Design and Characterisation of Personal Hygiene Gels Containing a Gypsophila Trichotoma Extract and Xanthium Strumarium Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents, Chemicals and Reagents
2.2. Plant Material and Extraction
2.3. Methods
2.3.1. Phytochemical Analysis
UHPLC Analysis of Saponins in EGT
GC-MS Analysis of EOXS
2.3.2. Preparation of EGT Test Solutions
2.3.3. Preparation of Liquid Handwashing Gels
2.3.4. pH Determination
2.3.5. Foam-Forming Ability and Foam Stability
2.3.6. Cleaning Capacity
2.3.7. Viscosity
2.3.8. Spreadability
2.3.9. Antibacterial Activity
3. Results and Discussion
3.1. Characterisation of EGT Solutions
3.2. Characterisation of Washing Gels
3.2.1. Preliminary Evaluation of Washing Gels
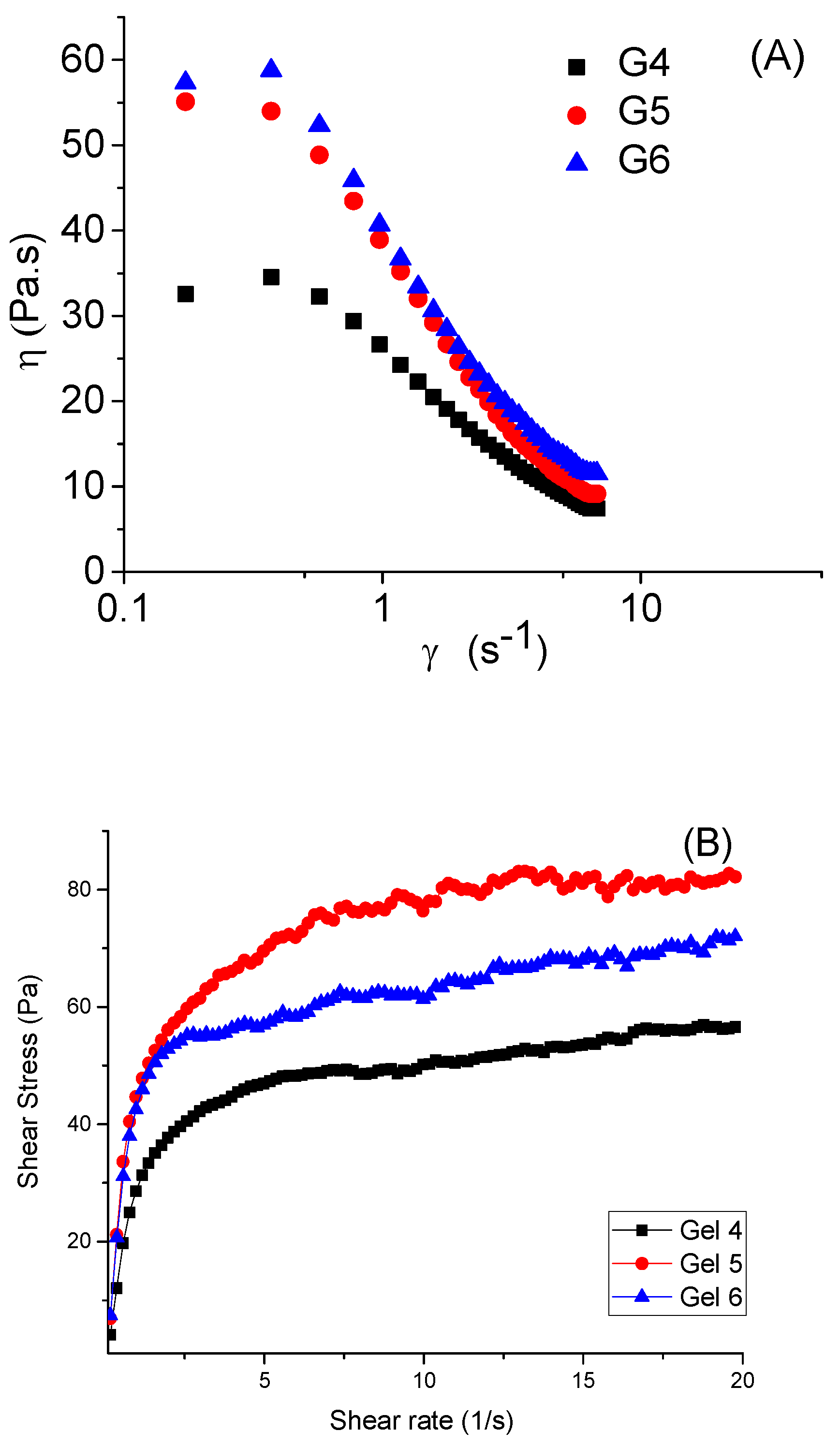
3.2.2. Evaluation of Optimised Washing Gels
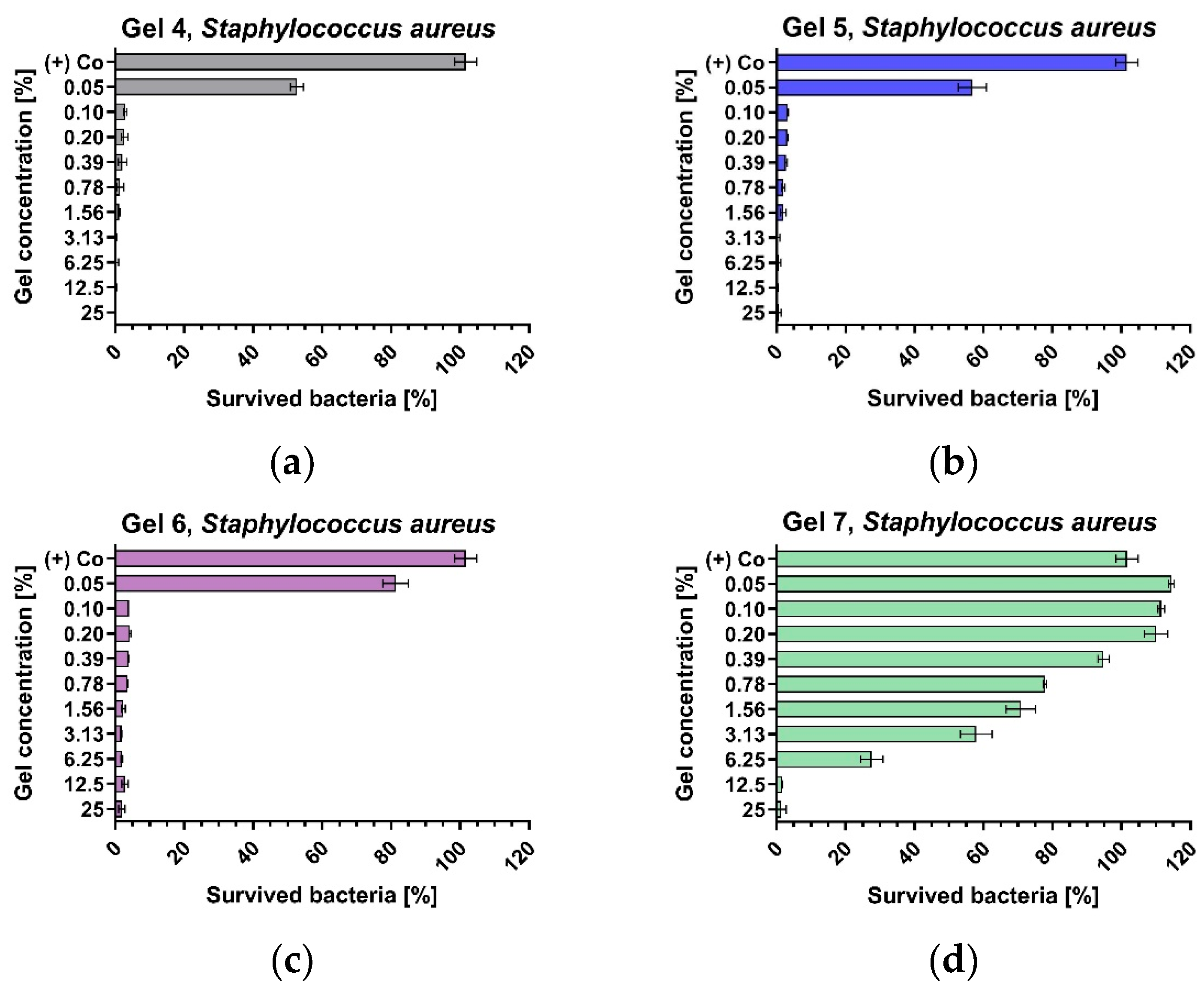
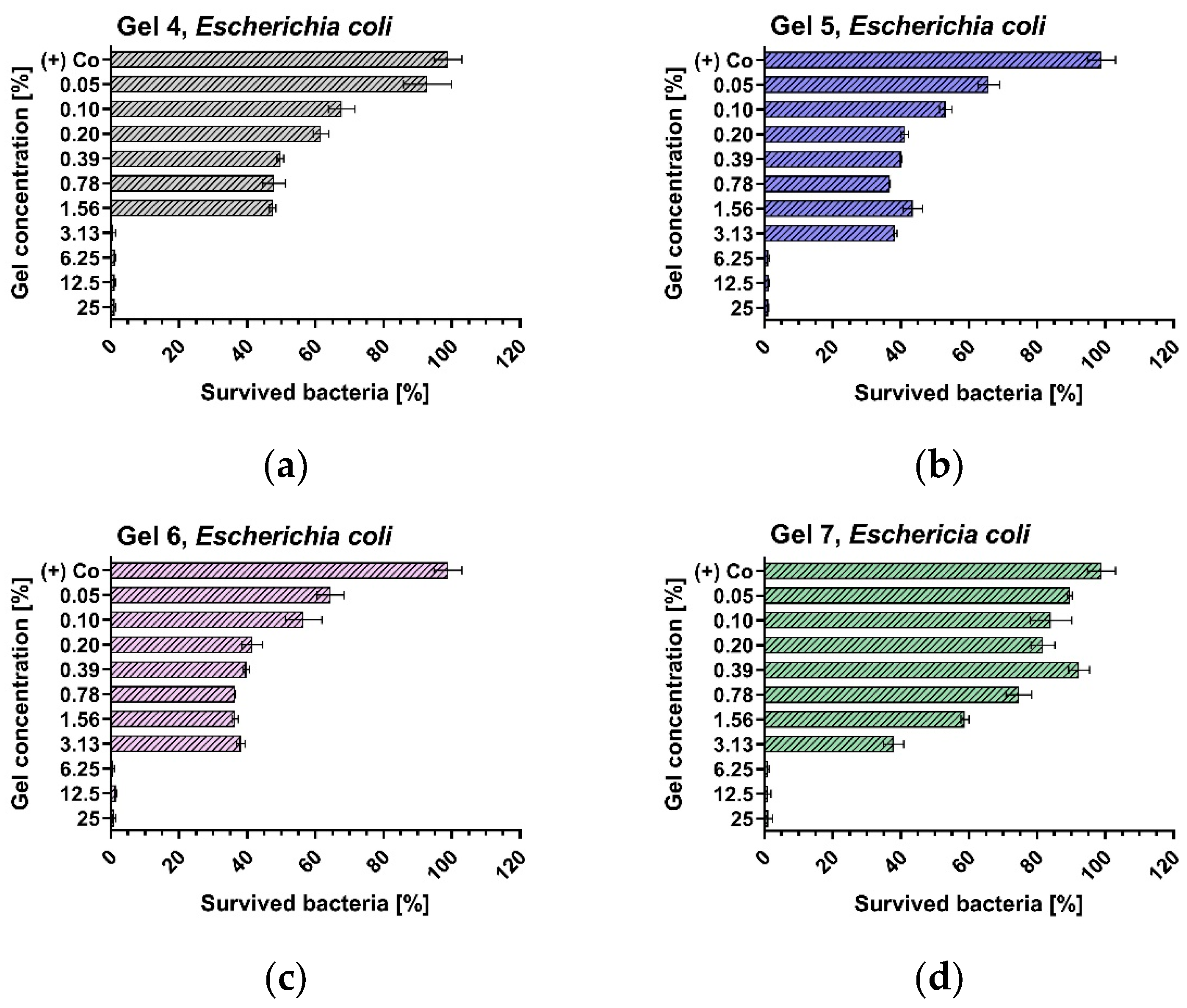
3.3. Antibacterial Activity of Optimised Gels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGT | Defatted Extract from Gypsophila trichotoma |
| EOXS | Essential Oil from Xanthium strumarium |
References
