The Oral Intake of Specific Bovine-Derived Bioactive Collagen Peptides Has a Stimulatory Effect on Dermal Matrix Synthesis and Improves Various Clinical Skin Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. The Test Product
2.2. In Vitro Tests
2.3. Gene Expression Studies
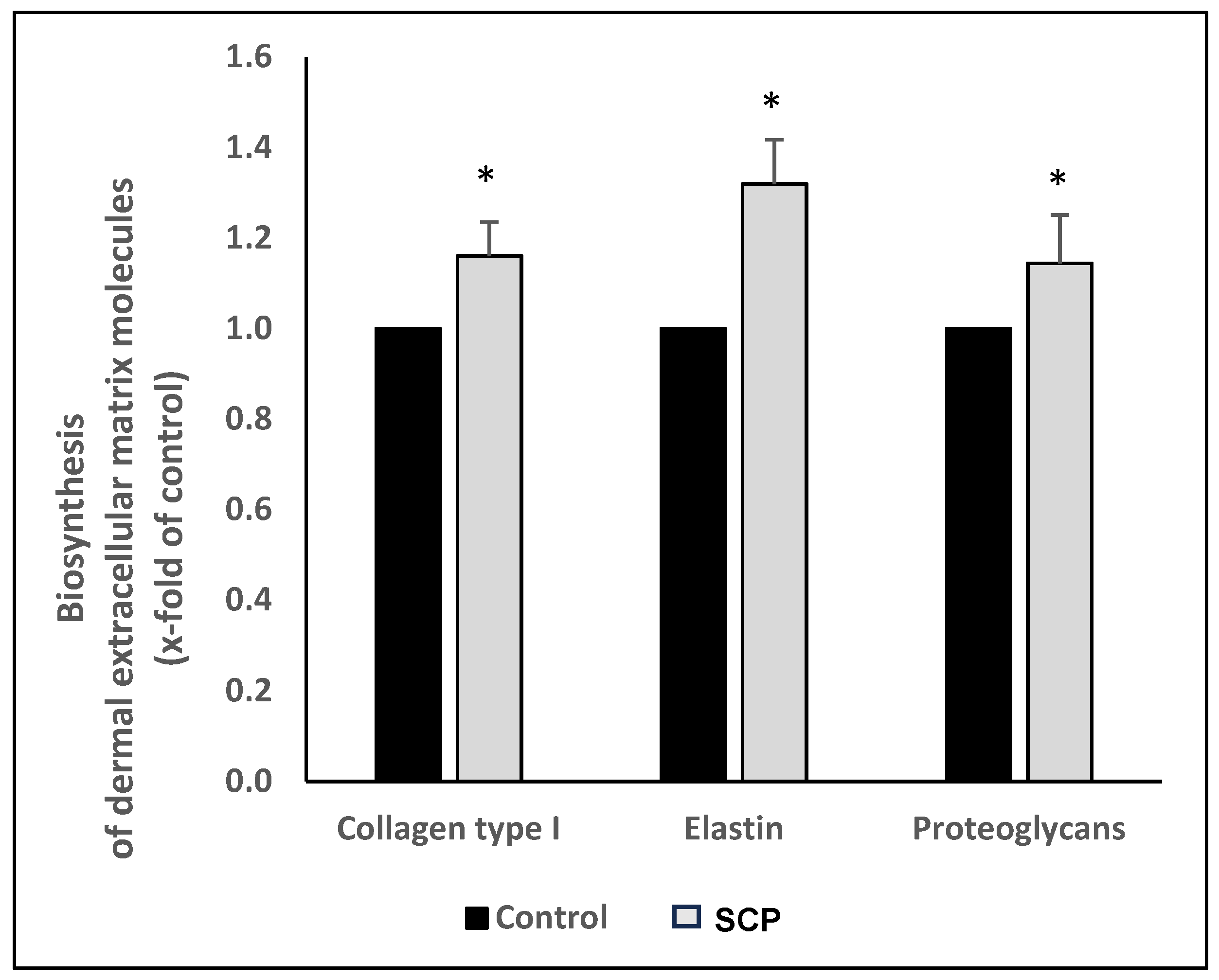
2.4. Measurement of the ECM Macromolecules
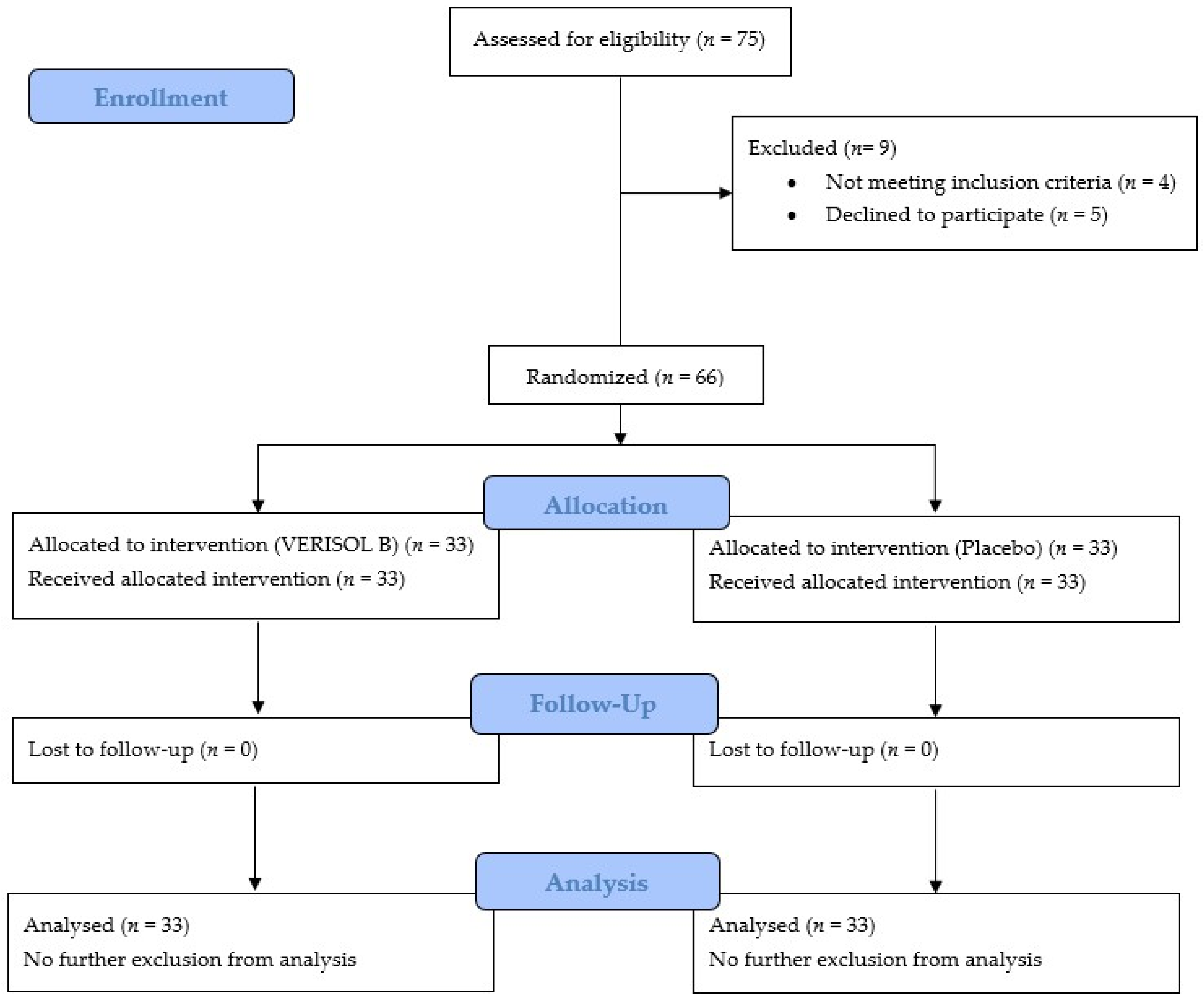
2.5. The Study Design of the Clinical Trial
2.6. The Inclusion and Exclusion Criteria
2.7. Subjects
2.8. Product Safety
2.9. Measurements
2.10. The Statistical Analysis
3. Results
3.1. The In Vitro Test
3.1.1. The Bioactivity of the SCPs
3.1.2. The Batch-to-Batch Analysis of the Test Product
3.2. The Clinical Trial
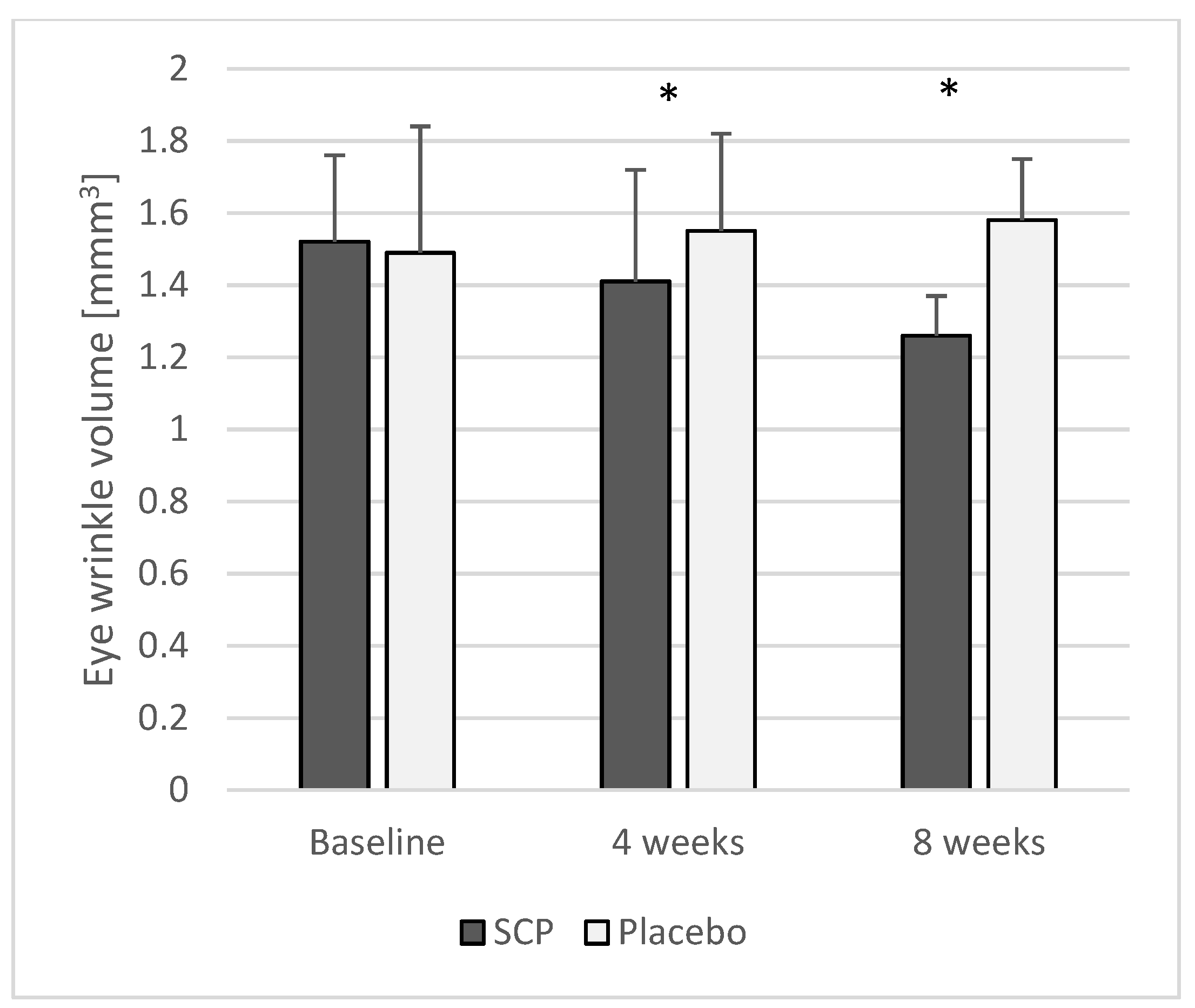
3.2.1. Eye Wrinkle Volume
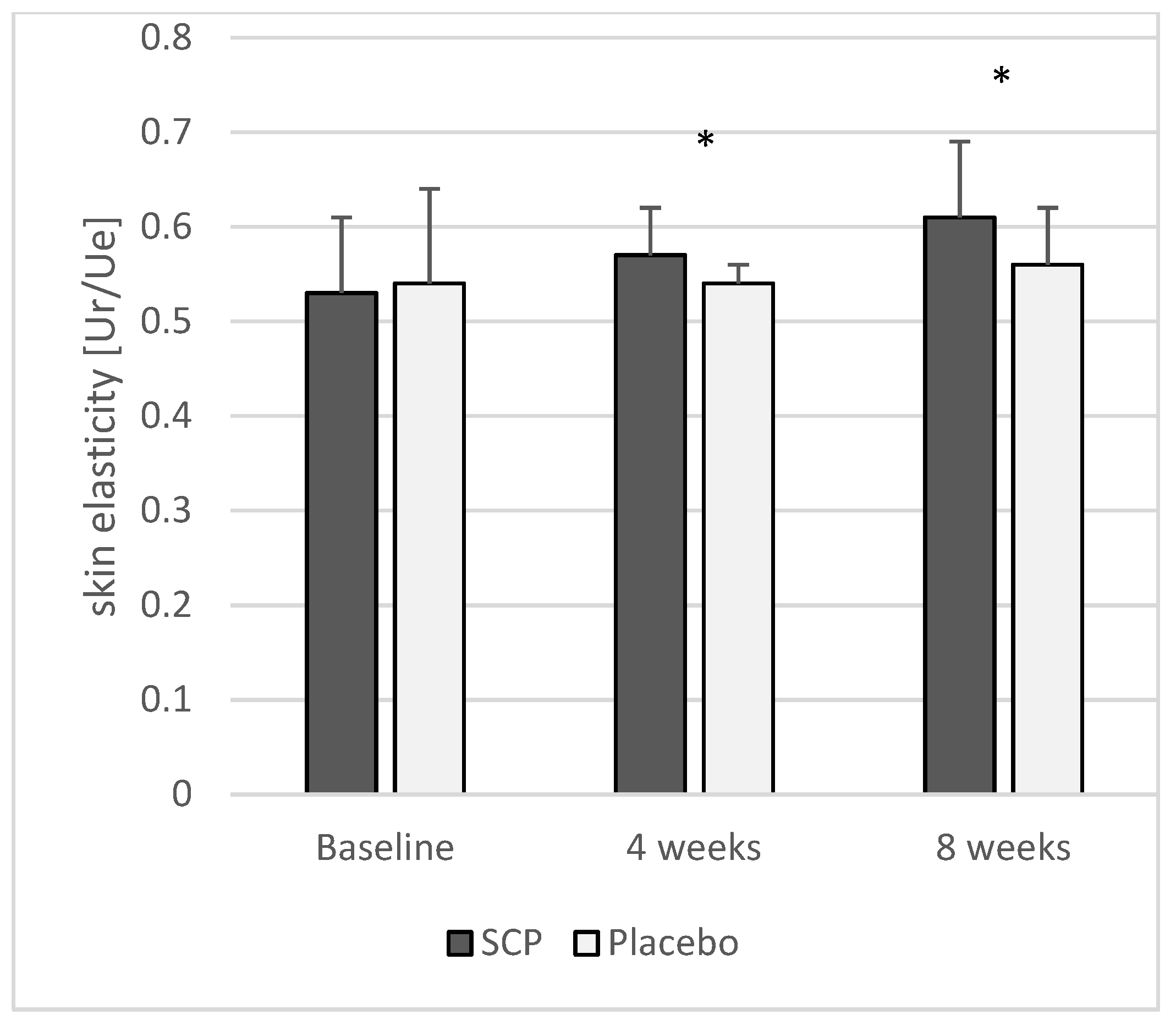
3.2.2. Skin Elasticity
3.2.3. Skin Hydration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic Acid |
| FCS | Fetal Calf Serum |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase |
| GCP | Good Clinical Practice |
| GRAS | Generally Recognized As Safe |
| ITT | Intention-to-Treat |
| kD | Kilodalton |
| KS | Kolmogorov–Smirnov |
| PP | Per-Protocol |
| RCT | Randomized Controlled Trial |
| RT-PCR | Real-Time Polymerase Chain Reaction |
| SCP | Specific Collagen Peptide |
| SPSS | Statistical Package for the Social Sciences |
| TM | Melting Temperature |
| UV | Ultraviolet |
References
- Smalls, L.K.; Randall Wickett, R.; Visscher, M.O. Effect of Dermal Thickness, Tissue Composition, and Body Site on Skin Biomechanical Properties. Skin Res. Technol. 2006, 12, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Agius, J.; Muscat-Baron, Y.; Brincat, M.P. Skin Ageing. Menopause Int. 2007, 13, 60–64. [Google Scholar] [CrossRef]
- Takema, Y.; Yorimoto, Y.; Kawai, M.; Imokawa, G. Age-Related Changes in the Elastic Properties and Thickness of Human Facial Skin. Br. J. Dermatol. 1994, 131, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Takema, Y.; Yorimoto, Y.; Kawai, M. The Relationship between Age-Related Changes in the Physical Properties and Development of Wrinkles in Human Facial Skin. J. Soc. Cosmet. Chem. 1995, 46, 163–173. [Google Scholar]
- Boelsma, E.; Hendriks, H.F.; Roza, L. Nutritional Skin Care: Health Effects of Micronutrients and Fatty Acids. Am. J. Clin. Nutr. 2001, 73, 853–864. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Cho, S. The Role of Functional Foods in Cutaneous Anti-Aging. J. Lifestyle Med. 2014, 4, 8–16. [Google Scholar] [CrossRef]
