Fmoc Chemistry; AJIPHASE®. Tetrahedron Lett. 2012, 53, 1936–1939. [CrossRef]
- Luo, Z.; Williams, J.; Read, R.W.; Curran, D.P. Fluorous Boc ((F)Boc) Carbamates: New Amine Protecting Groups for Use in Fluorous Synthesis. J. Org. Chem. 2001, 66, 4261–4266. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, T.; Song, W.; Liu, X.; Zhang, Z.; Yang, X.; Zhao, J. Synthesis Method for GHK. Tripeptide. Patent CN103665102A, 26 March 2014. [Google Scholar]
- Hu, L.; Xu, S.; Zhao, Z.; Yang, Y.; Peng, Z.; Yang, M.; Wang, C.; Zhao, J. Ynamides as Racemization-Free Coupling Reagents for Amide and Peptide Synthesis. J. Am. Chem. Soc. 2016, 138, 13135–13138. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, Z.; Lai, M.; Hu, L.; Zhao, J. Inverse Peptide Synthesis Using Transient Protected Amino Acids. J. Am. Chem. Soc. 2024, 146, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Aso, K.; Kodaka, H.; Fukushi, H.; Lee, H.-H. Trypsin-Catalyzed Synthesis of the Arginyl-Arginine Dipeptide Froml-Arginine Ethyl Ester. Biotechnol. Lett. 1992, 14, 451–454. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Hou, R.-Z.; Yang, Y.; Li, G.; Huang, Y.-B.; Wang, H.; Liu, Y.-J.; Xu, L.; Zhang, X.-Z. Synthesis of a Precursor Dipeptide of RGDS (Arg-Gly-Asp-Ser) Catalysed by the Industrial Protease Alcalase. Biotechnol. Appl. Biochem. 2006, 44, 73–80. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Xu, L.; Yang, X.-C.; Wu, X.-X.; Zhang, X.-Z. Lipase-Catalyzed Synthesis of Precursor Dipeptides of RGD in Aqueous Water-Miscible Organic Solvents. Prep. Biochem. Biotechnol. 2003, 33, 1–12. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Cai, Y.; Yang, S.; Wang, H.; Hou, R.-Z.; Xu, L.; Xiao-Xia, W.; Zhang, X.-Z. Synthesis of Tetrapeptide Bz-RGDS-NH2 by a Combination of Chemical and Enzymatic Methods. J. Biotechnol. 2006, 125, 311–318. [Google Scholar] [CrossRef]
- Yılmaz, F. Polymer Science; IntechOpen: London, UK, 2013; ISBN 978-953-51-0941-9. [Google Scholar]
- Yazawa, K.; Numata, K. Recent Advances in Chemoenzymatic Peptide Syntheses. Molecules 2014, 19, 13755–13774. [Google Scholar] [CrossRef]
- Sun, H.; He, B.-F.; Xu, J.; Wu, B.; Ouyang, P. Efficient Chemo-Enzymatic Synthesis of Endomorphin-1 Using Organic Solvent Stable Proteases to Green the Synthesis of the Peptide. Green Chem. 2011, 13, 1680–1685. [Google Scholar] [CrossRef]
- Heyland, J.; Antweiler, N.; Lutz, J.; Heck, T.; Geueke, B.; Kohler, H.-P.E.; Blank, L.M.; Schmid, A. Simple Enzymatic Procedure for L-Carnosine Synthesis: Whole-Cell Biocatalysis and Efficient Biocatalyst Recycling. Microb. Biotechnol. 2010, 3, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, K.; Ritala, A.; Suntio, T.; Blumer, S.; Holkeri, H.; Wahlström, E.H.; Baez, J.; Mäkinen, K.; Maria, N.A. Production of a Recombinant Full-Length Collagen Type I Alpha-1 and of a 45-kDa Collagen Type I Alpha-1 Fragment in Barley Seeds. Plant Biotechnol. J. 2009, 7, 657–672. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, Y.; Zhu, Z.; Gao, R. Recombinant Human Collagen Digestates Exhibit Higher Protective Effect on UVA-Damaged Skin Fibroblasts than Animal-Derived Collagens. J. Funct. Foods 2024, 113, 106035. [Google Scholar] [CrossRef]
- Hsiao, C.-D.; Wu, H.-H.; Malhotra, N.; Liu, Y.-C.; Wu, Y.-H.; Lin, Y.-N.; Saputra, F.; Santoso, F.; Chen, K.H.-C. Expression and Purification of Recombinant GHK Tripeptides Are Able to Protect against Acute Cardiotoxicity from Exposure to Waterborne-Copper in Zebrafish. Biomolecules 2020, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- De Leon Rodriguez, L.M.; Williams, E.T.; Brimble, M.A. Chemical Synthesis of Bioactive Naturally Derived Cyclic Peptides Containing Ene-Like Rigidifying Motifs. Chem. Eur. J. 2018, 24, 17869–17880. [Google Scholar] [CrossRef]