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, J.; Ma, J.; Yu, H.; Luo, L.; Wu, W.; Jin, L.; Yang, Q.; Lou, T.; Sun, D.; et al. Hand Sanitizer Gels: Classification, Challenges, and the Future of Multipurpose Hand Hygiene Products. Toxics 2023, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Bujak, T. Saponins as Natural Raw Materials for Increasing the Safety of Bodywash Cosmetic Use. J. Surfactants Deterg. 2018, 21, 767–776. [Google Scholar] [CrossRef]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, Classification and Occurrence in the Plant Kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Wimmerová, M.; Wimmer, Z. Saponins of Selected Triterpenoids as Potential Therapeutic Agents: A Review. Pharmaceuticals 2023, 16, 386. [Google Scholar] [CrossRef]
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and Stability of Emulsions Using a Natural Small Molecule Surfactant: Quillaja Saponin (Q-Naturale®). Food Hydrocoll. 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Petrova, A. Gypsophila trichotoma Wend.a. In Red Data Book of the Republic of Bulgaria, Vol. 1, Plants and Fungi; Peev, D., Golemanski, V., Eds.; Bulgarian Academy of Sciences: Sofia, Bulgaria, 2015. [Google Scholar]
- Vladimirov, V.; Aybeke, M.; Matevski, V.; Tan, K. New Floristic Records in the Balkans: 34. Phytol. Balc. 2017, 23, 413–444. [Google Scholar]