- Czajka, A.; Kania, E.M.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily Oral Supplementation with Collagen Peptides Combined with Vitamins and Other Bioactive Compounds Improves Skin Elasticity and Has a Beneficial Effect on Joint and General Wellbeing. Nutr. Res. 2018, 57, 97–108. [Google Scholar] [CrossRef]
- Liang, J.; Pei, X.; Zhang, Z.; Wang, N.; Wang, J.; Li, Y. The Protective Effects of Long-Term Oral Administration of Marine Collagen Hydrolysate from Chum Salmon on Collagen Matrix Homeostasis in the Chronological Aged Skin of Sprague-Dawley Male Rats. J. Food Sci. 2010, 75, H230–H238. [Google Scholar] [CrossRef]
- Lin, P.; Alexander, R.A.; Liang, C.-H.; Liu, C.; Lin, Y.-H.; Lin, Y.-H.; Chan, L.-P.; Kuan, C.-M. Collagen Formula with Djulis for Improvement of Skin Hydration, Brightness, Texture, Crow’s Feet, and Collagen Content: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Cosmet. Dermatol. 2021, 20, 188–194. [Google Scholar] [CrossRef]
- Morgado-Carrasco, D.; Gil-Lianes, J.; Jourdain, E.; Piquero-Casals, J. Oral Supplementation and Systemic Drugs for Skin Aging: A Narrative Review. Actas Dermosifiliogr. 2023, 114, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Meziane, S. On the Potential Role of the Antioxidant Couple Vitamin E/Selenium Taken by the Oral Route in Skin and Hair Health. Antioxidants 2022, 11, 2270. [Google Scholar] [CrossRef]
- Tarshish, E.; Hermoni, K.; Sharoni, Y.; Muizzuddin, N. Effect of Lumenato Oral Supplementation on Plasma Carotenoid Levels and Improvement of Visual and Experiential Skin Attributes. J. Cosmet. Dermatol. 2022, 21, 4042–4052. [Google Scholar] [CrossRef]
- Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, C.; Liu, K.; Feng, R.; Zhao, Y.; Zong, Y.; Du, R. Oral Administration of Deer Bone Collagen Peptide Can Enhance the Skin Hydration Ability and Antioxidant Ability of Aging Mice Induced by D-Gal, and Regulate the Synthesis and Degradation of Collagen. Nutrients 2024, 16, 1548. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Nutrition and Enhancing Youthful-Appearing Skin. Clin. Dermatol. 2010, 28, 400–408. [Google Scholar] [CrossRef]
