Unveiling the Biological Properties of Rosa rubiginosa L. Leaf Extract as a Bio-Functional Ingredient Based on 2D Cell-Based Models and In Vitro Assessments
Abstract
1. Introduction
2. Materials and Methods
2.1. Rosa rubiginosa L. Leaf Extract Preparation
2.2. Antioxidant Assays
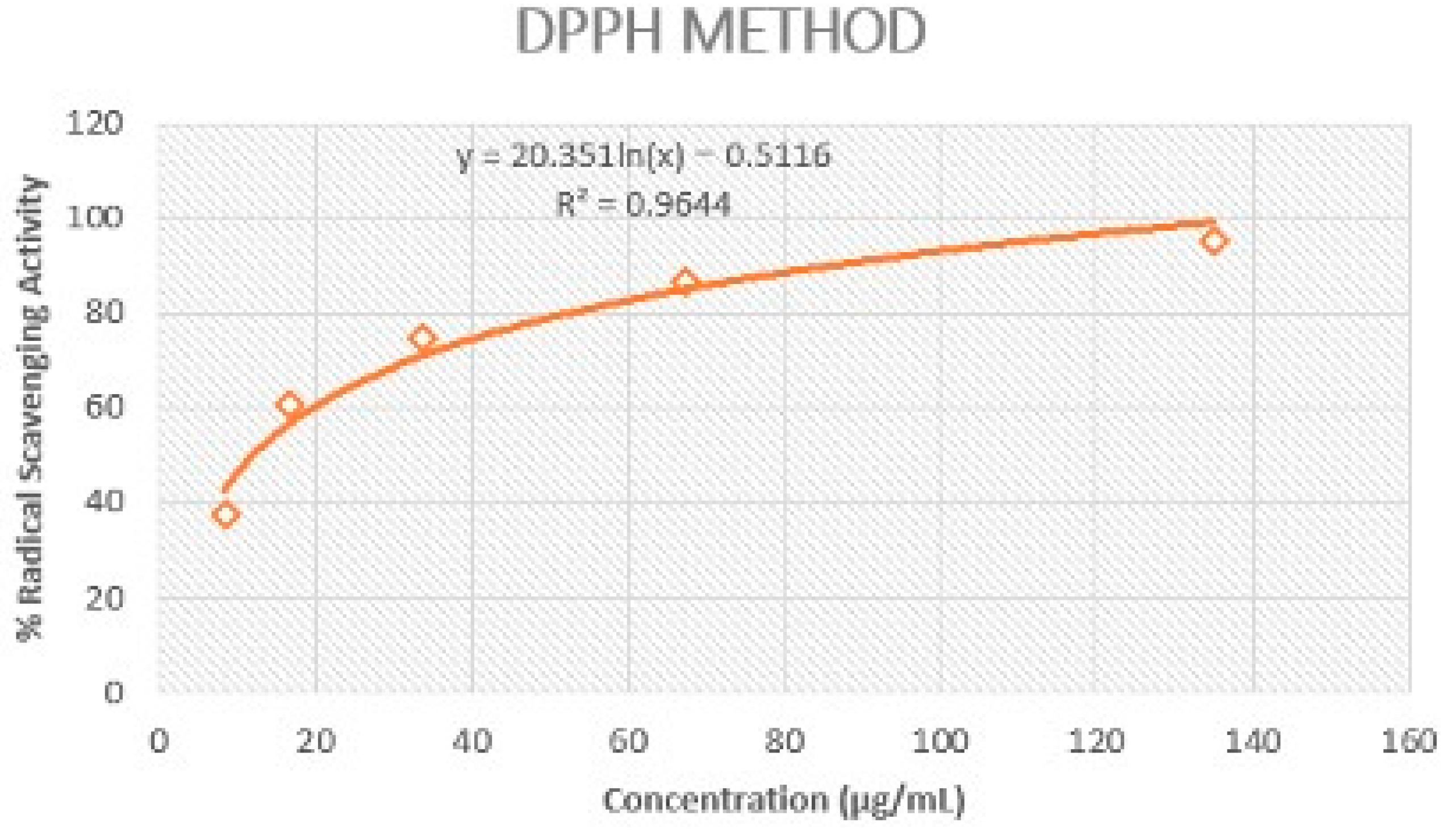
2.2.1. DPPH Method
2.2.2. Folin–Ciocalteu Method
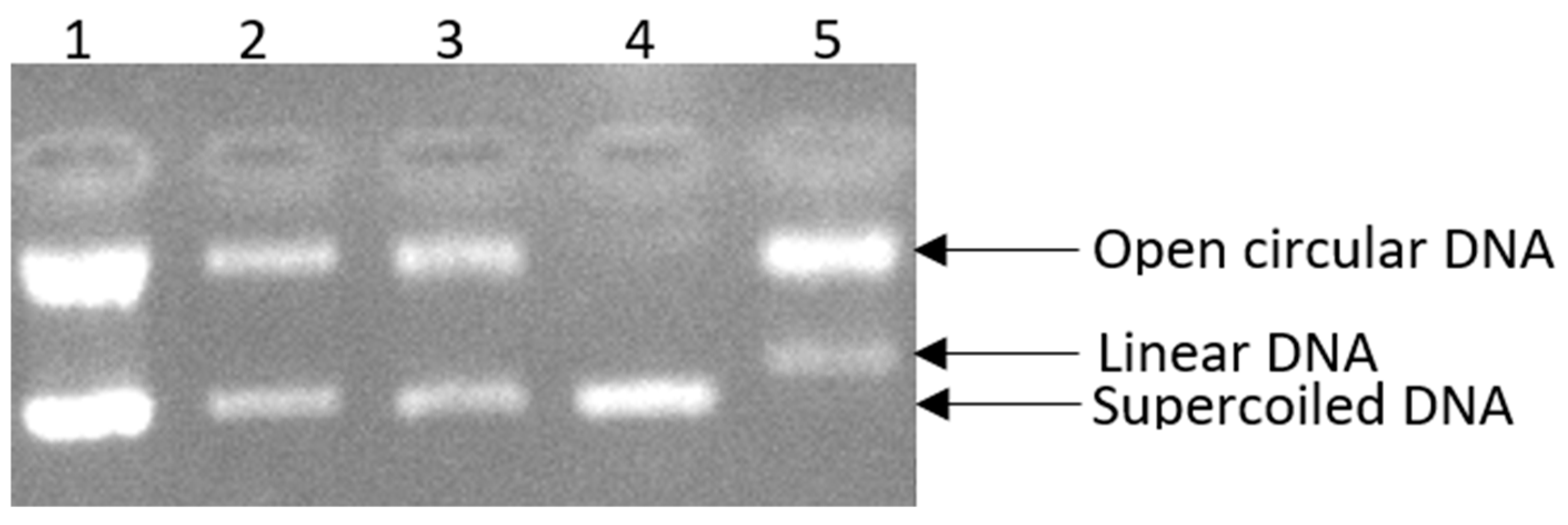
2.2.3. Prevention of Supercoiled Plasmid DNA Strand Breakage
2.3. Cell-Based Assessments
2.3.1. 2D Human Cell Cultures
2.3.2. UVA Irradiation Treatment
2.3.3. Cell Viability Assessments
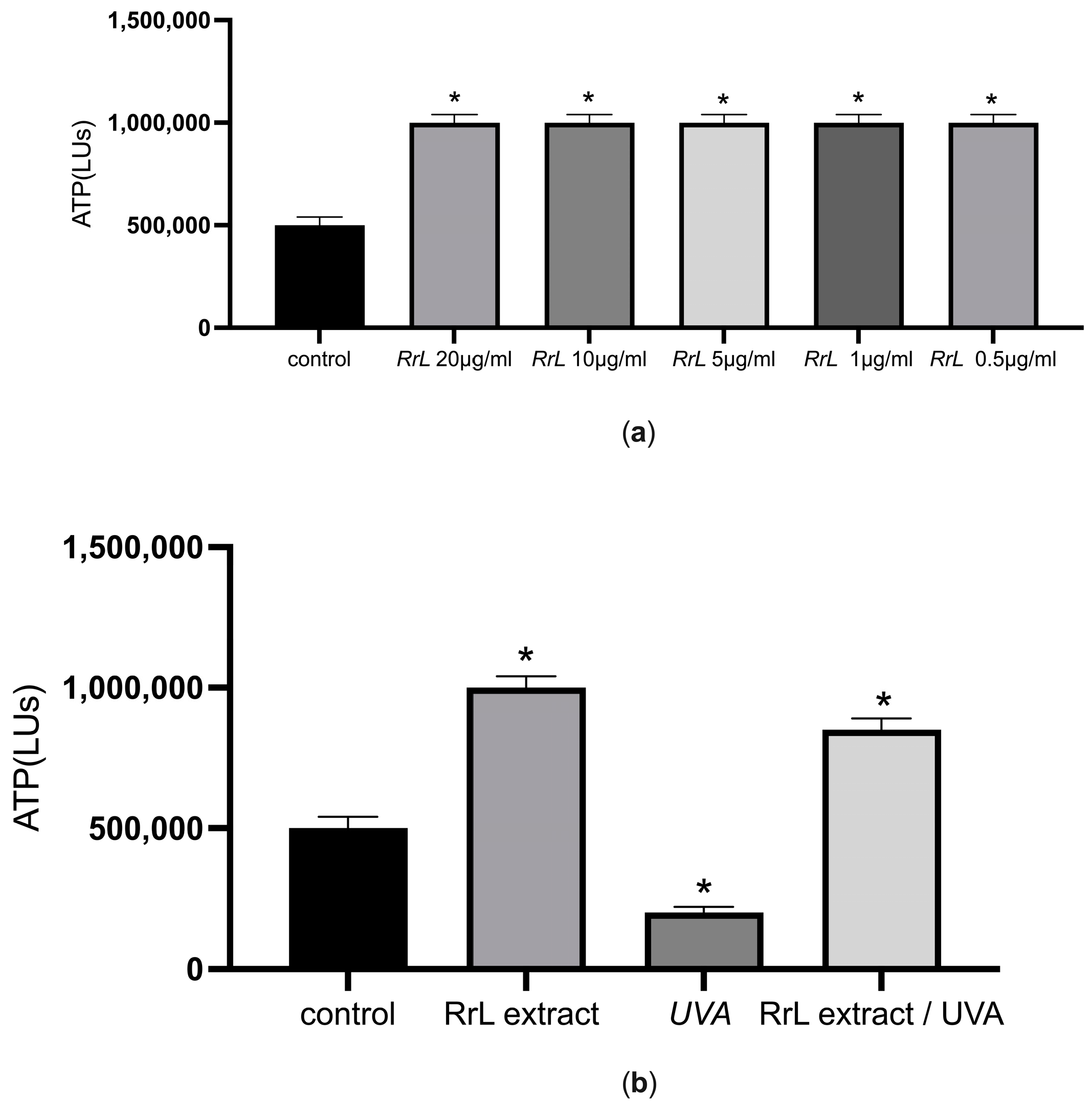
ATP Determination
2.4. RNA Isolation and cDNA Synthesis
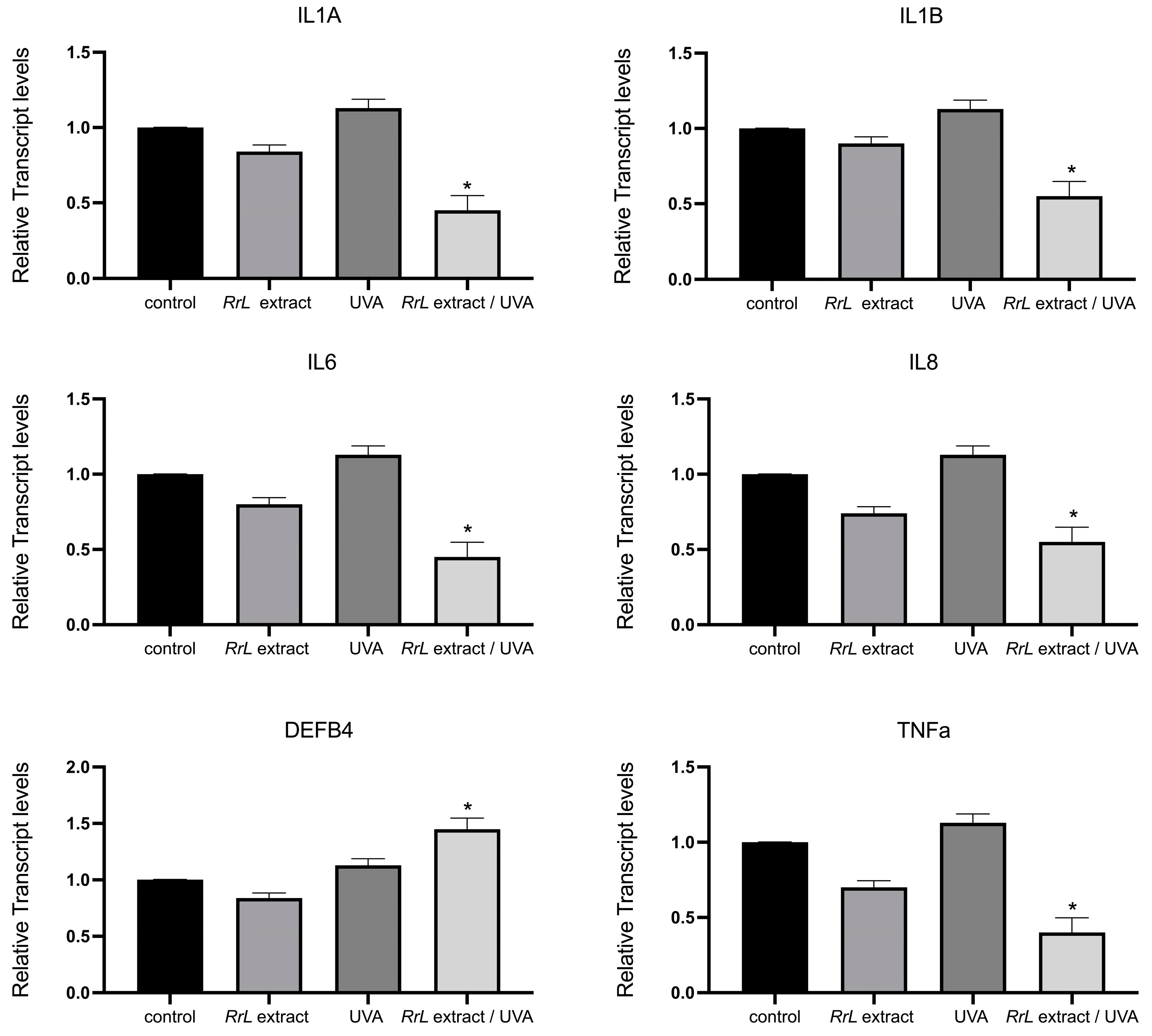
Reverse-Transcription PCR and Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. Antioxidant Assessments
3.1.1. DPPH and Folin–Ciocalteu Method
3.1.2. Prevention of Supercoiled Plasmid DNA Strand Breakage
3.2. In Vitro Assessments for Two-Dimensional Skin Models
3.2.1. ATP Assessments
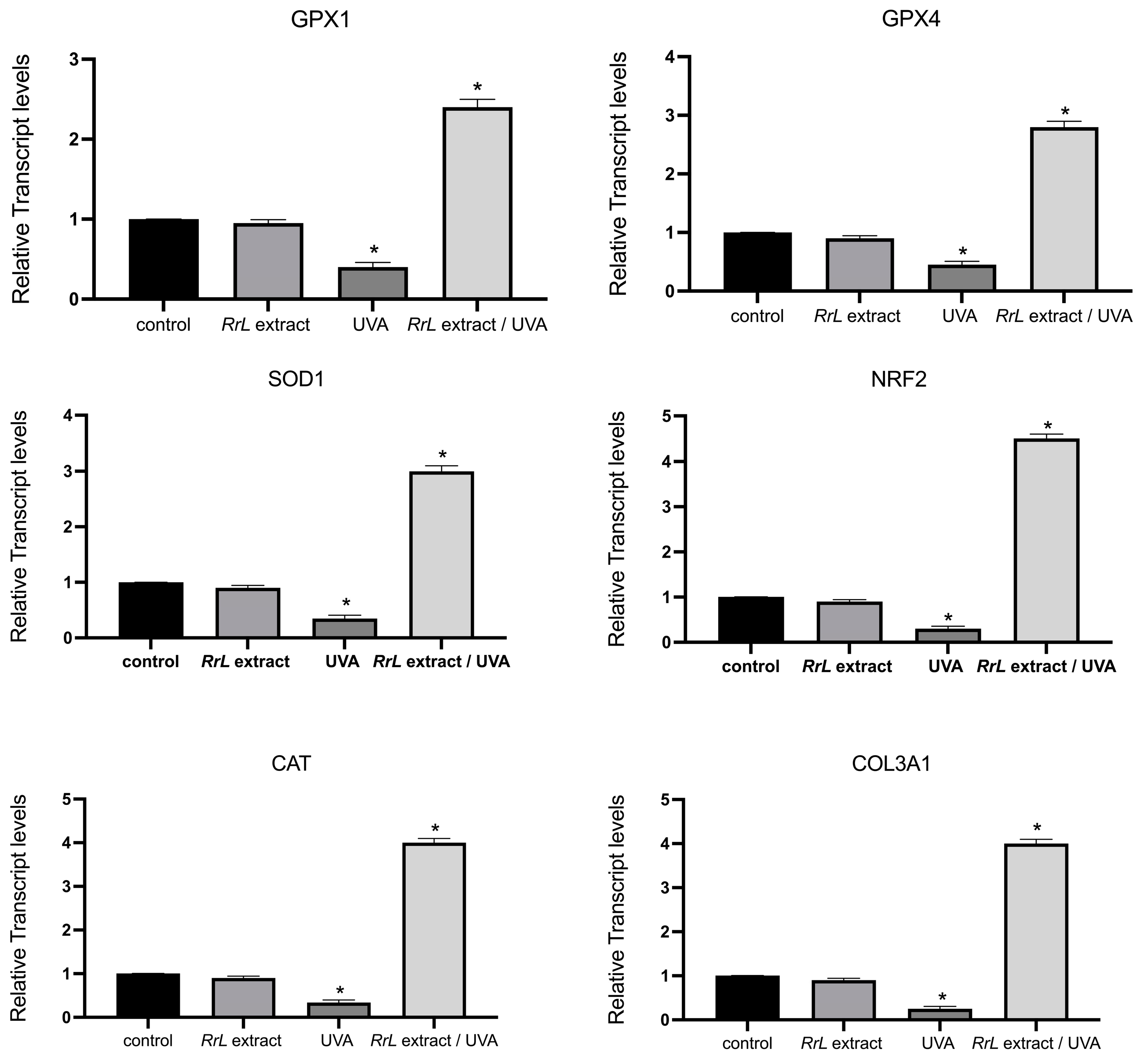
3.2.2. RT-PCR Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Quintero-Rincón, P.; Caballero-Gallardo, K. Aromatic Plants as Cosmeceuticals: Benefits and Applications for Skin Health. Planta 2024, 260, 132. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef] [PubMed]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound Identification of Selected Rose Species and Cultivars: An Insight to Petal and Leaf Phenolic Profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef]
- de Santana, F.B.; Gontijo, L.C.; Mitsutake, H.; Mazivila, S.J.; de Souza, L.M.; Borges Neto, W. Non-Destructive Fraud Detection in Rosehip Oil by MIR Spectroscopy and Chemometrics. Food Chem. 2016, 209, 228–233. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Rosa rubiginosa and Fraxinus oxycarpa Herbal Teas: Characterization of Phytochemical Profiles by Liquid Chromatography-Mass Spectrometry, and Evaluation of the Antioxidant Activity. New J. Chem. 2017, 41, 7681–7688. [Google Scholar] [CrossRef]
- Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865. [Google Scholar] [CrossRef]
- Son, D.-H.; Nam, M.-H.; Hong, C.-O.; Seol, H.-M.; Yang, J.-E.; Kim, Y.-B.; Kim, C.-T.; Lee, K.-W. 5-α Reductase Inhibitory Effect and Astringent Activity of Green Apple Rind Extract on Human Keratinocytes and Fibroblast Cells. Biosci. Biotechnol. Biochem. 2013, 77, 714–721. [Google Scholar] [CrossRef]
- Choi, E.-K.; Guo, H.; Choi, J.-K.; Jang, S.-K.; Shin, K.; Cha, Y.-S.; Choi, Y.; Seo, D.-W.; Lee, Y.-B.; Joo, S.-S.; et al. Extraction Conditions of White Rose Petals for the Inhibition of Enzymes Related to Skin Aging. Lab. Anim. Res. 2015, 31, 148. [Google Scholar] [CrossRef]
- Lee, M.; Nam, T.G.; Lee, I.; Shin, E.J.; Han, A.; Lee, P.; Lee, S.; Lim, T. Skin Anti-inflammatory Activity of Rose Petal Extract (Rosa gallica) through Reduction of MAPK Signaling Pathway. Food Sci. Nutr. 2018, 6, 2560–2567. [Google Scholar] [CrossRef]
- Maželienė, Ž.; Kirvaitienė, J.; Kaklauskienė, K.; Volskienė, R.; Aleksandravičienė, A. Antifungal and Antibacterial Activity of Aqueous and Ethanolic Extracts of Different Rosa rugosa Parts. Microbiol. Res. 2025, 16, 26. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of Polyphenols Extraction from Dried Chokeberry Using Maceration as Traditional Technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Velickovic, D.; Nikolova, M.; Ivancheva, S.; Stojanovic, J.; Veljkovic, V. Extraction of Flavonoids from Garden (Salvia officinalis L.) and Glutinous (Salvia glutinosa L.) Sage by Ultrasonic and Classical Maceration. J. Serbian Chem. Soc. 2007, 72, 73–80. [Google Scholar] [CrossRef]
