Abstract
The skin is continuously exposed to environmental changes, rendering it vulnerable to damage from external stressors that contribute to premature skin aging. This study aims to explore skin longevity pathways stimulated by a rose extract (RE) derived from petals. Human keratinocytes treated with RE exhibited a significant increase in NRF2 (NF-E2-related factor 2; ≈2–4% of induction) and LAMP2A (Lysosome-Associated Membrane Protein 2A; ≈6–12% of induction) levels. The presence of RE significantly mitigated the increase in carbonylation levels (≈34–37% of protection) and the number of labeled P16INK4A cells (≈60–72% of protection), associated with proliferation arrest, both induced by exposure to BAP (Benzo[a]pyrene) coupled with UV-A (Ultraviolet A) irradiation. The beneficial effects mediated by RE were inhibited by Compound C, a specific AMPK inhibitor (AMP-activated protein kinase). The involvement of the AMPK pathway in mediating the beneficial effects of RE has been confirmed by assessing its activation through the evaluation of its phosphorylation state which was significantly elevated in the presence of RE compared to the stress condition. In conclusion, the activation of the AMPK pathway enhances antioxidant defenses and promotes autophagy. This dual action, mediated by RE, helps protect skin cells from oxidative damage and senescence while maintaining proteostasis, skin integrity, and cellular proliferation under pollution-induced stress (BAP + UV-A). These findings highlight the potential in mitigating age-related skin changes through the modulation of longevity pathways.
1. Introduction
The skin is not only a vital protective barrier but also functions as a dynamic sensory organ, forming the primary line of defense against environmental aggressors. This complex organ is perpetually exposed to a myriad of environmental changes, making it susceptible to frequent damage from various external stressors. As a responsive shield, the skin actively counters and safeguards the body from these continual challenges, playing a critical role in maintaining overall health and well-being. To maintain its biological functions, the skin possesses the remarkable ability to repair and regenerate its components when damaged, involving mechanisms such as detoxification and the elimination of damaged proteins. Skin aging is a complex biological process influenced by both intrinsic factors, predominantly genetic, and extrinsic factors that affect an individual from conception to death, contributing to the biological and clinical signs of skin aging [1]. Environmental factors are key actors in premature aging, impairing skin homeostasis, which results in the accumulation of damages and a reduced ability to repair them. This decline is further contrasted by a cell’s internal energy sensor, the AMPK (AMP-activated protein kinase) pathway, which can trigger cellular processes that promote longevity. However, with age, AMPK function can become impaired, potentially contributing to the diminished ability of skin cells to maintain themselves [2]. Among environmental factors, Ultraviolet Radiation (UVR) is the main actor in skin dysfunction, inducing oxidative damage and harmful genetic modifications. The urban environment, in which the skin is exposed to pollutants (particulate matters, volatile organic compounds (VOCs), and polycyclic aromatic hydrocarbons (PAHs)), contributes to premature skin aging and reduces the functionality of the skin [3,4,5]. Other exogenous aggressors, such as dysbiosis (Cutibacterium acnes pathogenic subtypes or Staphylococcus aureus) [6] and brutal changes in environmental temperature are also known to be involved in oxidative stress induction within the skin. Oxidative stress results from an imbalance between the production and accumulation of oxygen reactive species (ROS) in cells and tissues and impaired ability of skin to detoxify these reactive products due to the accumulation of oxidative modification of macromolecules [7]. An accumulation of damaged proteins (proteostasis collapse) is associated with several age-related morbidities, such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, Parkinson’s disease, and cataract [8].
Among these modifications, protein carbonylation is a harmful irreversible modification identified as a major hallmark of severe oxidative damage related to oxidative disorders [9,10,11,12]. This modification is associated with protein dysfunction and misfolding and must be cleared to maintain skin homeostasis. Carbonylation can negatively affect, or totally abrogate, protein catalytic functions and may trigger the formation of potentially cytotoxic protein aggregates.
Antioxidant defenses, including enzymes, such as superoxide dismutases (SODs), glutathione peroxidases (GPXs), peroxiredoxins (PRDXs), and catalase (CAT), as well as non-enzymatic molecules, such as glutathione (GSH), protect skin proteins from oxidative stress. Among these antioxidant defenses, the NF-E2-related factor 2 (NRF2) is the master cytoprotective and antioxidant regulating the expression of ~250 genes by the antioxidant response element (ARE). Products of these genes are involved in antioxidant response, redox homeostasis, the detoxification of toxic compounds, mitochondrial biogenesis, and many other processes [9]. Once damaged by oxidative stress, the intracellular cellular components’ degradation can be mediated by autophagy.
Autophagy, essential for cell survival, is one of the clearance mechanisms involved in the degradation of damaged cellular components. The NRF2 target LAMP2A (Lysosome-Associated Membrane Protein 2A) [13] plays a significant role in chaperone-mediated autophagy (CMA) by facilitating the recognition, binding, translocation, and degradation of specific proteins targeted for lysosomal degradation. Its presence on the lysosomal membrane is essential for the efficient functioning of CMA, ensuring the removal of damaged proteins from the cell. The autophagy pathway is finely regulated and requires the activation of the AMP-activated protein kinase (AMPK) signaling pathway, which coordinates cell growth, autophagy, and metabolism.
Furthermore, a functional association between the AMPK and NRF2 pathways has been described to underlie the crosstalk between energy homeostasis and oxidative clearance upon AMPK activation upstream of NRF2 [3,14]. NRF2 and LAMP2A also play critical roles in mitochondrial function, ensuring both structural integrity and functional effectiveness [15,16].
In addition, previous work has shown that rose petal extracts contain large quantities of phenolic compounds, which have been described as stimulating antioxidant enzymes such as CAT and GPXs [17], but research on the detailed compositions of these extracts is lacking, and their characterization could lead us to obtain a better understanding of their overall activity.
In this study, rose extract was subjected to a chromatographic analysis coupled with mass spectrometry in order to annotate its major volatile and non-volatile compounds. The main objective was to identify the presence of compounds previously reported in the literature as being able to interfere with skin aging processes.
We evaluated the effects of a rose extract, obtained using a supercritical CO2 extraction process, on primary human keratinocytes, focusing on cellular resilience via antioxidant defenses, autophagy stimulation, and the modulation of the AMPK pathway. We show the effect of rose extract on the AMPK signaling pathway and that targeting this pathway may be a crucial strategy for promoting lifelong healthy skin.
2. Materials and Methods
2.1. Extraction from Rosa hybrid Flowers
Rosa hybrid “DELFLOBLA” is a variety of floribunda garden rose with the selection code number N° 09.8649.1 and is the result of breeding between the varieties “MEICHIBON” and “DELGRAMAUE” performed in 2009, which was first observed in Delbard nurseries in Malicorne, France, in 2010. The extraction of active compounds from rosa hybrid flowers was performed using a supercritical fluid extraction (SFE) method. Supercritical carbon dioxide (CO2) was used as the primary extraction fluid modified with an ethanol co-solvent. This co-solvent/active compound mixture was then used for GC-MS analysis tests and on cell cultures.
2.2. Volatile Molecules Analysis by GC-MS
Volatile chemical composition was determined by injecting 1 µL of the rose extract into GC-MS equipment using an HP 6890 GC system connected to an HP 5973 Mass Selective Detector equipped with a DB-5 MS capillary column (60 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies, Palo Alto, CA, USA). The column temperature was held at 60 °C for 3 min, programmed to increase at 5 °C/min to 310 °C, and then held at this temperature for 15 min. Helium was used as carrier gas with a flow rate of 2.3 mL/min and a split ratio of 20:1. The temperature of the injector was maintained at 290 °C. The MS was set to scan in the range of m/z 35–500 amu with an ionization energy of 70 eV. The chemical components were identified by a comparison of their retention indices and MS spectra with those reported in the literature and using Masshunter Qualitative Analysis software (B07.00, Agilent Technologies, USA) matching with standard reference databases (NIST23, Wiley275 and CNRS libraries). The retention index of each component was calculated relative to a standard mix of n-alkanes (C7–C40, Sigma-Aldrich, St. Louis, MO, USA), analyzed under identical experimental conditions. The concentration of the components was computed based on the percentage of their relative peak area (%).
2.3. Non-Volatile Molecules Analysis by UHPLC-MS
Non-volatile molecules were analyzed using Agilent Technologies Accurate-Mass ESI-Q-TOF LCMS 6530 with an LC 1290 Infinity system equipped with a DAD detector. Separation was carried out at 40 °C using a 120 EC-C18 column (3.0 × 100 mm, 2.7 μm; Agilent Technologies, Palo Alto, CA, USA). The CO2 extract was diluted in ethanol (1:10), and 1.5 μL was injected at the head of the column. The column was eluted at 0.7 mL/min with a solvent gradient using solvent A (water with formic acid 0.1% (v/v)) and solvent B (methanol with formic acid 0.1% (v/v)). After 1 min at 2%, the proportion of solvent B increased from 2% to 100% over 23 min, followed by a 3 min isocratic phase with 100% solvent B; the proportion decreased from 100% back to 2% in 0.5 min, and equilibration occurred with 2% of solvent B over 2.5 min until the end of the run at 30 min. Mass analyses were conducted in positive and negative mode in the range of 100–1500 m/z, with nebulization gas (nitrogen) at a flow of 10 L/min and 40 psg pressure. The capillary tension and the fragmentor were 3500 V and 100 V, respectively. Complementary tandem mass spectrometry analyses (MS/MS) were performed with a collision energy of 20 eV to elucidate molecular structures. The chemical components were identified using their structural data (UV and mass) and the SIRIUS software version 5.8.5 [18,19,20].
2.4. Primary Cultured Keratinocytes
Keratinocytes (Biopredic International, Saint-Grégoire, France) were cultured in OxiProteomics® medium at 37 °C in 5% CO2 humidified air. Cytotoxicity MTS assay (G542, Promega; Charbonnières-les-Bains, France) was carried out according to the manufacturer’s guidelines, and specific absorbance was recorded (Varioskan, Thermofisher™, Asnières-sur-Seine, France). The rose extract was diluted (w/v) in the medium at the defined doses for 48 h. 5-Aminoimidazole-4-carboxamide riboside (AICAR) at an amount of 0.5 mM was used as the positive inducer of the AMPK pathway. To confirm AMPK pathway activation, Compound C, a specific inhibitor of the AMPK pathway, was used at an amount of 10 µM. For “protective” property evaluation, cells were then exposed to Benzo[a]pyrene (BAP; CRM40071; 20 µM; Sigma-Aldrich-Merck KGaA, Darmstadt, Germany), diluted using Hank’s Balanced Salt Solution (HBSS), and irradiated with UV-A (LED source, emission peak at λ = 365 nm; 2,4 J/cm2) using the OxiProteomics® irradiation system after 48 h of contact with the rose extract. Just after the irradiation process, cells were transferred into a fresh culturing medium and incubated in optimal condition of culture for 2 h before sampling.
