Skin Cell Phototoxicity and Photoprotection Study of Agro-Derived Lignin and Nanocellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Lignin Fractions and Nanocellulose: Production and Tested Concentrations
2.2. Cell Culture
2.3. Phototoxicity Evaluation
2.4. Determination of Photoprotection Effect by Measuring Intracellular Reactive Oxygen Species (ROS)
3. Results
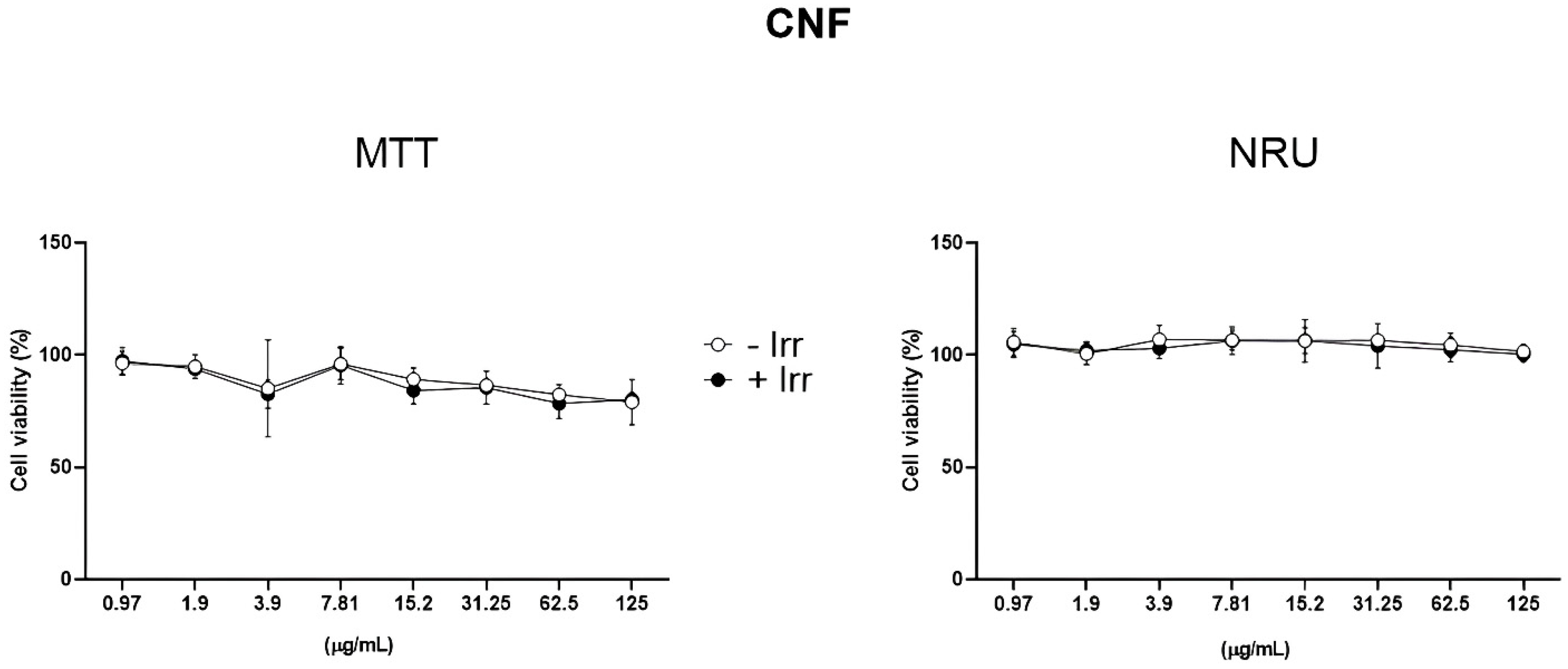
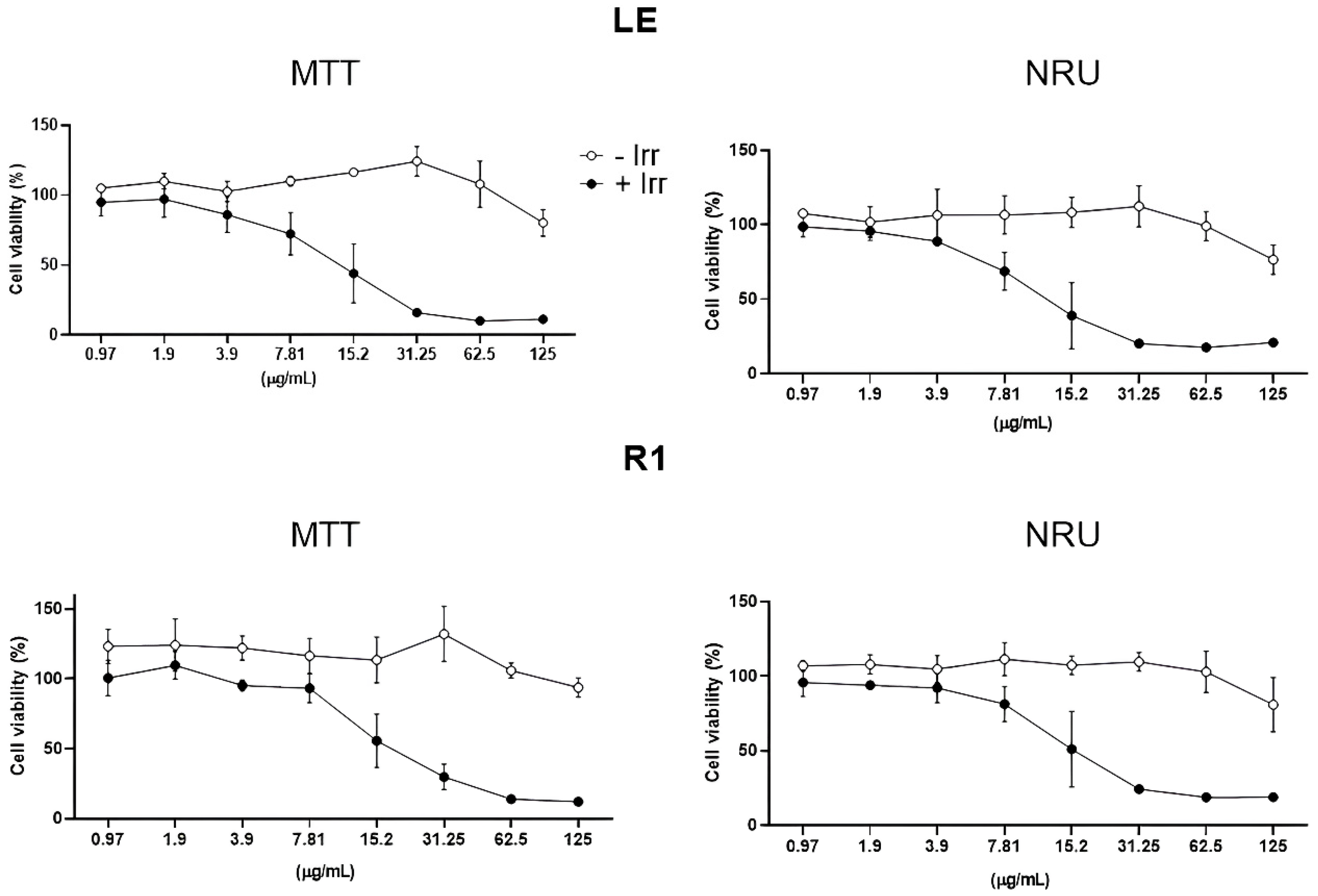
3.1. Phototoxicity Evaluation
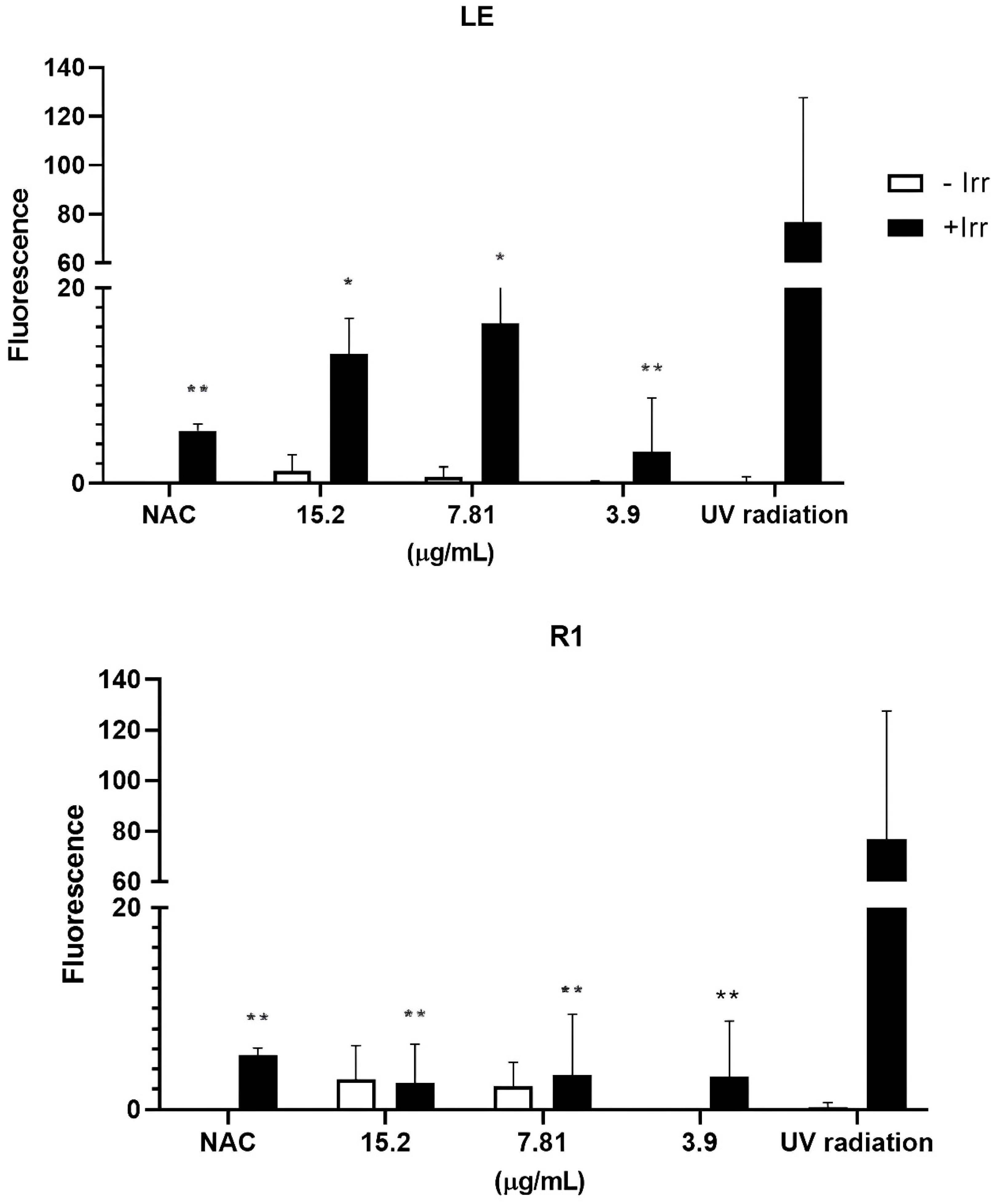
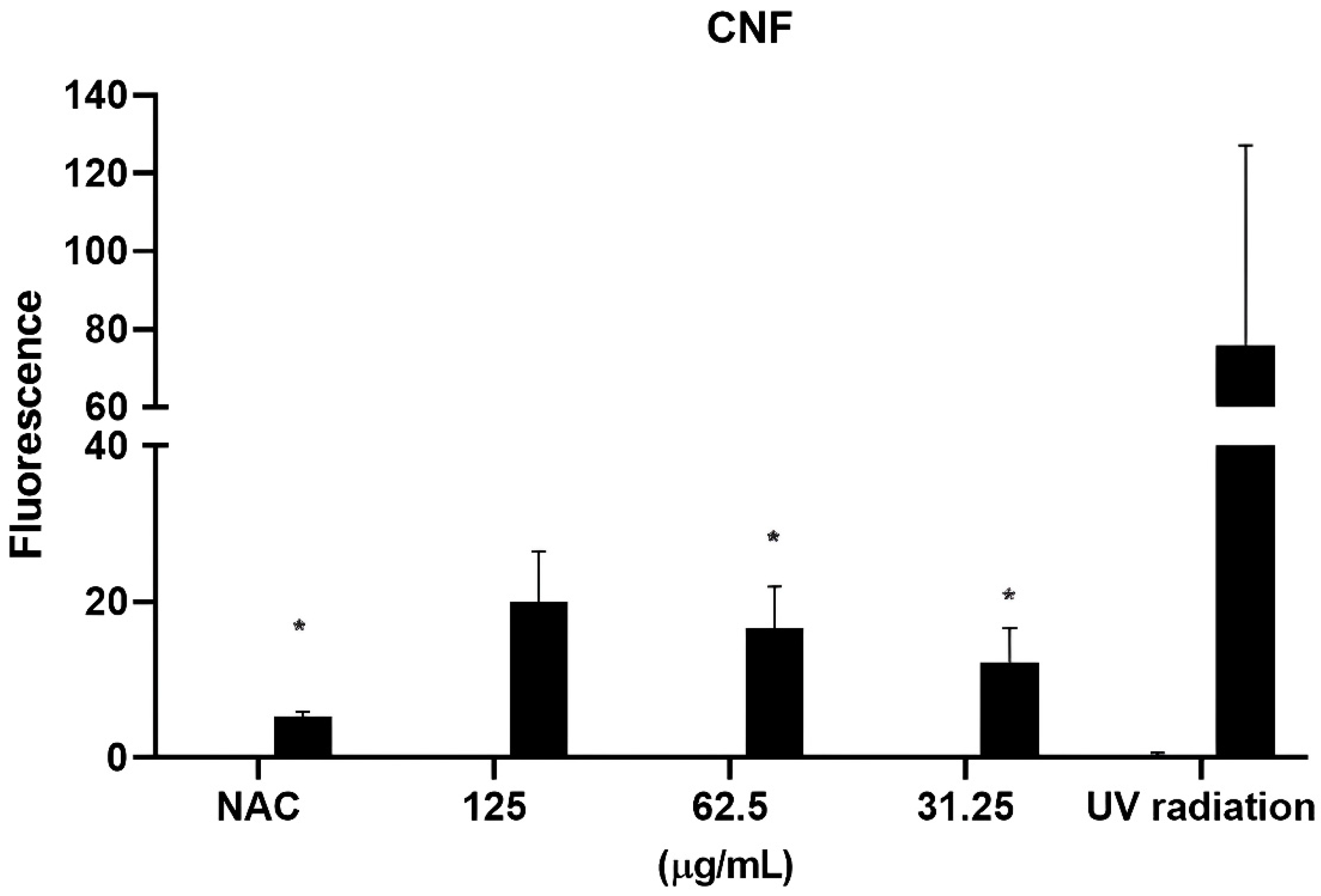
3.2. Evaluation of the Photoprotection Effect Measured by ROS Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | Ultraviolet irradiation |
| CNF | Cellulose nanofiber |
| LE | Lignin fraction LE |
| R1 | Lignin fraction R1 |
| DCF-DA | 2′,7′-dichlorofluorescein diacetate probe |
| SPF | Sun protection factor |
| ROS | Reactive oxygen species |
| TiO2 | Titanium dioxide |
| PIF | Photoirritation factor |
| HaCaT | Immortalized keratinocyte cell line |
| FBS | Fetal bovine serum |
| Glu | L-glutamine |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NRU | Neutral red uptake |
| PBS | Phosphate-buffered saline |
| DMSO | Dimethyl sulfoxide |
References
- Courtat, M.; Joyce, P.J.; Sim, S.; Sadhukhan, J.; Murphy, R. Towards credible, evidence-based environmental rating ecolabels for consumer products: A proposed framework. J. Environ. Manag. 2023, 336, 117684. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Jatrana, A.; Maan, S.; Sharma, P. Lignin modification and valorization in medicine, cosmetics, environmental remediation and agriculture: A review. Environ. Chem. Lett. 2022, 21, 2171–2197. [Google Scholar] [CrossRef]
