Physicochemical Properties and Composition of Peristomal Skin Care Products: A Narrative Review
Abstract
1. Introduction
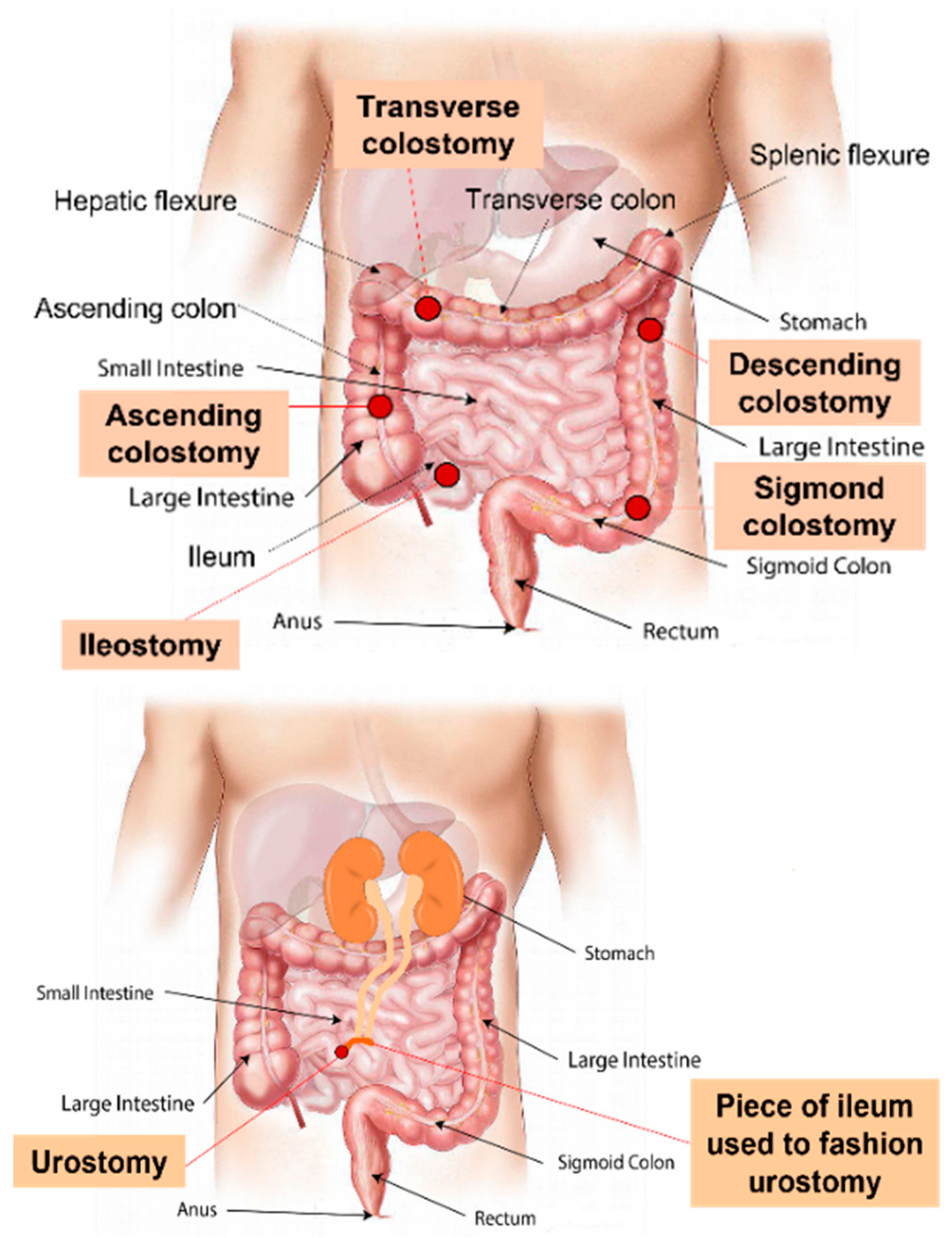
1.1. Stoma Definition, Classification, and Indications for Creation
1.2. Ostomy Appliance
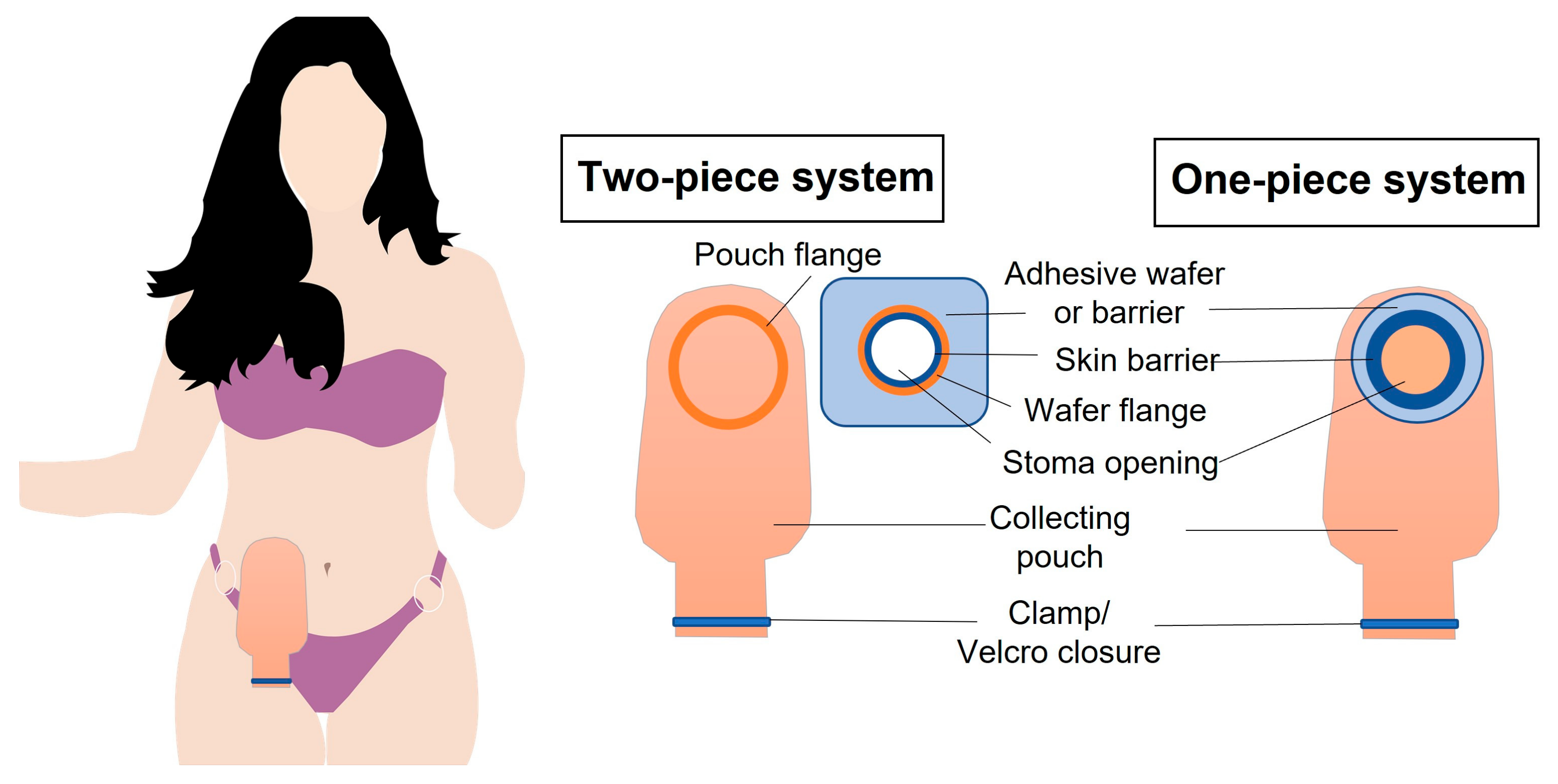
1.2.1. Ostomy Pouching Systems
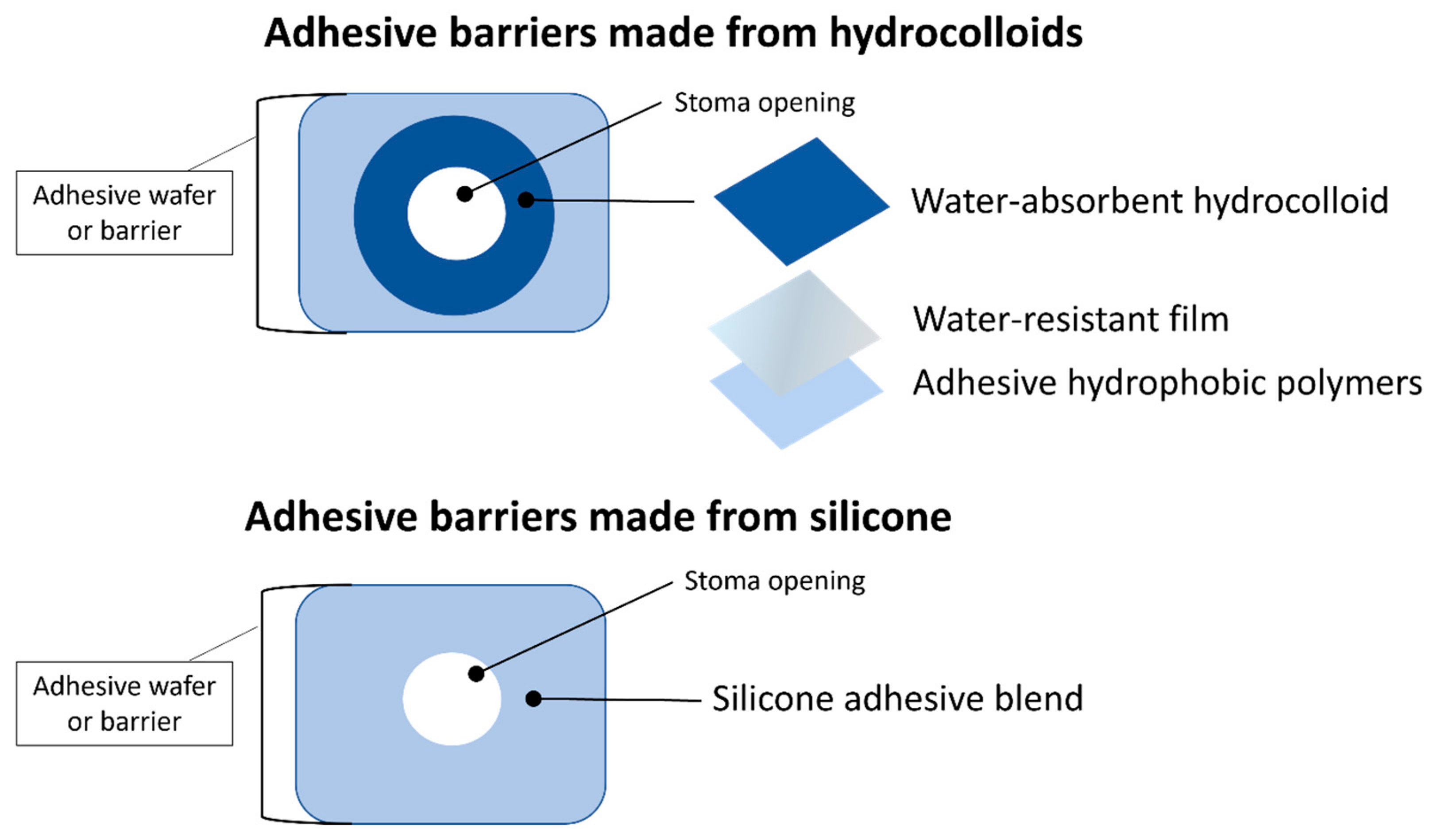
1.2.2. Appliance Adhesion and Absorption
1.3. Quality of Life of Patient with Stoma
1.3.1. Peristomal Skin Complications
1.3.2. Ostomy Appliance Issues
1.3.3. Stoma Issues
2. Materials and Methods
2.1. Aim and Objective