2.5. Protein Extraction and Western Blot
Keratinocytes were subjected to protein extraction in an aqueous buffer using the optimized OxiProteomics® buffer for Western blotting. Accurate measurement of protein concentration was carried out using the Bradford Protein Assay Dye Reagent (Bio-Rad™, Marnes-la-Coquette, France) according to the manufacturer’s guidelines. Extracted proteins were separated by high-resolution electrophoresis (SDS-PAGE—gradient 4–20%; Thermo Scientific™). After migration, proteins were transferred from gel to a 0.2 μm nitrocellulose membrane (Bio-Rad™). The membrane was washed in a Tris-buffered saline (TBS) solution at pH 7.6 with 0.1% of Tween (TBS-T) before being incubated for 30 min in TBS with 3% of Bovine Serum Albumin (TBS-BSA) for saturation. Just after the saturation step, the membrane was incubated at 4 °C overnight in a fresh TBS-BSA solution containing the primary antibodies (Table 1). The day after, the excess primary antibody was eliminated by washing with TBS-T solution, and then the membrane was incubated for 1 h with the secondary antibody coupled to a fluorophore (Table 1). Three (3) additional washes with TBS-T were performed to remove the excess secondary antibodies. The digital acquisition of images of P_AMPK, AMPK, P_ACC, and Actin was performed using the “iBright” system (Thermofisher™).

Table 1.
Antibodies used.
2.6. Detection, Visualization, and Quantification of Biomarkers
Keratinocytes were fixed with a solution containing 95% ethanol and 5% acetic acid. Oxidatively damaged (carbonylated) proteins were labeled using an OxiProteomics® fluorescent probe (Ex = 647 nm/Em = 650 nm) functionalized to specifically bind to carbonyl moieties and DAPI (4′,6-diamidino-2-phenylindole) for nuclear labeling in Phosphate-Buffered Saline, pH 7.4 (PBS). For immunodetection, a saturating step was carried out on the non-specific sites with a solution of PBS containing 3% BSA (PBS-BSA). Keratinocytes were incubated with diluted primary antibodies in a PBS-BSA solution (Table 1). The excess primary antibodies were eliminated with washing using a solution of PBS with 0.1% Tween (PBS-T), and then cells were then incubated for 1 h with the secondary antibody coupled to a fluorophore (Table 1) in PBS-BSA. The cellular nuclei were labeled using DAPI. Finally, the antibody and excess DAPI were removed with a sequence of washing steps with PBS-T. Fluorescent images were collected with an epi-fluorescent microscope (Thermofisher™, EVOS M5000 Imaging System) and analyzed with ImageJ software version 1.53 (U. S. National Institutes of Health, Bethesda, MD, USA).
A % of induction (compared to the experimental group of reference, namely the control or stress group) was obtained by using the following equation:
Induction (% group X vs. Reference group) = ((Biomarker_Levels_Group_X/Biomarker_Levels_Ref_Group) − 1) × 100
Also, a protective value (%) was obtained for the experimental groups using, as references, the control group, considered at maximum efficiency (100%), and the stress group, considered at minimum efficiency (0%).
Protection (% group X) = ((Biomarker_Levels_Group_Stress − Biomarker_Levels_Group_X)/(Biomarker_Levels_Group_Stress − Biomarker_Levels_Group_Control) × 100
2.7. Histogram Illustration and Statistical Analysis
The quantification of biomarkers was normalized in relation to the control (considered at 100%), finally obtaining a mean value and a standard deviation. Statistical analyses were carried out using the “GraphPad” software version 10.0.3 (La Jolla, CA, USA) by using a one-way ANOVA and Dunnett’s post hoc test for multi-comparison analyses (vs. the respective stress group) as well as Student’s binary t-test or Mann–Whitney comparisons between groups (confidence interval of 95%).
3. Results
3.1. Rose Extract Induces No Cytotoxic Effect on Primary Human Keratinocytes and Stimulates Antioxidant Defense and Autophagy
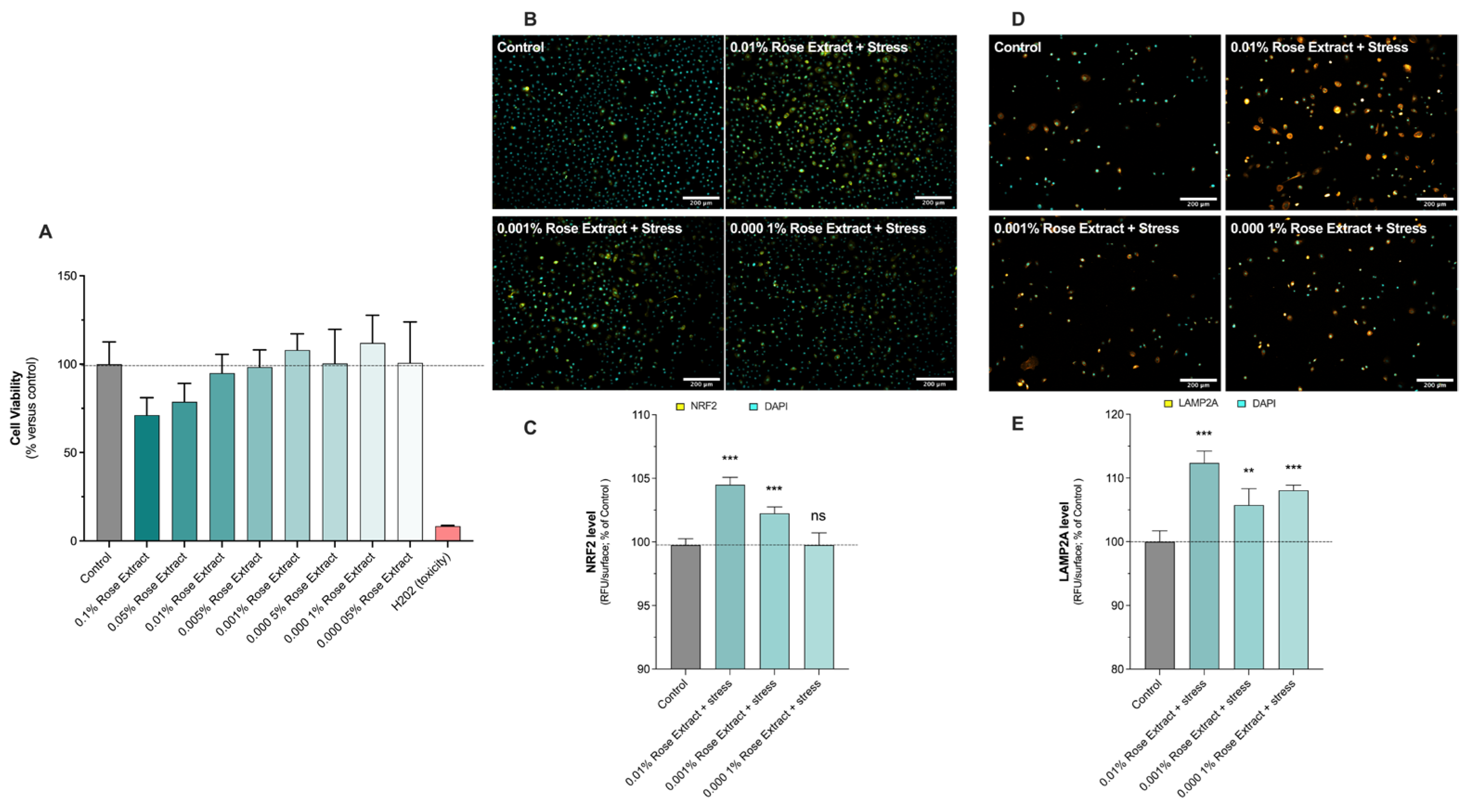
Initially, the cytotoxic impact of rose extract was assessed across concentrations ranging from 0.1% to 0.00005% (w/v) by exposing primary human keratinocytes for 48 h. Then, the effects of rose extract on primary keratinocytes after 48 h of contact, at basal levels, were explored by the evaluation of NRF2 (Figure 1B,C) and LAMP2A levels (Figure 1D,E), detected in situ (on cells) via epifluorescence microscopy.
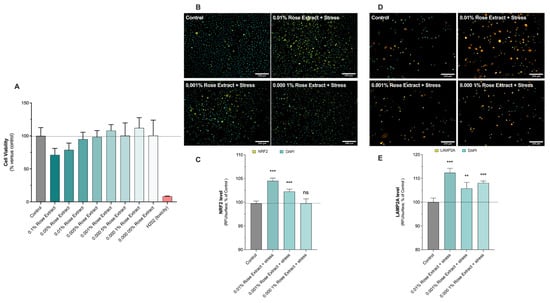
Figure 1.
Rose extract boosted antioxidative defense. (A) Cell viability upon rose extract contact was carried out using MTS assay. Keratinocytes were exposed for 48 h to rose extract, ranging from 0.00005% to 0.1%. Data are shown as mean +/− S.D. from 6 replicates per condition. Representative images of (B) NRF2 levels and (D) LAMP2A levels in keratinocytes upon UV-A irradiation and Benzopyrene (BAP) at 20 µM. Nuclei were stained in cyan (DAPI coloration). Scale bar, 200 µm. Quantification of (C) NRF2 levels and (E) LAMP2A levels of each experimental group is expressed as relative values (% vs. control) and shown as mean +/− S.D. from 4 replicates per condition. ***, p < 0.001; **, p < 0.01; ns, not significant. One-way ANOVA and Dunnett’s post hoc test were used for multi-comparisons vs. control (alpha = 0.05).
The presence of rose extract at concentrations of 0.01% (112.4 ± 1.8, 12% induction), 0.001% (105.7 ± 2.5, 6% induction), and 0.0001% (108.1 ± 0.8, 8% induction) showed stimulatory effects on detoxification mechanisms through increased levels of LAMP2A under basal conditions (100 ± 1.7). LAMP2A is a lysosomal protein involved in cell detoxification by hydrolyzing damaged proteins playing a crucial role in the process of chaperone-mediated autophagy (CMA), a selective form of autophagy that targets specific proteins for degradation within lysosomes. NRF2 is a transcriptional factor which is activated following cell damage to produce downstream target genes involved in cell defense and detoxification. Rose extract at concentrations of 0.01% (104.5 ± 0.6, 4% induction) and 0.001% (102.3 ± 0.5, 2% induction) significantly increased NRF2 levels under basal conditions (100 ± 0.5). Thus, the antioxidant and detoxification effects of rose extract were confirmed by the significant increase in NRF2 levels and the stimulation of detoxification mechanisms through elevated LAMP2A levels under basal conditions.