- Rosa, R.; Pini, M.; Cappucci, G.M.; Ferrari, A.M. Principles and indicators for assessing the environmental dimension of sustainability within green and sustainable chemistry. Curr. Opin. Green Sustain. Chem. 2022, 37, 100654. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative Antioxidant and Cytotoxic Effects of Lignins from Different Sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, S.K.; Chandna, S.; Kim, K.H.; Bhaumik, J. Lignin-based metal oxide nanocomposites for UV protection applications: A review. J. Clean. Prod. 2021, 317, 128300. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A Nature-Inspired Sun Blocker for Broadspectrum Sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Antunes, F.; Mota, I.F.; Fangueiro, J.F.; Lopes, G.; Pintado, M.; Costa, P.S. From Sugarcane to Skin: Lignin as a Multifunctional Ingredient for Cosmetic Application. Int. J. Biol. Macromol. 2023, 234, 123592. [Google Scholar] [CrossRef]
- Gagosian, V.S.C.; Claro, F.C.; de AP Schwarzer, A.C.; Cruz, J.V.; Thá, E.L.; da S Trindade, E.; Magalhães, W.L.; Pestana, C.B.; Leme, D.M. The Potential Use of Kraft Lignins as Natural Ingredients for Cosmetics: Evaluating Their Photoprotective Activity and Skin Irritation Potential. Int. J. Biol. Macromol. 2022, 222, 2535–2544. [Google Scholar] [CrossRef]