- Balabanova, V.; Zdraveva, P.; Kozuharova, E.; Krasteva, I.; Nikolov, S. A Possibility for Cultivation and Phytochemical Study of Endangered Gypsophila trichotoma Wend. Comptes Rendus L’académie Bulg. Sci. 2009, 62, 1247–1252. [Google Scholar]
- Kozuharova, E.; Balabanova, V.; Boev, V. In-Situ and Ex-Situ Morphological Characters of Gypsophila trichotoma Wend. Comptes Rendus L’académie Bulg. Des Sci. 2011, 64, 523–528. (In Bulgarian) [Google Scholar]
- Yotova, M.; Krasteva, I.; Nikolov, S. Triterpenoid Saponins from Genus Gypsophila L. (Caryophyllaceae). In Saponins: Properties, Applications and Health Benefits; Koh, R., Tay, I., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 99–122. [Google Scholar]
- Kamali, M.; Talebi, M.; Mottaghipisheh, J.; Sasani, E.; Mirshekari, B.M. An Updated Overview of Gypsophila Species: Phytochemical and Pharmacological Investigations. Fitoterapia 2024, 179, 106230. [Google Scholar] [CrossRef]
- Voutquenne-Nazabadioko, L.; Gevrenova, R.; Borie, N.; Harakat, D.; Sayagh, C.; Weng, A.; Thakur, M.; Zaharieva, M.; Henry, M. Triterpenoid Saponins from the Roots of Gypsophila trichotoma Wender. Phytochemistry 2013, 90, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, I.; Popov, I.; Balabanova, V.; Nikolov, S.; Pencheva, I. Phytochemical Study of Gypsophila trichotoma Wend. (Caryophyllaceae). Quim. Nova. 2008, 31, 1125–1126. [Google Scholar] [CrossRef]