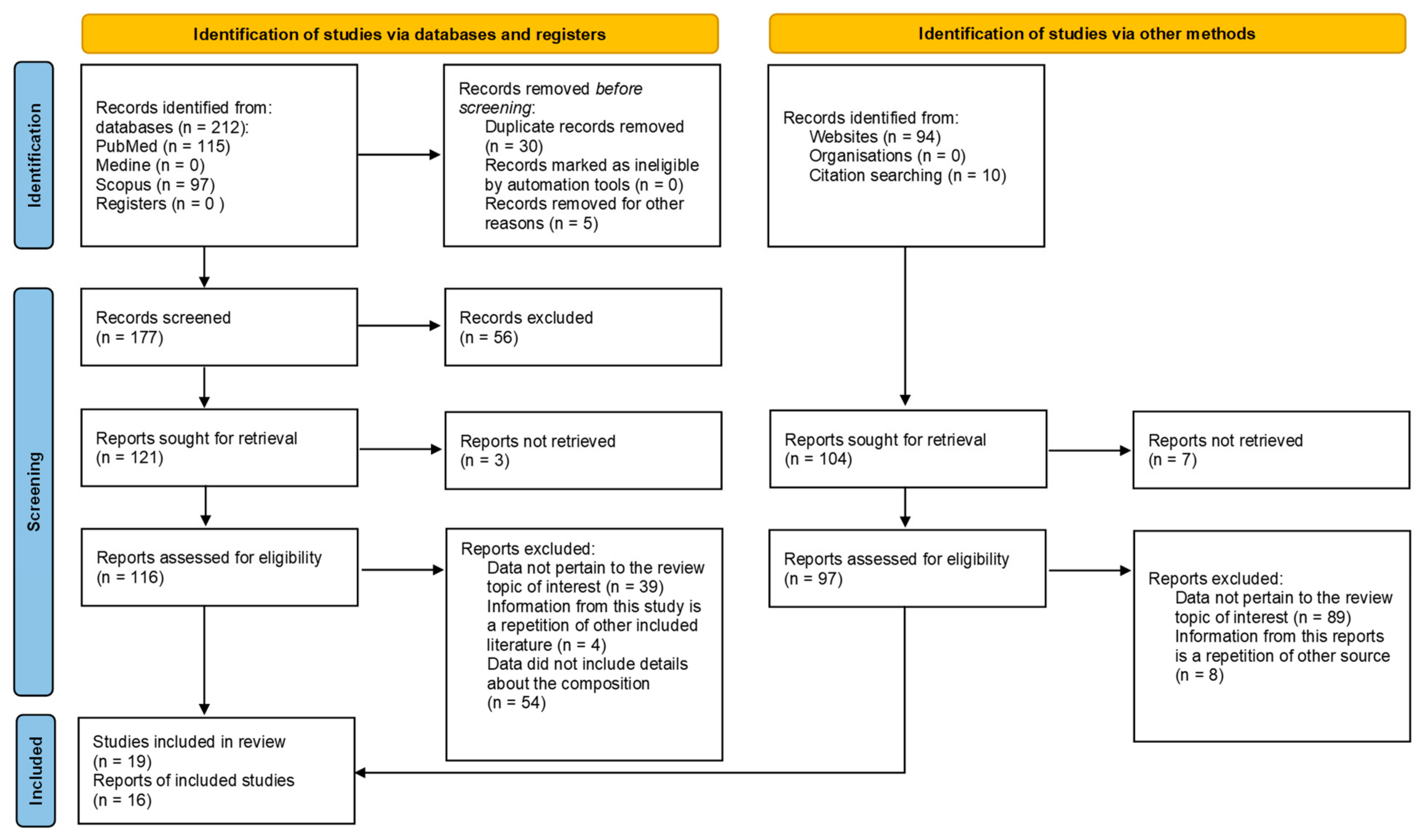
2.2. Research Strategy
2.3. Inclusion Criteria
3. Results
3.1. Ostomy Skin Care Products
3.1.1. Adhesive Remover
Alcohol- or Organic-Based Solvents
Oil-Based Solvents
Silicone-Based Removers
3.1.2. Skin Cleansing Formulations
3.1.3. Barrier Products
3.1.4. Filler Pastes
3.1.5. Soothing and Healing Formulations
3.1.6. Odour Eliminators
3.2. Avoidance and Management of Stoma Problems
4. Discussion
Study Limitation
5. Conclusions
6. Relevance to Clinical Practice
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAGR | Compound annual growth rate |
| DEA cocamide | Cocamide diethanolamine |
| DMDM Hydantoin | Dimethyl-Dimethyl hydantoin |
| MARSI | Medical adhesive-related skin injury |
| MASD | Moisture-associated skin damage |
| PIB | Polyisobutylene |
| PPG | Peristomal pyoderma gangrenosum |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| PSAs | Pressure-sensitive adhesives |
| PSCs | Peristomal skin complications |
| SIS | Styrene–isoprene–styrene |
References
- Allen, G. Understanding Stoma, Association of Surgical Technologists 2001. Available online: https://www.ast.org/pdf/206.pdf (accessed on 22 March 2023).
