Evaluation of Sebum Control and Safety for Daily Use of a Cosmetic Elastomer Formulated with Vegetable Oils from Peruvian Biodiversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation and Development of Cosmetic Elastomers
2.2. Study Design
2.3. Safety Evaluation Protocol for Cosmetic Use
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.3.3. Safety Analysis for Daily Use
2.4. Protocol of the in Vivo Sebum Control Study
2.4.1. Study Design
2.4.2. Study Population and Sample
2.4.3. Inclusion Criteria
2.4.4. Exclusion Criteria
2.4.5. In Vivo Instrumental Efficacy Evaluation
2.4.6. Instrumental Effectiveness Testing Procedures
2.5. Statistical Analysis
3. Results
3.1. Safety for Cosmetic Use
3.2. Sebum Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavinato, M. Cosmetics and Cosmeceuticals. In Encyclopedia of Biomedical Gerontology; Elsevier Inc.: Amsterdam, The Netherland, 2019; pp. 446–461. [Google Scholar]
- Wilhelm, K. Non Invasive Diagnostic Techniques in Clinical Dermatology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Endly, D.C.; Miller, R.A. Oily Skin: A review of treatment Options. J. Clin. Aesthet. Dermatol. 2017, 10, 49–55. [Google Scholar]
- Rosso, J.Q.D.; Kircik, L. The primary role of sebum in the pathophysiology of acne vulgaris and its therapeutic relevance in acne management. J. Dermatolog. Treat. 2024, 35, 2296855. [Google Scholar] [CrossRef] [PubMed]
- Downing, D.T.; Stewart, M.E.; Wertz, P.W.; Strauss, J.S. Essential fatty acids and acne. J. Am. Acad. Dermatol. 1986, 14, 221–225. [Google Scholar] [CrossRef]
- Hansen, H.S.; Jensen, B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and α-linolenate. Biochim. Biophys. Acta 1985, 834, 357–363. [Google Scholar] [PubMed]
- Breiden, B.; Sandhoff, K.; Feingold, K.R.; Elias, P. Biochimica et Biophysica Acta The role of sphingolipid metabolism in cutaneous permeability barrier formation. BBA Mol. Cell Biol. Lipids 2014, 1841, 441–452. [Google Scholar]
- Picardo, M.; Ottaviani, M.; Camera, E.; Mastrofrancesco, A. Sebaceous gland lipids. Derm. Endocronol. 2009, 1, 68–71. [Google Scholar]
- Jacobsen, E.; Billings, J.K.; Frantz, R.A.; Kinney, C.K.; Stewart, M.E.; Downing, D.T. Age-related changes in sebaceous wax ester secretion rates in men and women. J. Investig. Dermatol. 1985, 85, 483–485. [Google Scholar] [CrossRef]
- de Melo, M.O.; Maia Campos, P.M.B.G. Characterization of oily mature skin by biophysical and skin imaging techniques. Ski. Res. Technol. 2018, 24, 386–395. [Google Scholar]
- Youn, S.; Park, E.; Lee, D.; Huh, C.; Park, K. Clinical and Laboratoring Investigation Does facial sebum excretion really affect the development of acne? Br. J. Dermatol. 2005, 153, 919–924. [Google Scholar]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar]
- Eichenfield, D.; Sprague, J.; Eichenfield, L. Management of Acne Vulgaris: A Review. Available online: https://pubmed.ncbi.nlm.nih.gov/34812859/ (accessed on 10 December 2024).
