Abstract
The limited availability of primary normal human epidermal keratinocyte (NHEK) has hampered the large-scale implementation of skin models in biomedical, toxicological, and pharmaceutical research. Therefore, in this study, we aimed to establish an induced pluripotent stem cell (iPSC)-derived epidermal skin model that is not limited by donor type and cell lifespan, and evaluate whether it is equivalent to the primary NHEK-derived reconstructed epidermal skin model (RHE) for skin irritation testing. The results show that high expression of OCT4, SOX2, KLF4, c-MYC, and SSEA-4, TRA-1-60, TRA-1-81 indicated that iPSCs were successfully generated from human fibroblasts in vitro. The expression levels of ectoderm or KC marker genes CGB, IVL, KRT10, KRT14, TP63, and TBP were close to those of NHEKs. This result confirms that iPSCs were successfully differentiated into iPSC-KCs. The expression levels of iPSC-derived-RHE in FLG (60), AQP3 (151), CLDN1 (30.6), IVL (209), KRT5 (39.3), KRT10 (39.2), TSLP (99), IL-6 (53.1), IL-8 (79.4), and TNF-a (91.5) were significantly higher than those in RHE. These results indicate that iPSC-derived RHE has extremely strong vitality and renewal capacity. Meanwhile, there was no significant difference between iPSC-derived RHE and SkinEthic in predicting skin irritation, which means that our iPSC-derived RHE performed well in the test. iPSC-derived RHE can replace other skin models for skin irritation testing related to cosmetics. This technology has the potential to generate an unlimited number of genetically identical skin models and improve the reproducibility of experiments.
1. Introduction
The use of experimental animals in scientific research has long been a topic of controversy, with many advocating for a reduction in their use due to ethical concerns [1]. Furthermore, the biological differences between animal and human skin have highlighted the need for alternative models that can accurately mimic human skin [2]. In response to these societal urges, researchers have turned to developing innovative in vitro models of human skin.
One of the primary challenges in generating such models is the limited availability of primary NHEKs, which are essential cells for creating functional skin equivalents [3]. Primary NHEKs are a finite resource, and their harvesting often requires invasive procedures that can be detrimental to the donor’s health [4]. Moreover, primary NHEKs have a limited lifespan in culture, making it difficult to maintain a consistent supply of cells for large-scale research applications [5]. And their short lifespan leading to the use of various donors imposes issues with genetic variation. Immortalized cell lines could overcome these problems. However, few immortalized human keratinocyte cell lines are available, and most are unable to form fully stratified epithelia. Some have started to use human embryonic and induced pluripotent stem cells (hESCs/iPSCs) to generate skin cells for decades. The discovery of these pluripotent stem cells with multiple developmental potentials won the 2012 Nobel Prize in Physiology or Medicine [6].
Recent advances in iPSCs technology have offered a potential solution to this problem. iPSCs are a type of stem cell that can be generated from adult cells, such as skin or blood cells, and reprogrammed to exhibit pluripotency, the ability to differentiate into any cell type in the body [7]. This property makes iPSCs an attractive source for generating large quantities of KC, which can then be used to create skin equivalents.
Several studies have pointed out ways to differentiate keratinocytes. Studies have shown that KC can be obtained by inhibiting TGFβ, activating BMP and WNT pathways, and inhibiting NOTCH differentiation [8]. Another study also demonstrated differentiation of keratinocytes by precisely altering the activity of FGF, BMP, WNT, and TGFβ pathways in a minimal, chemically defined medium [9]. Therefore, this study simplified the differentiation process without affecting the efficiency of differentiation by summarizing the methods of keratinocytes in these studies and making corresponding adjustments.
iPSC-derived keratinocytes (iPSC-KCs) obtained by induced differentiation of iPSCs can be verified by comparison with typical gene and protein markers of NHEKs [10,11]. Typical markers of iPSC-KCs can usually be verified through qPCR technology, including typical markers of stem cell pluripotency OCT4 [12], SOX2 [13], KLF4, c-MYC, SSEA-4, TRA-1-60 and TRA-1-81 [14,15], and the typical markers of NHEK, KRT14 [16], AQP3 [17], and IVL [18,19]. The 3D skin model can usually verify the degree of water absorption of the model through the typical markers Filaggrin [20,21] and AQP3 [17]. Aquaporin 3 (AQP3) is not only highly permeable, but also an effective glycerol transporter. It plays an important role in barrier repair and moisturizing of the epidermis. AQP3 provides a short-circuit water circulation between the basal layer and the stratum corneum of epidermal cells, bringing circulating water into the epidermis, thereby ensuring a constant water content [22]. Filaggrin is a key protein required for the formation of the stratum corneum barrier. It retains skin moisture by binding water and is known as the natural moisturizing factor (NMF) [23]. The stability of the model in the efficacy test is then verified through frequent stimulation tests.
In this study, we aimed to develop a well-characterized iPSC-derived RHE and evaluate its equivalence to primary NHEK-derived RHE for skin irritation tests. By leveraging the potential of iPSCs, we aim to create a more sustainable, efficient, and reliable source of skin equivalents that can support the development of safer and more effective cosmetics, pharmaceuticals, and biomedical products.
2. Materials and Methods
2.1. Cell Culture
Normal human epidermal keratinocyte cells (NHEKs) and human fibroblasts were purchased from Guang-dong Biocell Co., Ltd. (Guangzhou, Guangdong, China) and cultured in EpiLife (MEPI500CA, Gibco, Thermo Fisher, Waltham, MA, USA) medium with HKGS (S0015, Gibco, Thermo Fisher, Waltham, MA, USA) and DMEM (REF: 11965092, Gibco, Thermo Fisher, Waltham, MA, USA) with 10% fetal bovine serum (FBS, REF: A5670401, Gibco, Thermo Fisher, Waltham, MA, USA) in a 5% CO2 incubator at 37 °C, respectively. The culture medium was changed every 2 days and used when the cell confluence reached 70% to 80%.
2.2. Generation of Human iPSCs
The reprogramming process was initiated by exogenous expression of transcription factors (OCT4, SOX2, KLF4, and c-MYC) using a lentiviral vector system [7]. The gene sequence is shown in Table 1. The resulting iPSCs were cultured in Essential 8™ Medium (Essential 8™ Medium with Essential 8™ Flex Supplement (50×), A28585-01, Gibco, Thermo Fisher, Waltham, MA, USA), which is a chemically defined medium that supports the growth and maintenance of pluripotent stem cells. iPSC markers SSEA-4, TRA-1-60, and TRA-1-81 were identified by flow cytometry [24]. The main steps required to reprogram human neonatal foreskin fibroblasts to generate iPSCs on vitronectin-coated dishes using the CytoTune™-iPS 2.0 Sendai Reprogramming Kit are as follows. Day 2: Human fibroblasts were cultured in 6-well plates using fibroblast medium (DMEM with 10% FBS). They were allowed to reach 30–60% confluency on the day of transduction (Day 0). Day 0: Cells were transduced with the CytoTune™ 2.0 Sendai reprogramming vector at the appropriate MOI. Cells were incubated overnight. Day 1: The medium was replaced with fresh complete fibroblast medium and the CytoTune™ 2.0 Sendai reprogramming vector was removed. Days 2–6: The medium was changed every other day. Day 7: Transduced cells were plated in fibroblast culture dishes. Day 8: The medium was changed to Complete Essential 8™ Medium. Days 9–28: The culture vessels were monitored for the emergence of iPSC colonies and the medium was changed daily. When iPSC colonies were ready for transfer, vital staining was performed and undifferentiated iPSCs were selected and transferred to fresh culture dishes for expansion.

Table 1.
Primer sequence information.