- Thakur, L.; Ghodasra, U.; Patel, N.; Dabhi, M. Novel Approaches for Stability Improvement in Natural Medicines. Pharmacogn. Rev. 2011, 5, 48. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Nunes, I.J.; Ferreira, W.V.; Tomasini, P.P.; Trindade, C.; Martins, C.C.; Wilhelm, E.A.; Oliboni, R.d.S.; Netz, P.A.; Stieler, R.; et al. Antioxidant and Anticancer Potential of the New Cu(II) Complexes Bearing Imine-Phenolate Ligands with Pendant Amine N-Donor Groups. Pharmaceutics 2023, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Tsakni, A.; Kyriakopoulou, E.; Letsiou, S.; Halvatsiotis, P.; Rigopoulos, H.; Vassilaki, N.; Houhoula, D. In Vitro Determination of Antimicrobial, Antioxidant and Antiviral Properties of Greek Plant Extracts. Microorganisms 2025, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Bioactivities and Antiradical Properties of Millet Grains and Hulls. J. Agric. Food Chem. 2011, 59, 9563–9571. [Google Scholar] [CrossRef]
- Tsakni, A.; Chatzilazarou, A.; Tsakali, E.; Tsantes, A.G.; Van Impe, J.; Houhoula, D. Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations 2023, 10, 373. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Wang, X.; Heraud, S.; Thépot, A.; Dos Santos, M.; Luo, Z. The Whitening Properties of the Mixture Composed of Pomegranate, Osmanthus and Olive and the Protective Effects Against Ultraviolet Deleterious Effects. Clin. Cosmet. Investig. Dermatol. 2021, 14, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Koldiri, E.; Beloukas, A.; Rallis, E.; Kefala, V. Deciphering the Effects of Different Types of Sunlight Radiation on Skin Function: A Review. Cosmetics 2024, 11, 80. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N.; Ouahab, A. LC–DAD/ESI–MS/MS Characterization of Phenolic Constituents in Rosa canina L. and Its Protective Effect in Cells. Biomed. Chromatogr. 2020, 34, e4961. [Google Scholar] [CrossRef]