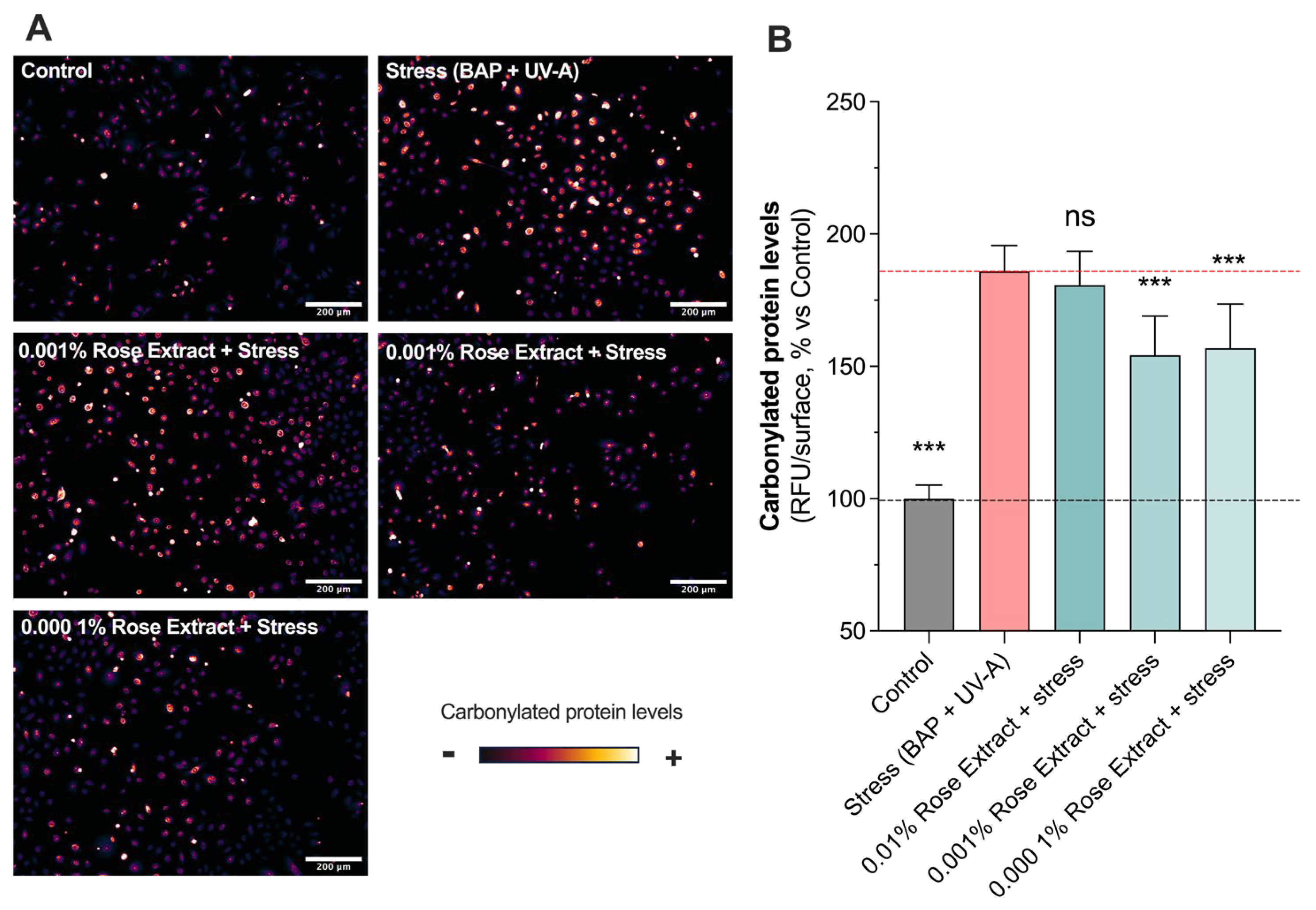
3.2. Treatment with Rose Extract Counteracts Pollution (BAP + UV-A)-Induced Oxidative Damage
The effects of rose extract on human primary keratinocytes exposed to oxidative damages such as those caused by pollution (BAP) and UV-A were assessed through in situ (on cell) detection, visualization, and the quantification of carbonylation levels. A significant increase in oxidative damage (185.9 ± 9.7) (carbonylation levels) was observed upon stress (BAP + UV-A) exposure when compared to the control group (100 ± 5.1) (Figure 2). Interestingly, rose extract at concentrations of 0.001% (154.2 ± 14.8; 37% protection) and 0.0001% (156.9 ± 16.7; 34% protection) counteracted stress-induced increases in the levels of carbonylation. The maintenance of proteostasis and the regulation of carbonylation levels in cells are pivotal for cellular health and functionality. Effective proteostasis ensures the balance and integrity of the proteome, preventing the accumulation of misfolded or damaged proteins. The beneficial effects of these processes also include enhanced cell viability, improved cellular responses to stress, and the overall promotion of longevity and skin health, spotlighting their vital role in cellular homeostasis.
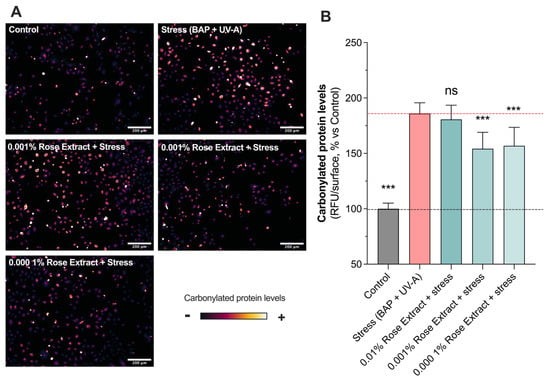
Figure 2.
Induced oxidative protein damage caused by Benzopyrene (BAP) and UV-A was counteracted by rose extract. Representative images of (A) oxidative damage (carbonyl levels, visualized in orange range) in keratinocytes upon BAP and UV-A irradiation at 20 µM. Scale bar, 200 µm. Quantification of (B) carbonyl levels of each experimental group are expressed as relative values (% vs. control) and shown as mean +/− S.D from at least 3 independent experiments. ***, p < 0.001; ns, not significant. One-way ANOVA and Dunnett’s post hoc test were used for multi-comparisons vs. stress group (alpha = 0.05).
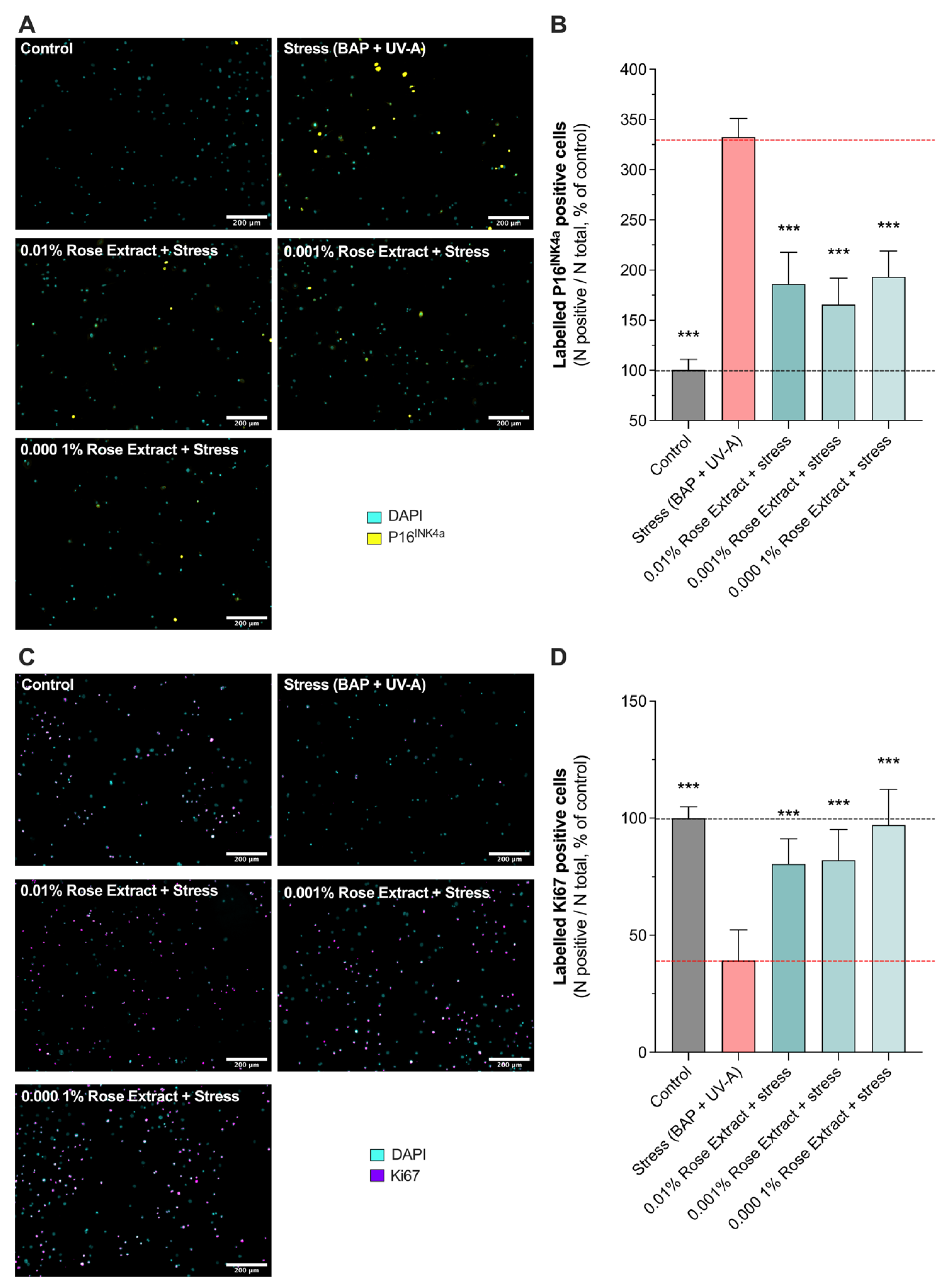
3.3. Treatment with Rose Extract Counteracts Pollution (BAP + UV-A)-Mediated Cellular Senescence and Cell Growth Arrest
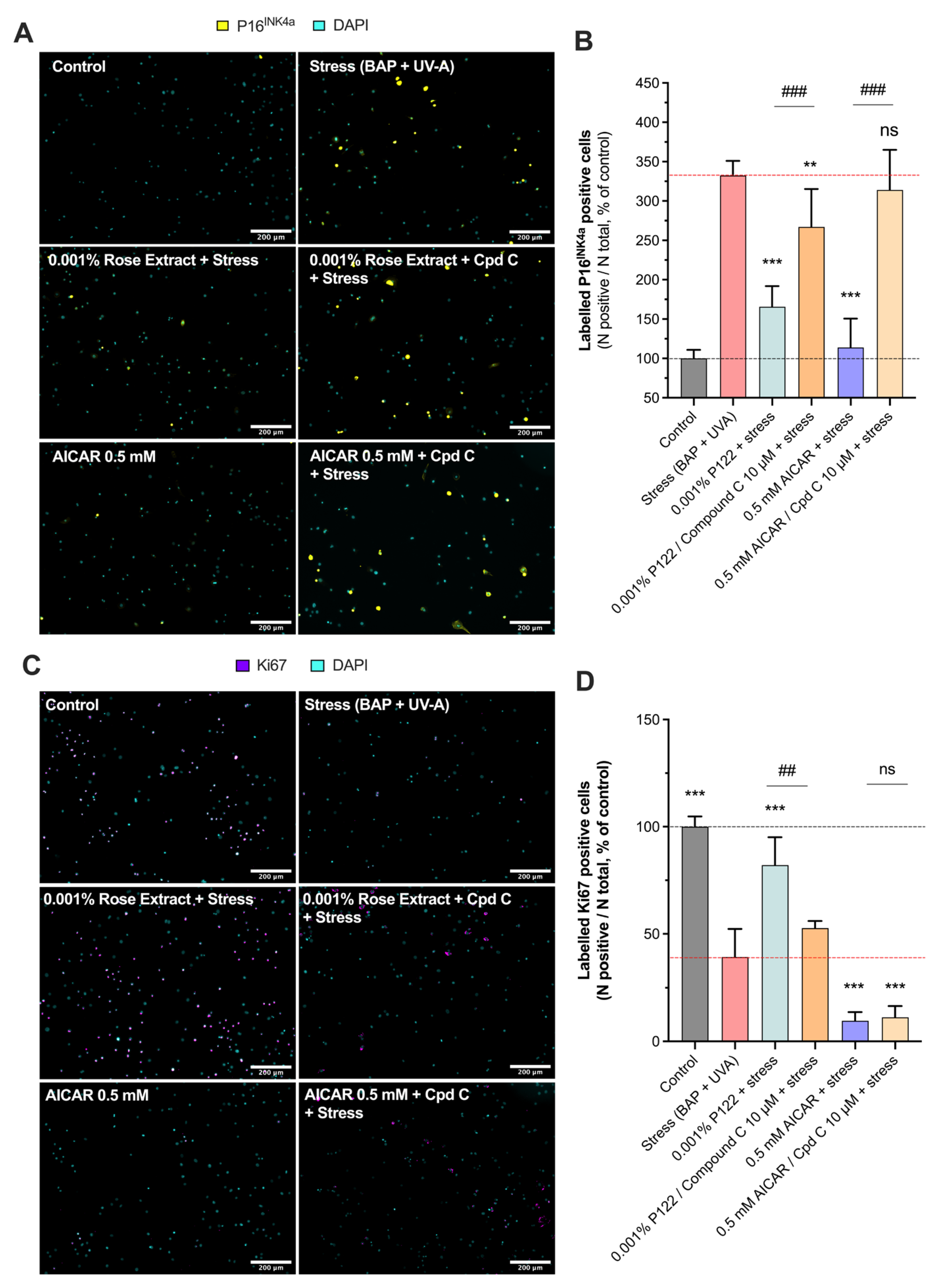
P16INK4A, a cell cycle regulator, is considered as a marker of cellular senescence as its expression was found to be significantly increased in senescent cells during natural aging or in age-related pathologies [21,22]. A significant increase in labeled P16INK4A cells (332.4 ± 18.61) was observed in keratinocytes exposed to BAP and UV-A when compared to the control (100 ± 10.7) (non-stressed group). Interestingly, rose extract at concentrations of 0.01% (186.0 ± 31.9 63% protection), 0.001% (165.6 ± 26.2; 72% protection), and 0.0001% (193.3 ± 25.7; 60% protection) significantly counteracted the increased number of labeled P16INK4A cells mediated by UV-A and pollution, suggesting