Abstract
Traditional herbal medicine, ethnopharmacology, and evidence-based phytotherapy inspire the development of botanical active ingredients for cosmetics. Ensuring their authenticity and quality is essential in guaranteeing the safety and efficacy of cosmetic formulations. However, the industry faces challenges related to adulteration and inconsistent verification practices. Adulteration can occur at both the crude raw material stage and during processing, involving misidentification, contamination, or the addition of unauthorized substances. This review emphasizes the need for robust authentication methods, including botanical identification, genetic testing, and phytochemical/metabolomic profiling. Analytical tools such as UV/VIS spectroscopy, HPTLC, GC-MS, HPLC/UHPLC, and isotope analysis provide complementary data for detecting and addressing adulteration. Adulteration jeopardizes product safety, efficacy, regulatory compliance, and consumer trust, while dilutions or substitutions erode the intended health benefits. A standardized, comprehensive approach across the supply chain—from raw material sourcing to extract manufacturing—is critical for maintaining the integrity of botanical ingredients. Cosmetovigilance and nutrivigilance are crucial aspects of ensuring product safety and compliance. This review presents a novel perspective by highlighting that, while the pharmaceutical and nutraceutical industries have long recognized the risks of botanical adulteration, awareness in the cosmetics industry remains limited. It further integrates recent advancements in metabolomic profiling, global regulatory challenges, and the economic implications of botanical adulteration in cosmetics. Future developments in AI-driven authentication technologies may represent a promising solution for addressing evolving challenges in product safety and traceability.
1. Introduction
Ensuring the authenticity and quality of botanical ingredients (i.e., lato sensu: plants, mosses, lichens, ferns, mushrooms, algae, and microalgae) is crucial in guaranteeing the quality and safety of cosmetic formulations. However, the increasing demand for botanical active ingredients in cosmetics has very likely led to a rise in cases of botanical adulteration. While some cosmetics companies and suppliers have robust systems in place to verify the authenticity and quality of botanical ingredients, this practice is not yet uniformly embraced across the industry. Additionally, even when companies follow established methods (e.g., some pharmacopeial approaches), these do not always ensure the absence of adulteration [1].
Thus, while crude botanical raw materials (BRMs), either powdered, cut, or whole, can benefit from visual and microscopic examination for their qualification, the corresponding processed products or PRMs (either powders or extracts obtained via various processes from a BRM), meanwhile, require extensive analytical approaches to ensure their integrity. Indeed, these extracts and powders fall under the designation of “botanical active ingredients” for cosmetics and are expected to comply with the purity of the BRM represented by a single botanical species (and sometimes a specific subspecies, a defined variety, a chemotype…) and the use of the correct part of the plant (leaf, flower, root…). Stringent requirements include appropriate extraction processes and no additional, unlisted, chemical compounds.
Two generic types of adulteration are possible: the first involves the initial raw material, and the second concerns the resulting extract or powder obtained through processing the BRM:
- (1)
- BRM adulteration, in fresh or dried botanicals, can be deliberate or not. It can occur due to inaccurate identification of the raw material during harvesting, leading to the use of an alternative botanical species. There is also the possibility of contamination with a totally different plant species or a mixture of species. BRM contamination can also occur due to harvesting-related factors, such as the presence of plants or plant pathogens, residues of phytosanitary products, etc. Moreover, inadequate post-harvest conditions, such as the development of molds, the presence of various pests, labeling inaccuracies, or intentional adulteration, can also contribute to the occurrence of these impurities [1]. In some cases, the specific plant species may be replaced by a substitute (e.g., other plant species, exhausted material from the same species), or adulterants may be added to mimic the appearance of the desired botanical (e.g., inert materials like sand, red plastic strips in saffron, or green-colored wheat bran in oregano).
- (2)
- Besides the alteration of BRM, the adulteration of transformed extracts (liquid or powdered) is the second type of adulteration; it is another potentially fraudulent practice with a higher prevalence, due to the higher commercial value of extracts compared to BRM. Moreover, by their very nature, transformed extracts are far more susceptible to deliberate adulteration. Firstly, the same adulterations are found as with unprocessed raw materials but with additional possibilities, even involving plants that are entirely different. Secondly, transformed extracts are subject to specific falsifications, such as the addition of endogenous substances (naturally occurring in the plant), exogenous substances (typically synthetic and foreign to the plant in question), or excessive dilutions with bulking agents and fillers like maltodextrin or starch. Under these circumstances, it is not rare to observe dilutions and deliberate addition of synthetic, inert, or active chemicals that compromise the integrity of the liquid extracts. Subsequently, any extract obtained from an adulterated BRM will itself be adulterated [2].
In this context, the term “fraud” or “fraudulent adulteration“ qualifies as an economically motivated adulteration (EMA), as defined by the FDA (https://www.fda.gov/food/compliance-enforcement-food/economically-motivated-adulteration-food-fraud (accessed on 20 March 2025)) in order to maximize gains by using deceiving techniques, whereas “adulteration” has a broader meaning, may be unintentional, and also encompasses a wide range of non-compliance (e.g., not complying with current Good Manufacturing Practices (cGMP) or label claims).