- Fascella, G.; Mammano, M.M.; D’Angiolillo, F. Leaf Methanolic Extracts from Four Sicilian Rose Species: Bioactive Compounds Content and Antioxidant Activity. Acta Hortic. 2019, 1232, 81–88. [Google Scholar] [CrossRef]
- Mourabit, Y.; El Hajjaji, S.; Taha, D.; Badaoui, B.; El Yadini, M.; Rusu, M.E.; Lee, L.-H.; Bouyahya, A.; Bourais, I. HPLC-DAD-ESI/MS Phytochemical Investigation, Antioxidant, and Antidiabetic Activities of Moroccan Rosa canina L. Extracts. Biocatal. Agric. Biotechnol. 2023, 52, 102817. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative Analysis of Bioactive Phenolic Compounds Composition from 26 Medicinal Plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive Compounds and Antioxidant Activity of Rosa canina L. Biotypes from Spontaneous Flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Alecu, A.; Albu, C.; Badea, G.-I.; Alionte, A.; Enache, A.-A.; Radu, G.-L.; Litescu, S.-C. Infrared Laser-Assisted Extraction of Bioactive Compounds from Rosa canina L. Int. J. Mol. Sci. 2025, 26, 992. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaur, M.; Sogi, D.S.; Purewal, S.S. A Comparative Study of Phytochemicals, Antioxidant Potential and in-Vitro DNA Damage Protection Activity of Different Oat (Avena sativa) Cultivars from India. J. Food Meas. Charact. 2019, 13, 347–356. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant, Anti-Inflammatory and DNA Scission Inhibitory Activities of Phenolic Compounds in Selected Onion and Potato Varieties. J. Funct. Foods 2013, 5, 930–939. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic Composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea Extracts and Assessment of Their Antioxidant Activity in Human Endothelial Cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Crouch, S.P.M.