a beneficial effect on stress-induced cellular senescence (Figure 3A,B). The effects of rose extract were also observed on the proliferation marker KI67 (Figure 3C,D). P16INK4A is a nuclear protein that is present during all active phases of the cell cycle (G1, S, G2, and mitosis) but absent in resting cells (G0 phase) [23]. Exposure to stress conditions (BAP + UV-A) significantly decreased the levels of KI67 (39.2 ± 13.1) in comparison to the control group (100 ± 4.7). The rose extract at concentrations of 0.01% (80.4 ± 10.7; 68% protection), 0.001% (82.1 ± 13.0; 70% protection), and 0.0001% (97.1 ± 15.1; 92% protection) preserved keratinocytes from undergoing cell growth arrest and maintained the proliferative (KI67-positive) bulk of cells, confirming its beneficial effects on cellular homeostasis under stress.
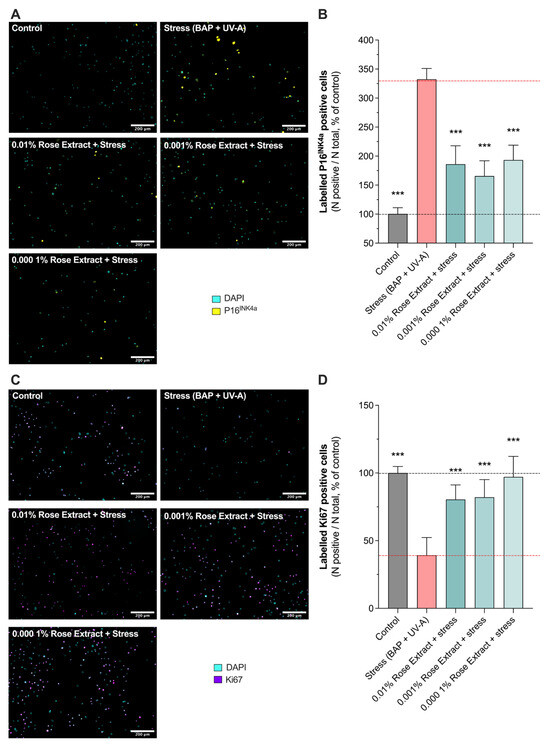
Figure 3.
Induced cellular senescence and cell growth arrest caused by Benzopyrene (BAP) and UV-A were counteracted by rose extract. Representative images of (A) labeled P16INK4A cells (yellow), and (C) labeled Ki67 cells (purple) in keratinocytes upon Benzopyrene (BAP) and UV-A irradiation at 20 µM. Nuclei were stained in cyan (DAPI coloration). Scale bar, 200 µm. Quantification of (B) labeled P16INK4A cells, and (D) labeled Ki67 cells of each experimental group are expressed as relative values (% vs. control) and shown as mean +/− S.D from at least 3 independent experiments. ***, p < 0.001. One-way ANOVA and Dunnett’s post hoc test for multi-comparisons vs. stress group (alpha = 0.05).
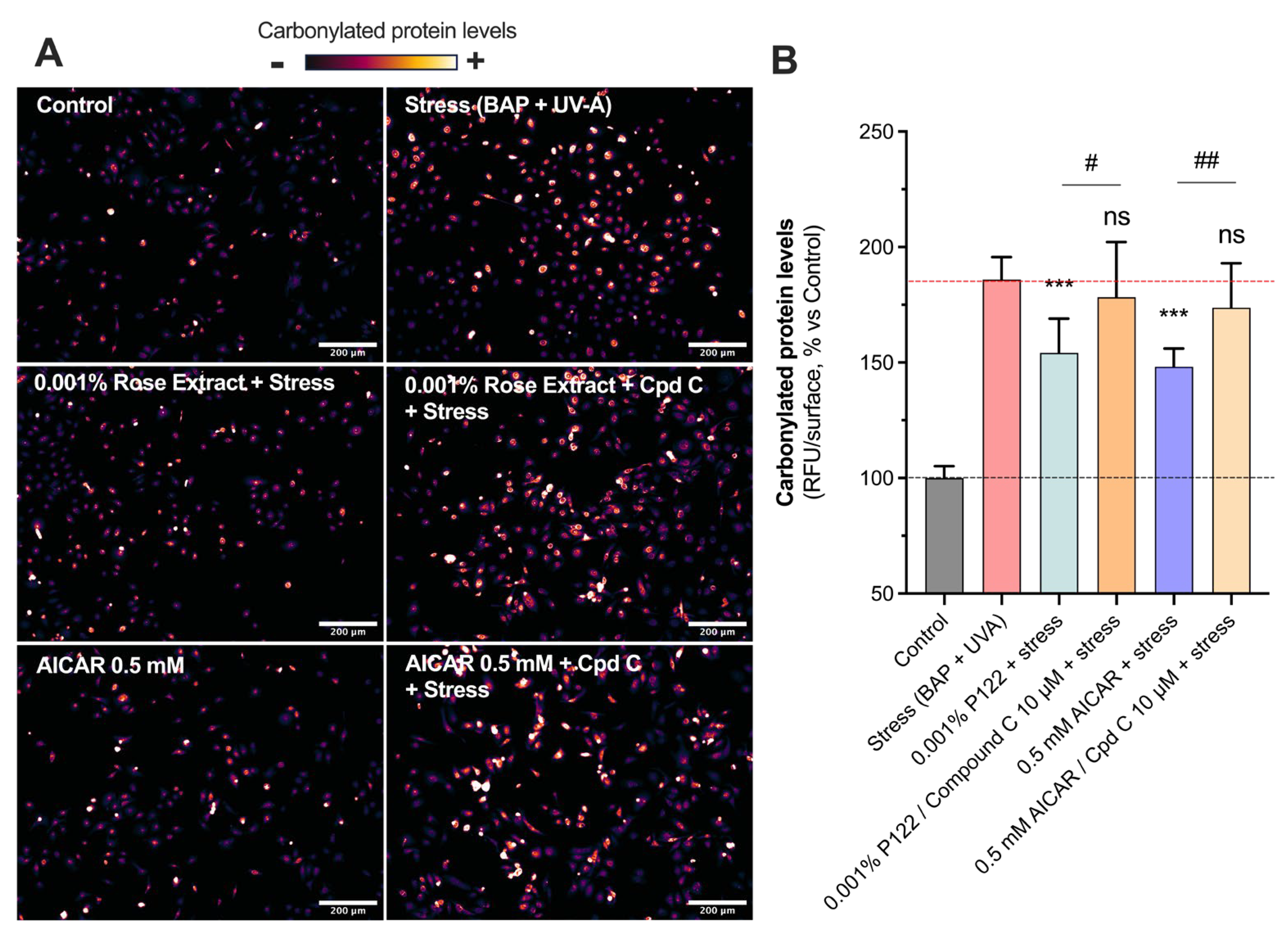
3.4. The Beneficial Antioxidant Effect of Rose Extract on Pollution (BAP + UV-A) Exposure Is Mediated by an AMPK-Dependent Mechanism
The AMPK pathway’s roles extend to influencing proteostasis, which encompasses the control of protein biogenesis, folding, trafficking, and degradation by maintaining cellular homeostasis, promoting autophagy, and preventing senescence [24,25]. Increased levels of carbonylated proteins (185.9 ± 9.7) mediated by stress were counteracted by rose extract at a concentration of 0.001% (154.2 ± 14.8) (Figure 4). The presence of Compound C, the specific inhibitor of AMPK, at 10 µM totally reversed the beneficial effects of 0.001% of rose extract (178.2 ± 23.9), suggesting that its positive impact is mediated via the AMPK pathway. The group treated with 0.5 mM of AICAR (an AMPK activator) displayed similar modulation (148.2 ± 7.8; 44% of protection) as that treated with rose extract, confirming the beneficial effects of AMPK pathway activation in preventing the oxidative stress-mediated increase in carbonyl levels. The inhibition of the AMPK pathway using 10 µM of Compound C fully blocked the beneficial effect mediated by AICAR (173.7 ± 19.3).
