Photoprotective Effect and Potential Mechanisms of Gardeniae Fructus Extract in UVB-Irradiated HaCaT Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Analysis of GFE
2.2. Cell Culture and UVB Irradiation
2.3. Detection of Reactive Oxygen Species (ROS), SOD, CAT, GSH-Px, and MDA
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Immunofluorescence Staining
2.6. Proteomic Analysis
2.7. Network Pharmacology
2.8. Statistical Analysis
3. Results
3.1. Analysis of GFE by UPLC-MS/MS
3.2. Effects of GFE on Antioxidants in HaCaT Cells
3.3. Effects of GFE on Anti-Inflammation in HaCaT Cells
3.4. Effects of GFE on Barrier Repair in Three-Dimensional Epidermal Models
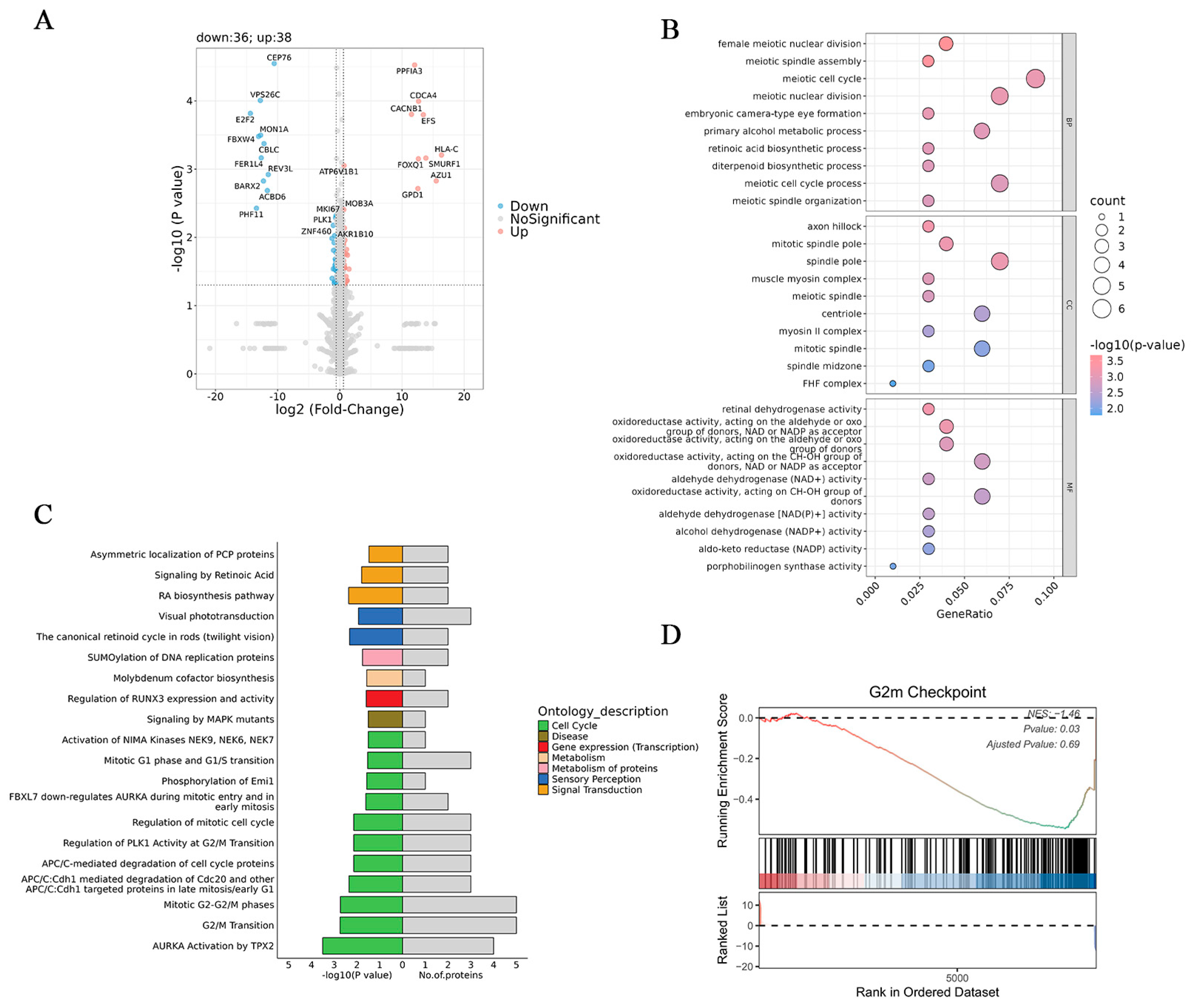
3.5. Proteomic Analysis
3.6. Network Pharmacology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Liu, T.; Jia, X.; Huang, X.; Qin, X.; Wang, X.; Sheng, J.; Xu, H. ATP supplementation suppresses UVB-induced photoaging in HaCaT cells via upregulation of expression of SIRT3 and SOD2. Ski. Res. Technol. 2023, 29, e13303. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.; Diepgen, T.L.; Green, A.C.; van der Pols, J.C.; Bernard, B.A.; Ly, F.; Bernerd, F.; et al. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019, 181, 916–931. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Lee, M.; Han, S.; Jun, W.; Jung, H.M.; Koo, Y.K.; Na, G.H.; Han, S.H.; Han, J.; et al. Enzyme-Treated Caviar Prevents UVB Irradiation-Induced Skin Photoaging. Mar. Drugs 2022, 20, 685. [Google Scholar] [CrossRef]