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The Use of ATP Bioluminescence as a Measure of Cell Proliferation and Cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Deters, A.M.; Schröder, K.R.; Hensel, A. Kiwi Fruit (Actinidia chinensis L.) Polysaccharides Exert Stimulating Effects on Cell Proliferation via Enhanced Growth Factor Receptors, Energy Production, and Collagen Synthesis of Human Keratinocytes, Fibroblasts, and Skin Equivalents. J. Cell. Physiol. 2005, 202, 717–722. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.; Mazzoni, L.; Forbes-Hernandez, T.; Gasparrini, M.; Gonzàlez-Paramàs, A.; Santos-Buelga, C.; Quiles, J.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-Rich Strawberry Extract Protects Human Dermal Fibroblasts against Hydrogen Peroxide Oxidative Damage and Improves Mitochondrial Functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef]
- Tsai, H.-Z.; Lin, R.-K.; Hsieh, T.-S. Drosophila Mitochondrial Topoisomerase III Alpha Affects the Aging Process via Maintenance of Mitochondrial Function and Genome Integrity. J. Biomed. Sci. 2016, 23, 38. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Panariello, L.; Danti, S.; Neri, S.; Fernandez-Avila, C.; Fusco, A.; Donnarumma, G.; et al. Properties and Skin Compatibility of Films Based on Poly(Lactic Acid) (PLA) Bionanocomposites Incorporating Chitin Nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.-B.; et al. Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Solá, P.; Mereu, E.; Bonjoch, J.; Casado-Peláez, M.; Prats, N.; Aguilera, M.; Reina, O.; Blanco, E.; Esteller, M.; Di Croce, L.; et al. Targeting Lymphoid-Derived IL-17 Signaling to Delay Skin Aging. Nat. Aging 2023, 3, 688–704. [Google Scholar] [CrossRef]