- Vitcheva, V.; Simeonova, R.; Krasteva, I.; Yotova, M.; Nikolov, S.; Mitcheva, M. Hepatoprotective Effects of Saponarin, Isolated from Gypsophila trichotoma Wend. on Cocaine-Induced Oxidative Stress in Rats. Redox Rep. 2011, 16, 56–61. [Google Scholar] [CrossRef]
- Krasteva, I.; Yotova, M.; Yosifov, D.; Benbassat, N.; Jenett-Siems, K.; Konstantinov, S. Cytotoxicity of Gypsogenic Acid Isolated from Gypsophila trichotoma. Pharmacogn. Mag. 2014, 10, 430–433. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology of Xanthium strumarium L.: A Review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef]
- Strother, J.L. Xanthium. In Flora of North America; Oxford University: New York, NY, USA, 2006. [Google Scholar]
- Aneva, I.Y.; Zhelev, P.; Stoyanov, S.S. Alien Species as a Part of Plant Composition in the Periphery of Agricultural Fields. Acta Zool. Bulg. Suppl. 2018, 11, 173–176. [Google Scholar]
- Kozuharova, E.; Ionkova, I.; Spadaro, V. Xanthium strumarium—A Potential Cheap Resource of Plant Substances for Medicinal Use. Flora Mediterr. 2019, 29, 93–102. [Google Scholar]
- Plants of the World Online. 2024. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:260893-1/general-information (accessed on 1 December 2024).