- Md Jaffri, J. Reactive Oxygen Species and Antioxidant System in Selected Skin Disorders. Malays. J. Med. Sci. 2023, 30, 7–20. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3947. [Google Scholar] [CrossRef]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef] [PubMed]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Angelopoulou, M.; Rizou, S.V.; Pratsinis, H.; Gorgoulis, V.G.; Kletsas, D. Activation of the JNKs/ATM-p53 axis is indispensable for the cytoprotection of dermal fibroblasts exposed to UVB radiation. Cell Death Dis. 2022, 13, 647. [Google Scholar] [CrossRef]
- Mayangsari, E.; Mustika, A.; Nurdiana, N.; Samad, N.A. Comparison of UVA vs UVB Photoaging Rat Models in Short-term Exposure. Med. Arch. 2024, 78, 88–91. [Google Scholar] [CrossRef]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Tan, H.; Ren, H.; Chai, J.; Zhai, C.; Li, T.; Zhou, X.; Lee, J.; Li, X.; Zhao, Y. Protective effect of ginseng berry saponin conversion products on skin photodamage caused by UVB in vitro and in vivo. Food Res. Int. 2024, 198, 115379. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Y.; An, Q.; Wang, D.; You, S.; Zhao, D.; Zhang, J.; Wang, C.; Li, M. Anti-Photoaging Effect of Rhodiola rosea Fermented by Lactobacillus plantarum on UVA-Damaged Fibroblasts. Nutrients 2022, 14, 2324. [Google Scholar] [CrossRef]