- Dreno, B.; Pecastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar]
- Lauriola, M.M.; Corazza, M. The Wild Market of Natural Cosmetics of Obscure Safety. Dermatology 2019, 235, 527–528. [Google Scholar]
- Zhu, J.; Van Reeth, I.; Johnson, B.K. The Beauty of Silicone in Hair Care Application. Dow Chem. Co. 2017, 388, 130–131. [Google Scholar]
- María, A.; Jáuregui, M.; Ureta, C.A.; Castañeda, B.; Caparó, F.L.; Mendoza, E.B.; Lucero, L.C.; Cèspedes, E.M. Estudio Nutricional de Plukenetia huayllabambana sp. nov. Rev. Soc. Química Perú 2013, 79, 47–56. [Google Scholar]
- Nocetti, D.; Núñez, H.; Puente, L.; Romero, F. Composition and biological effects of goldenberry byproducts: An overview. Soc. Chem. Ind. 2020, 100, 4335–4346. [Google Scholar]
- Ramadan, M.F.; Morse, J.-T. Oil Goldenberry (Physalis peruviana L.). J. Agric. Food Chem. 2003, 51, 969–974. [Google Scholar] [PubMed]
- Kluczkovski, A.M.; Martins, M.; Mundim, S.M.; Simões, H.; Nascimento, K.S.; Marinho, H.A.; Junior, A.K. Properties of Brazil nuts: A review. Afr. J. Biotechnol. 2015, 14, 642–648. [Google Scholar]
- Ministerio de Salud del Peru. Reglamento de Ensayos Clínicos; Instituto Nacional de Salud: Lima, Peru, 2018.
- Ministerio de Justicia y Derechos Humanos. Decreto Supremo No 003-2013-JUS; El Peruano: Lima, Peru, 2013.
- Mulyanti, Y.S.; Kasemchainan, B.; Mitra, P.P.; Evans, P.; Hartono, H. Evaluation of the Skin Irritation and Sensitization Potential of the Cussons Baby Sensicare Skin Range of Products in Healthy Volunteers. J. Cosmet. Dermatol. Sci. Appl. 2019, 9, 207–215. [Google Scholar] [CrossRef]
- Pongpairoj, K.; Ale, I.; Andersen, K.E.; Bruze, M.; Diepgen, T.L.; Elsner, P.U.; Goh, C.L.; Uk, M.; Goossens, A.; Jerajani, H.; et al. Proposed ICDRG Classification of the Clinical Presentation of Contact Allergy. Dermatitis 2016, 27, 248–258. [Google Scholar]
- Ivens, U.; Serup, J. Allergy patch test reading from photographic images: Disagreement on ICDRG grading but agreement on simplified tripartite reading. Ski. Res. Technol. 2007, 13, 110–113. [Google Scholar]