- Liang, J.; Dai, W.; Liu, C.; Wen, Y.; Chen, C.; Xu, Y.; Huang, S.; Hou, S.; Li, C.; Chen, Y.; et al. Gingerenone A Attenuates Ulcerative Colitis via Targeting IL-17RA to Inhibit Inflammation and Restore Intestinal Barrier Function. Adv. Sci. 2024, 11, 2400206. [Google Scholar] [CrossRef]
- Donnarumma, G.; Paoletti, I.; Fusco, A.; Perfetto, B.; Buommino, E.; de Gregorio, V.; Baroni, A. β-Defensins: Work in Progress. In Advances in Microbiology, Infectious Diseases and Public Health; Springer: Cham, Switzerland, 2015; pp. 59–76. [Google Scholar]
- Fusco, A.; Savio, V.; Cammarota, M.; Alfano, A.; Schiraldi, C.; Donnarumma, G. Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium. J. Immunol. Res. 2017, 2017, 6976935. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Gurjala, A.N.; Liu, W.R.; Mogford, J.E.; Procaccini, P.S.A.; Mustoe, T.A. Age-Dependent Response of Primary Human Dermal Fibroblasts to Oxidative Stress: Cell Survival, pro-Survival Kinases, and Entrance into Cellular Senescence. Wound Repair Regen. 2005, 13, 565–575. [Google Scholar] [CrossRef]
- Schroeder, P.; Pohl, C.; Calles, C.; Marks, C.; Wild, S.; Krutmann, J. Cellular Response to Infrared Radiation Involves Retrograde Mitochondrial Signaling. Free Radic. Biol. Med. 2007, 43, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Jubri, Z.; Rajab, N.; Rahim, K.; Yusof, Y.; Makpol, S. Gelam Honey Protects against Gamma-Irradiation Damage to Antioxidant Enzymes in Human Diploid Fibroblasts. Molecules 2013, 18, 2200–2211. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chang, C.-T.; Gowrisankar, Y.V.; Chen, X.-Z.; Lin, H.-C.; Yen, H.-R.; Yang, H.-L. Zerumbone Exhibits Antiphotoaging and Dermatoprotective Properties in Ultraviolet A-Irradiated Human Skin Fibroblast Cells via the Activation of Nrf2/ARE Defensive Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4098674. [Google Scholar] [CrossRef]