- Arshad, M.; Khan, Q.A. Ethnobotanical Study of Some Medicinal Plants of Rawal Town. Pak. J. Biol. Sci. 2000, 3, 1245–1246. [Google Scholar]
- Hussain, K.; Shahazad, A.; Zia-ul-Hussnain, S. An Ethnobotanical Survey of Important Wild Medicinal Plants of Hattar District Haripur, Pakistan. Ethnobot. Leafl. 2008, 2008, 5. [Google Scholar]
- Ali, H.; Qaiser, M. The Ethnobotany of Chitral Valley, Pakistan with Particular Reference to Medicinal Plants. Pak. J. Bot. 2009, 41, 2009–2041. [Google Scholar]
- Bhardwaj, R.; Dutta, S.; Sharma, K.C. Conserving Biodiversity of Medicinal Plants from Central Aravallis of Rajasthan, India. J. Env. Res. Dev. 2011, 6, 69–75. [Google Scholar]
- Yadav, D.K.; Jhariya, M.K.; Kumar, A.; Sinha, R. Documentation and Ethnobotanical Importance of Medicinal Plants Found in Sarguja District. J. Plant Dev. Sci. 2015, 7, 439–446. [Google Scholar]
- Ullah, M.; Khan, M.U.; Mahmood, A.; Malik, R.N.; Hussain, M.; Wazir, S.M.; Daud, M.; Shinwari, Z.K. An Ethnobotanical Survey of Indigenous Medicinal Plants in Wana District South Waziristan Agency, Pakistan. J. Ethnopharmacol. 2013, 150, 918–924. [Google Scholar] [CrossRef]
- Akhtar, N.; Rashid, A.; Murad, W.; Bergmeier, E. Diversity and Use of Ethno-Medicinal Plants in the Region of Swat, North Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 25. [Google Scholar] [CrossRef]
- Gilani, S.A.; Khan, A.M.; Qureshi, R.A.; Sherwani, S.K.; Ullah Khan, R.; Bokhari, T.Z. Ethnomedicinal Treatment of Common Gastrointestinal Disorders by Indigenous People in Pakistan. Adv. Biores 2014, 5, 42–49. [Google Scholar]
- Manandhar, N.P. Native Use of Herbal Drugs for Treatment of Skin Diseases in Nepal. In Plant-Derived Antimycotics; CRC Press: Boca Raton, FL, USA, 2024; pp. 429–439. [Google Scholar]
- Kunwar, R.M.; Acharya, R.P.; Chowdhary, C.L.; Bussmann, R.W. Medicinal Plant Dynamics in Indigenous Medicines in Farwest Nepal. J. Ethnopharmacol. 2015, 163, 210–219. [Google Scholar]
- Shkondrov, A.; Krasteva, I.; Kozuharova, E.; Ionkova, I. Chemical Composition of Essential Oil in Fruits of Xanthium strumarium L. Biotechnol. Biotechnol. Equip. 2021, 35, 1474–1479. [Google Scholar]
- Parveen, Z.; Mazhar, S.; Siddique, S.; Manzoor, A.; Ali, Z. Chemical Composition and Antifungal Activity of Essential Oil from Xanthium strumarium L. Leaves. Indian J. Pharm. Sci. 2017, 79, 316–321. [Google Scholar]
- Sarmah, M.; Bhola, R.K. Bio-Activity of Xanthium strumarium Extracts against Tea Mosquito Bug, Helopeltis Theivora. J. Plant Crop. 2014, 42, 40–47. [Google Scholar]
- Kejlová, K.; Jírová, D.; Bendová, H.; Gajdoš, P.; Kolářová, H. Phototoxicity of Essential Oils Intended for Cosmetic Use. Toxicol. Vitr. 2010, 24, 2084–2089. [Google Scholar] [CrossRef]
- Zazharskyi, V.V.; Brygadyrenko, V.V.; Boyko, O.O.; Bilan, M.V.; Zazharska, N.M. Antibacterial and Anthelmintic Activities of Xanthium strumarium (Asteraceae) Extracts. Regul. Mech. Biosyst. 2024, 15, 129–133. [Google Scholar]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.M.; Sharifi-Rad, M.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M.; Sharifi-Rad, R.; Raeisi, S. Phytochemical Compositions and Biological Activities of Essential Oil from Xanthium strumarium L. Molecules 2015, 20, 7034–7047. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Es, I.; Khaneghah, A.M.; Akbariirad, H. Global Regulation of Essential Oils. In Essential Oils in Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 327–338. ISBN 9781119149392. [Google Scholar]
- Yücekutlu, A. Determination of Plant Saponins and Some of Gypsophila Species: A Review of the Literature. Hacet. J. Biol. Chem. 2008, 36, 129–135. [Google Scholar]
- Available online: https://ifrafragrance.org/safe-use/library (accessed on 12 December 2024).
- Pradhan, A.; Bhattacharyya, A. Shampoos Then and Now: Synthetic versus Natural. J. Surf. Sci. Technol. 2014, 30, 59–76. [Google Scholar]
- Rai, S.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Characterization of Saponins from the Leaves and Stem Bark of Jatropha curcas L. for Surface-Active Properties. Heliyon 2023, 9, e15807. [Google Scholar]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R.G.d.S. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar]
- ISO 20776-1:2019 Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices Part 1: Broth Micro-Dilution Reference Method for Testing the in Vitro Activity of Antimicrobial Agents. Available online: https://www.iso.org/standard/70464.html (accessed on 1 December 2024).