- Morss-Walton, P.; Yi, J.Z.; Gunning, M.; McGee, J.S. Ostomy 101 for dermatologists: Managing peristomal skin diseases. Dermatol. Ther. 2021, 34, e15069. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, S. Care of the stoma: Complications and treatments. Br. J. Community Nurs. 2018, 23, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Hill, B. Stoma care: Procedures, appliances, and nursing consideration. Br. J. Nurs. 2020, 29, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Information Brochure: Ileostomy Hints&Tips. Available online: https://www.dansac.com/en-gb/stoma-care-clinical-education/patient-education/hints-and-tips-series (accessed on 22 March 2023).
- StomaLife Foundation—Information Materials: What is a Stoma? Available online: https://stomalife.pl/o-stomii/co-to-jest-stomia/ (accessed on 22 March 2023).
- Jones, M. Fundamentals of peristomal skin care. Wound Essent. 2016, 11, 51–54. [Google Scholar]
- Burch, J. Peristomal skin care and the use of accessories to promote skin health. Br. J. Nurs. 2011, 20, S4, S6, S8 passim. [Google Scholar] [CrossRef]
- Maydick-Youngberg, D. A Descriptive Study to Explore the Effect of Peristomal Skin Complications on Quality of Life of Adults With a Permanent Ostomy. Ostomy Wound Manag. 2017, 63, 10–23. [Google Scholar]
- Montesinos Gálvez, A.C.; Jódar Sánchez, F.; Alcántara Moreno, C.; Pérez Fernández, A.J.; Benítez García, R.; Coca López, M.; Bienvenido Ramírez, M.P.; Cabrera López, M.; Vázquez Burrero, L.; Jurado Berja, P.; et al. Value-Based Healthcare in Ostomies. Int. J. Environ. Res. 2020, 17, 5879. [Google Scholar] [CrossRef]
- Burch, J.; Sica, J. Stoma care accessories: An overview of a crowded market. Br. J. Nurs. 2005, 10, 25–31. [Google Scholar] [CrossRef]
- Rudoni, C. Accessories or necessities? Br. J. Nurs. 2009, 18, 1106–1112. [Google Scholar] [CrossRef]
- Stoma Care Products. Available online: https://www.bladderandbowel.org/bowel/stoma/stoma-care-products/ (accessed on 22 March 2023).
- Burch, J. Essential care for patients with stomas. Nurs. Times 2011, 107, 12–14. [Google Scholar]
- Nazarko, L. Caring for a patient with a urostomy in a community setting. Br. J. Community Nurs. 2010, 13, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Your Guide to Ostomy Adhesives—Conplast Company Information Materials. Available online: https://www.coloplast.co.za/Documents/Stoma/Guide_to_Ostomy_Adhesives.pdf (accessed on 22 March 2023).
- Coloplast Website—Products for Stoma Patients. Available online: https://www.coloplast.co.uk/products/ (accessed on 22 March 2023).
- Convatec Website—Products for Stoma Patients. Available online: https://www.convatec.com/en-gb/products/stoma-care/ (accessed on 22 March 2023).
- Salts Healthcare Website—Products for Stoma Patients. Available online: https://www.salts.co.uk/en-gb/products (accessed on 22 March 2023).
- TrioHealthcare Website—Products for Stoma Patients. Available online: https://trioostomycare.com/product-range/ (accessed on 22 March 2023).
- Dansac Website—Products for Stoma Patients. Available online: https://www.dansac.co.uk/en-gb/products.aspx (accessed on 22 March 2023).
- Wellandmedical Website—Products for Stoma Patients. Available online: https://wellandmedical.com/aurum-profile-ostomy-range/ (accessed on 22 March 2023).
- Hollister Website—Products for Stoma Patients. Available online: https://www.hollister.com/en/products/ostomy-care-products (accessed on 22 March 2023).
- B. Braun Website—Products for Stoma Patients. Available online: https://www.bbraun.com/en/products-and-solutions/therapies/ostomy-care.html (accessed on 22 March 2023).