- Bagheri Miyab, K.; Alipoor, E.; Vaghardoost, R.; Saberi Isfeedvajani, M.; Yaseri, M.; Djafarian, K.; Hosseinzadeh-Attar, M.J. The Effect of a Hydrolyzed Collagen-Based Supplement on Wound Healing in Patients with Burn: A Randomized Double-Blind Pilot Clinical Trial. Burns 2020, 46, 156–163. [Google Scholar] [CrossRef]
- Venzin, C.; Jacot, V.; Berdichevsky, A.; Karol, A.A.; Seliktar, D.; von Rechenberg, B.; Nuss, K.M. Biocompatibility of Pegylated Fibrinogen and Its Effect on Healing of Full-Thickness Skin Defects: A Preliminary Study in Rats. J. Biotechnol. Biomater. 2016, 6, online. [Google Scholar] [CrossRef]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral Administration of Marine Collagen Peptides Prepared from Chum Salmon (Oncorhynchus Keta) Improves Wound Healing Following Cesarean Section in Rats. Food Nutr. Res. 2015, 59, 26411. [Google Scholar] [CrossRef]
- Zague, V. A New View Concerning the Effects of Collagen Hydrolysate Intake on Skin Properties. Arch. Dermatol. Res. 2008, 300, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Matsumoto, H.; Ito, K.; Iwai, K.; Sato, K. Comparison of Quantity and Structures of Hydroxyproline-Containing Peptides in Human Blood after Oral Ingestion of Gelatin Hydrolysates from Different Sources. J. Agric. Food Chem. 2007, 55, 1532–1535. [Google Scholar] [CrossRef]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of Food-Derived Collagen Peptides in Human Blood after Oral Ingestion of Gelatin Hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral Administration of (14)C Labeled Gelatin Hydrolysate Leads to an Accumulation of Radioactivity in Cartilage of Mice (C57/BL). J. Nutr. 1999, 129, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Schunck, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral Intake of Specific Bioactive Collagen Peptides Reduces Skin Wrinkles and Increases Dermal Matrix Synthesis. Skin Pharmacol. Physiol. 2014, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.; Chaudagne, C.; Bonté, F.; Meybeck, A. In Vitro Biosynthesis of Type I and III Collagens by Human Dermal Fibroblasts from Donors of Increasing Age. Mech. Ageing Dev. 1994, 73, 179–187. [Google Scholar] [CrossRef]
- Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.I.; Sotelo, C.G. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Mar. Drugs 2018, 16, 144. [Google Scholar] [CrossRef]