2.3. Differentiation of iPSCs into Keratinocytes
The method for differentiating iPSCs into keratinocytes to obtain iPSC-KCs was optimized several times. First, hEB medium (DMEM/F12 medium (REF: 21331020, Gibco, Thermo Fisher, Waltham, MA, USA) supplemented with 10 µM rock inhibitor Y27632 (REF: ab120129, Gibco, Thermo Fisher, Waltham, MA, USA), 200 mM L-glutamine/glutaMAX (REF: 35050061, Gibco, Thermo Fisher, Waltham, MA, USA), Knockout Serum (REF: 10828028, Gibco, Thermo Fisher, Waltham, MA, USA), NEAA (REF: 11140050, Gibco, Thermo Fisher, Waltham, MA, USA), and 0.1 mM 2-Mercaptoethanol (REF: 21985023, Gibco, Thermo Fisher, Waltham, MA, USA) was used to culture the iPSCs. iPSCs were cultured into EB spheres at 1000 cells per microwell in a pre-made AggreWell™ 400 24-well plate (REF: 34411, stemcell, Vancouver, BC, Canada) using DMEM/F12 medium. After sphering, the EB spheres were transferred to a new 6-well plate coated with vitronectin (VTN, REF: A14700, Thermo Fisher, Waltham, MA, USA) for subsequent differentiation culture. On the first day, hEB medium was used. On the second day, differentiation medium A (hEB medium supplemented with 10 ng/mL bone morphogenetic protein 4 (BMP4, REF: PHC9534, Gibco, Thermo Fisher, Waltham, MA, USA) and 1 µM/L all-trans retinoic acid (RA, REF: 554720, Sigma, St Louis, MO, USA) was used.
EBs were generated by culturing in a cell culture incubator for 2 days. They were not removed during this period. On day 3, VTN-coated or bottomed culture dishes or plates were prepared. EBs were collected in a 15 mL centrifuge tube and 5 mL of PBS was added. EBs were precipitated at the bottom of the tube (approximately 5–10 min). The PBS was carefully aspirated and about 1 mL was left at the bottom of the tube. The above steps were repeated twice for a total of three washes. EBs were placed in a 60 mm culture dish with a pre-coated bottom of collagen IV. A total of 6 mL of hEB medium was added to the dish. EBs were cultured in the incubator overnight. On days 4–7, the medium was aspirated to remove unattached EBs. Then, 6 mL of hEB medium containing 25 ng/mL BMP4 and 1 μM RA was added. On days 8–14, the medium was changed to differentiation medium B (EpiLife medium (REF: MEPI500CA, Gibco, Thermo Fisher, Waltham, MA, USA) supplemented with 25 ng/mL BMP4, 1 μM RA, and HKGS (REF: S0015, Gibco, Thermo Fisher, Waltham, MA, USA). On days 15–24, the medium was changed to a keratinocyte medium (EpiLife medium supplemented with HKGS). iPSC-KCs were obtained by passage on day 30.
2.4. Reconstructed iPSC-Derived RHE and RHE
The differentiated keratinocytes were used to create a reconstructed iPSC-derived RHE and RHE models using a modified protocol based on the SkinEthic system [25]. The resulting skin model was composed of multiple layers of keratinocytes, mimicking the architecture of human epidermis. iPSC-derived RHE and RHE models were constructed using iPSC-KC and NHEK, respectively. The construction method was the same. Complete EpiLife growth medium and cell culture inserts (REF: MEPI500CA, Thermo Fisher, Waltham, MA, USA) were used to establish the model. A total of 140 μM CaCl2 (C7902-500G, Sigma, St Louis, MO, USA), HKGS (REF: S0015, Thermo Fisher, Waltham, MA, USA), 10 ng/mL keratinocyte growth factor (104-07, PrimeGene, Shanghai, China), and 50 μg/mL ascorbic acid (A4544-25G, Sigma, USA) were added to EpiLife medium to prepare the complete growth medium. Collage type I (REF: 354236, Sigma, St Louis, MO, USA) was diluted 1:100 in D-PBS (Thermo Fisher, Waltham, MA, USA) in the inserts according to standard protocols and placed at room temperature for 1 h. Cells were seeded at a density of 7.5 × 104 cells/cm2 in a 0.5 mL growth medium on the pre-coated inserts. The 0.5 mL growth medium was added to the chamber below the insert. The fresh growth medium was replaced every other day for 4 days. The inserts were then transferred to the support frame and the medium was changed to CnT-Prime 3D medium (CnT-PR-3D, Cellntec, Bern, Kanton Bern, Switzerland) for 14 days of air-liquid culture (1 mL CnT-Prime 3D was added to the chamber below). CnT-Prime 3D medium was changed every 3 days. After 14 days of air-liquid culture, a testable model was obtained. The medium was changed to EpiLife medium supplemented with a stimulation cocktail (10 ng/mL poly I:C, 50 ng/mL rHuIL-4, 10 ng/mL rHuTNF-a, 50 ng/mL rHuIL-13, Prime-Gene, Shanghai, China) [26]. Half of the tissue was collected for RNA extraction, and the other half was used for immunohistochemical staining. All non-sterile reagents were filtered through a 0.22 μm filter (Millipore Express PES Membrane filter Unit, 0.22 μm, Merck Millipore Ltd., Rahway, NJ, Germany).
2.5. Quantitative Real-Time PCR Analysis
Total RNA from tissues was extracted according to the recommended protocol of TRIZOL reagent (Life Technologies, Carlsbad, CA, USA). Qubit 3.0 (Thermo Fisher, Waltham, MA, USA) was used to detect the concentration and quality of RNA. A PrimeScript RT reagent kit (Thermo Fisher, Waltham, MA, USA) was used to synthesize cDNA. A Light Cycler 96 system (Roche, Mannheim, Germany) and FastStart Essential DNA Green Master (Cat. 06402712001, Roche, Mannheim, Germany) were used to measure the changes in mRNA levels according to the manufacturer’s recommended protocol. The primers for FLG, AQP3, CLDN1, IVL, KRT5, KRT10, TSLP, IL-6, IL-8, TNF-a, and glyceraldehyde-3-phosphate dehydrogenase (GADPH) are listed in Table 1. The delta cycle threshold method was used to analyze the relevant data, and the relative expression of each gene was normalized to the Ct of GAPDH and calculated according to the 2-DDCT method.
2.6. Immunohistofluorescence Assay
iPSC-derived RHE and RHE were fixed by ice-cold methanol for 20 min. The treated iPSC-derived RHE and RHE sections were blocked with 5% bovine serum albumin (BSA) buffer for 60 min. iPSC-derived RHE and RHE sections were incubated with primary antibodies (FLG 1:500, AQP3 1:500, Abcam, Cambridge, UK) overnight at 4 °C, and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:1000; Invitrogen, Waltham, MA, USA) was used for incubation at 37 °C in the dark for 2 h. A total of 4,6-diamidino-2-phenylindole (DAPI, 1:1000, Thermo Fisher, Waltham, MA, USA) was used to stain the cell nuclei at room temperature. A fluorescence microscope (EVOS FL Auto, Life Technology, Carlsbad, CA, USA) was used to observe the immunohistofluorescence images. EVOS Viewer Imaging Software 1.7 (Thermo Fisher, Waltham, MA, USA) was used for analysis.
2.7. Skin Irritation Test
A series of test chemicals with known irritation potentials were selected for evaluation in our reconstructed skin model. These included sodium lauryl sulfate (SLS), triton X-100, and benzalkonium chloride. The skin irritation test was performed according to the OECD TG 439 guidelines [27]. Briefly, the test chemicals were applied to the skin model for a predetermined duration, after which the viability of the keratinocytes was assessed using the MTT assay. To validate our iPSC-derived reconstructed skin model, we compared its performance with that of the commercially available SkinEthic system. The SkinEthic system is a well-established in vitro skin model that has been widely used for skin irritation testing and cosmetic research [25].
2.8. Statistical Analyses
All statistical analyses were performed using SPSS 25.0 (IBM Corporation, Armonk, NY, USA). The mean values shown were calculated based on data from at least three independent replicates. Data were analyzed using the Duncan test. p values less than 0.05 were considered statistically significant.