- Guo, S.; Bao, L.; Li, C.; Sun, J.; Zhao, R.; Cui, X. Antiviral activity of iridoid glycosides extracted from Fructus Gardeniae against influenza A virus by PACT-dependent suppression of viral RNA replication. Sci. Rep. 2020, 10, 1897. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, M.R.; Lee, A.R.; Seo, B.I.; Park, H.J.; Roh, S.S. Improvement of Inflammation through Antioxidant Pathway of Gardeniae Fructus 50% EtOH Extract (GE) from Acute Reflux Esophagitis Rats. BioMed Res. Int. 2020, 2020, 4826176. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Ahn, S.H.; Nguyen, U.T.; Yang, I.J.; Shin, H.M. Geniposide, a Principal Component of Gardeniae Fructus, Protects Skin from Diesel Exhaust Particulate Matter-Induced Oxidative Damage. Evid. -Based Complement. Altern. Med. 2021, 2021, 8847358. [Google Scholar] [CrossRef]
- De Tollenaere, M.; Chapuis, E.; Martinez, J.; Paulus, C.; Dupont, J.; Don Simoni, E.; Robe, P.; Sennelier-Portet, B.; Auriol, D.; Scandolera, A.; et al. Gardenia jasminoides Extract, with a Melatonin-like Activity, Protects against Digital Stress and Reverses Signs of Aging. Int. J. Mol. Sci. 2023, 24, 4948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lai, Q.; Li, Y.; Liu, Y.; Yang, M. Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J. Ethnopharmacol. 2017, 196, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hino, S.; Horie, N.; Shimoyama, T.; Kaneko, T.; Kusama, K.; Sakagami, H. Anti-UV activity of Kampo medicines and constituent plant extracts: Re-evaluation with skin keratinocyte system. Vivo 2014, 28, 571–578. [Google Scholar]