- Zague, V.; do Amaral, J.B.; Rezende Teixeira, P.; de Oliveira Niero, E.L.; Lauand, C.; Machado-Santelli, G.M. Collagen Peptides Modulate the Metabolism of Extracellular Matrix by Human Dermal Fibroblasts Derived from Sun-Protected and Sun-Exposed Body Sites. Cell Biol. Int. 2018, 42, 95–104. [Google Scholar] [CrossRef]
- Chai, H.-J.; Li, J.-H.; Huang, H.-N.; Li, T.-L.; Chan, Y.-L.; Shiau, C.-Y.; Wu, C.-J. Effects of Sizes and Conformations of Fish-Scale Collagen Peptides on Facial Skin Qualities and Transdermal Penetration Efficiency. J. Biomed. Biotechnol. 2010, 2010, 757301. [Google Scholar] [CrossRef]
- Choi, F.D.; Sung, C.T.; Juhasz, M.L.W.; Mesinkovsk, N.A. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J. Drugs Dermatol. 2019, 18, 9–16. [Google Scholar]
- Barati, M.; Jabbari, M.; Navekar, R.; Farahmand, F.; Zeinalian, R.; Salehi-Sahlabadi, A.; Abbaszadeh, N.; Mokari-Yamchi, A.; Davoodi, S.H. Collagen Supplementation for Skin Health: A Mechanistic Systematic Review. J. Cosmet. Dermatol. 2020, 19, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral Supplementation of Specific Collagen Peptides Has Beneficial Effects on Human Skin Physiology: A Double-Blind, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef]
- Oesser, S.; Schunk, M.; Proksch, E. Positive Effect of Fish-Derived Bioactive Collagen Peptides on Skin Health. Int. J. Nutraceuticals Funct. Foods Nov. Foods 2020, 1, 127–133. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.-Y.; Huang, Y.-L.; Pu, C.-M.; Kang, Y.-N.; Hoang, K.D.; Chen, K.-H.; Chen, C. Effects of Oral Collagen for Skin Anti-Aging: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2080. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of the Health Aspects of Gelatin as a Food Ingredient; Federation of American Societies for Experimental Biology: Bethesda, MD, USA; Life Sciences Research Office, Food and Drug Administration: Washington, DC, USA, 1975.
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 3.0). Available online: https://www.randomizer.org/about/ (accessed on 29 January 2013).
- 7 Final Report on the Safety Assessment of Hydrolyzed Collagen. J. Am. Coll. Toxicol. 1985, 4, 199–221. [CrossRef]
- Segger, D.; Schönlau, F. Supplementation with Evelle Improves Skin Smoothness and Elasticity in a Double-Blind, Placebo-Controlled Study with 62 Women. J. Dermatol. Treat. 2004, 15, 222–226. [Google Scholar] [CrossRef]