3. Results
3.1. Induction of Human iPSCs from Fibroblasts
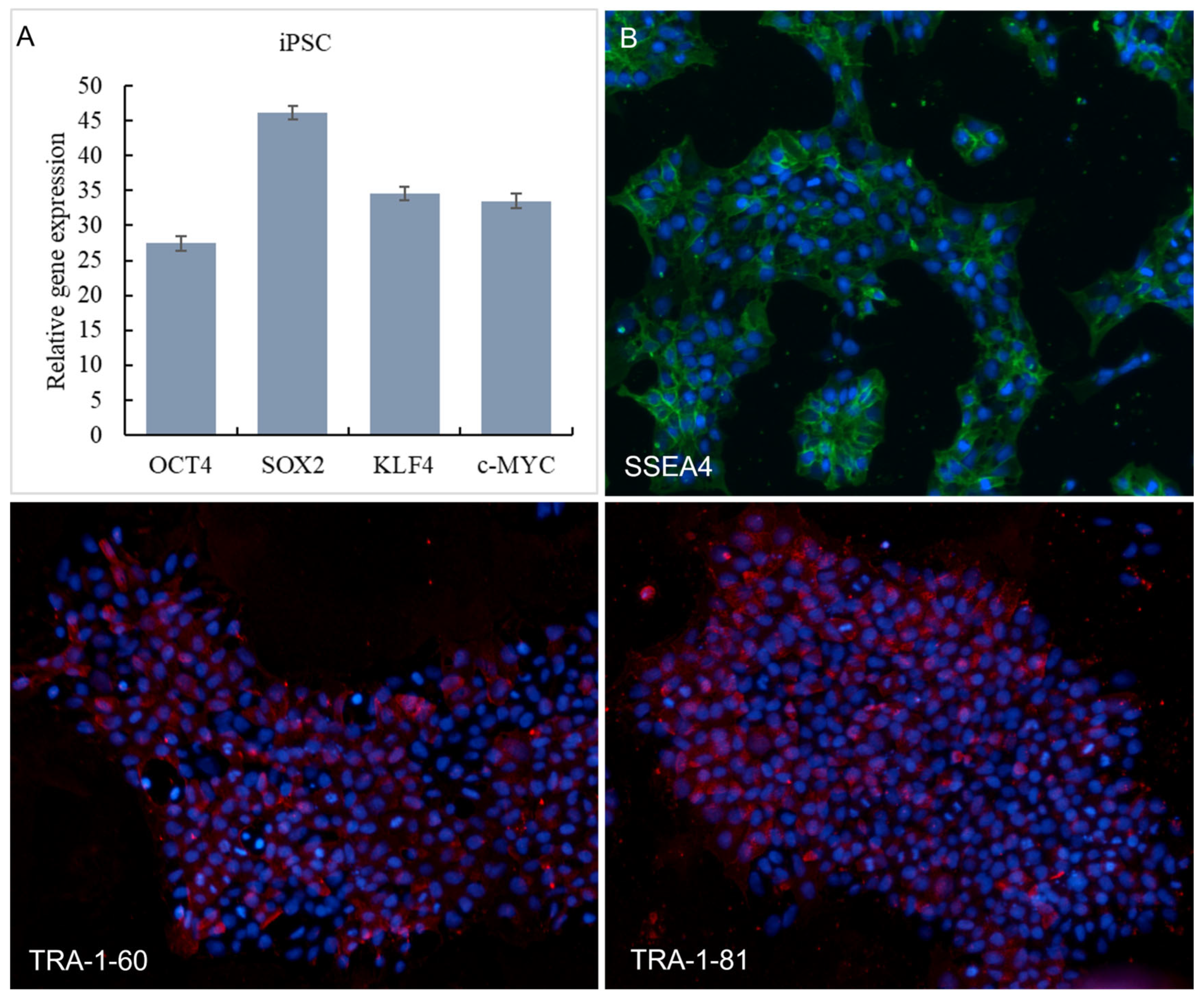
We successfully induced human iPSCs from fibroblasts using a lentiviral vector system, as evidenced by the expression of pluripotency markers OCT4, SOX2, KLF4, and c-MYC. Expression of pluripotency markers by the iPSC was further confirmed using qPCR analyzing OCT4, SOX2, KLF4, and c-MYC gene expression (Figure 1A). SSEA-4, TRA-1-60, and TRA-1-81 are used to identify the pluripotency of stem cells. The immunofluorescence staining results of SSEA-4, TRA-1-60, and TRA-1-81 of iPSC are shown in Figure 1B. As can be seen from Figure 1B, iPSC was significantly expressed on SSEA-4, TRA-1-60, and TRA-1-81.
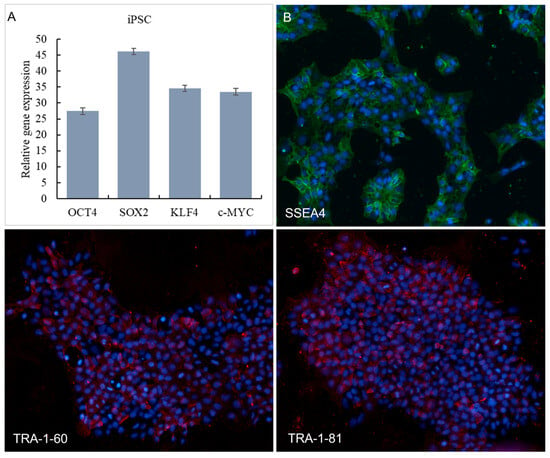
Figure 1.
Expression of iPSCs in OCT4, SOX2, KLF4 and c-MYC in qPCR (A). Immunofluorescence staining results of SSEA-4 (Red signals), TRA-1-60 (Green signals) and TRA-1-81 (Green signals) protein expression (B). Nucleus were stained with DAPI (Blue signals).
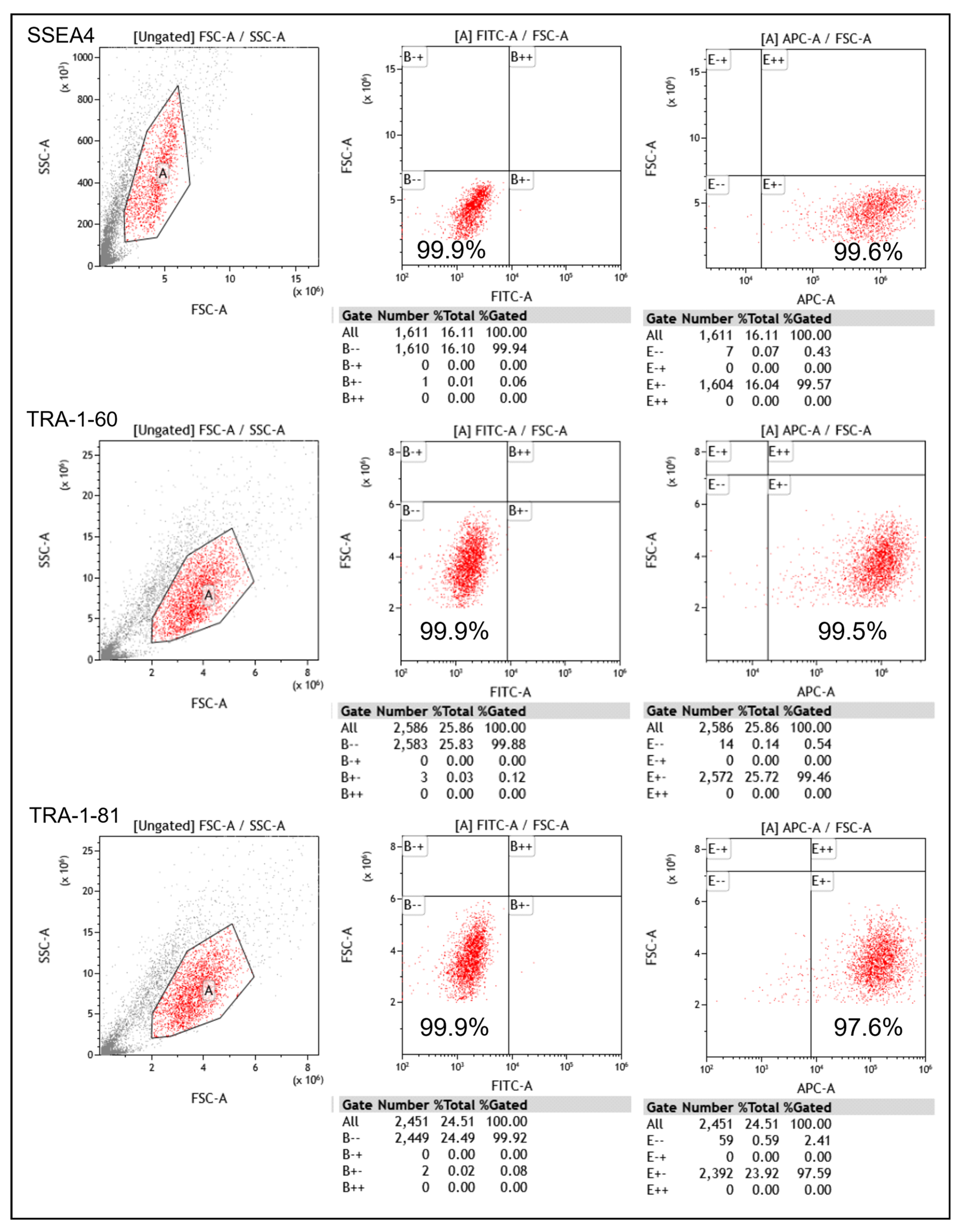
The pluripotency of the generated iPSCs was further assessed using flow cytometry. The results show that the iPSCs expressed high levels of SSEA-4, TRA-1-60, and TRA-1-81, which are commonly used markers for pluripotent stem cells (Figure 2). These findings confirm the successful induction of iPSCs from fibroblasts.