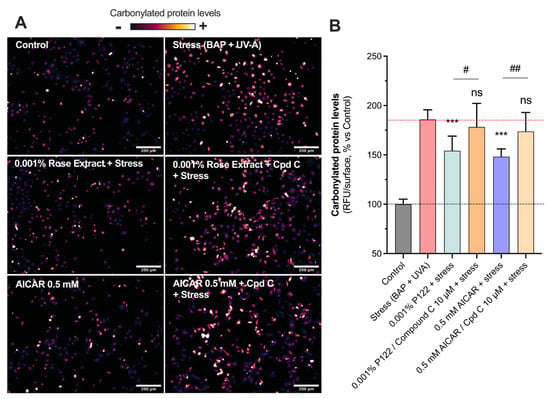
Figure 4.
Rose extract counteracted oxidative stress via AMPK-dependent mechanism: Representative images of (A) oxidative damage (carbonyl levels, visualized in orange range) in keratinocytes upon Benzopyrene (BAP) and UV-A irradiation at 20 µM. Scale bar, 200 µm. Quantification of (B) carbonyl levels of each experimental group are expressed as relative values (% vs. control) and shown as mean +/− S.D from at least 3 independent experiments. ***, p < 0.001; ns, not significant. One-way ANOVA and Dunnett’s post hoc test were used for multi-comparisons vs. stress group (alpha = 0.05). ##, p < 0.01; #, p < 0.05; ns, not significant. Student’s t-test was used for binary-comparisons (alpha = 0.05).
3.5. The Anti-Cell Senescence and Cell Growth Arrest Effect of Rose Extract Are Mediated by the AMPK Pathway
The involvement of the AMPK pathway in rose extract-mediated anti-senescence and cell proliferation effects was investigated (Figure 5). Rose extract at a concentration of 0.001% significantly reduced (165.6 ± 26.2) the number of labeled P16INK4A cells (332.4 ± 18.6), indicating its anti-senescent properties. This beneficial effect was blocked by Compound C (267.0 ± 48.3), a specific AMPK inhibitor, suggesting that the anti-senescent effects of rose extract are mediated through the AMPK pathway. Similarly, AICAR at 0.5 mM also mitigated the stress-induced increase in labeled P16INK4A cells (113.9 ± 36.6), and this effect was reversed by Compound C (314.0 ± 51.0), further confirming the involvement of the AMPK pathway (Figure 5A,B).
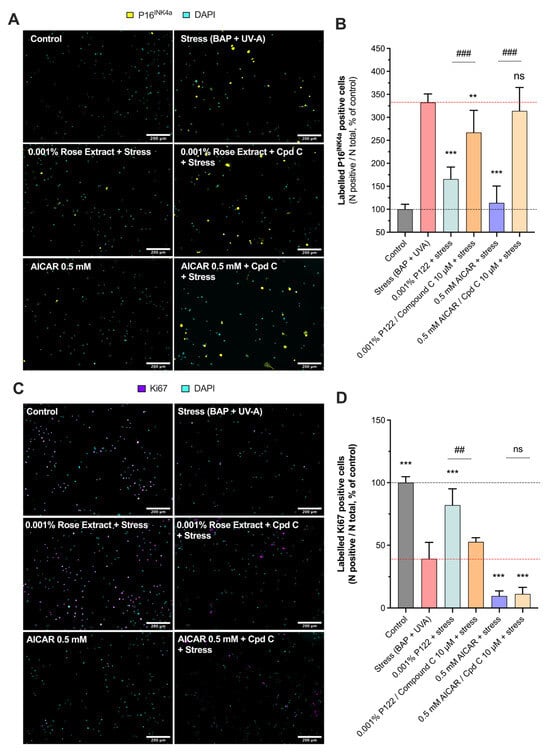
Figure 5.
Rose extract counteracts UVA- and pollution-mediated senescence, and cell growth arrest is dependent on AMPK pathway. Representative images of (A) labeled P16INK4A cells (yellow) and (C) labeled Ki67 cells (purple) in keratinocytes upon Benzopyrene (BAP) and UV-A irradiation at 20 µM. Nuclei were stained in cyan (DAPI coloration). Scale bar, 200 µm. Quantification of (B) labeled P16INK4A cells and (D) labeled Ki67 cells of each experimental group is expressed as relative values (% vs. control) and shown as mean +/− S.D from at least 3 independent experiments. ***, p < 0.001; **, p < 0.01; ns, not significant. One-way ANOVA and Dunnett’s post hoc test were used for multi-comparisons vs. stress group (alpha = 0.05). ###, p < 0.001; ##, p < 0.01; ns, not significant. Student’s t-test was used for binary comparisons (alpha = 0.05).
In addition, rose extract at a concentration of 0.001% prevented the stress-induced decrease in the number of KI67-positive cells (82.1 ± 13.1), indicating its role in promoting cell proliferation. This effect was also counteracted by Compound C (52.7 ± 3.4), reinforcing the role of AMPK activation in this process. However, AICAR at 0.5 mM exhibited anti-proliferative properties (9.6 ± 4.1) that were not affected by Compound C (11.2 ± 5.2), suggesting that AICAR’s anti-proliferative effects operate through an AMPK-independent mechanism (Figure 5C,D). This aligns with previous findings that AICAR has AMPK-independent anti-proliferative properties [26,27].
3.6. Rose Extract Stimulates the AMPK Pathway, Causing a Beneficial Effect
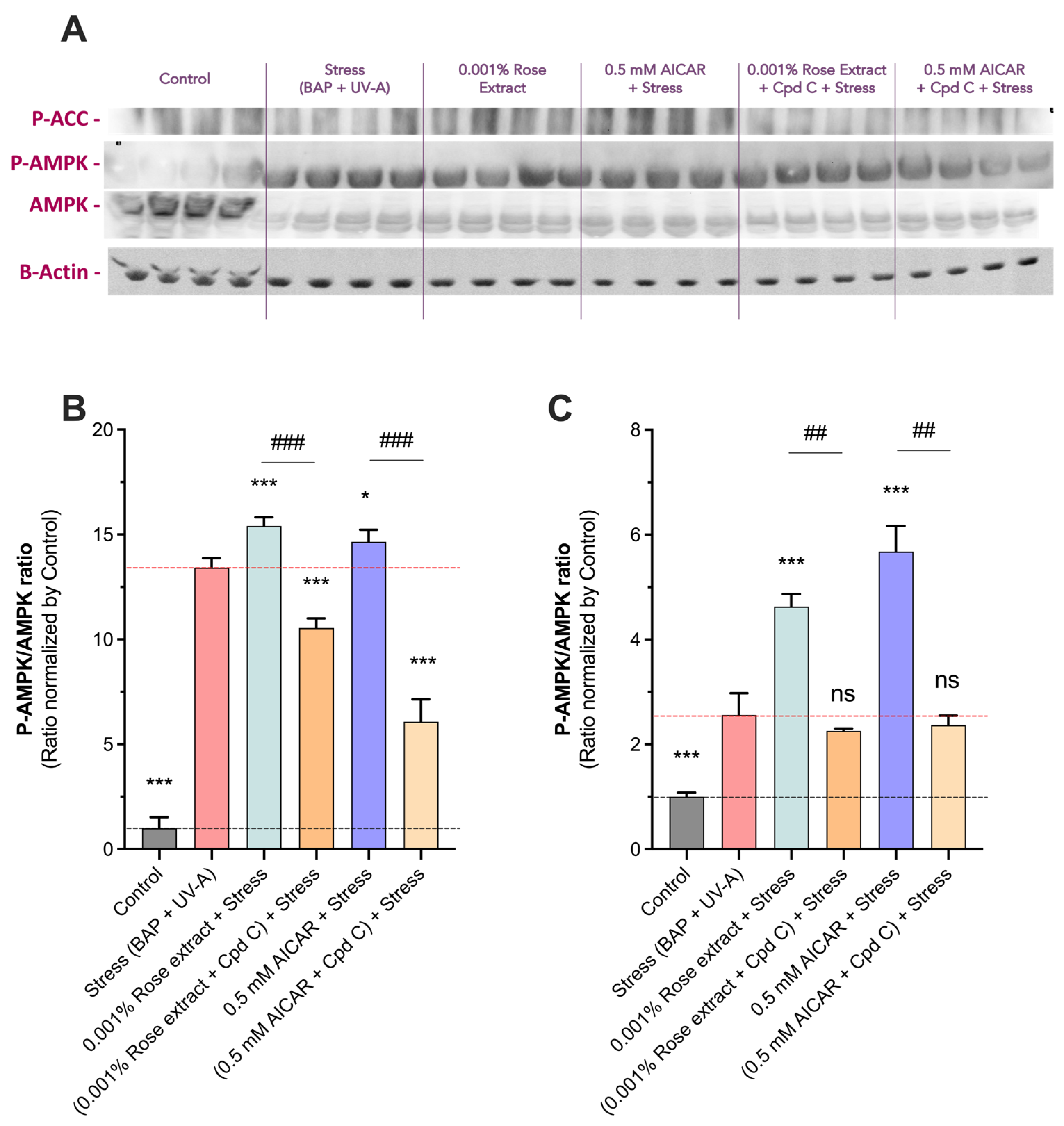
To confirm the activation of the AMPK pathway by rose extract, we assessed the ratio of P-AMPK to total AMPK (P-AMPK/AMPK) and the levels of P-ACC (a downstream target of AMPK) via a Western blot analysis (Figure 6). Quantification revealed a significant increase in the P-AMPK/AMPK ratio in the stress group (13.4 ± 0.5) compared to the control (1 ± 0.5). Notably, treatment with 0.001% rose extract further elevated this ratio (15.4 ± 0.4, 15% induction), indicating enhanced AMPK pathway activation. Similar results were observed with AICAR at 0.5 mM (14.7 ± 0.6, 10% induction), supporting the activation of the AMPK pathway. The stimulatory effects on the AMPK pathway were fully negated by Compound C, a specific AMPK inhibitor, confirming the specificity of rose extract (10.6 ± 0.5) and AICAR (6.1 ± 1.1) in inducing AMPK activation. Additionally, both rose extract (4.63 ± 0.7) and AICAR (5.7 ± 1.4) significantly increased the P-ACC levels, an effect that was also totally counteracted by Compound C (2.2 ± 0.1 and 2.4 ± 0.4, respectively). These findings provide strong evidence that rose extract activates the AMPK pathway and influences downstream phosphorylation events, demonstrating its potential role in cellular defense mechanisms.