- Segger, D.; Matthies, A.; Saldeen, T. Supplementation with Eskimo Skin Care Improves Skin Elasticity in Women. A Pilot Study. J. Dermatol. Treat. 2008, 19, 279–283. [Google Scholar] [CrossRef]
- Krueger, N.; Luebberding, S.; Oltmer, M.; Streker, M.; Kerscher, M. Age-Related Changes in Skin Mechanical Properties: A Quantitative Evaluation of 120 Female Subjects. Skin Res. Technol. 2011, 17, 141–148. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of Age and Regional Differences on Skin Elasticity as Measured by the Cutometer. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef]
- Batisse, D.; Bazin, R.; Baldeweck, T.; Querleux, B.; Lévêque, J.-L. Influence of Age on the Wrinkling Capacities of Skin. Skin Res. Technol. 2002, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Frei, V.; Perrier, E.; Orly, I.; Huc, A.; Augustin, C.; Damour, O. Activation of Fibroblast Metabolism in a Dermal and Skin Equivalent Model: A Screening Test for Activity of Peptides. Int. J. Cosmet. Sci. 1998, 20, 159–173. [Google Scholar] [CrossRef]
- Paul, C.; Maumus-Robert, S.; Mazereeuw-Hautier, J.; Guyen, C.N.; Saudez, X.; Schmitt, A.M. Prevalence and Risk Factors for Xerosis in the Elderly: A Cross-Sectional Epidemiological Study in Primary Care. Dermatology 2011, 223, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, Y.; Edwards, C.; Pearse, A.; Matsumoto, K.; Kawai, M.; Marks, R. Degenerative Alterations of Dermal Collagen Fiber Bundles in Photodamaged Human Skin and UV-Irradiated Hairless Mouse Skin: Possible Effect on Decreasing Skin Mechanical Properties and Appearance of Wrinkles. J. Investig. Dermatol. 2001, 117, 1458–1463. [Google Scholar] [CrossRef]
- Sumino, H.; Ichikawa, S.; Abe, M.; Endo, Y.; Ishikawa, O.; Kurabayashi, M. Effects of Aging, Menopause, and Hormone Replacement Therapy on Forearm Skin Elasticity in Women. J. Am. Geriatr. Soc. 2004, 52, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Vuillermoz, B.; Wegrowski, Y.; Contet-Audonneau, J.-L.; Danoux, L.; Pauly, G.; Maquart, F.-X. Influence of Aging on Glycosaminoglycans and Small Leucine-Rich Proteoglycans Production by Skin Fibroblasts. Mol. Cell. Biochem. 2005, 277, 63–72. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin Collagen through the Lifestages: Importance for Skin Health and Beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Zague, V.; de Freitas, V.; da Costa Rosa, M.; de Castro, G.Á.; Jaeger, R.G.; Machado-Santelli, G.M. Collagen Hydrolysate Intake Increases Skin Collagen Expression and Suppresses Matrix Metalloproteinase 2 Activity. J. Med. Food 2011, 14, 618–624. [Google Scholar] [CrossRef]