- Shin, D.; Lee, S.; Huang, Y.H.; Lim, H.W.; Lee, Y.; Jang, K.; Cho, Y.; Park, S.J.; Kim, D.D.; Lim, C.J. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm. Biol. 2018, 56, 176–182. [Google Scholar] [CrossRef]
- Dong, J.; Li, B.; Xu, P.; Kong, L.; Zhang, W.; Wu, D.; Tian, Y.; Wang, X. Skin barrier repair efficacy and safety evaluation of an extract from Gardenia jasminoides J. Ellis. Nat. Prod. Res. 2024, 1–7. [Google Scholar] [CrossRef]
- Ke, H.; Zhang, X.; Liang, S.; Zhou, C.; Hu, Y.; Huang, Q.; Wu, J. Study on the anti-skin aging effect and mechanism of Sijunzi Tang based on network pharmacology and experimental validation. J. Ethnopharmacol. 2024, 333, 118421. [Google Scholar] [CrossRef]
- Zong, K.; Xu, K.; Zhang, X.; Wang, P.; Wang, Z.; Yang, S.; Li, H.; Ke, H.; He, S.; Hu, Y.; et al. Explorating the mechanism of Huangqin Tang against skin lipid accumulation through network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 313, 116581. [Google Scholar] [CrossRef]
- Baek, J.; Kim, J.H.; Park, J.; Kim, D.H.; Sa, S.; Han, J.S.; Kim, W. 1-Kestose Blocks UVB-Induced Skin Inflammation and Promotes Type I Procollagen Synthesis via Regulating MAPK/AP-1, NF-κB and TGF-β/Smad Pathway. J. Microbiol. Biotechnol. 2024, 34, 911–919. [Google Scholar] [CrossRef]