- Black, P. Stoma care: Finding the most appropriate appliance. Br. J. Nurs. 1995, 4, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A. Foreword: The prospects of new silicone-based biomaterial technologies in stoma care. Br. J Nurs. 2021, 30, 5–6. [Google Scholar] [CrossRef]
- Sica, J. Helping ostomates choose the right appliance for their stoma. Gastrointest. Nurs 2018, 16, 20–22. [Google Scholar] [CrossRef]
- Berry, J. Assessing the value of silicone and hydrocolloid products in stoma care. Br. J. Nur. 2007, 16, 772–778. [Google Scholar] [CrossRef]
- Fumarola, S.; Allaway, R.; Callaghan, R.; Collier, M.; Downie, F.; Geraghty, J.; Kiernan, S.; Spratt, F. Overlooked and underestimated: Medical adhesive-related skin injuries. Best practice consensus document on prevention. J. Wound Care 2020, 29, S1–S24. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Colwell, J.C.; Pittman, J.; Raizman, R.; Salvadalena, G. A randomized controlled trial determining variances in ostomy skin conditions and the economic impact (ADVOCATE trial). J. Wound Ostomy Cont. Nurs. 2018, 45, 37–42. [Google Scholar] [CrossRef]
- Szewczyk, M.T.; Majewska, G.; Cabral, M.V.; Hölzel-Piontek, K. The effects of using a moldable skin barrier on peristomal skin condition in persons with an ostomy: Results of a prospective, observational, multinational study. Ostomy Wound Manag. 2014, 60, 16–26. [Google Scholar]
- Liu, G.; Chen, Y.; Luo, J.; Liu, A.; Tang, X. The application of a moldable skin barrier in the self-Care of Elderly Ostomy Patients. Gastroenterol. Nurs. 2017, 40, 117–120. [Google Scholar] [CrossRef]
- More, S. The Practice, Nurses Guide to New Stoma Care Products. Nursing in General Practice 38–40. Available online: https://www.lenus.ie/bitstream/handle/10147/582817/art6.pdf (accessed on 22 March 2023).
- Bobowska, K. Problemy Psychologiczne, Społeczne i Medyczne Pacjentów ze Stomią. Available online: https://www.coloplast.pl/opieka-stomijna/profesjonalnie-o-stomii/dzielmy-sie-doswiadczeniem/ (accessed on 22 March 2023).
- Petersén, C.; Carlsson, E. Life with a stoma—coping with daily life: Experiences from focus group interviews. J. Clin. Nurs. 2021, 30, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Jayarajah, U.; Samarasekera, D.N. Psychological adaptation to alteration of body image among stoma patients: A descriptive study. Indian J. Psychol. Med. 2017, 39, 63–68. [Google Scholar] [CrossRef]
- Intimate Life—Ostomy Information Brochure. SaltsHealthcare Company. Available online: https://stomalife.pl/zycie-intymne-ze-stomia (accessed on 22 March 2023).
- Rolls, N.; Yssing, C.; Bøgelund, M.; Håkan-Bloch, J.; de Fries Jensen, L. Utilities associated with stoma-related complications: Peristomal skin complications and leakages. J. Med. Econ. 2022, 25, 1005–1014. [Google Scholar] [CrossRef]
- Black, P.K. Hiden problems of stoma care. Br. J. Nurs. 1994, 3, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.; Wilson, K.; Bertinara, A.; Amos, L. Educating patients in stoma care. Gastrointest. Nurs 2019, 28, S4–S5. [Google Scholar] [CrossRef]
- Zalewska, E. Skóra a Stomia. Sposoby Postępowania ze Zmianami Skórnymi. Available online: https://stomalife.pl/magazyn-po-prostu-zyj/zycie-ze-stomia/skora-a-stomia-sposoby-postepowania-ze-zmianami-skornymi/ (accessed on 22 March 2023).
- Bazaliński, D.; Sałacińska, I.; Więch, P.; Kózka, M. Life satisfaction and self-efficacy in patients with stoma. Prog. Health Sci. 2014, 4, 22–30. [Google Scholar]
- Villa, G.; Mandarina, M.; Della Giovanna, G.; Marzo, E.; Manara, D.F.; Vellone, E. A literature review about self-care on ostomy patients and their caregivers. J. Urol. Nurs. 2019, 13, 75–80. [Google Scholar] [CrossRef]
- Rosenberg, A.; McGee, M. Patient education for stoma patients. Semin. Colon Rectal Surg. 2023, 34, 100952. [Google Scholar] [CrossRef]
- Kan, H.K.; Choudhary, M. Stoma Self-care: Knowledge and Practices among Ostomates with Intestinal Stoma. South Asian J. Cancer 2024, 1–6. [Google Scholar] [CrossRef]
- Altuntas, Y.E.; Kement, M.; Gezen, C.; Eker, H.H.; Aydin, H.; Sahin, F.; Okkabaz, N.; Oncel, M. The role of group education on quality of life in patients with a stoma. Eur. J. Cancer Care 2012, 21, 776–781. [Google Scholar] [CrossRef]
- Bird, A.; Wilson, K.; Bertinara, A. Educating patients in stoma care. Gastrointest. Nurs. 2019, 17, 18–22. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.J.; Oh, D.N.; Kim, J. Comparison of standardized peristomal skin care and crusting technique in prevention of peristomal skin problems in ostomy patients. J. Korean Acad. Nurs. 2011, 41, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, A.K.; Burcharth, J.; Rosenberg, J. Patient education has a positive effect on patients with a stoma: A systematic review. Color. Dis. 2013, 15, e276–e283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Zhu, L. The Impact of Stoma Management Education on the Self-care Abilities of Individuals with an Intestinal Stoma. Gastrointest. Nurs. 2023, 21, 14. [Google Scholar] [CrossRef]