- Matsuda, N.; Koyama, Y.; Hosaka, Y.; Ueda, H.; Watanabe, T.; Araya, T.; Irie, S.; Takehana, K. Effects of Ingestion of Collagen Peptide on Collagen Fibrils and Glycosaminoglycans in the Dermis. J. Nutr. Sci. Vitaminol. 2006, 52, 211–215. [Google Scholar] [CrossRef]
- Tanaka, M.; Koyama, Y.; Nomura, Y. Effects of Collagen Peptide Ingestion on UV-B-Induced Skin Damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral Administration of Marine Collagen Peptides from Chum Salmon Skin Enhances Cutaneous Wound Healing and Angiogenesis in Rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef]
- Lin, P.; Hua, N.; Hsu, Y.-C.; Kan, K.-W.; Chen, J.-H.; Lin, Y.-H.; Lin, Y.-H.; Kuan, C.-M. Oral Collagen Drink for Antiaging: Antioxidation, Facilitation of the Increase of Collagen Synthesis, and Improvement of Protein Folding and DNA Repair in Human Skin Fibroblasts. Oxid. Med. Cell. Longev. 2020, 2020, 8031795. [Google Scholar] [CrossRef] [PubMed]
- Prokopová, A.; Pavlačková, J.; Mokrejš, P.; Gál, R. Collagen Hydrolysate Prepared from Chicken By-Product as a Functional Polymer in Cosmetic Formulation. Molecules 2021, 26, 2021. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Rangel, M.A.R.; Oliveira, C.R.; da Silva Olímpio, F.R.; Aimbire, F.; Mateus-Silva, J.R.; Chaluppe, F.A.; Vieira, R.P. Hydrolyzed Collagen Induces an Anti-Inflammatory Response That Induces Proliferation of Skin Fibroblast and Keratinocytes. Nutrients 2022, 14, 4975. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, D.; Park, S.-H.; Jung, J.; Cho, W.; Yu, A.R.; Lee, J. Fish Collagen Peptide (Naticol®) Protects the Skin from Dryness, Wrinkle Formation, and Melanogenesis Both In Vitro and In Vivo. Prev. Nutr. Food Sci. 2022, 27, 423–435. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Bouvret, E.; Le Faouder, J.; Roux, V.; Macian, N.; Pickering, G.; Wittrant, Y. Benefits of Circulating Human Metabolites from Fish Cartilage Hydrolysate on Primary Human Dermal Fibroblasts, an Ex Vivo Clinical Investigation for Skin Health Applications. Nutrients 2022, 14, 5027. [Google Scholar] [CrossRef]
- Bauza, E.; Oberto, G.; Berghi, A.; Dal, C.F.; Domloge, N. Collagen-like Peptide Exhibits a Remarkable Antiwrinkle Effect on the Skin When Topically Applied: In Vivo Study. Int. J. Tissue React. 2004, 26, 105–111. [Google Scholar]