- Jang, A.Y.; Choi, J.; Rod-In, W.; Choi, K.Y.; Lee, D.H.; Park, W.J. In Vitro Anti-Inflammatory and Skin Protective Effects of Codium fragile Extract on Macrophages and Human Keratinocytes in Atopic Dermatitis. J. Microbiol. Biotechnol. 2024, 34, 940–948. [Google Scholar] [CrossRef]
- He, H.; Huang, W.; Xiong, L.; Ma, C.; Wang, Y.; Sun, P.; Shi, D.; Li, L.; Yan, H.; Wu, Y. FUNDC1-mediated mitophagy regulates photodamage independently of the PINK1/Parkin-dependent pathway. Free Radic. Biol. Med. 2024, 225, 630–640. [Google Scholar] [CrossRef]
- Tang, Z.; Tong, X.; Huang, J.; Liu, L.; Wang, D.; Yang, S. Research progress of keratinocyte-programmed cell death in UV-induced Skin photodamage. Photodermatol. Photoimmunol. Photomed. 2021, 37, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Di, F.; Li, L.; Pu, C.; Wang, C.; Zhang, J. Anti-Photodamage Effect of Agaricus blazei Murill Polysaccharide on UVB-Damaged HaCaT Cells. Int. J. Mol. Sci. 2024, 25, 4676. [Google Scholar] [CrossRef]
- Milutinov, J.; Pavlović, N.; Ćirin, D.; Atanacković Krstonošić, M.; Krstonošić, V. The Potential of Natural Compounds in UV Protection Products. Molecules 2024, 29, 5409. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In vivo SPF from multifunctional sunscreen systems developed with natural compounds-A review. J. Cosmet. Dermatol. 2021, 20, 729–737. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Rosado, C.; Tokunaga, V.K.; Sauce, R.; de Oliveira, C.A.; Sarruf, F.D.; Parise-Filho, R.; Maurício, E.; de Almeida, T.S.; Velasco, M.V.R.; Baby, A.R. Another Reason for Using Caffeine in Dermocosmetics: Sunscreen Adjuvant. Front. Physiol. 