- Jo, H.; Meinhardt, N.; Wu, Y.; Kulkarni, S.; Hu, X.; Low, K.E.; Davies, P.L.; DeGrado, W.F.; Greenbaum, D.C. Development of α-Helical Calpain Probes by Mimicking a Natural Protein-Protein Interaction. J. Am. Chem. Soc. 2012, 134, 17704–17713. [Google Scholar] [CrossRef]
- West, C.W.; Rich, D.H. Novel Cyclic Tripeptides and Substituted Aromatic Amino Acids via Ruthenium-Activated S(N)Ar Reactions. Org. Lett. 1999, 1, 1819–1822. [Google Scholar] [CrossRef]
- Blackwell, H.E.; Grubbs, R.H. Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis. Angew. Chem. Int. Ed. Engl. 1998, 37, 3281–3284. [Google Scholar] [CrossRef]
- Islam, M.S.; Junod, S.L.; Zhang, S.; Buuh, Z.Y.; Guan, Y.; Zhao, M.; Kaneria, K.H.; Kafley, P.; Cohen, C.; Maloney, R.; et al. Unprotected Peptide Macrocyclization and Stapling via a Fluorine-Thiol Displacement Reaction. Nat. Commun. 2022, 13, 350. [Google Scholar] [CrossRef]
- Wieland, T.; Faulstich, H. Fifty Years of Amanitin. Experientia 1991, 47, 1186–1193. [Google Scholar] [CrossRef]
- Krunic, A.; Vallat, A.; Mo, S.; Lantvit, D.D.; Swanson, S.M.; Orjala, J. Scytonemides A and B, Cyclic Peptides with 20S Proteasome Inhibitory Activity from the Cultured Cyanobacterium Scytonema Hofmanii. J. Nat. Prod. 2010, 73, 1927–1932. [Google Scholar] [CrossRef]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the Marine Sponge Callyspongia Aerizusa: Cyclic Peptides with Antitubercular Activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.; Xie, Z.; Xie, C.; Zhan, C.; Hu, X.; Shen, Q.; Wei, X.; Su, B.; Wang, J.; et al. D-Peptides as Recognition Molecules and Therapeutic Agents. Chem. Rec. 2016, 16, 1772–1786. [Google Scholar] [CrossRef]
- Collins, J.M.; Porter, K.A.; Singh, S.K.; Vanier, G.S. High-Efficiency Solid Phase Peptide Synthesis (HE-SPPS). Org. Lett. 2014, 16, 940–943. [Google Scholar] [CrossRef]
- Giesler, R.J.; Erickson, P.W.; Kay, M.S. Enhancing Native Chemical Ligation for Challenging Chemical Protein Syntheses. Curr. Opin. Chem. Biol. 2020, 58, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cistrone, P.A.; Bird, M.J.; Flood, D.T.; Silvestri, A.P.; Hintzen, J.C.J.; Thompson, D.A.; Dawson, P.E. Native Chemical Ligation of Peptides and Proteins. Curr. Protoc. Chem. Biol. 2019, 11, e61. [Google Scholar] [CrossRef]
- Agouridas, V.; El Mahdi, O.; Diemer, V.; Cargoët, M.; Monbaliu, J.-C.M.; Melnyk, O. Native Chemical Ligation and Extended Methods: Mechanisms, Catalysis, Scope, and Limitations. Chem. Rev. 2019, 119, 7328–7443. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.C.; Milton, S.C.; Kent, S.B. Total Chemical Synthesis of a D-Enzyme: The Enantiomers of HIV-1 Protease Show Reciprocal Chiral Substrate Specificity. Science 1992, 256, 1445–1448. [Google Scholar] [CrossRef]
- Chang, H.-N.; Liu, B.-Y.; Qi, Y.-K.; Zhou, Y.; Chen, Y.-P.; Pan, K.-M.; Li, W.-W.; Zhou, X.-M.; Ma, W.-W.; Fu, C.-Y.; et al. Blocking of the PD-1/PD-L1 Interaction by a D-Peptide Antagonist for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2015, 54, 11760–11764. [Google Scholar] [CrossRef]