- Maia Campos, P.M.B.G.; Melo, M.O.; Siqueira César, F.C. Topical Application and Oral Supplementation of Peptides in the Improvement of Skin Viscoelasticity and Density. J. Cosmet. Dermatol. 2019, 18, 1693–1699. [Google Scholar] [CrossRef]
- Sangsuwan, W.; Asawanonda, P. Four-Weeks Daily Intake of Oral Collagen Hydrolysate Results in Improved Skin Elasticity, Especially in Sun-Exposed Areas: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Dermatol. Treat. 2021, 32, 991–996. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A Randomized, Triple-Blind, Placebo-Controlled, Parallel Study to Evaluate the Efficacy of a Freshwater Marine Collagen on Skin Wrinkles and Elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A Antagonizes Decreased Cell Growth and Elevated Collagen-Degrading Matrix Metalloproteinases and Stimulates Collagen Accumulation in Naturally Aged Human Skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.R.; Hammon, K.A.; Gafner, A.; Dahl, A.; Guttman, N.; Fong, M.; Schauss, A.G. Novel Hydrolyzed Chicken Sternal Cartilage Extract Improves Facial Epidermis and Connective Tissue in Healthy Adult Females: A Randomized, Double-Blind, Placebo-Controlled Trial. Altern. Ther. Health Med. 2019, 25, 12–29. [Google Scholar]
- Marini, A.; Grether-Beck, S.; Jaenicke, T.; Weber, M.; Burki, C.; Formann, P.; Brenden, H.; Schönlau, F.; Krutmann, J. Pycnogenol® Effects on Skin Elasticity and Hydration Coincide with Increased Gene Expressions of Collagen Type I and Hyaluronic Acid Synthase in Women. Skin Pharmacol. Physiol. 2012, 25, 86–92. [Google Scholar] [CrossRef]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M. Barrier Recovery and Influence of Irritant Stimuli in Skin Treated with a Moisturizing Cream. Contact Dermat. 1997, 36, 256–260. [Google Scholar] [CrossRef]
- Draelos, Z.D. Active Agents in Common Skin Care Products. Plast. Reconstr. Surg. 2010, 125, 719–724. [Google Scholar] [CrossRef]

| Gene | Forward Sequences (5′-3′) | Reverse Sequences (3′-5′) | Annealing (°C) | Accession |
|---|---|---|---|---|
| GAPDH | GCTCTCTGCTCCTCCTGTTC | ACTCCGACCTTCACCTTCC | 63.0 | NG_007073.2 |
| Collagen type I | AATGGTGCTCCTGGTATTGC | ACCAGGTTCACCGCTGTTAC | 59.0 | NM_000088 |
| Decorin | TGATTTGGGTCTGGACAAAG | TGCCCAGTTCTATGACAATC | 60.0 | AF_491944.2 |