2019, 10, 519. [Google Scholar] [CrossRef]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Liu, S.; Biu, A.M.; Han, Y.; Jin, X.; Liang, C.; Liu, Y.; Li, J.; Fang, S.; et al. A comprehensive review of ethnopharmacology, chemical constituents, pharmacological effects, pharmacokinetics, toxicology, and quality control of gardeniae fructus. J. Ethnopharmacol. 2024, 320, 117397. [Google Scholar] [CrossRef]
- Sun, K.X.; Chen, Y.Y.; Li, Z.; Zheng, S.J.; Wan, W.J.; Ji, Y.; Hu, K. Genipin relieves diabetic retinopathy by down-regulation of advanced glycation end products via the mitochondrial metabolism related signaling pathway. World J. Diabetes 2023, 14, 1349–1368. [Google Scholar] [CrossRef]
- Li, H.; Yang, D.H.; Zhang, Y.; Zheng, F.; Gao, F.; Sun, J.; Shi, G. Geniposide suppresses NLRP3 inflammasome-mediated pyroptosis via the AMPK signaling pathway to mitigate myocardial ischemia/reperfusion injury. Chin. Med. 2022, 17, 73. [Google Scholar] [CrossRef]
- Chen, Y.I.; Cheng, Y.W.; Tzeng, C.Y.; Lee, Y.C.; Chang, Y.N.; Lee, S.C.; Tsai, C.C.; Chen, J.C.; Tzen, J.T.; Chang, S.L. Peroxisome proliferator-activated receptor activating hypoglycemic effect of Gardenia jasminoides Ellis aqueous extract and improvement of insulin sensitivity in steroid induced insulin resistant rats. BMC Complement. Altern. Med. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Guo, J.; Li, Z.; Xu, X.; Wang, J.; Zhai, L.; Liu, J.; Sun, G.; Wang, F.; Xu, Y.; et al. Screening and Research on Skin Barrier Damage Protective Efficacy of Different Mannosylerythritol Lipids. Molecules 2022, 27, 4648. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, A.; Gniadecki, R.; Calay, D.; Wulf, H.C. TNF-alpha impairs the S-G2/M cell cycle checkpoint and cyclobutane pyrimidine dimer repair in premalignant skin cells: Role of the PI3K-Akt pathway. J. Investig. Dermatol. 2008, 128, 2069–2077. [Google Scholar] [CrossRef]
- Chilampalli, C.; Guillermo, R.; Zhang, X.; Kaushik, R.S.; Young, A.; Zeman, D.; Hildreth, M.B.; Fahmy, H.; Dwivedi, C. Effects of magnolol on UVB-induced skin cancer development in mice and its possible mechanism of action. BMC Cancer 2011, 11, 456. [Google Scholar] [CrossRef]
- Chen, W.J.; Bao, T.Z.; Chen, K.; Zhu, C.M.; Wan, F.; Tan, Y.L.; Yan, F. [Effects of geniposide on SNP-induced apoptosis of chondrocyte and cell cycle]. China J. Orthop. Traumatol. 2013, 26, 232–235. [Google Scholar]
- Liu, F.J.; Chen, X.Y.; Yang, J.; Zhao, Z.; Wang, Q.L.; Li, P.; Jiang, Y.; Li, H.J. Revealing active components and action mechanism of Fritillariae Bulbus against non-small cell lung cancer through spectrum-effect relationship and proteomics. Phytomedicine 2023, 110, 154635. [Google Scholar] [CrossRef]

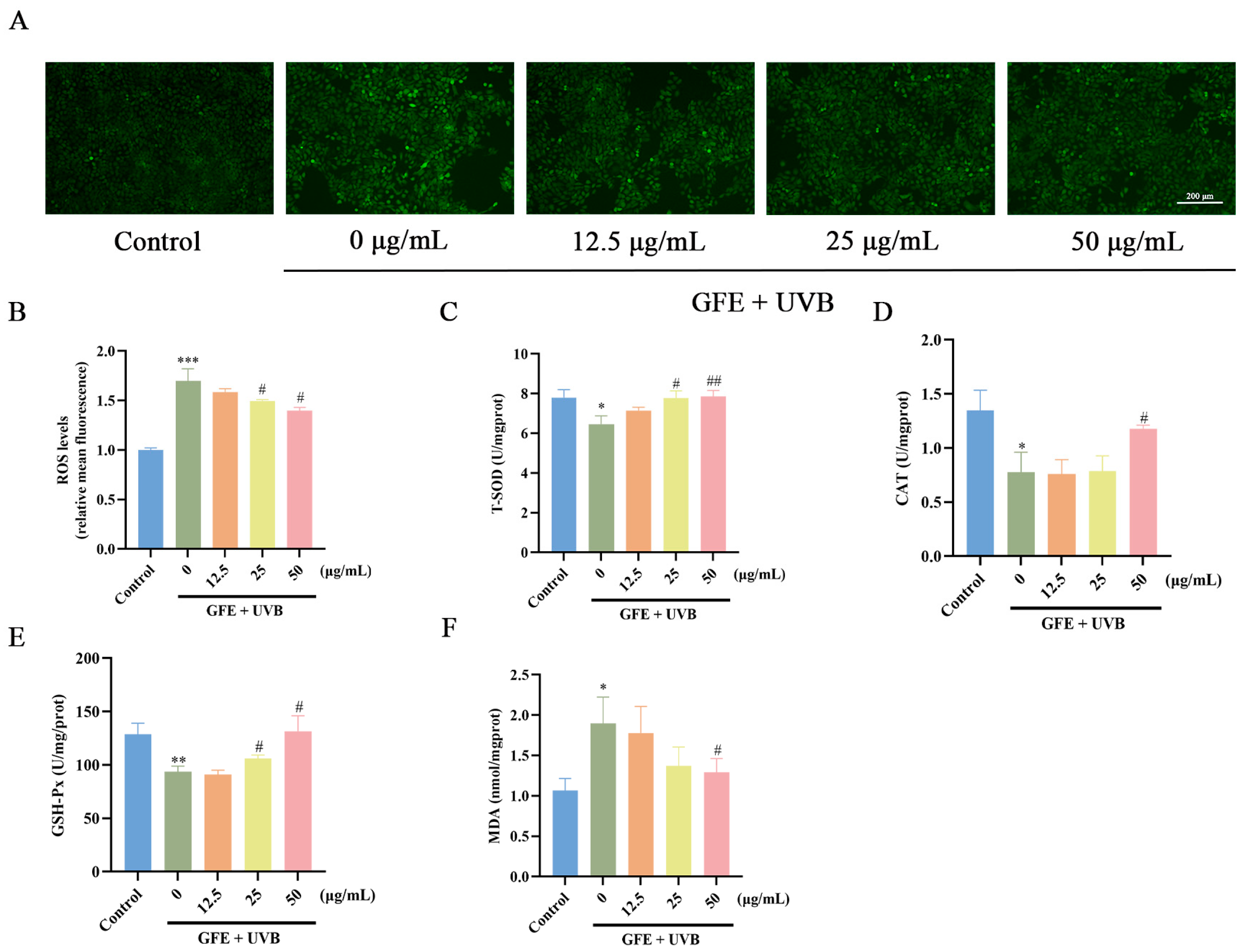