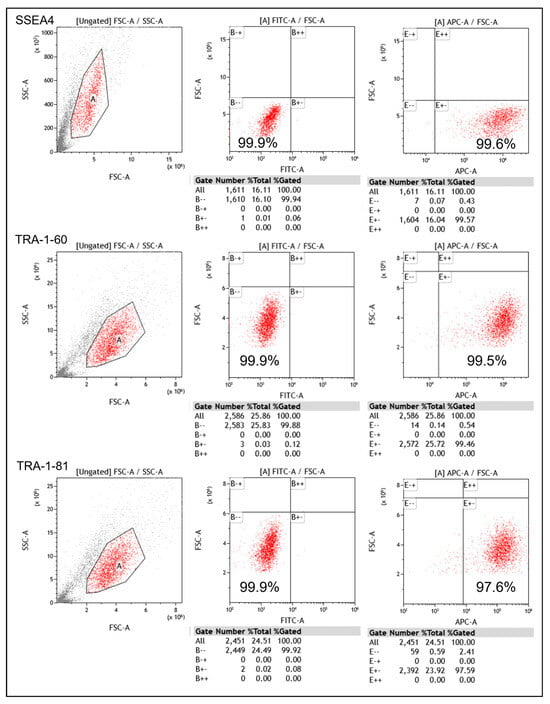
Figure 2.
Expression of iPSCs in SSEA-4, TRA-1-60, and TRA-1-81 in flow cytometry (A: a region with high similarity).
3.2. Differentiation from iPSCs into Keratinocytes
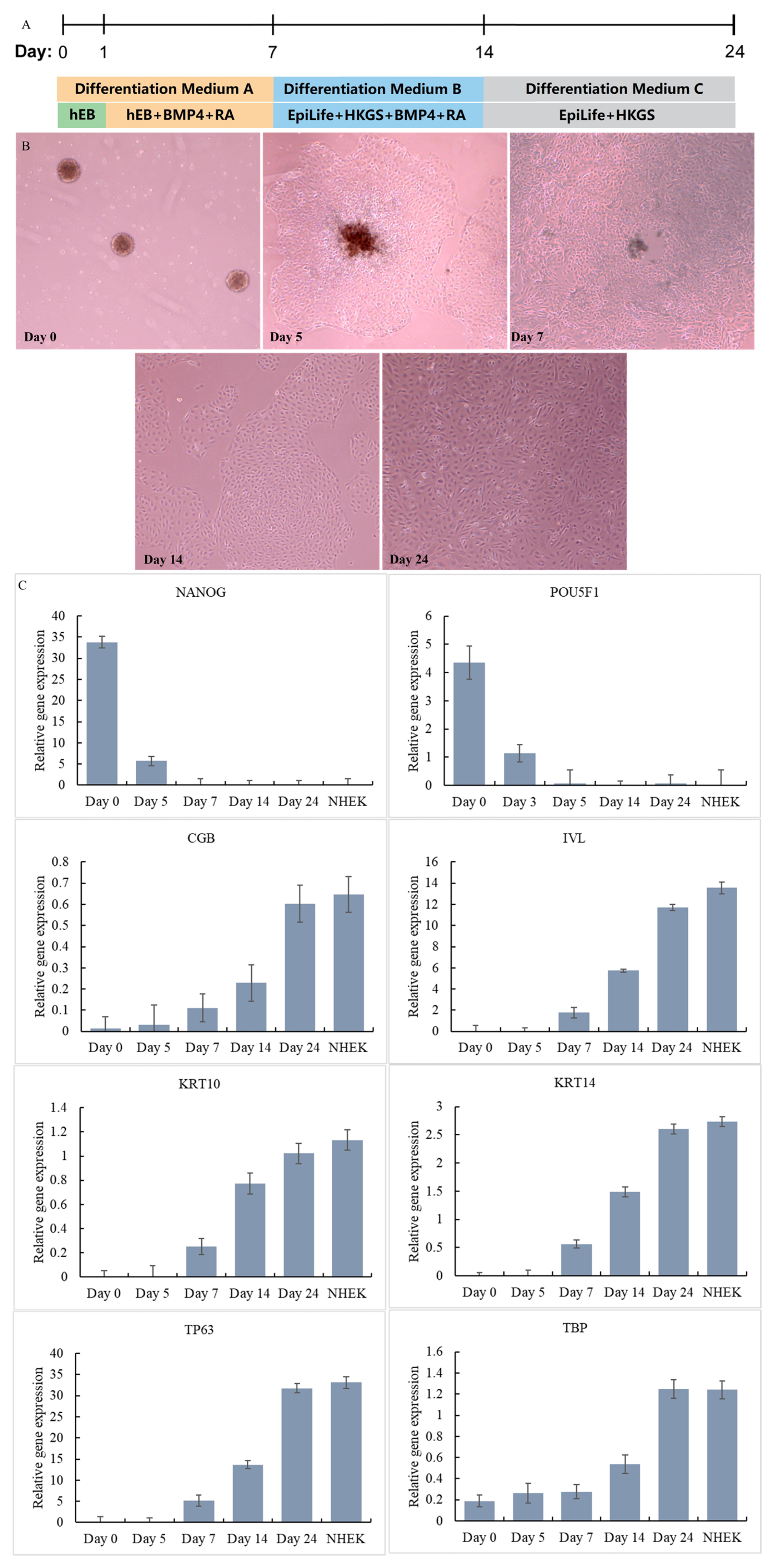
To generate keratinocytes from the iPSCs, we employed a three-stage differentiation protocol. In the first stage, iPSCs were treated with Y27632, BMP4, and RA for 7 days to induce the expression of keratinocyte-specific markers [28]. The resulting cells were then transferred to EpiLife medium with HKGS, BMP4 and RA, a specialized medium designed to support the growth and differentiation of keratinocytes. Finally, the medium was replaced with EpiLife medium with HKGS, and the differentiated cells were screened through a passage to obtain iPSC-KC with higher vitality. The flow chart of the differentiation method is shown in Figure 3A. The differentiation of each stage is shown in Figure 3B. iPSC was induced to differentiate into IPSC-KCs through a three-stage differentiation process.