- Ratanasumarn, N.; Chitprasert, P. Cosmetic Potential of Lignin Extracts from Alkaline-Treated Sugarcane Bagasse: Optimization of Extraction Conditions Using Response Surface Methodology. Int. J. Biol. Macromol. 2020, 153, 138–145. [Google Scholar] [CrossRef]
- Li, S.X.; Li, M.F.; Bian, J.; Wu, X.F.; Peng, F.; Ma, M.G. Preparation of Organic Acid Lignin Submicrometer Particle as a Natural Broad-Spectrum Photo-Protection Agent. Int. J. Biol. Macromol. 2019, 132, 836–843. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Sunscreen Performance of Lignin from Different Technical Resources and Their General Synergistic Effect with Synthetic Sunscreens. ACS Sustain. Chem. Eng. 2016, 4, 4029–4035. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Iqbal, A.; Shen, C.C.; Bhawani, S.A.; Adam, F. Synthesis of Lignin Based Composites of TiO2 for Potential Application as Radical Scavengers in Sunscreen Formulation. BMC Chem. 2019, 13, 17. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Lavoine, N.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Controlled Release of Chlorhexidine Digluconate Using β-Cyclodextrin and Microfibrillated Cellulose. Colloids Surf. B Biointerfaces 2014, 121, 196–205. [Google Scholar] [CrossRef]
- Rai, R.; Dhar, P. Biomedical engineering aspects of nanocellulose: A review. Nanotechnology 2022, 33, 362001. [Google Scholar] [CrossRef]
- Hinton, A.N.; Goldminz, A.M. Feeling the Burn: Phototoxicity and Photoallergy. Dermatol. Clin. 2020, 38, 165–175. [Google Scholar] [CrossRef]