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 1 November 2005; pp. 11–12. [Google Scholar]
- Chen, S.; Kord, A. UHPLC Method Development. In Ultra-High Performance Liquid Chromatography and Its Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1–30. ISBN 9781118533956. [Google Scholar]
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Esmaeili, A.; Rustaiyan, A.; Akbari, M.T.; Moazami, N.; Masoudi, S.; Amiri, H. Composition of the Essential Oils of Xanthium strumarium L. and Cetaurea solstitialis L. from Iran. J. Essent. Oil Res. 2006, 18, 427–429. [Google Scholar]
- El-Gawad, A.A.; Elshamy, A.; El Gendy, A.E.-N.; Gaara, A.; Assaeed, A. Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal PH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Malysa, K. Simple and Generally Applicable Method of Determination and Evaluation of Foam Properties. J. Surfactants Deterg. 2003, 6, 69–74. [Google Scholar] [CrossRef]
- Schad, T.; Preisig, N.; Blunk, D.; Piening, H.; Drenckhan, W.; Stubenrauch, C. Less Is More: Unstable Foams Clean Better than Stable Foams. J. Colloid Interface Sci. 2021, 590, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; He, W.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Effect of PH and Xanthan Gum on Emulsifying Property of Ovalbumin Stabilized Oil-in Water Emulsions. LWT 2021, 147, 111621. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Giri, T.K.; Nakhate, K.T.; Kashyap, P.; Tripathi, D.K. Xanthan Gum and Its Derivatives as a Potential Bio-Polymeric Carrier for Drug Delivery System. Curr. Drug Deliv. 2013, 10, 587–600. [Google Scholar] [CrossRef]
- Ćirin, D.; Milutinov, J.; Krstonošić, V. Occurrence of Alkyl Glucosides in Rinse-off Cosmetics Marketed as Hypoallergenic or for Sensitive Skin. Toxicol. Ind. Health 2024, 40, 306–311. [Google Scholar] [CrossRef]
- Brunchi, C.-E.; Bercea, M.; Morariu, S.; Dascalu, M. Some Properties of Xanthan Gum in Aqueous Solutions: Effect of Temperature and PH. J. Polym. Res. 2016, 23, 123. [Google Scholar] [CrossRef]
- Ali, I.; Shah, L.A. Rheological Investigation of the Viscoelastic Thixotropic Behavior of Synthesized Polyethylene Glycol-Modified Polyacrylamide Hydrogels Using Different Accelerators. Polym. Bull. 2021, 78, 1275–1291. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Moon, R.J.; Hubbe, M.A.; Bortner, M.J. Rheological Aspects of Cellulose Nanomaterials: Governing Factors and Emerging Applications. Adv. Mater. 2021, 33, 2006052. [Google Scholar] [CrossRef]
- Patel, M.C.; Ayoub, M.A.; Hassan, A.M.; Idress, M.B. A Novel ZnO Nanoparticles Enhanced Surfactant Based Viscoelastic Fluid Systems for Fracturing under High Temperature and High Shear Rate Conditions: Synthesis, Rheometric Analysis, and Fluid Model Derivation. Polymers 2022, 14, 4023. [Google Scholar] [CrossRef]