| Biglycan | CCTCCAGGTGGTCTATCTGC | CATCAGGATGTGTGGCTGTG | 58.0 | AH_002674.2 |
| Elastin | AAGGTGGCTGCCAAAGC | ACTCCTCCAAGTGGGAACTG | 60.0 | NM_00501 |
| mRNA Expression | p-Value | |
|---|---|---|
| Collagen type I | 2.06 ± 0.17 | <0.05 |
| Elastin | 1.17 ± 0.12 | <0.05 |
| Decorin | 1.15 ± 0.21 | <0.05 |
| Biglycan | 1.30 ± 0.13 | <0.05 |
| Batch No. | 1 | 2 | 3 | 4 | p-Value |
|---|---|---|---|---|---|
| Collagen type I | 1.22 ± 0.09 | 1.19 ± 0.06 | 1.20 ± 0.15 | 1.19 ± 0.09 | n.s. |
| Proteoglycans | 1.19 ± 0.09 | 1.17 ± 0.09 | 1.18 ± 0.09 | 1.22 ± 0.06 | n.s. |
| Elastin | 1.26 ± 0.14 | 1.34 ± 0.14 | 1.34 ± 0.30 | 1.31 ± 0.37 | n.s. |
| Group | n | 0 ± SD | 4 ± SD | p-Value * (After 4 Weeks) | 8 ± SD | p-Value * (After 8 Weeks) | |
|---|---|---|---|---|---|---|---|
| Eye wrinkle volume (mm3) | SCPs Placebo | 33 33 | 1.52 ± 0.24 1.49 ± 0.35 | 1.41 ± 0.31 1.55 ± 0.27 | <0.05 | 1.26 ± 0.11 1.58 ± 0.17 | <0.05 |
| Skin elasticity (Ur/Ue) | SCPs Placebo | 33 33 | 0.53 ± 0.08 0.54 ± 0.10 | 0.57 ± 0.05 0.54 ± 0.02 | <0.05 | 0.61 ± 0.08 0.56 ± 0.06 | <0.05 |
| Skin hydration (AU) | SCPs Placebo | 33 33 | 38 ± 3.9 37 ± 4.4 | 46 ± 2.9 39 ± 4.7 | <0.05 | 49 ± 4.2 39 ± 5.3 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proksch, E.; Zdzieblik, D.; Oesser, S. The Oral Intake of Specific Bovine-Derived Bioactive Collagen Peptides Has a Stimulatory Effect on Dermal Matrix Synthesis and Improves Various Clinical Skin Parameters. Cosmetics 2025, 12, 79. https://doi.org/10.3390/cosmetics12020079
Proksch E, Zdzieblik D, Oesser S. The Oral Intake of Specific Bovine-Derived Bioactive Collagen Peptides Has a Stimulatory Effect on Dermal Matrix Synthesis and Improves Various Clinical Skin Parameters. Cosmetics. 2025; 12(2):79. https://doi.org/10.3390/cosmetics12020079
Chicago/Turabian StyleProksch, Ehrhardt, Denise Zdzieblik, and Steffen Oesser. 2025. "The Oral Intake of Specific Bovine-Derived Bioactive Collagen Peptides Has a Stimulatory Effect on Dermal Matrix Synthesis and Improves Various Clinical Skin Parameters" Cosmetics 12, no. 2: 79. https://doi.org/10.3390/cosmetics12020079
APA StyleProksch, E., Zdzieblik, D., & Oesser, S. (2025). The Oral Intake of Specific Bovine-Derived Bioactive Collagen Peptides Has a Stimulatory Effect on Dermal Matrix Synthesis and Improves Various Clinical Skin Parameters. Cosmetics, 12(2), 79. https://doi.org/10.3390/cosmetics12020079