- Zelga, P.; Kluska, P.; Zelga, M.; Piasecka-Zelga, J.; Dziki, A. Patient-Related Factors Associated With Stoma and Peristomal Complications Following Fecal Ostomy Surgery: A Scoping Review. J. Wound Ostomy Cont. Nurs. 2021, 48, 415–430. [Google Scholar] [CrossRef]
- Stelton, S. Stoma and Peristomal Skin Care: A Clinical Review. Am. J. Nurs. 2019, 119, 38–45. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Pappalardo, C.; Scardigno, A.; Maida, A.; Ricciardi, R.; Calabrň, G.E. Peristomal Skin Complications in Ileostomy and Colostomy Patients: What We Need to Know from a Public Health Perspective. Int. J. Environ. Res. Public Health 2023, 20, 79. [Google Scholar] [CrossRef]
- Black, P. Peristomal skin care: An overview of available products. Br. J. Nurs. 2007, 16, 1049–1056. [Google Scholar] [CrossRef][Green Version]
- Voegeli, D.; Karlsmark, T.; Eddes, E.H.; Hansen, H.D.; Zeeberg, R.; Håkan-Bloch, J.; Hedegaard, C.J. Factors influencing the incidence of peristomal skin complications: Evidence from a multinational survey on living with a stoma. Gastrointest. Nurs. 2020, 18, S31–S38. [Google Scholar] [CrossRef]
- Maglio, A.; Malvone, A.P.; Scaduto, V.; Brambilla, D.; Denti, F.C. The frequency of early stomal, peristomal and skin complications. Br. J. Nurs. 2021, 30, 1272–1276. [Google Scholar] [CrossRef]
- Burch, J.; Marsden, J.; Boyles, A.; Martin, N.; Voegeli, D.; McDermott, B.; Maltby, E. Keep it simple: Peristomal skin health, quality of life and wellbeing. Best practice consensus document on skin health. Br. J. Nurs. 2021, 30 (Suppl. 6), 5–24. [Google Scholar] [CrossRef] [PubMed]
- Taneja, C.; Netsch, D.; Rolstad, B.S.; Inglese, G.; Lamerato, L.; Oster, G. Clinical and economic burden of peristomal skin complications in patients with recent ostomies. J. Wound Ostomy Cont. Nurs. 2017, 44, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Alvey, B.; Beck, D.E. Peristomal dermatology. Clin. Colon Rectal Surg. 2008, 21, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo de Faria, M.; Ferreira, M.B.G.; Marques dos Santos, F.M.; Vieira Bessa, R.M.; Barbosa, M.H. Prevention of medical adhesive-related skin injury during patient care: A scoping review. Int. J. Adv. Nurs. Stud. 2022, 4, 100078. [Google Scholar] [CrossRef]
- Peristomal Skin Care—Information Brochure, SaltsHealthcare Company. Available online: https://stomalife.pl/o-stomii/poradniki-dla-stomikow/ (accessed on 22 March 2023).
- Peristomal Skin Complications: Clinical Resource Guide, Wound, Ostomy, and Continence Nurses Society 2015. Available online: https://cdn.ymaws.com/member.wocn.org/resource/resmgr/document_library/Peristomal_Skin_Complication.pdf (accessed on 22 March 2023). [CrossRef]
- Afifi, L.; Sanchez, I.M.; Wallace, M.M.; Braswell, S.F.; Ortega-Loayza, A.G.; Shinkai, K. Diagnosis and management of peristomal pyoderma gangrenosum: A systematic review. J. Am. Acad. Dermatol. 2018, 78, 1195–1204.e1. [Google Scholar] [CrossRef]
- Living with an Ostomy—Common Issues, Convatec Company Information Materials. Available online: https://www.convatec.com/ostomy-care/living-with-an-ostomy/common-issues/ (accessed on 22 March 2023).
- Stoma Problems, ColostomyUK Fundation—Information Materials. Available online: https://www.colostomyuk.org/information/stoma-problems/pancaking/ (accessed on 22 March 2023).
- Nortoft, E.; Mthombeni, F.; Hakan-Bloch, J. Pmd50 comparing the ability of two innovative ostomy bags to prevent ballooning in a real-life setting. Value Health 2019, 22 (Suppl. 3), S678. [Google Scholar] [CrossRef]
- Problems You May Experience with a Stoma, SaltsHealthcare Company Information Materials. Available online: https://www.salts.co.uk/en-gb/your-stoma/living-with-a-stoma/problems-you-may-experience (accessed on 22 March 2023).
- Koc, U.; Karaman, K.; Gomceli, I.; Dalgic, T.; Ozer, I.; Ulas, M.; Ercan, M.; Bostanci, E.; Akoglu, M. A Retrospective Analysis of Factors Affecting Early Stoma Complications. Ostomy Wound Manag. 2017, 63, 28–32. [Google Scholar]
- Krishnamurty, D.M.; Blatnik, J.; Mutch, M. Stoma Complications. Clin. Colon Rectal Surg. 2017, 30, 193–200. [Google Scholar] [CrossRef]
- Díaz, C.; Hernández, S.M.; Muñoz, B.M.; Sánchez Crisol, I.; Pérez Marfil, M.N.; Montoya-Juárez, R.; Montoro, C.H. Stoma care nurses’ perspectives on the relative significance of factors influencing ostomates’ quality of life. Gastrointest. Nurs. 2018, 16, 28–33. [Google Scholar] [CrossRef]
- Burch, J. Preoperative care of patients undergoing stoma formation: What the nurse needs to know. Nurs. Stand. 2017, 31, 40–43. [Google Scholar] [CrossRef]
- Regulation (EU) 2023/607 of the European Parliament and of the Council of 15 March 2023 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards the transitional provisions for certain medical devices and in vitro diagnostic medical devices (Text with EEA relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023R0607&qid=1744018054269 (accessed on 7 April 2025).