- Lin, H.-R.; Sung, K.C.; Vong, W.-J. In Situ Gelling of Alginate/Pluronic Solutions for Ophthalmic Delivery of Pilocarpine. Biomacromolecules 2004, 5, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Stubenrauch, C.; Drenckhan, W. Cleaning Solid Surfaces with Liquid Interfaces and Foams: From Theory to Applications. Curr. Opin. Colloid Interface Sci. 2024, 72, 101818. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Kashi, M.E.; Shakeri, A. Natural Products against Gram-Negative Bacteria: Promising Antimicrobials in Future Complementary Medicine. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Di Vito, M.; Smolka, A.; Proto, M.R.; Barbanti, L.; Gelmini, F.; Napoli, E.; Bellardi, M.G.; Mattarelli, P.; Beretta, G.; Sanguinetti, M.; et al. Is the Antimicrobial Activity of Hydrolates Lower than That of Essential Oils? Antibiotics 2021, 10, 88. [Google Scholar] [CrossRef]
- Fan, J.; Jin, S.; Gilmartin, L.; Toth, I.; Hussein, W.M.; Stephenson, R.J. Advances in Infectious Disease Vaccine Adjuvants. Vaccines 2022, 10, 1120. [Google Scholar] [CrossRef]
- Ahmad, A.; Elisha, I.L.; van Vuuren, S.; Viljoen, A. Volatile Phenolics: A Comprehensive Review of the Anti-Infective Properties of an Important Class of Essential Oil Constituents. Phytochemistry 2021, 190, 112864. [Google Scholar] [CrossRef]
- Cappiello, F.; Loffredo, M.R.; Del Plato, C.; Cammarone, S.; Casciaro, B.; Quaglio, D.; Mangoni, M.L.; Botta, B.; Ghirga, F. The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics 2020, 9, 325. [Google Scholar] [CrossRef]

| Gel | EGT (%) | Coco Glycoside (%) | EOXS (%) | Xanthan Gum (%) | Glycerine (%) | Deionised Water (%) |
|---|---|---|---|---|---|---|
| G1 | 10 | - | - | 0.2 | 7 | 82.8 |
| G2 | 20 | - | - | 0.4 | 7 | 72.6 |
| G3 | 30 | - | - | 0.6 | 7 | 62.4 |
| G4 | 10 | 5 | 0.2 | 0.6 | 7 | 77.2 |
| G5 | 20 | 5 | 0.5 | 0.6 | 7 | 73.9 |
| G6 | 30 | 5 | 1.0 | 0.6 | 7 | 63.4 |
| G7 | - | 5 | - | 0.6 | 7 | 87.4 |
| Parameter | Gypsogenic Acid | Gypsogenin |
|---|---|---|
| Mean peak area ± SD | 20.0910 | 33.9863 |
| Peak area, %RSD | 2.78 | 2.33 |
| Mean tR, min ± SD | 17.05 ± 0.2 | 18.66 ± 0.2 |
| tR, %RSD | 0.15 | 0.21 |
| Mean theoretical plates ± SD | 477,458 ± 173 | 344,687 ± 132 |
| Resolution, mean ± SD | 2.0 ± 0.0 | 2.2 ± 0.0 |
| Resolution, %RSD | 0.23 | 0.24 |
| Parameter | Value | |
|---|---|---|
| Gypsogenic Acid | Gypsogenin | |
| Range of linearity, mg/mL | 0.2–3.0 | 0.1–2.0 |
| Slope | 13.617 | 34.821 |
| Y-intercept | −1.6129 | −9.3945 |
| Coefficient of correlation r2 | 0.9980 | 0.9973 |
| Analytical Marker | Theoretical Concentration, mg/mL | Concentration Found, mg/mL | Recovery, % ± SD | %RSD | Average Recovery, % |
|---|---|---|---|---|---|
| Gysogenic acid | 1.000 | 1.050 | 105.0 ± 3.5 | 3.5 | 100.2 ± 1.5 |
| 0.500 | 0.501 | 100.2 ± 1.3 | 4.1 | ||
| 0.100 | 0.099 | 99.9 ± 3.0 | 2.1 | ||
| Gypsogenin | 1.000 | 0.999 | 99.9 ± 2.8 | 4.0 | 100.5 ± 1.6 |
| 0.500 | 0.498 | 99.6 ± 3.2 | 2.7 | ||
| 0.100 | 0.102 | 102.0 ± 3.5 | 1.5 |
| Analytical Marker | Concentration of the Standard Solution, mg/mL | Concentration Found (Intraday) *, mg/mL | Intraday Variance **, %RSD | Concentration Found (Interday) *, mg/mL | Interday Variance ***, %RSD | |
|---|---|---|---|---|---|---|
| Analysis Series 1 | Analysis Series 2 | |||||
| Gysogenic acid | 1.0000 | 1.0502 | 1.0498 | 1.4 | 1.0501 | 2.9 |