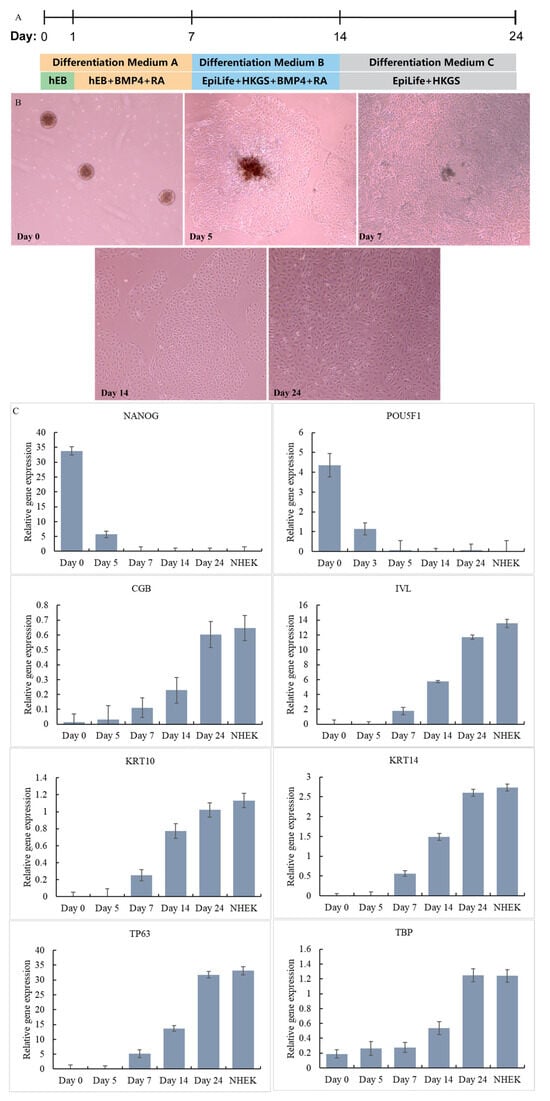
Figure 3.
Differentiation steps of iPSC-KC (A). Differentiation process of iPSC-KC (B). Expression of signature genes at different stages of iPSC-KC differentiation (C).
The cells at different time points were harvested and the differentiation of iPSCs was monitored. The cells on Day 0, Day 5, Day 7, Day 14, and Day 24 were harvested and tested for the expression of stem cell and KC gene markers by qPCR together with NHEK cells. As shown in Figure 3C, at Day 0, the iPSC marker genes NANOG and POU5F1 were highly expressed [24]. However, there was no expression of the ectoderm or KC marker genes CGB, IVL, KRT10, KRT14, TP63, and TBP. Starting from Day 7, the iPSC marker genes NANOG and POU5F1 were no longer expressed. The ectoderm or KC marker genes CGB, IVL, KRT10, KRT14, TP63, and TBP all began to be expressed, and the expression levels gradually increased from Day 7 to Day 24. On Day 24, the expression of ectoderm or KC marker genes CGB (iPSC-KC: 0.6, NHEK: 0.65), IVL (iPSC-KC: 11.7, NHEK: 13.5), KRT10 (iPSC-KC: 1.02, NHEK: 1.13), KRT14 (iPSC-KC: 2.6, NHEK: 2.73), TP63 (iPSC-KC: 31.8, NHEK: 33.1), and TBP (iPSC-KC: 1.25, NHEK: 1.24) reached a level close to that of NHEK. This result confirms that on Day 24, iPSCs had successfully differentiated into iPSC-KCs. This method has a shorter construction time than the method of Dubau et al. [24], which used hair follicle-derived keratinocytes to reprogram into induced pluripotent stem cells (construction time 35 days).
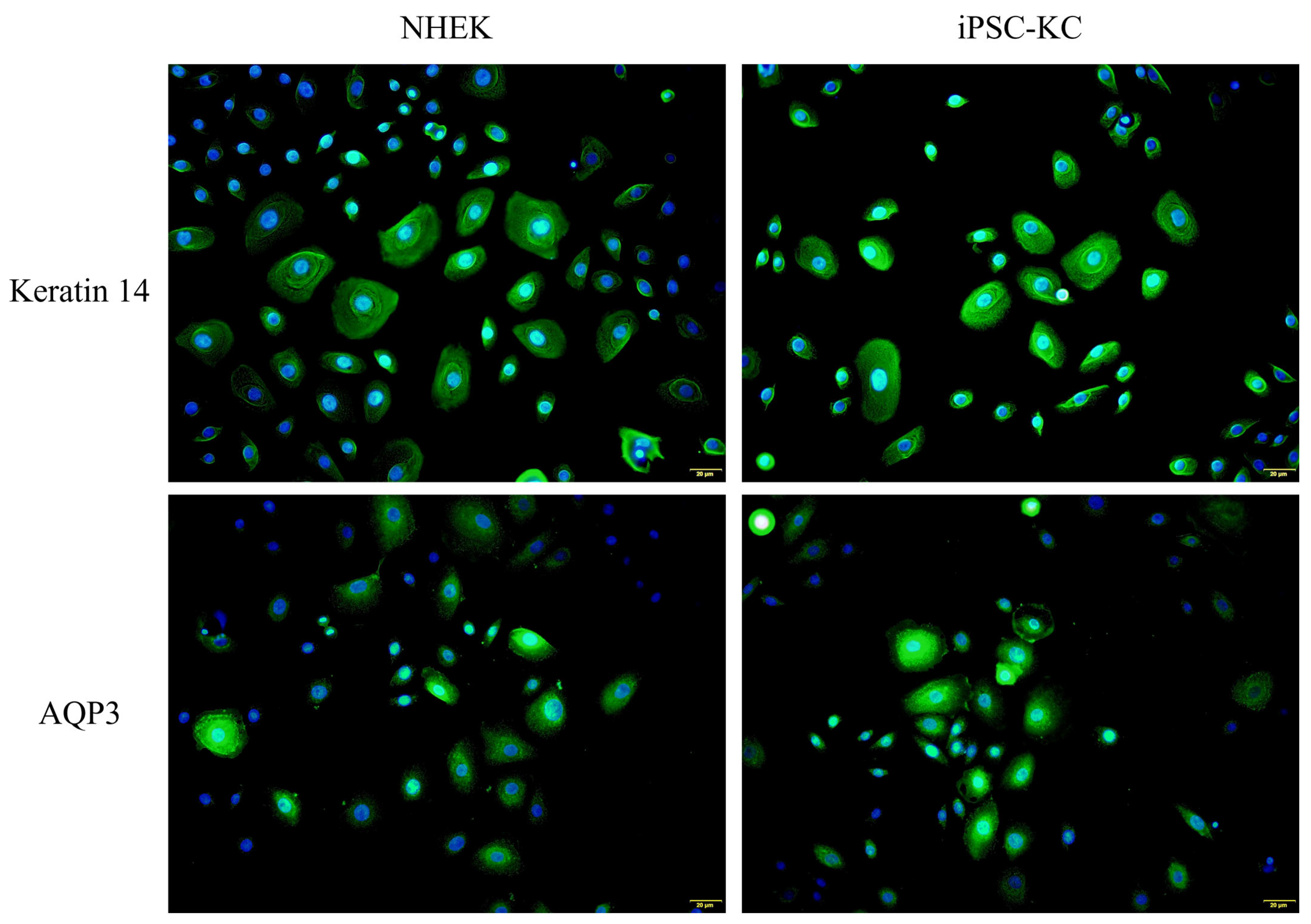
In addition, Keratin 14 and AQP3 immunofluorescence staining were performed simultaneously on iPSC-KC and NHEK cells harvested on Day 24. As shown in Figure 4, the fluorescence expression of iPSC-KC on Keratin 14 and AQP3 was similar to that of NHEK. This result also confirms the successful differentiation of iPSC-KC.
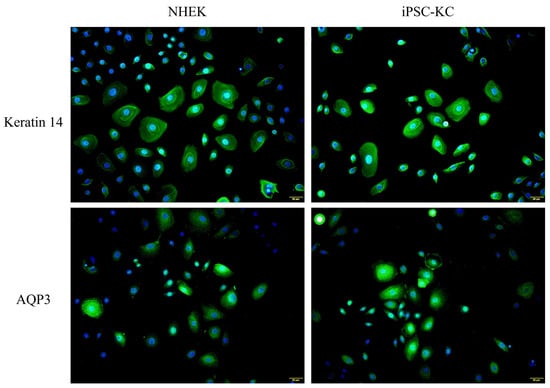
Figure 4.