- Traub, E.F. Evaluation of Dermal Sensitivity. Arch. Derm. Syphilol. 2015, 69, 399–409. [Google Scholar]
- Gupta, V.; Sharma, V.K. Skin typing: Fitzpatrick grading and others. Clin. Dermatol. 2019, 37, 430–436. [Google Scholar]
- Crowther, J.M. Method for quantification of oils and sebum levels on skin using the Sebumeter®. Int. J. Cosmet. Sci. 2016, 38, 210–216. [Google Scholar]
- Anurukvorakun, O.; Numnim, S. Development and Clinical Efficacy Evaluation of Facial Toner Containing Houttuynia cordata Thunb. Cosmetics 2023, 10, 133. [Google Scholar] [CrossRef]
- Kozlowska, J.; Kaczmarkiewicz, A.; Stachowiak, N.; Sionkowska, A. Evaluation of sebostatic activity of Juniperus communis fruit oil and Pelargonium graveolens oil compared to niacinamide. Cosmetics 2017, 4, 36. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Y.; Dong, M.; Wang, C.; Sun, Y.; Su, N.; Liu, J.; Zheng, H.; Yang, X.; Li, J.; et al. Moisturizing and antisebum effect of cosmetic application on facial skin. J. Cosmet. Dermatol. 2007, 6, 172–177. [Google Scholar]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar]
- Rodríguez-Cruz, M.; Tovar, A.R.; del Prado, M.; Torres, N. Mecanismos moleculares de acción de los ácidos grasos poliinsaturados y sus beneficios en la salud. Rev. Investig. Clin. 2005, 57, 457–472. [Google Scholar]
- Qidwai, A.; Pandey, M.; Pathak, S.; Kumar, R.; Dikshit, A. The emerging principles for acne biogenesis: A dermatological problem of puberty. Hum. Microbiome J. 2017, 4, 7–13. [Google Scholar]
- Briganti, S.; Mosca, S.; Di Nardo, A.; Flori, E.; Ottaviani, M. New Insights into the Role of PPARγ in Skin Physiopathology. Biomolecules 2024, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- López-estebaranz, J.L.; Herranz-pinto, P.; Dréno, B. Consenso español para establecer una clasificación y un algoritmo de tratamiento del acn. Actas Dermo-Sifiliogr. 2017, 108, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Mccusker, M.M.; Grant-kels, J.M. Healing fats of the skin: The structural and immunologic roles of the ω-6 and ω-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, M.; Zhang, L. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Greydanus, D.E.; Azmeh, R.; Cabral, M.D.; Dickson, C.A.; Patel, D.R. Acne in the first three decades of life: An update of a disorder with profound implications for all decades of life. Disease-a-Month 2021, 67, 101103. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. In vivo confocal Raman microscopic determination of depth pro fi les of the stratum corneum lipid organization in fl uenced by application of various oils. J. Dermatol. Sci. 2017, 87, 183–191. [Google Scholar] [CrossRef]
- Choe, C.; Darvin, M.E. Analysis of Human and Porcine Skin in vivo/ex vivo for Penetration of Selected Oils by Confocal Raman Microscopy. Ski. Pharmacol. Physiol. 2015, 28, 318–330. [Google Scholar] [CrossRef]
- Čižinauskas, V.; Elie, N.; Briedis, A.B.; Ci, V. Fatty acids penetration into human skin ex vivo: A TOF-SIMS analysis approach Fatty acids penetration into human skin ex vivo: A TOF-SIMS analysis approach. Biointerphases 2017, 12, 011003. [Google Scholar] [CrossRef]
- Sakuma, T.H.; Maibach, H.I. Oily skin: An overview. Skin Pharmacol. Physiol. 2012, 25, 227–235. [Google Scholar] [CrossRef]
- Liang, T.; Liao, S. Inhibition of steroid 5x-reductase by specific aliphatic unsaturated fatty acids. Biochem. J. 1992, 285, 557–562. [Google Scholar] [CrossRef]
- Dobrev, H. Clinical and instrumental study of the efficacy of a new sebum control cream. J. Cosmet. Dermatol. 2007, 2, 113–118. [Google Scholar]
- Khan, B.A.; Akhtar, N. Clinical and sebumetric evaluation of topical emulsions in the treatment of acne vulgaris. Adv. Dermatol. Allergol. 2014, 4, 229–234. [Google Scholar]
- Pongsakornpaisan, P.; Lourith, N.; Kanlayavattanakul, M. Anti-sebum efficacy of guava toner: A split-face, randomized, single-blind placebo-controlled study. J. Cosmet. Dermatol. 2019, 18, 1737–1741. [Google Scholar] [PubMed]
- El-domyati, M.; Medhat, W. Skin Aging: An Immunohistochemical Evaluation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–17. [Google Scholar]