| 0.5000 | 0.5012 | 0.5009 | 1.6 | 0.5002 | 1.7 | |
| 0.1000 | 0.9989 | 0.9979 | 2.1 | 0.0998 | 2.3 | |
| Gypsogenin | 1.000 | 0.9998 | 0.9958 | 1.2 | 0.9996 | 2.5 |
| 0.500 | 0.4981 | 0.4990 | 1.3 | 0.4995 | 1.9 | |
| 0.100 | 0.1020 | 0.1010 | 1.1 | 0.1012 | 2.2 | |
| EGT Solution | EGT (%) | Saponin Concentration (%) | pH | Foam Height (cm) | R5 (%) | Cleaning Ability (%) |
|---|---|---|---|---|---|---|
| S1 | 10 | 0.4 | 6.8 | 4.5 ± 0.1 | 44 ± 1.1 | 90 ± 0.5 |
| S2 | 20 | 0.8 | 6.7 | 5 ± 0.3 | 51 ± 2.1 | 85 ± 1.3 |
| S3 | 30 | 1.2 | 6.5 | 6 ± 0.2 | 63 ± 2.8 | 97 ± 0.8 |
| Formulation Code | EGT (%) | Coco Glucoside (%) | EOXS (%) | Xanthan Gum (%) | pH | Viscosity (Pa.s) | Spreadability (cm) | Foam Height (cm) | R5 (%) | Cleaning Ability (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | 10 | - | - | 0.2 | 5.2 ± 0.6 | 24 ± 3.5 | 8.6 ± 0.9 | 3.8 ± 0.3 | 39 ± 2.1 | 76 ± 3.2 |
| G2 | 20 | - | - | 0.4 | 5.0 ± 0.8 | 40 ± 3.1 | 7.7 ± 0.5 | 5.0 ± 0.4 | 48 ± 1.4 | 82 ± 1.8 |
| G3 | 30 | - | - | 0.6 | 4.8 ± 0.5 | 43 ± 1.1 | 7.5 ± 1.2 | 6.0 ± 1.1 | 51 ± 2.2 | 88 ± 2.5 |
| G4 | 10 | 5 | 0.2 | 0.6 | 5.8 ± 0.5 | 44 ± 2.8 | 7.4 ± 0.2 | 12.0 ± 1.2 | 97 ± 2.1 | 98 ± 1.1 |
| G5 | 20 | 5 | 0.5 | 0.6 | 5.7 ± 0.5 | 45 ± 0.5 | 7.3 ± 0.6 | 13.0 ± 1.1 | 98 ± 0.9 | 98 ± 0.6 |
| G6 | 30 | 5 | 1.0 | 0.6 | 5.6 ± 0.5 | 45 ± 1.2 | 7.1 ± 0.8 | 15.0 ± 1.3 | 98 ± 1.1 | 99 ± 0.8 |
| G7 | - | 5 | - | 0.6 | 7.8 ± 0.5 | 44 ± 2.1 | 7.6 ± 0.6 | 13.0 ± 2.1 | 95 ± 2.1 | 97 ± 1.6 |
| Gels | Staphylococcus aureus, ATCC® 29213TM | Escherichia coli, ATCC® 35218 TM | ||
|---|---|---|---|---|
| MIC [%] | MBC [%] | MIC [%] | MBC [%] | |
| G4 | 0.100 | 3.125 | 3.125 | 3.125 |
| G5 | 0.100 | 3.125 | 6.250 | 6.250 |
| G6 | 0.100 | 3.125 | 6.250 | 6.250 |
| G7 | 6.250 | 12.500 | 6.250 | 6.250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkondrov, A.; Momekova, D.; Zaharieva, M.M.; Najdenski, H.; Kozuharova, E.; Krasteva, I. Design and Characterisation of Personal Hygiene Gels Containing a Gypsophila Trichotoma Extract and Xanthium Strumarium Essential Oil. Cosmetics 2025, 12, 65. https://doi.org/10.3390/cosmetics12020065
Shkondrov A, Momekova D, Zaharieva MM, Najdenski H, Kozuharova E, Krasteva I. Design and Characterisation of Personal Hygiene Gels Containing a Gypsophila Trichotoma Extract and Xanthium Strumarium Essential Oil. Cosmetics. 2025; 12(2):65. https://doi.org/10.3390/cosmetics12020065
Chicago/Turabian StyleShkondrov, Aleksandar, Denitsa Momekova, Maya Margaritova Zaharieva, Hristo Najdenski, Ekaterina Kozuharova, and Ilina Krasteva. 2025. "Design and Characterisation of Personal Hygiene Gels Containing a Gypsophila Trichotoma Extract and Xanthium Strumarium Essential Oil" Cosmetics 12, no. 2: 65. https://doi.org/10.3390/cosmetics12020065
APA StyleShkondrov, A., Momekova, D., Zaharieva, M. M., Najdenski, H., Kozuharova, E., & Krasteva, I. (2025). Design and Characterisation of Personal Hygiene Gels Containing a Gypsophila Trichotoma Extract and Xanthium Strumarium Essential Oil. Cosmetics, 12(2), 65. https://doi.org/10.3390/cosmetics12020065