- Mandal, K.; Uppalapati, M.; Ault-Riché, D.; Kenney, J.; Lowitz, J.; Sidhu, S.S.; Kent, S.B.H. Chemical Synthesis and X-ray Structure of a Heterochiral {D-Protein Antagonist plus Vascular Endothelial Growth Factor} Protein Complex by Racemic Crystallography. Proc. Natl. Acad. Sci. USA 2012, 109, 14779–14784. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like Peptide-1 Receptor: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of Glucose-Dependent Insulinotropic Polypeptide and Truncated Glucagon-like Peptide 1 In Vitro and In Vivo by Dipeptidyl Peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Shao, S.-C.; Kuo, S.; Yang, C.-Y.; Chen, H.-Y.; Chan, Y.-Y.; Ou, H.-T. Comparative Effectiveness of Dulaglutide versus Liraglutide in Asian Type 2 Diabetes Patients: A Multi-Institutional Cohort Study and Meta-Analysis. Cardiovasc. Diabetol. 2020, 19, 172. [Google Scholar] [CrossRef]
- Drucker, D.J.; Dritselis, A.; Kirkpatrick, P. Liraglutide. Nat. Rev. Drug Discov. 2010, 9, 267–268. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Guglielmi, D.A.S.; Martinelli, A.M.; Rissi, N.C.; Cilli, E.M.; Soares, C.P.; Chiavacci, L.A. Synthesis of the Peptide Ac-Wahx-KTTKS and Evaluation of the Ability to Induce In Vitro Collagen Synthesis. Protein Pept. Lett. 2016, 23, 544–547. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Kloska, A.; Zdrowowicz, M.; Wieczorek, R.; Makowska, J. Modification of Amino-Acid Sequence of Cosmetic Peptide Eyeseryl Enhances the Affinity towards Copper(II) Ion. Polyhedron 2022, 222, 115948. [Google Scholar] [CrossRef]
- Lim, S.H.; Sun, Y.; Thiruvallur Madanagopal, T.; Rosa, V.; Kang, L. Enhanced Skin Permeation of Anti-Wrinkle Peptides via Molecular Modification. Sci. Rep. 2018, 8, 1596. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef]
- Kunjulakshmi, R.; Kumar, A.; Vinod Kumar, K.; Sengupta, A.; Kundal, K.; Sharma, S.; Pawar, A.; Krishna, P.S.; Alfatah, M.; Ray, S.; et al. AagingBase: A Comprehensive Database of Anti-Aging Peptides. Database 2024, 2024, baae016. [Google Scholar] [CrossRef]
- Jones, R.R.; Castelletto, V.; Connon, C.J.; Hamley, I.W. Collagen Stimulating Effect of Peptide Amphiphile C16–KTTKS on Human Fibroblasts. Mol. Pharm. 2013, 10, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Abu Samah, N.H.; Heard, C.M. Topically Applied KTTKS: A Review. Int. J. Cosmet. Sci. 2011, 33, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Tałałaj, U.; Uścinowicz, P.; Bruzgo, I.; Surażyński, A.; Zaręba, I.; Markowska, A. The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity. Molecules 2019, 24, 3698. [Google Scholar] [CrossRef]
- Park, H.; An, E.; Cho Lee, A.-R. Effect of Palmitoyl-Pentapeptide (Pal-KTTKS) on Wound Contractile Process in Relation with Connective Tissue Growth Factor and α-Smooth Muscle Actin Expression. Tissue Eng. Regen. Med. 2017, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Hsu, C.-C.; Chung, C.-Y.; Lin, M.-S.; Li, S.-L.; Pang, J.-H.S. The Pentapeptide KTTKS Promoting the Expressions of Type I Collagen and Transforming Growth Factor-β of Tendon Cells. J. Orthop. Res. 2007, 25, 1629–1634. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Liu, T.; Zhao, Y.; Sun, X.; Lu, B.; Zhang, J.; Liu, Z.; Zhang, J. Pioneering Ionic Liquids in Neuro-Soothing: Enhanced Transdermal Delivery of Collagen Peptides and Their Synergistic Anti-Aging Functions. Mater. Today Bio 2025, 31, 101527. [Google Scholar] [CrossRef]
- Sarkar, P.; Li, Z.; Ren, W.; Wang, S.; Shao, S.; Sun, J.; Ren, X.; Perkins, N.G.; Guo, Z.; Chang, C.-E.A.; et al. Inhibiting Matrix Metalloproteinase-2 Activation by Perturbing Protein–Protein Interactions Using a Cyclic Peptide. J. Med. Chem. 2020, 63, 6979–6990. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Clemente, J.; Jodas, G.; Gil, A.; Fernández-Ballester, G.; Ponsati, B.; Gutierrez, L.; Pérez-Payá, E.; Ferrer-Montiel, A. A Synthetic Hexapeptide (Argireline) with Antiwrinkle Activity. Int. J. Cosmet. Sci. 2002, 24, 303–310. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.-A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