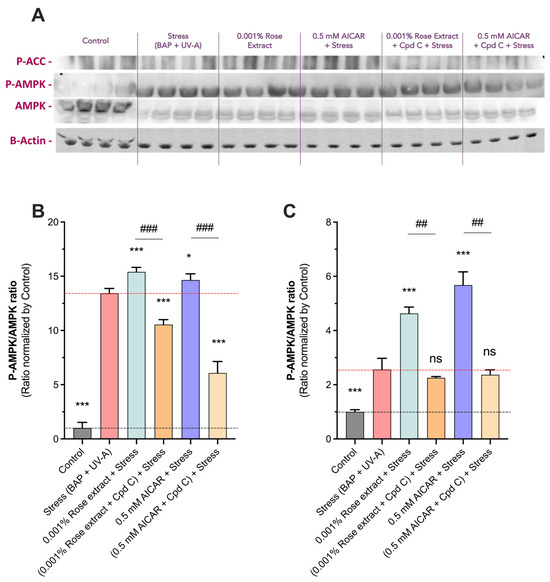
Figure 6.
Rose extract mediates beneficial effect in AMPK-dependent manner. (A) Representative images of Western blots of P-ACC, P-AMPK, AMPK, and Actin in keratinocytes upon Benzopyrene (BAP) and UV-A irradiation at 20 µM. Semi quantification of (B) P-AMPK/AMPK ratio and (C) à P_ACC/Actin ratio of each experimental group is expressed as relative values and shown as mean +/− S.D from at least 3 independent experiments. ***, p < 0.001; *, p < 0.05 ns, not significant. One-way ANOVA and Dunnett’s post hoc test were used for multi-comparisons vs. stress group (alpha = 0.05). ###, p < 0.001; ##, p < 0.01; ns, not significant. Student’s t-test was used for binary comparisons (alpha = 0.05).
3.7. Volatile and Non-Volatile Compositions of Rose Extract
The characterization of rose extract using hyphenated techniques enabled us to detect 166 different compounds (22 in the LC/MS experiment and 144 in the GC/MS experiment). Transforming GC/MS and LC-MS data into an annotated metabolite profile is a major challenge. Despite the intricacy and complexity of the sample, several key metabolites were successfully annotated with clarity: 16 non-volatile and 107 volatile compounds. These compounds belong to different primary and secondary metabolites, such as tannins, flavonoids, and organic acids for non-volatile compounds and monoterpenes, sesquiterpenes, and fatty acid derivatives for volatile compounds (tables available in Supplementary Materials).
4. Discussion
Twelve hallmarks of aging have been characterized, including DNA instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, disabled macroautophagy, chronic inflammation, and dysbiosis [1,8]. Loss of proteostasis and proteome integrity is a key element of aging. Rosa species, in addition to their global growth, contain elements such as phenolic acid, flavonols, and anthocyanins that have been identified to exhibit beneficial effects on skin biofunction, addressing inflammation and aging [28].
Despite there being limited knowledge about the effects of rose extracts on skin aging, studies have shown that rose extract can stimulate the activity and levels of catalase (CAT) and glutathione peroxidases (GPXs), thereby confirming its antioxidant properties [17]. However, further investigation is needed to clarify additional biological properties of rose extract, particularly in relation to the key hallmarks of aging such as antioxidant defense and autophagy. First, NRF2, a cytoprotective and antioxidant transcription factor regulating ~250 antioxidant downstream target genes, was investigated following 48 h of contact with rose extract at concentrations of 0.01%, 0.001%, and 0.0001% in human keratinocytes. The presence of rose extract significantly increased the levels of NRF2, suggesting its stimulatory effect on antioxidant defense. Premature aging and loss of longevity result from the accumulation of misfolded, oxidized, glycated, or ubiquitinylated proteins upon exposure to exogenous stressors [10,12,29]. To overcome these accumulations of damaged proteins, the degradation of intracellular components by the lysosome can be achieved through lysosome vesicles via chaperone-mediated autophagy and LAMP2A, which facilitates the translocation of damaged proteins into the lysosome lumen [30,31]. The impact of rose extract on autophagy has not been established and needs to be addressed. The presence of rose extract at concentrations of 0.01%, 0.001%, and 0.0001% for 48 h significantly increased the levels of LAMP2A in human keratinocytes, suggesting that rose extract could boost autophagy pathways favoring detoxification. Interestingly, enhanced autophagic activity is associated with improvement in the skin’s healthy lifespan (longevity) [32]. Thus, rose extract was characterized for the first time as a key stimulatory factor of antioxidant defense and the detoxification process (autophagy) associated with skin longevity. Taken together, these cellular processes are part of the hallmarks of aging [8,29], and rose extract is addressed as an antioxidant and detoxifying ingredient. To dive deeper into the biological properties of rose extract, the effects on proteostasis were examined. Both UV-A irradiation and BAP are stressors related as oxidative inducers [3,10,33] associated with skin dysfunction, inducing oxidative damage and harmful genetic modification, contributing to premature skin aging [3,4,5]. One early, direct, and irreversible modification induced by ROS, RNS, RXS, and reactive aldehydes is the carbonylation of proteins that consists of the formation of reactive aldehyde or ketone residues on proteins [10,34]. This harmful irreversible modification process has been identified as a major hallmark of severe oxidative damage associated with protein dysfunction and misfolding related to premature skin aging [10,35]. To assess the antioxidant properties of rose extract, keratinocytes were pre-exposed to the rose extract for 48 h before being subjected to stress induced by BAP and UV-A irradiation. Rose extract at various concentrations significantly protected proteins from oxidative damage (carbonyl levels) induced by BAP and UV-A irradiation, confirming its antioxidant and proteostasis-restoring properties.
Senescence is the primary aging process at the cell level that is elicited by acute or chronic damage [8]. Senescent cells are characterized by the loss of proliferative capacity, mitochondrial dysfunction, significantly altered patterns of expression, and the secretion of bioactive molecules [22,36]. P16INK4A expression is strongly increased in aged tissues of rodents and underscores the significance of senescence as a hallmark of aging and a driver of age-related diseases. The removal of P16INK4A -positive senescent cells has been shown to delay the progression of age-related pathologies, extending both premature and natural aging lifespans [21,37]. Rose extract has the ability to mitigate cellular senescence assessed by quantifying labeled P16INK4A cells in cultured human keratinocytes exposed to BAP and UVA irradiation. As expected, stress UV-A and pollution significantly increased the number of labeled P16INK4A cells, which was contrasted by the rose extract at various concentrations. In addition to being directly related to aging, P16INK4A is a tumor suppressor and cell cycle regulator. P16INK4A inhibits the cyclin-dependent kinases CDK4/6 and CDK2 that directly control the cell cycle, which implicate them in cellular proliferation-dependent mechanisms such as tissue regeneration (renewal), aging, and cellular senescence. Therefore, the ability of rose extract to counteract stress-induced reductions in cell proliferation was investigated. Stress induced by BAP + UV-A significantly decreased the number of labeled KI67 cells (proliferative cells), an effect mitigated by rose extract at different concentrations. This study sheds light on the anti-senescent effects and tissue regeneration properties of rose extract, expanding its potential beyond antioxidant effects and its beneficial effect on on proteostasis.
The AMPK signaling pathway is crucial for maintaining proteome integrity (proteostasis), enhancing protein stability, and removing damaged proteins through proteasomal degradation or autophagy. Additionally, AMPK is involved in preventing cell senescence, promoting cell growth, and regulating mitochondrial biogenesis to control mitochondrial redox homeostasis [2,38]. In senescent cells, AMPK is typically inactivated. The activation of AMPK, such as by metformin, significantly reduces the development of senescent cells, while inhibition of AMPK by Compound C accelerates it [24]. This study aimed to elucidate the pathway through which rose extract exerts its beneficial effects, focusing on the AMPK pathway.
To investigate this, Compound C, a selective AMPK inhibitor, was used to counteract the effects of rose extract at a concentration of 0.001%. Additionally, AICAR, an AMPK activator, was used alone or in combination with Compound C to confirm AMPK activation in cultured human keratinocytes exposed to BAP and UV-A irradiation. The beneficial effects of rose extract at a concentration of 0.001% on reducing carbonyls were counteracted by Compound C, indicating that rose extract mediates its antioxidant effects via an AMPK-dependent mechanism. AICAR exhibited similar effects to rose extract, and these effects were also counteracted by Compound C, further confirming the involvement of the AMPK pathway. Therefore, rose extract appears to act through an AMPK-dependent mechanism to address early oxidative events and the loss of proteostasis associated with oxidative protein modifications like carbonyls. Interestingly, while AMPK activation has been associated with mitigating oxidative stress, these findings also reveal a direct link between AMPK activation and the prevention of or reduction in protein carbonylation.