- Kim, Y.U.; Gi, H.; Jeong, E.K.; Han, S.; Seo, W.-Y.; Kim, Y.J.; Lee, S.B.; Kim, K. Development of a Highly Effective Recombinant Protein from Human Collagen Type III Alpha 1 (COL3A1) to Enhance Human Skin Cell Functionality. BMB Rep. 2024, 57, 424–429. [Google Scholar] [CrossRef]
- Neumann, U.; Louis, S.; Gille, A.; Derwenskus, F.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Anti-Inflammatory Effects of Phaeodactylum tricornutum Extracts on Human Blood Mononuclear Cells and Murine Macrophages. J. Appl. Phycol. 2018, 30, 2837–2846. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-Inflammatory Effect of Strawberry Extract against LPS-Induced Stress in RAW 264.7 Macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, S.-J.; Yi, E.-J.; Park, S.-J.; Jin, X.; Nguyen, T.T.M.; Yi, G.-S.; Jeon, Y.-J.; Yi, T.-H. Enzyme-Assisted Rosa Davurica Mitigates UV-Induced Skin Photodamage by Modulating Apoptosis through Nrf2/ARE and MAPK/NF-ΚB Pathways. J. Photochem. Photobiol. B 2025, 263, 113098. [Google Scholar] [CrossRef]
- Abdelkader, M.-A.E.; Mediatrice, H.; Lin, D.; Lin, Z.; Aggag, S.A. Mitigating Oxidative Stress and Promoting Cellular Longevity with Mushroom Extracts. Foods 2024, 13, 4028. [Google Scholar] [CrossRef]
- Park, B.; Hwang, E.; Seo, S.A.; Zhang, M.; Park, S.-Y.; Yi, T.-H. Dietary Rosa Damascena Protects against UVB-Induced Skin Aging by Improving Collagen Synthesis via MMPs Reduction through Alterations of c-Jun and c-Fos and TGF-Β1 Stimulation Mediated Smad2/3 and Smad7. J. Funct. Foods 2017, 36, 480–489. [Google Scholar] [CrossRef]
- Faur, C.-A.; Zăhan, M.; Bunea, C.I.; Hârșan, E.; Bora, F.-D.; Bunea, A. Antiproliferative and Biochemical Evaluation of Rose Extracts: Impact on Tumor and Normal Skin Cells. Front. Plant Sci. 2024, 15, 1477243. [Google Scholar] [CrossRef]