- Giménez-Arnau, A.M.; Deza, G.; Bauer, A.; Johnston, G.A.; Mahler, V.; Schuttelaar, M.L.; Sanchez-Perez, J.; Silvestre, J.F.; Wilkinson, M.; Uter, W. Natural does. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 664–671. [Google Scholar]
- Keen, M.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar]
- Tabee, E.; Azadmard-Damirchi, S.; Jägerstad, M.; Dutta, P.C. Effects of α-tocopherol on oxidative stability and phytosterol oxidation during heating in some regular and high-oleic vegetable oils. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 857–867. [Google Scholar]


| Phase | Trade Name | INCI Name | %Purity/Origin | Amount% w/w |
|---|---|---|---|---|
| A | MSS-500/3H | Silica | 100.0/South PlainfieldUSA | 24.80 |
| B | Super Sacha inchi | Plukenetia huayllabambana seed oil | 100.0/Amazon—Peru | 23.70 |
| Aguaymanto | Physalis peruviana seed oil | 100.0/Huanuco—Peru | 43.00 | |
| Castaña de Indias | Bertholletia excelsa seed oil | 100.0/Madre de Dios—Peru | 7.500 | |
| Vitamina E-Alfa tocoferol acetato | Tocopherol | 98.6/Ludwigshafen—Germany | 1.00 |
| Mdil | Result |
|---|---|
| MdiI = 0.0 | Nonirritating, very good skin compatibility |
| MdiI < 0.2 | Nonirritating, good skin compatibility |
| 0.2 < MdiI < 0.5 | Slightly irritating, regular skin compatibility |
| 0.5 < MdiI < 1 | Moderately irritating, poor skin compatibility |
| >MdiI 1 | Irritating, very poor skin compatibility |
| Results of the Skin Irritation Potential | |
|---|---|
| Total number of participants | 24 |
| Σ skin reactions (Idil) | 0.0 |
| Mean skin irritation index (Mdil) | 0.00 |
| Final outcome (Mdil) | Nonirritating, very good skin compatibility |
| Control n = 6 | 2 h w/o Product | 2 h w/Product | 4 h w/o Product | 4 h w/Product | 5 h w/o Product | 5 h w/Product |
|---|---|---|---|---|---|---|
| Average | 122.17 | 72.58 | 178.17 | 117.67 | 184.42 | 123.17 |
| Max | 150.00 | 93.00 | 212.00 | 151.50 | 214.50 | 176.50 |
| Min | 96.50 | 60.50 | 143.50 | 70.50 | 130.50 | 72.50 |
| SE | 8.69 | 5.03 | 10.38 | 11.67 | 13.62 | 15.94 |
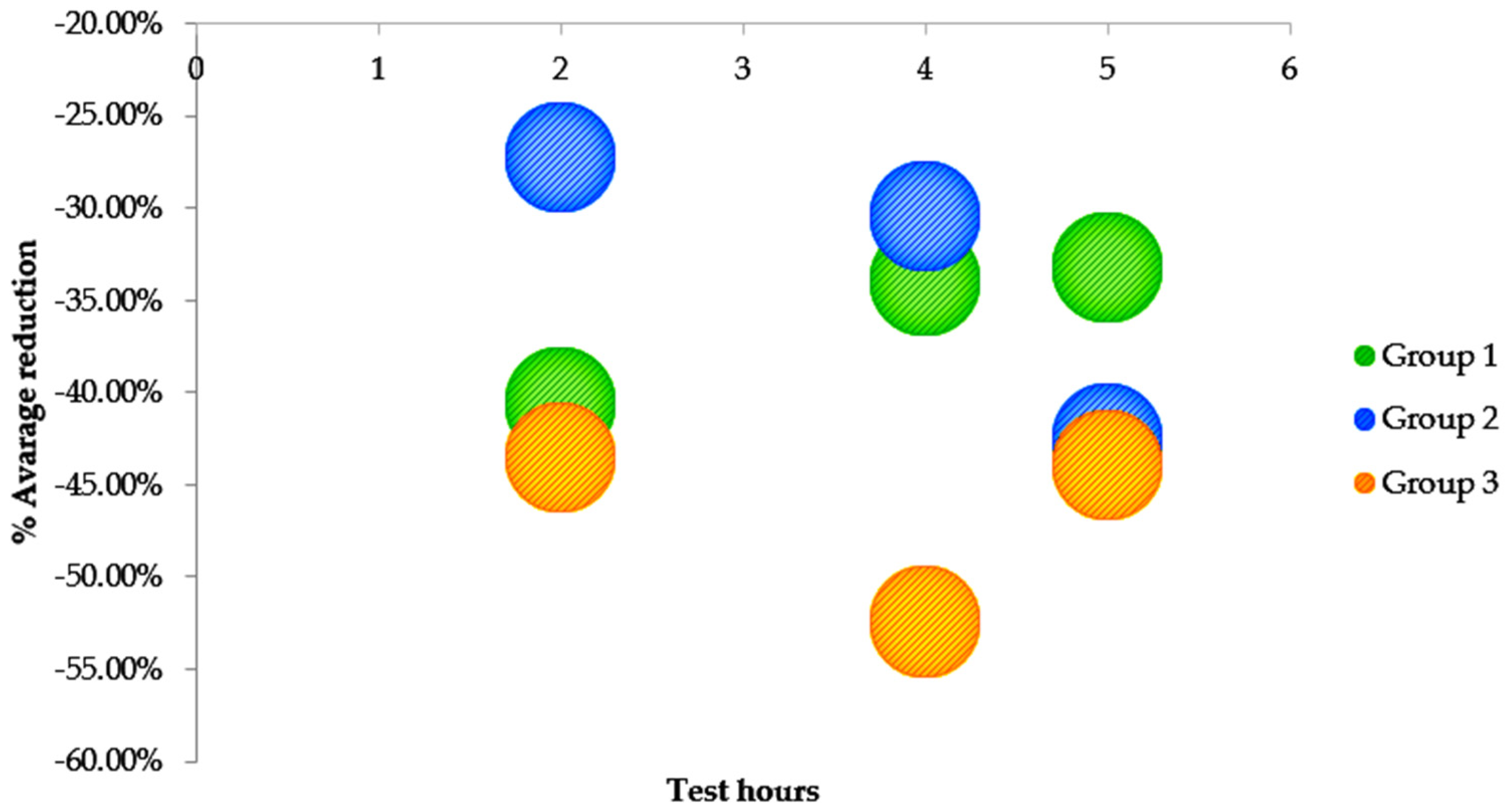