- Sica, J.; Burch, J. 12 Appliances. In Stoma Care, 2nd ed.; Burch, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 183–199. [Google Scholar]
- Cressey, B.D.; Belum, V.R.; Scheinman, P.; Silvestri, D.; McEntee, N.; Livingston, V.; Lacouture, M.E.; Zippin, J.H. Stoma care products represent a common and previously underreported source of peristomal contact dermatitis. Contact Dermat. 2017, 76, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Dupont Company, Silicone—Information Brochure. Available online: https://www.dupont.com/products/Liveo-silicone-adhesives.html (accessed on 22 March 2023).
- Swift, T.; Westgate, G.; Van Onselen, J.; Lee, S. Developments in silicone technology for use in stoma care. Br J Nurs. 2020, 29, S6–S15. [Google Scholar] [CrossRef] [PubMed]
- Stoma Care Accessory Items for Adults Preferred Prescribing List (PPL), Conventry&Warwickhire Area Prescribing Committee, Clinical Guideline—CG02. Available online: https://www.covwarkformulary.nhs.uk/docs/chapter01/CG025-Stoma%20Accessories%20PPL.pdf (accessed on 22 March 2023).
- Marseille Soap Characteristics—Website Manufacture. Available online: https://ufranciszka.pl/marseille-soap-with-palm-oil-marseille-soaps-natural-soaps (accessed on 22 March 2023).
- Cleanser, Coloplast Information Materials. Available online: https://produkty.coloplast.pl/coloplast/leczenie-ran/comfeel-zmywacz/ (accessed on 22 March 2023).
- Skin Lotion, Dansac Information Materials. Available online: https://www.dansac.co.uk/en-gb/products/ostomy-care-products/accessories/skin-lotion/dansac-skin-lotion (accessed on 22 March 2023).
- Guide to Ostomy Skin Cleansers. Available online: https://www.veganostomy.ca/guide-to-ostomy-skin-cleansers/ (accessed on 22 February 2023).
- Fore, J. A review of skin and effects of aging on skin structure and function. Ostomy Wound Manage. 2006, 52, 24–35. [Google Scholar] [PubMed]
- Cutting, K. Silicone as skin adhesives. J. Community Nurs. 2006, 20, 36–37. [Google Scholar]
- Kulawik-Pióro, A.; Drabczyk, A.K.; Kruk, J.; Wróblewska, M.; Winnicka, K.; Tchórzewska, J. Thiolated Silicone Oils as New Components of Protective Creams in the Prevention of Skin Diseases. Materials 2021, 14, 4723. [Google Scholar] [CrossRef]
- Description of Products Available on Market to Patients with Stoma. Available online: https://dlapacjenta.pl/kosmetyki-stomijne.html (accessed on 22 March 2023).
- Cronin, E. Silicone-based stoma accessories in clinical practice. Br. J. Nurs. 2016, 25 (Suppl. 5), S28–S34. [Google Scholar] [CrossRef]
- White, M. Using silicone technology to maintain healthy skin in stoma care. Br. J. Nurs. 2014, 23, 1190–1193. [Google Scholar] [CrossRef][Green Version]
- Mojsiewicz-Pieńkowska, K.; Łukasiak, J. Polidimetylosiloksany jako substancja czynna preparatów farmaceutycznych. Farm. Pol. 2005, 61, 1041–1044. [Google Scholar]
- O’Flynn, S.K. Protecting peristomal skin: A guide to conditions and treatments. Gastrointest. Nurs. 2016, 7, 14–19. [Google Scholar]
- O’Flynn, S.K. Peristomal skin damage: Assessment prevention and treatment. Br. J. Nurs. 2019, 28, 6–12. [Google Scholar] [CrossRef]
- Ostomy Care And Accessories Market Size, Share & Trends Analysis Report By Product (Bags, Accessories), By Application (Colostomy, Ileostomy, Urostomy), By End-use (Home Care Settings, Hospitals), By Region, And Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/stoma-care-ostomy-care-accessories-market (accessed on 22 March 2023).
- Study of the Needs of Ostomates in the Area of Stoma Equipment Supply. Report from a Nationwide Study Prepared on Behalf of the STOMALife Foundation and the POL-ILKO Association. Available online: https://stomalife.pl/wp-content/uploads/2022/08/Raport_Badania_STOMALife_2022_ver.FINAL_.pdf (accessed on 9 October 2024).