- Ohtake, H.; Tokuyoshi, Y.; Iyama, Y.; Nukaga, T.; Nishida, H.; Ohtake, T.; Hirota, M.; Yamada, K.; Seto, Y.; Sato, H.; et al. Reactive Oxygen Species (ROS) Assay-Based Photosafety Screening for Complex Ingredients: Modification of the ROS Assay Protocol. J. Toxicol. Sci. 2022, 47, 483–492. [Google Scholar] [CrossRef]
- Stoudmann, N.; Nowack, B.; Som, C. Prospective Environmental Risk Assessment of Nanocellulose for Europe. Environ. Sci. Nano 2019, 6, 2520–2531. [Google Scholar] [CrossRef]
- OECD. Test No. 432: In Vitro 3T3 NRU Phototoxicity Test; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2019; Section 4. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Aliabadi, M.; Chee, B.S.; Matos, M.; Cortese, Y.J.; Nugent, M.J.D.; de Lima, T.A.M.; Magalhães, W.L.E.; de Lima, G.G. Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage. Polymers 2020, 12, 2807. [Google Scholar] [CrossRef] [PubMed]
- Claro, F.C.; Matos, M.; Jordão, C.; Avelino, F.; Lomonaco, D.; Magalhães, W.L.E. Enhanced Microfibrillated Cellulose-Based Film by Controlling the Hemicellulose Content and MFC Rheology. Carbohydr. Polym. 2019, 218, 307–314. [Google Scholar] [CrossRef] [PubMed]
- de Lima, G.G.; Ferreira, B.D.; Matos, M.; Pereira, B.L.; Nugent, M.J.D.; Hansel, F.A.; Magalhães, W.L.E. Effect of Cellulose Size-Concentration on the Structure of Polyvinyl Alcohol Hydrogels. Carbohydr. Polym. 2020, 245, 116612. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.M.G.; de Sousa Carvalho, E.H.; da Silva Pereira, B.; Petersohn, E.; Magalhães, W.L.E.; Moura, R.B.P.; Taveira, S.F.; de Cademartori, P.H.G.; Jacumazo, J.; de Freitas, R.A.; et al. Testing the Ecotoxicity of Nanofibrillated Kraft-Bleached Pulp for Use in Nanotechnology Products. Cellulose 2024, 32, 519–534. [Google Scholar] [CrossRef]
- Martínez, V.; Galbiati, V.; Corsini, E.; Martín-Venegas, R.; Vinardell, M.P.; Mitjans, M. Establishment of an in Vitro Photoassay Using THP-1 Cells and IL-8 to Discriminate Photoirritants from Photoallergens. Toxicol. In Vitro 2013, 27, 1920–1927. [Google Scholar] [CrossRef]
- Baccarin, T.; Mitjans, M.; Ramos, D.; Lemos-Senna, E.; Vinardell, M.P. Photoprotection by Punica Granatum Seed Oil Nanoemulsion Entrapping Polyphenol-Rich Ethyl Acetate Fraction against UVB-Induced DNA Damage in Human Keratinocyte (HaCaT) Cell Line. J. Photochem. Photobiol. B 2015, 153, 127–136. [Google Scholar] [CrossRef]
- Gordobil, O.; Oberemko, A.; Saulis, G.; Baublys, V.; Labidi, J. In Vitro Cytotoxicity Studies of Industrial Eucalyptus Kraft Lignins on Mouse Hepatoma, Melanoma and Chinese Hamster Ovary Cells. Int. J. Biol. Macromol. 2019, 135, 353–361. [Google Scholar] [CrossRef]
- Gil-Chávez, G.J.; Padhi, S.S.P.; Pereira, C.V.; Guerreiro, J.N.; Matias, A.A.; Smirnova, I. Cytotoxicity and biological capacity of sulfur-free lignins obtained in novel biorefining process. Int. J. Biol. Macromol. 2019, 136, 697–703. [Google Scholar] [CrossRef]