To further explore the role of the AMPK pathway in the mechanism of action of rose extract, its effects on the number of labeled P16INK4A cells and proliferative (Ki67) cells induced by stress were assessed in the presence or absence of the AMPK inhibitor Compound C. Interestingly, Compound C significantly blocked the rose extract-mediated reduction in the number of labeled P16INK4A cells and the restoration of Ki67 cells. Additionally, AICAR displayed a similar pattern of action to rose extract for the P16INK4A biomarker, which was also counteracted by Compound C. However, AICAR completely blocked the proliferation of keratinocytes, and this antiproliferative effect was not reversed by Compound C, suggesting an AMPK-independent mechanism [26,27]. This suggests that the anti-senescent and tissue regeneration properties of rose extract are mediated by an AMPK-dependent mechanism. To ultimately confirm the activation of the AMPK pathway by rose extract, the phosphorylation of AMPK at T183/T172 (normalized by total AMPK) and a downstream target of the AMPK pathway, phospho-ACC at S79 (normalized by Actin), were analyzed. The presence of rose extract at a concentration of 0.001% and AICAR significantly increased the P-AMPK/AMPK ratio and the P-ACC/ACC ratio compared to the stressed condition. These elevations in P-AMPK and P-ACC, indicative of AMPK pathway activation, were nullified in the presence of Compound C. In summary, these findings confirm that rose extract mediates its antioxidant, anti-senescent, and tissue regeneration properties through an AMPK-dependent mechanism.
The analysis of the rose extract using coupled methods revealed a wide range of phenolic compounds and the presence of other fatty acid derivatives and terpene compounds, which contribute to their characteristic aroma, flavor, and biological activity. Rose petal extract, rich in phenolic compounds, has been previously described to boost antioxidant enzymes like CAT and GPXs [14]; moreover, sesquiterpene compounds have not yet been identified to interfere with the AMPK pathway [39]. Rose petal extract contains terpenes, such as limonene, β-myrcene, linalool, camphor, and γ-terpineol, which are known to display potential biological effects, including anti-inflammatory, antioxidant, and antimicrobial properties. These effects may be linked to the activation of AMP-activated protein kinase (AMPK) [39,40]. Alcohols including 1-hexanol, 2-hexenol, 3-hexenol, 2-ethyl-1-hexanol, benzyl alcohol, terpinen-4-ol, and linalool are well known for their therapeutic properties [17,41]. For instance, linalool, a monoterpene alcohol, has shown anti-inflammatory effects, which could be linked to AMPK modulation to reduce inflammation [42]. Sesquiterpenes such as ocimene and α-murolene have anti-inflammatory and antioxidant properties and play a role in regulating energy metabolism and potentially influencing the AMPK pathway. These compounds reduce markers of oxidative stress and modulate inflammatory processes, which are linked to AMPK activation.
Fatty acids such as linoleic acid and oleic acid have well-established effects on lipid metabolism regulation and inflammation. These polyunsaturated fatty acids can influence the activation of the AMPK pathway, playing a role in managing energy metabolism and inflammation. For example, linoleic acid has been shown to activate AMPK, thus modifying cellular energy balance [43,44].
In addition, resveratrol, another plant compound, is known to activate the AMPK pathway, counteracting stress-mediated increases in the number of senescent cells [45,46].
The effects of various plant extracts on the AMPK pathway in cosmetics have been well characterized. The extract of Lycium barbarum (goji berries) is a potent modulator of lipid synthesis in keratinocytes through AMPK activation and SIRT1 signaling. This leads to an increase in the expression of key lipid synthesis genes, strengthening epidermal barrier function [47]. Flavonoid-rich plant extracts have been shown to possess anti-aging effects on the skin. These compounds exhibit antioxidant, anti-inflammatory, and antimicrobial properties, helping to protect skin from the effects of aging [48]. Tea extract (Camellia sinensis), particularly the saponins derived from tea seeds, has been shown to suppress sebum production by regulating the AMPK/mTOR pathway, favoring antiphotoaging, stress resistance, neuroprotection, autophagy, and lipid production [49,50]. Like rose extract, other plant extracts that activate the AMPK pathway hold significant potential in the development of skincare products aiming to improve skin health. Their incorporation into formulations can help reinforce the barrier function, reduce inflammation, and prevent skin aging.
In terms of AMPK activation by rose petal extract, while many of these compounds are known to exhibit biological activities, some may influence AMPK activation indirectly through their effects on oxidative stress, inflammation, or metabolic processes. However, further detailed studies are required to clarify the specific role of these compounds in AMPK activation as their effects may vary depending on concentration, combinations, and the biological systems involved.
5. Conclusions
This study provides new insights into the protective mechanisms mediated by AMPK activation in the context of oxidative stress. Interestingly, these findings reveal a novel link between AMPK activation and the preservation of protein carbonylation, particularly under treatment with rose extract. This suggests that AMPK activation may play a critical role in maintaining protein integrity by reducing oxidative protein modifications.
Moreover, this study highlights the beneficial effects of rose extract on antioxidant defense and autophagy, which protect skin cells from oxidative damage, senescence, and the loss of proliferation induced by pollution (BAP and UV-A). Notably, this is the first time that the activation of the AMPK pathway by rose extract has been observed, underscoring its crucial role in mediating these beneficial effects.
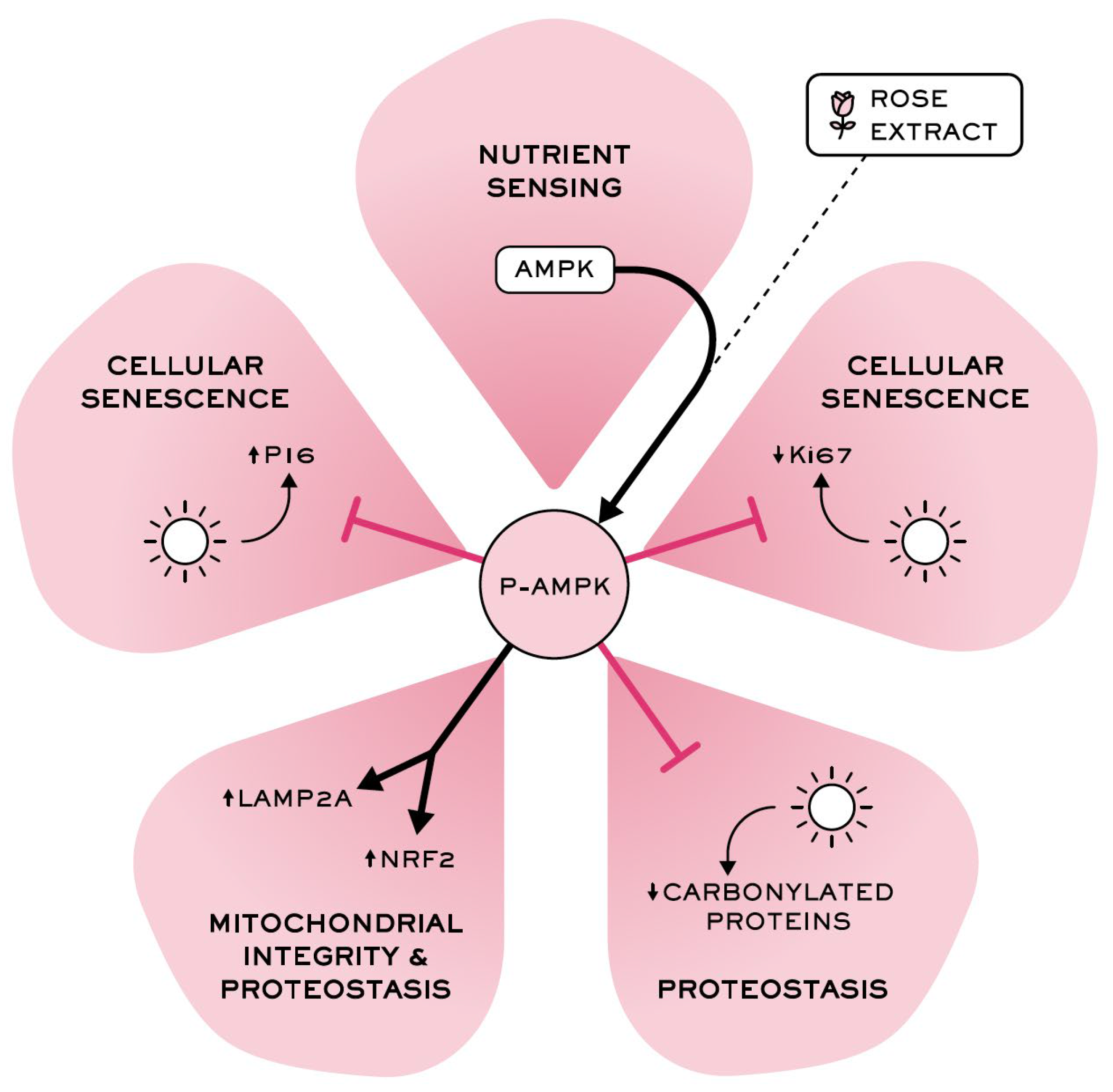
Overall, these results confirm that rose extract plays a significant role in maintaining proteome integrity and stability (cellular proteostasis) and in promoting the removal of damaged proteins through the stimulation of autophagy (Figure 7), suggesting its potential to promote healthy skin aging by targeting longevity pathways.
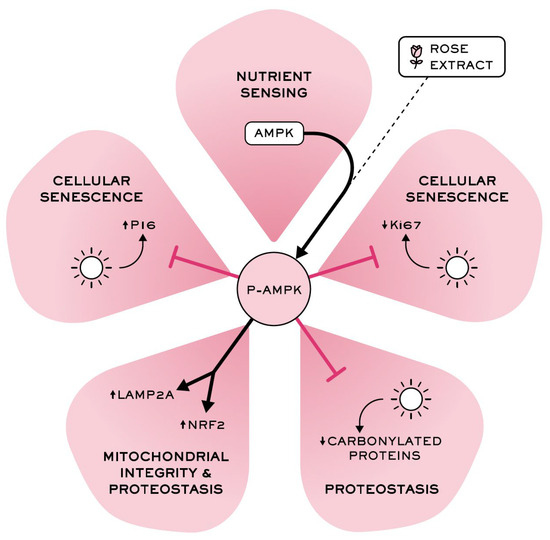
Figure 7.
Rose extract, a stimulatory AMPK pathway ingredient, counteracts skin damage caused by UVA irradiation and pollution-mediated oxidative damages, counteracts cellular senescence, and maintains epidermal integrity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cosmetics12020057/s1, Table S1. Chromatographic and mass spectral characteristics of compounds annotated by LC-ESI-MS in CO2 extract; Table S2. Volatile chemical composition of CO2 extract (abundance: %area).