- Radrezza, S.; Carini, M.; Baron, G.; Aldini, G.; Negre-Salvayre, A.; D’Amato, A. Study of Carnosine’s Effect on Nude Mice Skin to Prevent UV-A Damage. Free Radic. Biol. Med. 2021, 173, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.B.; Scarlato, G.C.G.; Cavalini, D.F.; Shiguemoto, G.E. Humanin, MOTS-c and Physical Exercise: A New Perspective. Biomed. Res. Rev. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural Skin-Whitening Compounds for the Treatment of Melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef]
- Marini, A.; Farwick, M.; Grether-Beck, S.; Brenden, H.; Felsner, I.; Jaenicke, T.; Weber, M.; Schild, J.; Maczkiewitz, U.; Köhler, T.; et al. Modulation of Skin Pigmentation by the Tetrapeptide PKEK: In Vitro and in Vivo Evidence for Skin Whitening Effects. Exp. Dermatol. 2012, 21, 140–146. [Google Scholar] [CrossRef]
- Ghodsi, R.; Kheirouri, S. Carnosine and Advanced Glycation End Products: A Systematic Review. Amino Acids 2018, 50, 1177–1186. [Google Scholar] [CrossRef]
- del Marmol, V.; Solano, F.; Sels, A.; Huez, G.; Libert, A.; Lejeune, F.; Ghanem, G. Glutathione Depletion Increases Tyrosinase Activity in Human Melanoma Cells. J. Investig. Dermatol. 1993, 101, 871–874. [Google Scholar] [CrossRef]
- Sonthalia, S.; Jha, A.K.; Lallas, A.; Jain, G.; Jakhar, D. Glutathione for Skin Lightening: A Regnant Myth or Evidence-Based Verity? Dermatol. Pract. Concept. 2018, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Kim, J.; Jeong, M.K.; Lee, Y.M.; Chung, Y.J.; Kim, E.M. Whitening Effect of Novel Peptide Mixture by Regulating Melanosome Biogenesis, Transfer and Degradation. Korean J. Physiol. Pharmacol. 2021, 25, 15–26. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of Topical Hyaluronic Acid for Skin Quality and Signs of Skin Aging: From Literature Review to Clinical Evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef] [PubMed]
- Dal Farra, C.; Domloge, N.; Botto, J.-M. Peptide for Activating Aquaporin Synthesis. Patent WO2009112645A1, 17 September 2009. [Google Scholar]
- Pickart, L.; Margolina, A. Regenerative and Protective Actions of the GHK-Cu Peptide in the Light of the New Gene Data. Int. J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Vilcinskas, A. Short Antimicrobial Peptides as Cosmetic Ingredients to Deter Dermatological Pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 8847–8855. [Google Scholar] [CrossRef]
- Kamysz, W.; Okrój, M.; Łukasiak, J. Novel Properties of Antimicrobial Peptides. Acta Biochim. Pol. 2003, 50, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pöppel, A.-K.; Vogel, H.; Wiesner, J.; Vilcinskas, A. Antimicrobial Peptides Expressed in Medicinal Maggots of the Blow Fly Lucilia Sericata Show Combinatorial Activity against Bacteria. Antimicrob. Agents Chemother. 2015, 59, 2508–2514. [Google Scholar] [CrossRef]
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337. [Google Scholar] [CrossRef]
- Commissioner, O. of the Modernization of Cosmetics Regulation Act of 2022 (MoCRA). Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra (accessed on 17 April 2025).
- UNION, PEAN. Union Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union 2009, 342, 59–209. [Google Scholar]
- Zuo, Q.; Song, X.; Yan, J.; Bao, G.; Li, Y.; Shen, J.; He, Z.; Hu, K.; Sun, W.; Wang, R. Triazination/IEDDA Cascade Modular Strategy Installing Pyridines/Pyrimidines onto Tyrosine Enables Peptide Screening and Optimization. J. Am. Chem. Soc. 2025, 147, 9576–9589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).