- Basil, N. Stoma care: The market in products lets patients down. BMJ 2013, 347, f6129. [Google Scholar] [CrossRef] [PubMed]
- Swift, T. Peristomal skin complications: New materials needed to ease the ostomy care market. Br. J. Dermatol. 2023, 188, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.H. An overview of stoma care accessory products. Gastrointest. Nurs. 2017, 15, 25–34. [Google Scholar] [CrossRef]
- Aqil, M.; Chaudhuri, A.; Qadir, A. Herbal cosmeceuticals: New opportunities in cosmetology. Trends Phytochem. Res. 2020, 4, 117–142. [Google Scholar]
- Boyles, A. Stoma and peristomal skin complications: Predisposing factors and management. Gastrointest. Nurs. 2010, 8, 26–36. [Google Scholar] [CrossRef]
- van der Storm, S.L.; Hensen, N.; Schijven, M.P. Patient satisfaction with stoma care and their expectations on mobile apps for supportive care. Color. Dis. 2023, 25, 1852–1862. [Google Scholar] [CrossRef]
| Issue | Appliance and Accessory Solutions and Treatments | Other Solutions | Sources |
|---|---|---|---|
| Peristomal skin issues | |||
| Candida | Apply antifungal powder to the affected area, apply skin barrier film over the antifungal, and let it dry completely before attaching the pouching system | Avoid excess moisture on the peristomal skin by thoroughly drying the pouching system Use antifungal drugs such as miconazole, clotrimazole, and allylamines If compromised, consider a dermatology review | [2,3,48,63,90,91] |
| Contact dermatitis | Exercise care when using accessory products like adhesives, tapes, adhesive removers, and skin cleansers, as well as antiperspirants or deodorants, as they may act as potential allergens Use barrier film, protective powders, and seals | Review the appliance and resize the aperture; check the patient’s technique Choose a non-adhesive pouching system until the dermatitis has healed | [2,3,22,48,63,90,91] |
| Mucocutaneous separation | Apply protective powder, paste, and/or seal for superficial cases | Prevent leakage to reduce the risk of infection Use alginate dressing for deeper wounds | [3,48,90,91] |
| Folliculitis | Apply adhesive remover to avoid pulling hair from follicles, and use a skin sealant to protect the skin Use antibacterial soap when changing the pouching system and cleansing the skin | Shave regularly, avoid straight-edge razors, clean and completely dry the skin prior to using a new pouching system Discuss your problem with your primary healthcare provider regarding a prescription for a topical antibiotic powder | [48,63,90,91] |
| Pyoderma gangrenosum | None | Steroid treatment and/or dermatological review | [2,3,48,63,90,91] |
| Skin creases | Fill with filler paste, strip paste, or a seal | Check technique/correct appliance | [3,48,63,90] |
| Skin stripping | Use adhesive remover to prevent irritation of peristomal skin Apply barrier films or powder to the lesion during each pouching system change | Teach the patient proper techniques for applying/removing the pouching system, tapes, and any adhesive products Consider using the “crusting” technique for severe skin stripping | [3,22,48,63,90,91] |
| Sore skin | Use protective powder and then apply barrier film or cream | Adjust the aperture size and evaluate the patient’s technique | [2,3,22,90,91] |
| Wet skin | To dry the area, apply protective powder and then use barrier film | Use a cool hairdryer at a distance | [2,3,48,90,91] |
| Ostomy appliance issues | |||
| Allergies | Use an alternative adhesive remover, skin barrier, or appliance with a different flange material | Consider reducing accessory use | [48] |
| Pouch leakage | Choose a proper fitting pouching system and use accessories (pastes or seals) to fill in areas of the skin where leakage could occur | Empty the pouch before it is half-full Gas should be released before the pouch fills with air | [48] |
| Ballooning | Evaluate the appliance for potential improvements, such as upgrading the filter, adding a vent, or opting for a drainable pouch | Employ dietary modifications | [48] |
| Pancaking | Use stickers for coating the appliance filter | Enhance fluid intake, lubrication, or tissues within the appliance | [3,48] |
| Odour | Deodorant | Employ dietary changes and more frequent appliance changes | [40,48] |
| Watery output | Place thickening sachets in the appliance | Use thickening medications, rehydration solutions, and low-fibre diets, and increase intake of starches and carbohydrates | [40,48] |
| Stoma issues | |||
| Bleeding | Better cut the appliance aperture | Use direct pressure, cauterisation, sutures, and silver nitrate | [48] |
| Granulomas | Better cut the appliance aperture; ensure proper fit of the pouching system Use stoma barrier paste at the mucocutaneous junction | Refrain from wearing tight-fitting clothing or belts over the stoma area Cauterise with silver nitrate | [3,48,63] |
| Necrosis | Use a transparent and/or two-piece appliance to allow observation | Refashion the stoma | [48] |
| Parastomal hernia | Level the skin, use flexible convex appliances and hernia supports such as belts and garments, and review the appliance | Perform light abdominal exercises, avoid physical strain, and have a balanced diet | [3,48] |
| Prolapse | Use stoma caps and shields, supportive garments and belts, barrier film, and alternative appliances designed to adjust to the stoma’s changing size | Wear clothes that do not catch or drag the stoma | [3,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulawik-Pióro, A.; Miastkowska, M.; Bialik-Wąs, K.; Zelga, P.; Piotrowska, A. Physicochemical Properties and Composition of Peristomal Skin Care Products: A Narrative Review. Cosmetics 2025, 12, 74. https://doi.org/10.3390/cosmetics12020074
Kulawik-Pióro A, Miastkowska M, Bialik-Wąs K, Zelga P, Piotrowska A. Physicochemical Properties and Composition of Peristomal Skin Care Products: A Narrative Review. Cosmetics. 2025; 12(2):74. https://doi.org/10.3390/cosmetics12020074
Chicago/Turabian StyleKulawik-Pióro, Agnieszka, Małgorzata Miastkowska, Katarzyna Bialik-Wąs, Piotr Zelga, and Anna Piotrowska. 2025. "Physicochemical Properties and Composition of Peristomal Skin Care Products: A Narrative Review" Cosmetics 12, no. 2: 74. https://doi.org/10.3390/cosmetics12020074
APA StyleKulawik-Pióro, A., Miastkowska, M., Bialik-Wąs, K., Zelga, P., & Piotrowska, A. (2025). Physicochemical Properties and Composition of Peristomal Skin Care Products: A Narrative Review. Cosmetics, 12(2), 74. https://doi.org/10.3390/cosmetics12020074