Author Contributions
O.G. conducted the biological analyses, interpreted the results, and wrote the paper. A.C. and L.B. co-guided the research, interpreted the results, and contributed to writing and reviewing the manuscript. G.A. and S.J. co-guided the project and reviewed the manuscript. G.C. and M.B. performed the extract content analyses and contributed to manuscript writing. A.F.B. and M.A.B. co-guided the study, supervised the research, and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study involved the use of biological samples derived from human subjects, in accordance with regulations and ethical standards, approved by the French Minister of Higher Education and Research (AC-2022-5147, last approval date 3 January 2023). Biopredic International holds all regulatory permits for the collection, processing, transfer and export of human tissues and cell-based products to be used exclusively for research (AC-2023-5431, last approval date 19 July 2023).
Informed Consent Statement
Confirmation of written informed consent from donor has been collected and conserved by Biopredic International complying strictly with the ethical rules for donation and collection of human tissues, in view of research use only.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to confidentiality agreements.
Conflicts of Interest
Olivier Gouin, Andrea Cavagnino and Martin A. Baraibar are employed by Oxiproteomics. Gayané Azadiguian, Sibylle Jäger, and Annie F. Black are employed by L’Oréal. Lionel Breton is employed by CILIA Consulting. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Y.; Yin, J.; Huang, M.; Luo, F. Targeting AMPK Signaling by Polyphenols: A Novel Strategy for Tackling Aging. Food Funct. 2023, 14, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.-P.; Ahmed, E.K.; Baraibar, M.A.; Friguet, B. Proteome Oxidative Modifications and Impairment of Specific Metabolic Pathways During Cellular Senescence and Aging. Proteomics 2020, 20, e1800421. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial Barrier Hypothesis: Effect of the External Exposome on the Microbiome and Epithelial Barriers in Allergic Disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Robinson, O.; Chadeau-Hyam, M.; Dehghan, A.; Mudway, I.; Dagnino, S. What Is New in the Exposome? Environ. Int. 2020, 143, 105887. [Google Scholar] [CrossRef]
- Azzimonti, B.; Ballacchino, C.; Zanetta, P.; Cucci, M.A.; Monge, C.; Grattarola, M.; Dianzani, C.; Barrera, G.; Pizzimenti, S. Microbiota, Oxidative Stress, and Skin Cancer: An Unexpected Triangle. Antioxidants 2023, 12, 546. [Google Scholar] [CrossRef]
- Md Jaffri, J. Reactive Oxygen Species and Antioxidant System in Selected Skin Disorders. Malays. J. Med. Sci. 2023, 30, 7–20. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Friguet, B. Oxidative Proteome Modifications Target Specific Cellular Pathways during Oxidative Stress, Cellular Senescence and Aging. Exp. Gerontol. 2013, 48, 620–625. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Ladouce, R.; Friguet, B. Proteomic Quantification and Identification of Carbonylated Proteins upon Oxidative Stress and during Cellular Aging. J. Proteom. 2013, 92, 63–70. [Google Scholar] [CrossRef]
- Le Boulch, M.; Ahmed, E.K.; Rogowska-Wrzesinska, A.; Baraibar, M.A.; Friguet, B. Proteome Oxidative Carbonylation during Oxidative Stress-Induced Premature Senescence of WI-38 Human Fibroblasts. Mech. Ageing Dev. 2018, 170, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Arias, E.; Díaz-Carretero, A.; Cuervo, A.M.; Cuadrado, A. Transcription Factor NFE2L2/NRF2 Modulates Chaperone-Mediated Autophagy through the Regulation of LAMP2A. Autophagy 2018, 14, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The Crosstalk between Nrf2 and AMPK Signal Pathways Is Important for the Anti-Inflammatory Effect of Berberine in LPS-Stimulated Macrophages and Endotoxin-Shocked Mice. Antioxid. Redox Signal 2014, 20, 574–588. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The Emerging Role of Nrf2 in Mitochondrial Function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Liu, X.; Liao, X.; Rao, X.; Wang, B.; Zhang, J.; Xu, G.; Jiang, X.; Qin, X.; Chen, C.; Zou, Z. The Lysosomal Membrane Protein LAMP-2 Is Dispensable for PINK1/Parkin-Mediated Mitophagy. FEBS Lett. 2020, 594, 823–840. [Google Scholar] [CrossRef]
- Ng, T.B.; Gao, W.; Li, L.; Niu, S.M.; Zhao, L.; Liu, J.; Shi, L.S.; Fu, M.; Liu, F. Rose (Rosa rugosa)-Flower Extract Increases the Activities of Antioxidant Enzymes and Their Gene Expression and Reduces Lipid Peroxidation. Biochem. Cell Biol. 2005, 83, 78–85. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy Robert P. Adams. J. Am. Soc. Mass. Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching Molecular Structure Databases with Tandem Mass Spectra Using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-Positive Senescent Cells Delays Ageing-Associated Disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Alessio, N.; Aprile, D.; Cappabianca, S.; Peluso, G.; Di Bernardo, G.; Galderisi, U. Different Stages of Quiescence, Senescence, and Cell Stress Identified by Molecular Algorithm Based on the Expression of Ki67, RPS6, and Beta-Galactosidase Activity. Int. J. Mol. Sci. 2021, 22, 3102. [Google Scholar] [CrossRef]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. AMPK Activation Protects Cells from Oxidative Stress-Induced Senescence via Autophagic Flux Restoration and Intracellular NAD+ Elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in Autophagy. Front. Physiol. 2023, 13, 2994. [Google Scholar]
- Philippe, C.; Pinson, B.; Dompierre, J.; Pantesco, V.; Viollet, B.; Daignan-Fornier, B.; Moenner, M. AICAR Antiproliferative Properties Involve the AMPK-Independent Activation of the Tumor Suppressors LATS 1 and 2. Neoplasia 2018, 20, 555–562. [Google Scholar] [CrossRef]
- Albrecht, D.; Ceschin, J.; Dompierre, J.; Gueniot, F.; Pinson, B.; Daignan-Fornier, B. Chemo-Genetic Interactions Between Histone Modification and the Antiproliferation Drug AICAR Are Conserved in Yeast and Humans. Genetics 2016, 204, 1447–1460. [Google Scholar] [CrossRef]
- Shin, E.J.; Han, A.; Lee, M.; Song, Y.-R.; Lee, K.M.; Nam, T.-G.; Lee, P.; Lee, S.-Y.; Lim, T.-G. Extraction Conditions for Rosa Gallica Petal Extracts with Anti-Skin Aging Activities. Food Sci. Biotechnol. 2019, 28, 1439–1446. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Jeong, D.; Qomaladewi, N.P.; Lee, J.; Park, S.H.; Cho, J.Y. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. J. Investig. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. The Coming of Age of Chaperone-Mediated Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Raz, Y.; Guerrero-Ros, I.; Maier, A.; Slagboom, P.E.; Atzmon, G.; Barzilai, N.; Macian, F. Activation-Induced Autophagy Is Preserved in CD4+ T-Cells in Familial Longevity. J. Gerontol. Ser. A 2017, 72, 1201–1206. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparło, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and Pathophysiology of Protein Carbonylation, Nitration and Chlorination in Age-Related Brain Diseases and Aging. Aging 2018, 10, 868–901. [Google Scholar] [CrossRef]
- Nyström, T. Role of Oxidative Carbonylation in Protein Quality Control and Senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef]
- Low, E.; Alimohammadiha, G.; Smith, L.A.; Costello, L.F.; Przyborski, S.A.; von Zglinicki, T.; Miwa, S. How Good Is the Evidence That Cellular Senescence Causes Skin Ageing? Ageing Res. Rev. 2021, 71, 101456. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally Occurring p16Ink4a-Positive Cells Shorten Healthy Lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Smitha Grace, S.R.; Chandran, G.; Chauhan, J.B. Terpenoids: An Activator of “Fuel-Sensing Enzyme AMPK” with Special Emphasis on Antidiabetic Activity. In Plant and Human Health, Volume 2: Phytochemistry and Molecular Aspects; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 227–244. ISBN 978-3-030-03344-6. [Google Scholar]
- The Potential Therapeutic Value of Terpenes. Available online: https://accscience.com/journal/ITPS/7/3/10.36922/itps.0332 (accessed on 11 February 2025).
- Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Sierra, I.; Câmara, J.S. Volatilomic Fingerprinting from Edible Flowers. Unravelling Some Impact Compounds Behind Its Attractiveness. Food Biosci. 2022, 50, 102188. [Google Scholar] [CrossRef]
- Tamilmani, P.; Sathibabu Uddandrao, V.V.; Chandrasekaran, P.; Saravanan, G.; Brahma Naidu, P.; Sengottuvelu, S.; Vadivukkarasi, S. Linalool Attenuates Lipid Accumulation and Oxidative Stress in Metabolic Dysfunction-Associated Steatotic Liver Disease via Sirt1/Akt/PPRA-α/AMPK and Nrf-2/HO-1 Signaling Pathways. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102231. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Z.; Riethoven, J.-J.; Xia, Y.; Miner, J.; Fromm, M. Conjugated Linoleic Acid Activates AMP-Activated Protein Kinase and Reduces Adiposity More Effectively When Used with Metformin in Mice. J. Nutr. 2009, 139, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Steinberg, G.R.; Chen, Z.-P.; Kemp, B.E.; Febbraio, M.A. Fatty Acids Stimulate AMP-Activated Protein Kinase and Enhance Fatty Acid Oxidation in L6 Myotubes. J. Physiol. 2006, 574, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol Prevents Oxidative Stress-Induced Senescence and Proliferative Dysfunction by Activating the AMPK-FOXO3 Cascade in Cultured Primary Human Keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Weikel, K.A.; Cacicedo, J.M.; Ido, Y. Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application. Nutrients 2017, 9, 751. [Google Scholar] [CrossRef]
- Lee, S.-H.; Seo, H.-S.; Seo, S.J.; Kim, C.-D.; Hong, S.-P. Screening of Plant-Derived Natural Extracts to Identify a Candidate Extract Capable of Enhancing Lipid Synthesis in Keratinocytes. Ann. Dermatol. 2022, 34, 331–339. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.-C.; Deng, J.-M.; Yu, M.; Zouboulis, C.C.; Wang, G.-L.; Wang, J. Tea (Camellia sinensis) Seed Saponins Act as Sebosuppression Agents via the AMPK/mTOR Pathway. J. Cosmet. Dermatol. 2025, 24, e16793. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).