Expression of NHEK and iPSC-KC on Keratin 14 (Green signals) and AQP3 (Green signals). Nucleus were stained with DAPI (Blue signals).
3.3. Reconstructed Epidermal Skin Model and Immunofluorescence Staining
We developed a reconstructed epidermal skin model using the iPSC-derived keratinocytes (iPSC-derived-RHE). The generated skin model exhibited a multilayered epidermis with a distinct cornified layer at the surface, similar to human epidermis. This indicates that the iPSC-derived keratinocytes had differentiated and organized into a functional epidermal tissue.
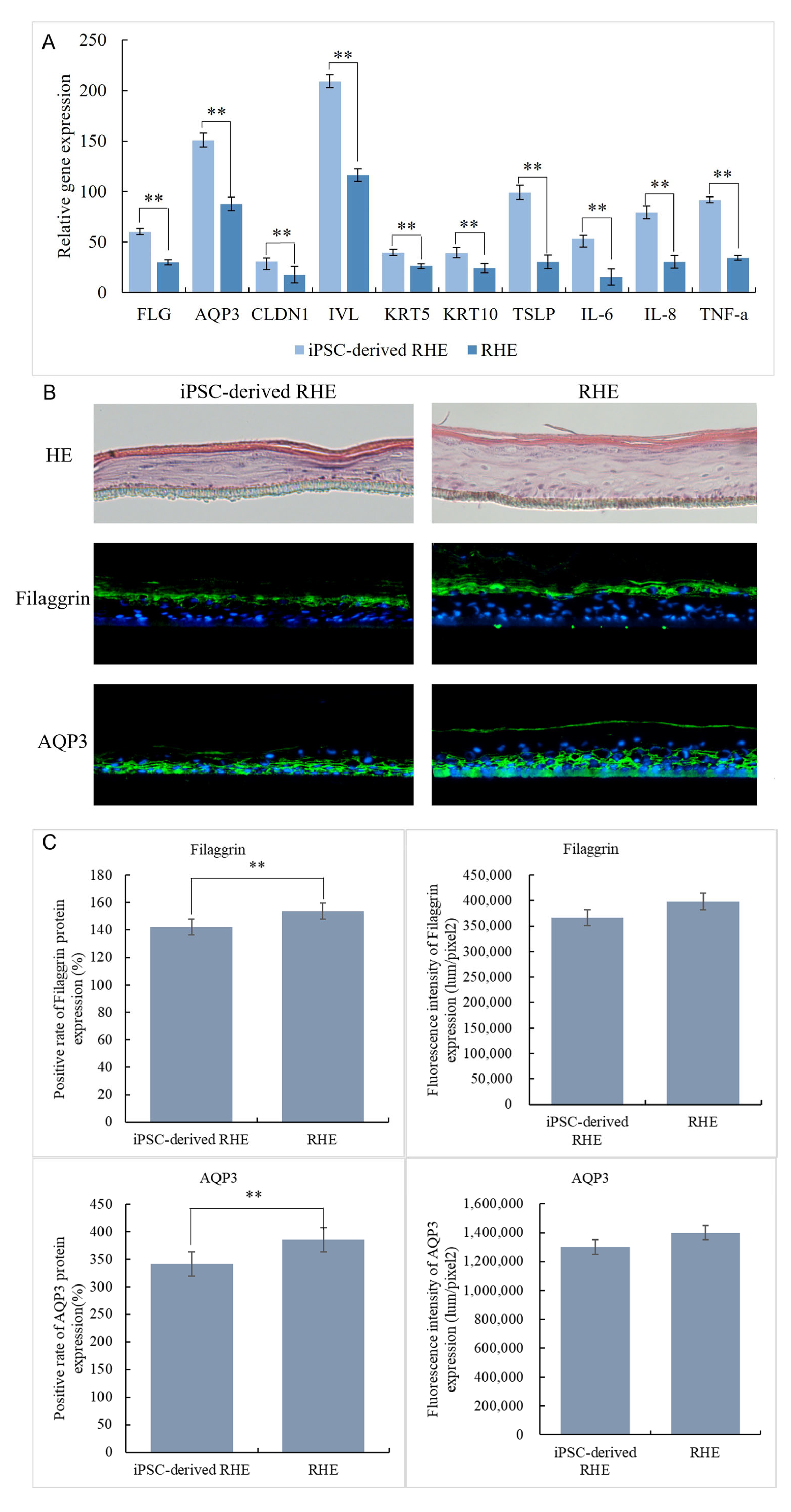
The iPSC-derived-RHE and NHEK-constructed RHE were harvested together to extract RNA and tested for expression of FLG, AQP3, CLDN1, IVL, KRT5, KRT10, TSLP, IL-6, IL-8, and TNF-a genes by qPCR. The results are shown in Figure 5A. The expression levels of iPSC-derived-RHE in FLG (60), AQP3 (151), CLDN1 (30.6), IVL (209), KRT5 (39.3), KRT10 (39.2), TSLP(99), IL-6(53.1), IL-8(79.4), and TNF-a(91.5) were significantly higher than those in RHE (FLG (29.9), AQP3 (87.7), CLDN1 (17.8), IVL (116), KRT5 (26.2), KRT10 (24.3), TSLP(30.4), IL-6(15.3), IL-8(30.4), and TNF-a(34.5)). This may be because iPSC-derived-RHE is constructed from iPSC-KC, which has extremely strong vitality and renewal ability like newborn baby skin cells. This makes the iPSC-derived-RHE constructed by the iPSC-KC thinner in structure; it also has a weaker skin barrier [29,30]. The HE staining results in Figure 5B intuitively confirm this conclusion. The weaker skin barrier also resulted in IPSC-Derivation-RHE being more sensitive to and expressing more inflammation-related genes such as TSLP, IL-6, IL-8, and TNF-a in a stage of rapid growth. Therefore, genes at this stage are highly expressed.
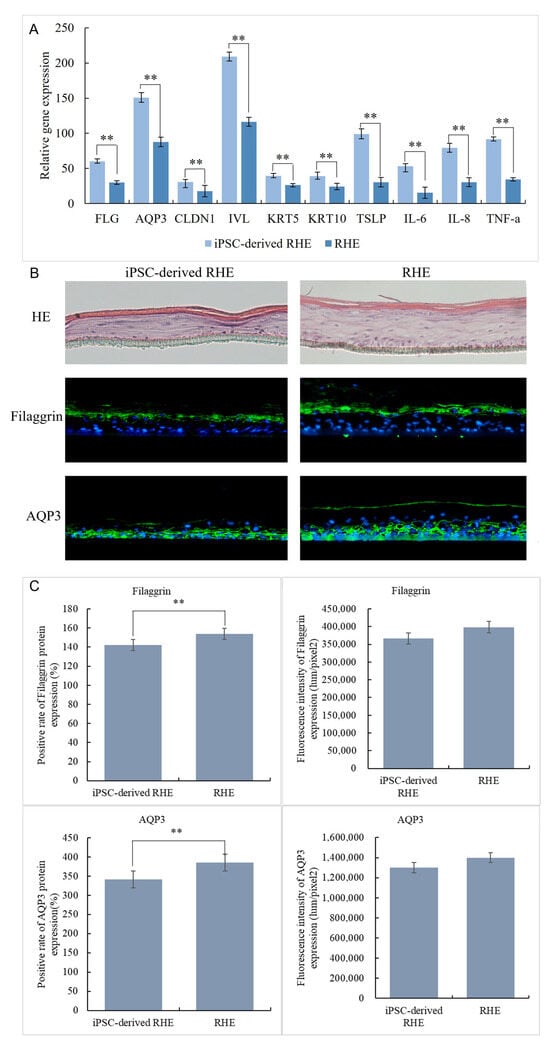
Figure 5.
iPSC-derived gene expression of RHE and RHE (A), HE staining results and expression on Filaggrin (Green signals) and AQP3 (Green signals) of iPSC-derived RHE and RHE (B), nucleus were stained with DAPI (Blue signals), immunofluorescence staining results of iPSC-derived RHE and RHE (C). ** Compared with the NC group, p < 0.01.