- Ventura, C.; Pinto, F.; Lourenço, A.F.; Ferreira, P.J.T.; Louro, H.; Silva, M.J. On the toxicity of cellulose nanocrystals and nanofibrils in animal and cellular models. Cellulose 2020, 27, 5509–5544. [Google Scholar] [CrossRef]
- Ventura, C.; Lourenço, A.F.; Sousa-Uva, A.; Ferreira, P.J.T.; Silva, M.J. Evaluating the Genotoxicity of Cellulose Nanofibrils in a Co-Culture of Human Lung Epithelial Cells and Monocyte-Derived Macrophages. Toxicol. Lett. 2018, 291, 173–183. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In Vitro Biological Responses to Nanofibrillated Cellulose by Human Dermal, Lung and Immune Cells: Surface Chemistry Aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lian, H.; Xia, C.; Deng, J.; Li, X.; Zhang, C. Mechanistic insights and applications of lignin-based ultraviolet shielding composites: A comprehensive review. Int. J. Biol. Macromol. 2024, 280, 135477. [Google Scholar] [CrossRef]
- Zhang, Y.; Naebe, M. Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Zhao, L.; Ouyang, X.; Ma, G.; Qian, Y.; Qiu, X.; Ruan, T. Improving Antioxidant Activity of Lignin by Hydrogenolysis. Ind. Crops Prod. 2018, 125, 228–235. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Thá, E.L.; Matos, M.; Avelino, F.; Lomonaco, D.; Rodrigues-Souza, I.; Gagosian, V.S.C.; Cestari, M.M.; Magalhães, W.L.E.; Leme, D.M. Safety Aspects of Kraft Lignin Fractions: Discussions on the in Chemico Antioxidant Activity and the Induction of Oxidative Stress on a Cell-Based in Vitro Model. Int. J. Biol. Macromol. 2021, 182, 977–986. [Google Scholar] [CrossRef]