- Shin, E.J.; Han, A.-R.; Lee, M.-H.; Song, Y.-R.; Lee, K.M.; Nam, T.-G.; Lee, P.; Lee, S.-Y.; Lim, T.-G. Extraction Conditions for Rosa Gallica Petal Extracts with Anti-Skin Aging Activities. Food Sci. Biotechnol. 2019, 28, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Son, S.-R.; Choi, Y.J.; Kim, Y.; Ahn, S.-Y.; Jang, D.S.; Lee, S. Rosarugosides A and D from Rosa rugosa Flower Buds: Their Potential Anti-Skin-Aging Effects in TNF-α-Induced Human Dermal Fibroblasts. Plants 2024, 13, 1266. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Hao, Y.; Chen, Y.; Yu, S.; Qu, L. Chemical Constituents and Bioactivities of Fermented Rose (from Yunnan) Extract. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letsiou, S.; Tsakni, A.; Antonopoulos, D.; Tsoupras, A.; Houhoula, D. Unveiling the Biological Properties of Rosa rubiginosa L. Leaf Extract as a Bio-Functional Ingredient Based on 2D Cell-Based Models and In Vitro Assessments. Cosmetics 2025, 12, 62. https://doi.org/10.3390/cosmetics12020062
Letsiou S, Tsakni A, Antonopoulos D, Tsoupras A, Houhoula D. Unveiling the Biological Properties of Rosa rubiginosa L. Leaf Extract as a Bio-Functional Ingredient Based on 2D Cell-Based Models and In Vitro Assessments. Cosmetics. 2025; 12(2):62. https://doi.org/10.3390/cosmetics12020062
Chicago/Turabian StyleLetsiou, Sophia, Aliki Tsakni, Dionysis Antonopoulos, Alexandros Tsoupras, and Dimitra Houhoula. 2025. "Unveiling the Biological Properties of Rosa rubiginosa L. Leaf Extract as a Bio-Functional Ingredient Based on 2D Cell-Based Models and In Vitro Assessments" Cosmetics 12, no. 2: 62. https://doi.org/10.3390/cosmetics12020062
APA StyleLetsiou, S., Tsakni, A., Antonopoulos, D., Tsoupras, A., & Houhoula, D. (2025). Unveiling the Biological Properties of Rosa rubiginosa L. Leaf Extract as a Bio-Functional Ingredient Based on 2D Cell-Based Models and In Vitro Assessments. Cosmetics, 12(2), 62. https://doi.org/10.3390/cosmetics12020062