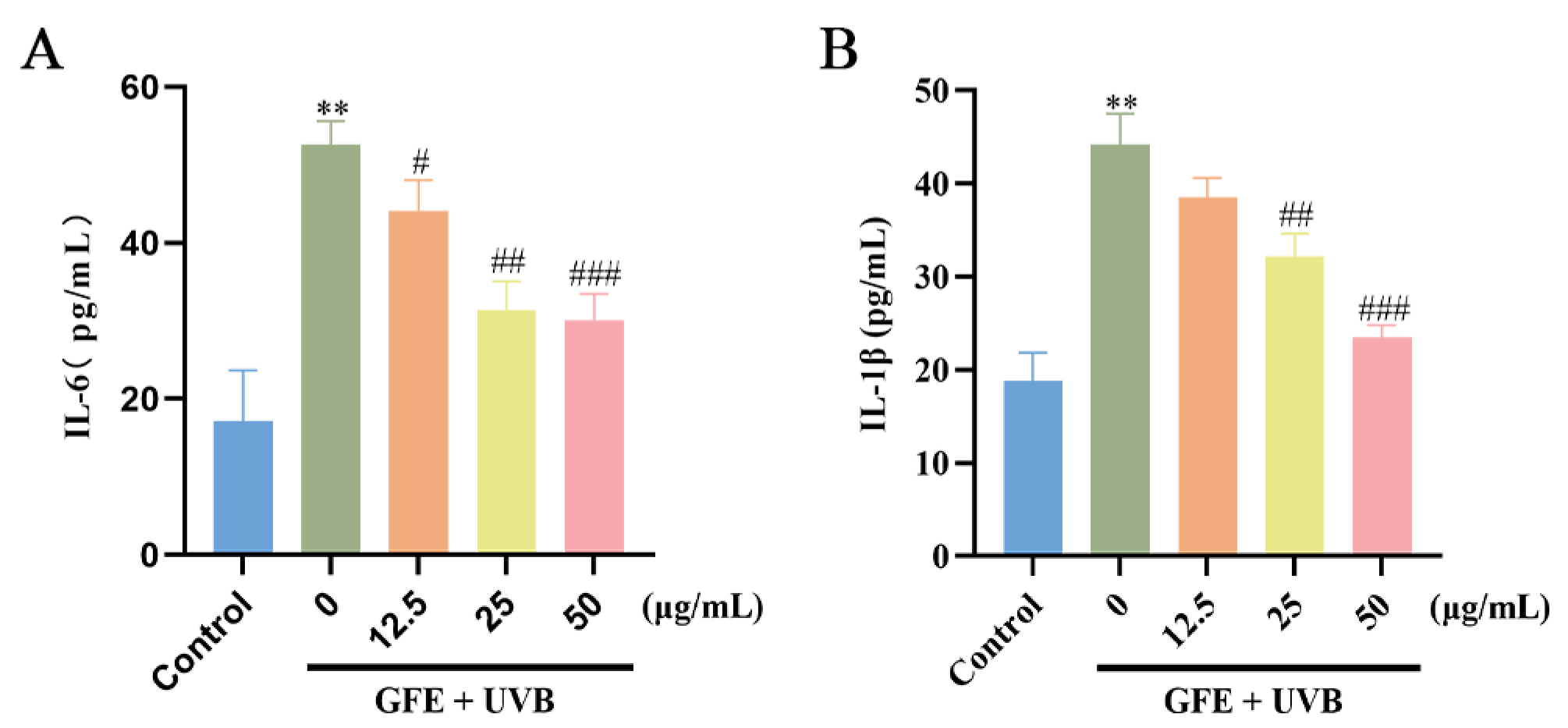
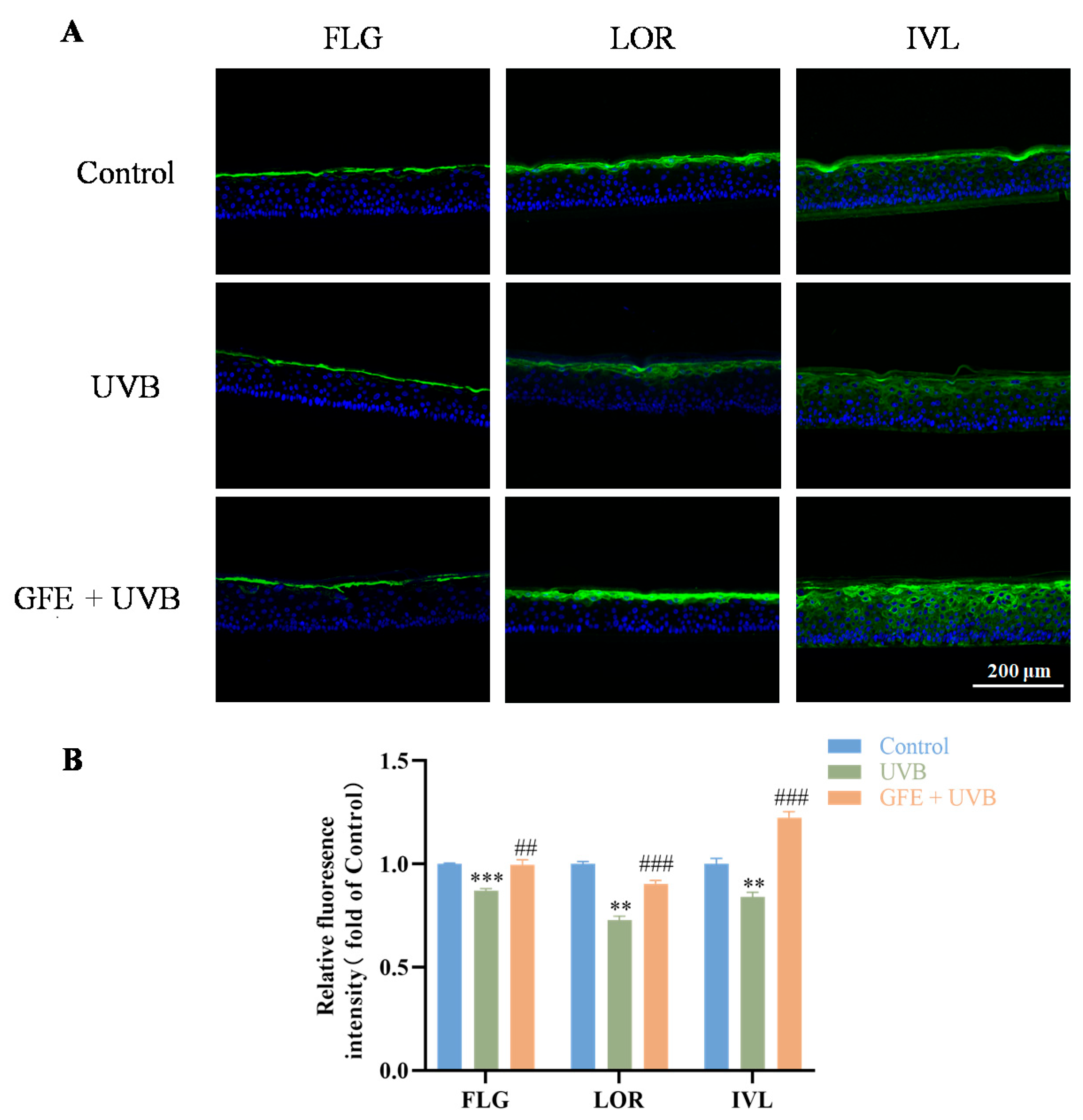
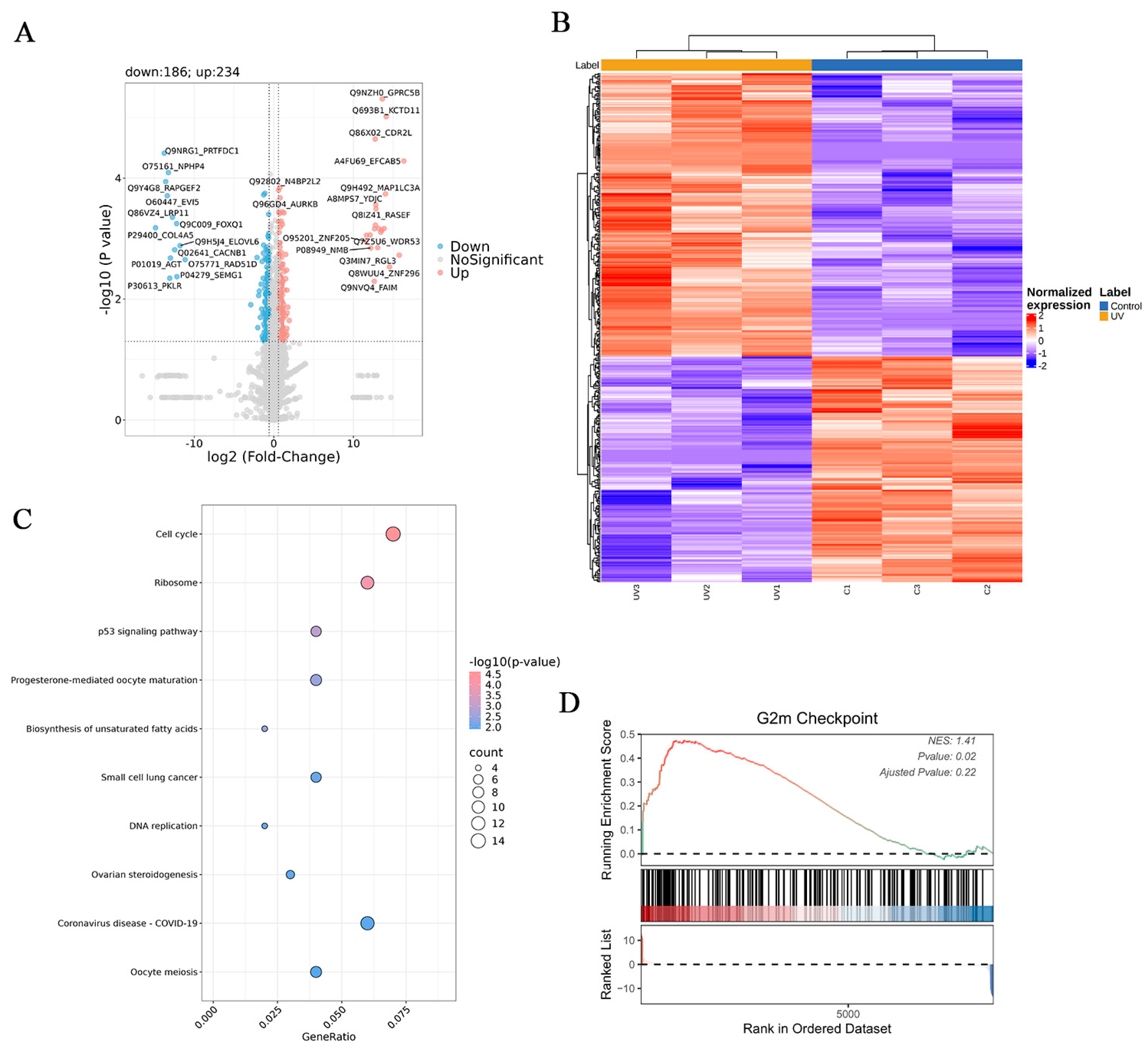

| No. | Target | Degree Centrality | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|
| 1 | IL1B | 14 | 0.302057308 | 0.7 |
| 2 | MMP9 | 11 | 0.124122346 | 0.6 |
| 3 | TP53 | 10 | 0.138510101 | 0.636363636 |
| 4 | BCL2 | 10 | 0.079234179 | 0.617647059 |
| 5 | SIRT1 | 8 | 0.187934962 | 0.583333333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, K.; Li, X.; Zhou, F.; Dong, J.; Huang, Q.; Wu, J. Photoprotective Effect and Potential Mechanisms of Gardeniae Fructus Extract in UVB-Irradiated HaCaT Cells. Cosmetics 2025, 12, 72. https://doi.org/10.3390/cosmetics12020072
Zong K, Li X, Zhou F, Dong J, Huang Q, Wu J. Photoprotective Effect and Potential Mechanisms of Gardeniae Fructus Extract in UVB-Irradiated HaCaT Cells. Cosmetics. 2025; 12(2):72. https://doi.org/10.3390/cosmetics12020072
Chicago/Turabian StyleZong, Kaile, Xiang Li, Fangni Zhou, Junzi Dong, Qing Huang, and Jianxin Wu. 2025. "Photoprotective Effect and Potential Mechanisms of Gardeniae Fructus Extract in UVB-Irradiated HaCaT Cells" Cosmetics 12, no. 2: 72. https://doi.org/10.3390/cosmetics12020072
APA StyleZong, K., Li, X., Zhou, F., Dong, J., Huang, Q., & Wu, J. (2025). Photoprotective Effect and Potential Mechanisms of Gardeniae Fructus Extract in UVB-Irradiated HaCaT Cells. Cosmetics, 12(2), 72. https://doi.org/10.3390/cosmetics12020072