| % reduction | −40.59% | −33.96% | −33.21% | |||
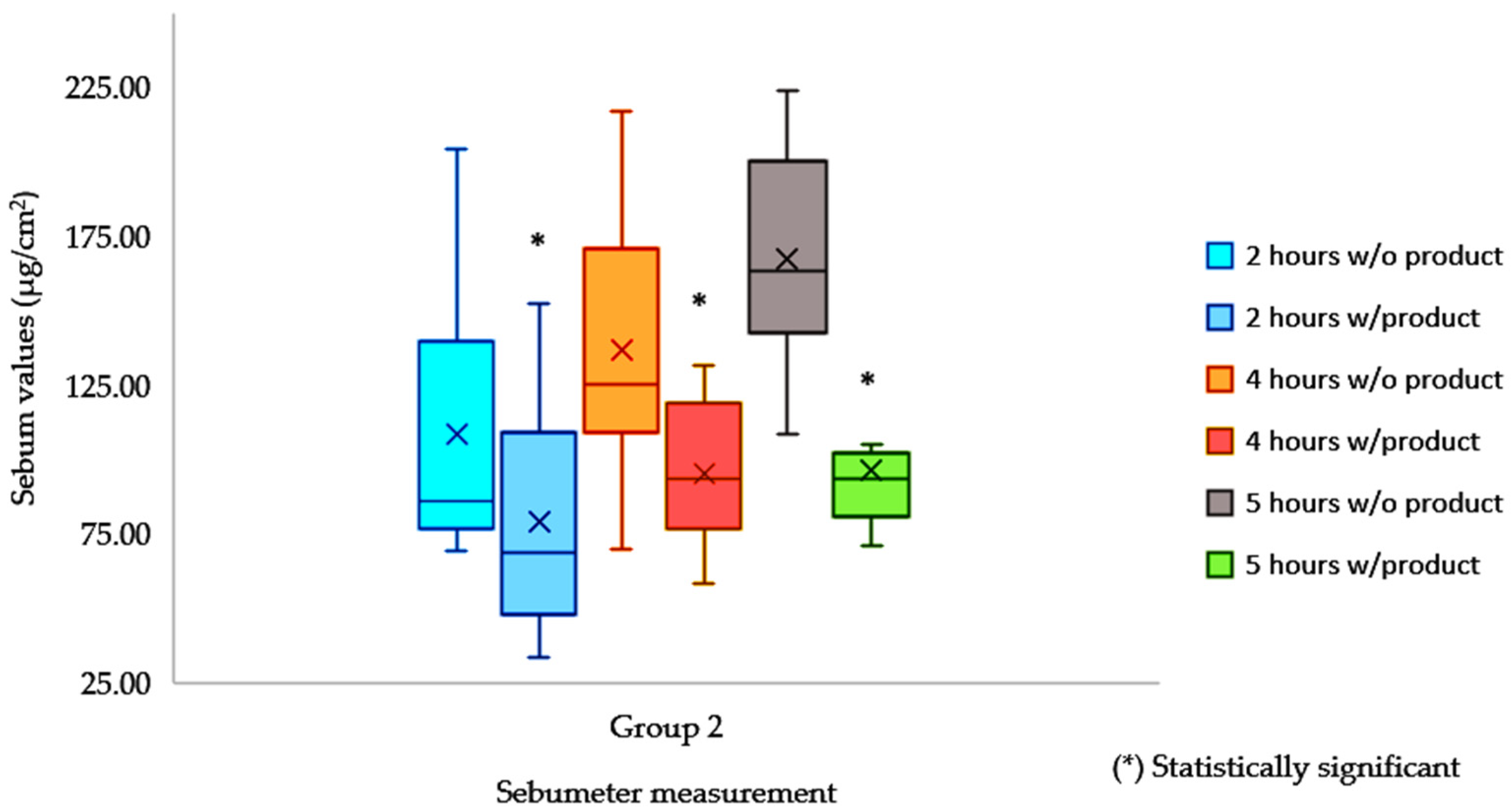
| Control n = 8 | 2 h w/o Product | 2 h w/Product | 4 h w/o Product | 4 h w/Product | 5 h w/o Product | 5 h w/Product |
|---|---|---|---|---|---|---|
| Average | 108.38 | 78.88 | 136.88 | 95.25 | 167.38 | 96.19 |
| Max | 152.00 | 204.00 | 131.50 | 217.00 | 146.00 | 224.00 |
| Min | 33.50 | 69.00 | 58.00 | 70.00 | 71.00 | 108.50 |
| SE | 14.04 | 16.36 | 8.65 | 15.98 | 8.03 | 13.00 |
| % reduction | −27.22% | −30.41% | −42.53% | |||
| Control n = 10 | 2 h w/o Product | 2 h w/Product | 4 h w/o Product | 4 h w/Product | 5 h w/o Product | 5 h w/Product |
|---|---|---|---|---|---|---|
| Average | 63.30 | 112.00 | 70.45 | 148.10 | 77.35 | 138.00 |
| Max | 167.00 | 92.50 | 230.00 | 96.00 | 174.50 | 94.50 |
| Min | 34.00 | 40.00 | 48.00 | 37.00 | 71.00 | 65.00 |
| SE | 12.95 | 5.68 | 16.09 | 6.83 | 11.30 | 3.38 |
| % reduction | −43.48% | −52.43% | −43.95% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozada, P.; Victoria-Tinoco, L.; Muñoz, A.M.; Rojas, J. Evaluation of Sebum Control and Safety for Daily Use of a Cosmetic Elastomer Formulated with Vegetable Oils from Peruvian Biodiversity. Cosmetics 2025, 12, 66. https://doi.org/10.3390/cosmetics12020066
Lozada P, Victoria-Tinoco L, Muñoz AM, Rojas J. Evaluation of Sebum Control and Safety for Daily Use of a Cosmetic Elastomer Formulated with Vegetable Oils from Peruvian Biodiversity. Cosmetics. 2025; 12(2):66. https://doi.org/10.3390/cosmetics12020066
Chicago/Turabian StyleLozada, Patricia, Lourdes Victoria-Tinoco, Ana María Muñoz, and Jorge Rojas. 2025. "Evaluation of Sebum Control and Safety for Daily Use of a Cosmetic Elastomer Formulated with Vegetable Oils from Peruvian Biodiversity" Cosmetics 12, no. 2: 66. https://doi.org/10.3390/cosmetics12020066
APA StyleLozada, P., Victoria-Tinoco, L., Muñoz, A. M., & Rojas, J. (2025). Evaluation of Sebum Control and Safety for Daily Use of a Cosmetic Elastomer Formulated with Vegetable Oils from Peruvian Biodiversity. Cosmetics, 12(2), 66. https://doi.org/10.3390/cosmetics12020066