The immunofluorescence staining results of Filaggrin and AQP3 (Figure 5B,C) more intuitively show the structural differences between iPSC-derived-RHE and RHE. From the staining results, we can see that the Filaggrin positivity rate of iPSC-derived-RHE is 142%, while that of RHE is 154%. This result indicates that the granular layer and stratum corneum of iPSC-derived-RHE are thinner than those of RHE. However, the Filaggrin fluorescence intensity of iPSC-derived-RHE was 366,228 lum/pixel2, and that of RHE was 398,274 lum/pixel2, and the difference was not significant. This indicates that both the granular layer and stratum corneum of iPSC-derived-RHE are denser and more compact. Regarding the expression of AQP3, the positive rate of iPSC-derived-RHE was 340.9%, and the positive rate of RHE was 385%. This indicates that the water activity of iPSC-derived-RHE from the basal layer to the granular layer is extremely strong. Similarly, there was no significant difference between the fluorescence intensity of iPSC-derived-RHE and that of RHE. This indicates that iPSC-derived-RHE has more dense water channels in fewer skin layers.
3.4. Skin Irritation Tests
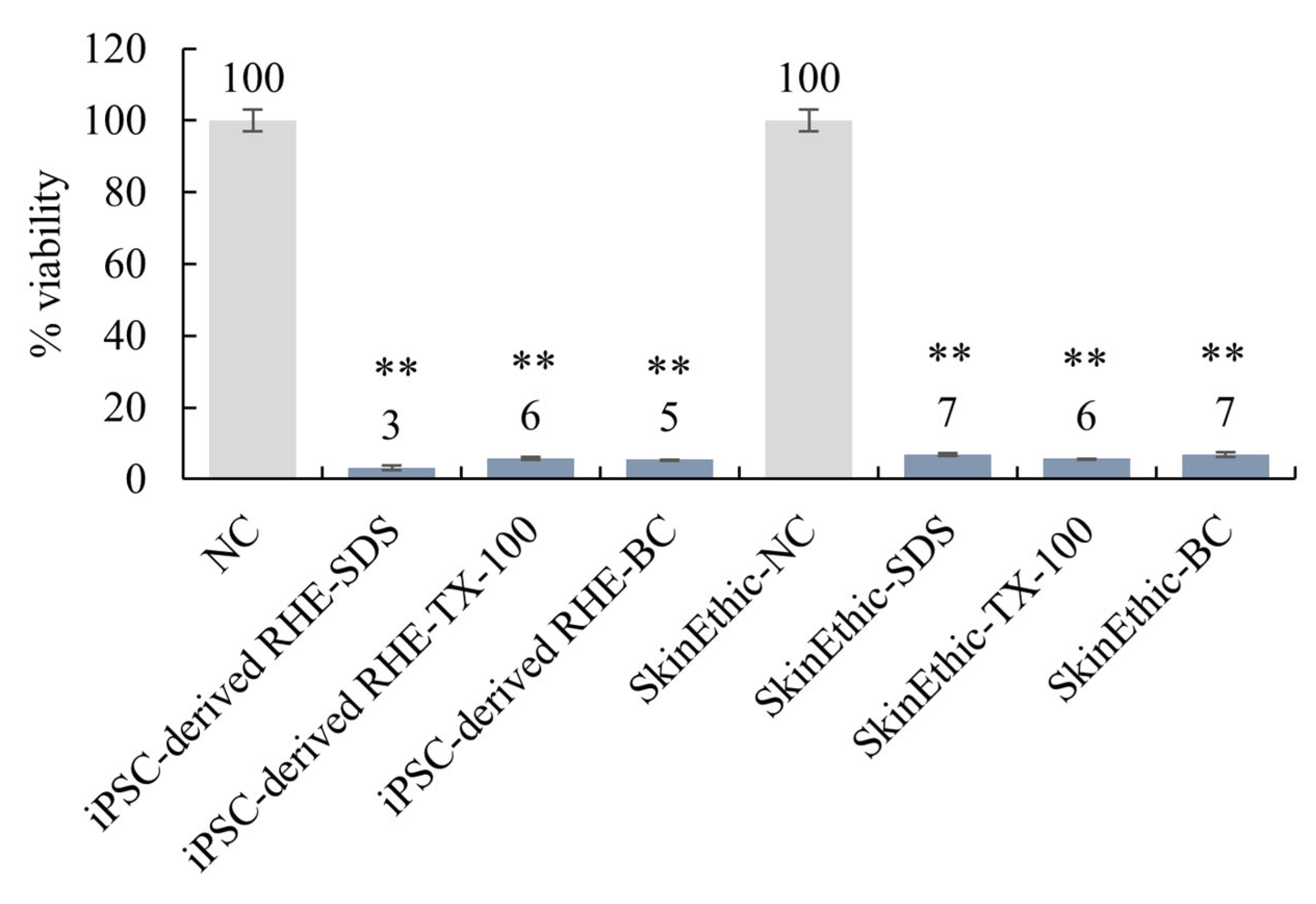
Several test chemicals with known irritation potentials were applied to the surface of the reconstructed skin model for skin irritation tests. The MTT assay was used to evaluate the viability of the keratinocytes after exposure to the test chemicals. The results show that the iPSC-derived-RHE predicted skin irritations in a manner similar to the commercially available SkinEthic system (Figure 6). Specifically, the skin model correctly predicted the irritation potential of 5% sodium dodecyl sulphate (SDS), 1% Triton X-100 (TX-100), and 1% benzalkonium chloride (BC). Methods refer to OECD Test Guideline No. 439.
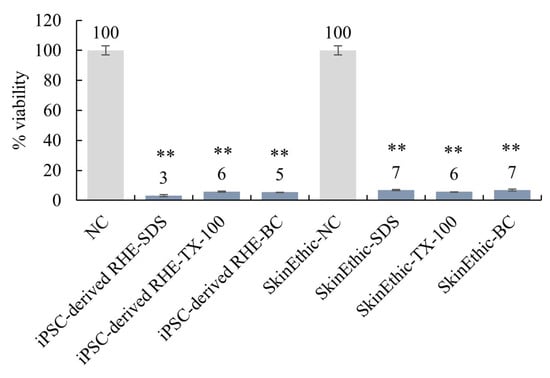
Figure 6.
iPSC-derived-RHE were compared to commercially available SkinEthic systems for skin irritation testing. ** Compared with the NC group, p < 0.01.
These findings demonstrate the successful development of a reconstructed epidermal skin model using iPSC-derived keratinocytes, which can be used for skin irritation testing and cosmetic research. The results also highlight the potential of this system as an alternative to animal testing and human clinical trials.
4. Discussion
iPSC technology can generate an unlimited number of genetically identical skin models that mimic the complexity of human skin very well, making it a promising new tool in the fields of dermatology and cosmetics.
In a previous study, Dubau et al. [24] also used iPSC-derived KC and FB to construct a full-thickness skin model containing both the dermis and epidermis. However, the model construction method was relatively complex and took a long time to build (25 days). The iPSC-derived RHE constructed in this study only contains the epidermis, and the construction method is simple and takes less time (18 days). iPSC-derived RHE is more suitable for dermatology research and cosmetic testing fields that require more batch and repetitive testing of the epidermis.
In this study, we demonstrated the successful development of a reconstructed epidermal skin model using iPSC-KC, which showed no significant differences from the commercially available SkinEthic system in predicting skin irritations. This indicates that our iPSC-derived RHE is a reliable and effective tool for skin irritation testing.
The use of iPSC-derived epidermal skin models offers several advantages over traditional skin models. Firstly, they can be generated in unlimited quantities, eliminating the limitations imposed by donor variability and cell lifespan. Secondly, they can be used to study specific skin diseases or conditions without the need for multiple donors. Finally, they can be employed to test the safety and efficacy of cosmetic products and pharmaceuticals, reducing the need for animal testing and human clinical trials.