- Morsella, M.; D’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef]
- Ishibashi, K.-I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in Ti02 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]

| MTT | NRU | |
|---|---|---|
| LE | 10.0 | 8.8 |
| R1 | 6.4 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.V.; Maddaleno, A.S.; Gava, J.S.; Magalhães, W.L.E.; de Oliveira, D.P.; Leme, D.M.; Mitjans, M.; Vinardell, M.P. Skin Cell Phototoxicity and Photoprotection Study of Agro-Derived Lignin and Nanocellulose. Cosmetics 2025, 12, 61. https://doi.org/10.3390/cosmetics12020061
Cruz JV, Maddaleno AS, Gava JS, Magalhães WLE, de Oliveira DP, Leme DM, Mitjans M, Vinardell MP. Skin Cell Phototoxicity and Photoprotection Study of Agro-Derived Lignin and Nanocellulose. Cosmetics. 2025; 12(2):61. https://doi.org/10.3390/cosmetics12020061
Chicago/Turabian StyleCruz, Juliana Varella, Adriana Solange Maddaleno, Julia Salles Gava, Washington Luiz Esteves Magalhães, Danielle Palma de Oliveira, Daniela Morais Leme, Montserrat Mitjans, and Maria Pilar Vinardell. 2025. "Skin Cell Phototoxicity and Photoprotection Study of Agro-Derived Lignin and Nanocellulose" Cosmetics 12, no. 2: 61. https://doi.org/10.3390/cosmetics12020061
APA StyleCruz, J. V., Maddaleno, A. S., Gava, J. S., Magalhães, W. L. E., de Oliveira, D. P., Leme, D. M., Mitjans, M., & Vinardell, M. P. (2025). Skin Cell Phototoxicity and Photoprotection Study of Agro-Derived Lignin and Nanocellulose. Cosmetics, 12(2), 61. https://doi.org/10.3390/cosmetics12020061