The use of immortalized cell lines has been proposed as an alternative to primary cell lines, but few immortalized NHEK cell lines are available, and most do not form a fully stratified epithelium. In contrast, iPSCs have been used to generate skin cells for decades, and their potential in this field is vast.
iPSC-derived RHE also has some limitations, including the long differentiation and culture time of iPSC-KCs, and the stability needs to be further optimized. This is also a problem to be solved in subsequent research.
5. Conclusions
In this study, we successfully constructed an iPSC-derived RHE that is not limited by donor type and cell lifespan by inducing differentiation of KC from iPSC. Compared with RHE, iPSC-derived RHE has higher expression of FLG, AQP3, CLDN1, IVL, KRT5, KRT10, TSLP, IL-6, IL-8, and TNF-a. At the same time, in the skin irritation comparison experiment with SkinEthic, the irritation potential of SLS, TX-100, and BC was correctly predicted. The potential of iPSC-derived RHE as a substitute for traditional skin models is confirmed. The model uses iPSC technology and is expected to be widely used in the fields of dermatology research and cosmetic testing. The development of this more accurate and reliable in vitro model could reduce the need for animal testing and human clinical trials.
Author Contributions
Conceptualization, methodology, formal analysis, data curation, writing—original draft preparation, T.X.; software, validation and investigation, W.Q.; writing—review and editing and project administration, T.J.; supervision, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting this article’s conclusions are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Pigeon Manufacturing (Shanghai) Co, Ltd. for financial and sample support.
Conflicts of Interest
Author Tong Xie, Wu Qiao, Tinghan Jia, Ken Kaku was employed by the company Pigeon Manufacturing (Shanghai) Co., Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Stephens, M.L.; Goldberg, A.M.; Rowan, A.N. The first forty years of the alternatives approach: Refining, reducing, and replacing the use of laboratory animals. In The State of the Animals; Salem, D.J., Rowan, A.N., Eds.; Humane Society Press: Washington, DC, USA, 2001; pp. 121–135. [Google Scholar]
- Schmook, F.P.; Meingassner, J.G.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.P.; Niehues, H.; Rikken, G.; van Vlijmen-Willems, I.M.; van de Zande, G.W.; Zeeuwen, P.L.; Schalkwijk, J.; van den Bogaard, E.H. Immortalized N/TERT keratinocytes as an alternative cell source in 3D human epidermal models. Sci. Rep. 2017, 7, 11838. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Lee, C. Immortalization of primary keratinocytes and its application to skin research. Biomol. Ther. 2015, 23, 391. [Google Scholar] [CrossRef] [PubMed]
- Strudwick, X.L.; Lang, D.L.; Smith, L.E.; Cowin, A.J. Combination of low calcium with Y-27632 rock inhibitor increases the proliferative capacity, expansion potential and lifespan of primary human keratinocytes while retaining their capacity to differentiate into stratified epidermis in a 3D skin model. PLoS ONE 2015, 10, e0123651. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Zhong, H.; Ren, Z.; Wang, X.; Miao, K.; Ni, W.; Meng, Y.; Lu, L.; Wang, C.; Liu, W.; Deng, C.-X. Stagewise keratinocyte differentiation from human embryonic stem cells by defined signal transduction modulators. Int. J. Biol. Sci. 2020, 16, 1450. [Google Scholar] [CrossRef]
- Tchieu, J.; Zimmer, B.; Fattahi, F.; Amin, S.; Zeltner, N.; Chen, S.; Studer, L. A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell 2017, 21, 399–410.e397. [Google Scholar] [CrossRef]
- Li, A.; Simmons, P.J.; Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 3902–3907. [Google Scholar] [CrossRef]
- Green, H.; Easley, K.; Iuchi, S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15625–15630. [Google Scholar] [CrossRef]
- Zangrossi, S.; Marabese, M.; Broggini, M.; Giordano, R.; D’Erasmo, M.; Montelatici, E.; Intini, D.; Neri, A.; Pesce, M.; Rebulla, P. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells 2007, 25, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Montserrat, N.; Aasen, T.; Gonzalez, F.; Rodríguez-Pizà, I.; Vassena, R.; Raya, A.; Boué, S.; Barrero, M.J.; Corbella, B.A. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell 2009, 5, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Drews, K.; Matz, P.; Adjaye, J. Generation of iPSC lines from primary human amniotic fluid cells. Stem Cell Res. 2015, 15, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.-L.; Munoz, A.; Nalula, G.; Santostefano, K.E.; Sanghez, V.; Sanchez, G.; Terada, N.; Mattis, A.N.; Iacovino, M.; Iribarren, C. Evaluation of commonly used ectoderm markers in iPSC trilineage differentiation. Stem Cell Res. 2019, 37, 101434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, M.; Zhang, L.-J. Keratin 6, 16 and 17—Critical barrier alarmin molecules in skin wounds and psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Sougrat, R.; Gobin, R.; Verbavatz, J.-M.; Morand, M.; Gondran, C.; Barré, P.; Bonté, F.; Dumas, M. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Investig. Dermatol. 2002, 118, 678–685. [Google Scholar] [CrossRef]
- Ghahary, A.; Marcoux, Y.; Karimi-Busheri, F.; Tredget, E.E. Keratinocyte differentiation inversely regulates the expression of involucrin and transforming growth factor β1. J. Cell. Biochem. 2001, 83, 239–248. [Google Scholar] [CrossRef]
- Schmidt, A.D. Understanding the Role of Involucrin in Skin Inflammation. Ph.D. Thesis, Washington University in St. Louis, St. Louis, MI, USA, 2022. [Google Scholar]
- Kim, Y.; Lim, K.-M. Skin barrier dysfunction and filaggrin. Arch. Pharmacal Res. 2021, 44, 36–48. [Google Scholar] [CrossRef]
- Kezic, S.; Jakasa, I. Filaggrin and skin barrier function. Ski. Barrier Funct. 2016, 49, 1–7. [Google Scholar]
- Itoh, A.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J. Gastroenterol. Hepatol. 2003, 18, 203–210. [Google Scholar] [CrossRef]
- Kezic, S.; Kemperman, P.M.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, W.I.; Puppels, G.J.; Caspers, P.J. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef] [PubMed]
- Dubau, M.; Tripetchr, T.; Mahmoud, L.; Kral, V.; Kleuser, B. Advancing skin model development: A focus on a self-assembled, induced pluripotent stem cell-derived, xeno-free approach. J. Tissue Eng. 2024, 15, 20417314241291848. [Google Scholar] [CrossRef] [PubMed]
- Andres, E.; Barry, M.; Hundt, A.; Dini, C.; Corsini, E.; Gibbs, S.; Roggen, E.; Ferret, P.J. Preliminary performance data of the RHE/IL-18 assay performed on SkinEthic™ RHE for the identification of contact sensitizers. Int. J. Cosmet. Sci. 2017, 39, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Xie, T.; Lu, J.; Jia, T.; Kaku, K. Identification of potential hub genes associated with atopic dermatitis-like recombinant human epidermal model using integrated transcriptomic and proteomic analysis. Biomol. Biomed. 2024, 24, 89. [Google Scholar] [CrossRef]
- Guideline, P.-B.T. OECD guideline for the testing of chemicals. Hershberger 2001, 601, 858. [Google Scholar]
- Sah, S.K.; Kanaujiya, J.K.; Chen, I.-P.; Reichenberger, E.J. Generation of keratinocytes from human induced pluripotent stem cells under defined culture conditions. Cell. Reprogramming 2021, 23, 1–13. [Google Scholar] [CrossRef]
- Visscher, M.O.; Carr, A.N.; Winget, J.; Huggins, T.; Bascom, C.C.; Isfort, R.; Lammers, K.; Narendran, V. Biomarkers of neonatal skin barrier adaptation reveal substantial differences compared to adult skin. Pediatr. Res. 2021, 89, 1208–1215. [Google Scholar] [CrossRef]
- Ågren, J.; Zelenin, S.; Håkansson, M.; Eklöf, A.-C.; Aperia, A.; Nejsum, L.N.; Nielsen, S.; Sedin, G. Transepidermal water loss in developing rats: Role of aquaporins in the immature skin. Pediatr. Res. 2003, 53, 558–565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).