To summarize, intentional adulteration will occur as a consequence of efforts to minimize manufacturing expenses and/or conceal the use of low-quality ingredients, with the aim of achieving an optimal balance between maximizing profitability and minimizing the detectability of adulteration. Another goal of adulteration is to boost or introduce an activity through the unclaimed addition of pharmaceutical drugs, further compromising the integrity and safety of the products [2]. Numerous studies and reports have extensively documented these practices and, in this review, a small selection of representative examples will be highlighted.
This review intends to address the issue of botanical adulteration in the cosmetics industry, especially in the field of active ingredients, by providing a comprehensive analysis of the types of adulteration, the regulatory gaps that allow such practices to proliferate, and the pertinence of the methods used to authenticate botanical materials.
2. Traditional Medicines and Evidence-Based Phytotherapy as a Source of Inspiration for Cosmetic Active Ingredients
Plants have a long history of traditional use aimed at facilitating the optimal expression of the human body’s physical and mental capacities. The utilization of herbal medicine today encompasses two distinct aspects.
On one hand, traditional practices imbued with empiricism have developed over centuries or millennia within Traditional Medicine systems [3] associated with specific geographical regions, cultures, and distinctive histories (e.g., Indian Ayurveda [4], Indian Siddha Traditional Medicine [5], Tibetan Sowa-Rigpa Medicine [6], Ancient and Traditional Chinese Medicine [7], Japanese Kampo Traditional Medicine [8], Korean Traditional Medicine [9], Indonesian Jamu traditional herbal medicine [10], Perso–Arabic Yunani system of Medicine [11], Native American Medicine [12], South American Medicine [13], Traditional African Medicine [14], Traditional Aboriginal Medicine [15], and Traditional European Herbal Medicine [16]). This rich traditional heritage provides valuable insights into inspiring modern cosmetics, particularly in the development of active ingredients with expected specific potential benefits for the skin, hair, or nails. Many botanical extracts traditionally used for healing can also deliver targeted skincare advantages (Table 1). For example, certain plants such as Angelica sinensis (Dong Quai) and Scutellaria baicalensis (Baikal skullcap), employed in Traditional Chinese Medicine, are known for their anti-inflammatory properties, which may be useful for soothing irritated skin in cosmetic formulations. Plant species like Centella asiatica, which has a rich history in Ayurvedic medicine, have been scientifically validated for their role in skin rejuvenation and skin regeneration (often associated with wound-healing properties) [17], making them ideal for anti-aging products. Furthermore, traditional plants such as green tea (Camellia sinensis), utilized in Chinese, Japanese, and Korean traditional practices, have strong antioxidant effects, which help in neutralizing free radicals and protecting skin from environmental damage [18]. Adaptogens like Ashwagandha (Withania somnifera), originating from Indian Ayurvedic medicine, are now popular in cosmetics for their potential to enhance skin resilience to stressors, thereby promoting overall skin health. However, it is important to keep in mind that under EU Cosmetics Regulation 1223/2009, cosmetic products are not intended to exert pharmacological or therapeutic effects.

Table 1.
Harnessing heritage knowledge: active compounds from traditional medicines benefiting modern cosmetics and their alleviating properties.
On the other hand, the scientific approach, based on phytochemistry and pharmacognosy, involves scientific and clinical studies illustrating the pharmacological activity of plant metabolites, with the aim of documenting their potential therapeutic administration in humans, in the context of pharmaceutical or phototherapeutic developments, that will be prescribed by physicians. Whereas traditional approaches and practices are generally focused on individual health, the scientific and evidence-based approaches tend to focus on conditions and diseases, as well as clinical and population studies. Tradition, however, nourishes science: the evidence-based scientific pharmacognostic approach to medical phytotherapy is regularly enriched by data from ethnobotany and ethnopharmacology, and investigates the current traditional uses of plant materials worldwide to rigorously assess their efficacy and the absence of toxicity, as well as establish indications, dosages, and manufacturing standards [28].
However, while this convergence of tradition (traditional medicines) and science (medical and scientific phytotherapy) provides a robust foundation and inspiration in the development of botanical extracts dedicated to cosmetics applications, the lack of dedicated standards or detailed monographs for plant-based ingredients introduces the risk of significant variability in the quality and authenticity of botanical extracts used in formulations.
3. Botanical Material Adulteration
Pharmacopoeias and corresponding monographs play a pivotal role in ensuring the quality, safety, and regulatory compliance of pharmaceutical products—including those derived from botanical raw materials—by providing well-established quality, purity, and safety standards and guidelines. These monographs serve as critical benchmarks for both whole plants and processed botanical raw materials (PRMs), ensuring consistency and consumer trust. However, it is important to note that pharmacopeial guidelines allow for a minimal tolerance of contamination, often at a small percentage, to account for practical variability in production processes (e.g., the European pharmacopoeia permits a maximum of 5% foreign elements).
The dietary supplement industry has adopted these pharmacopeial approaches for their botanical raw materials. However, the lack of standardized methods dedicated specifically to PRMs (such as powdered raw materials and extracts obtained via various extraction approaches), has led to the global emergence of inconsistent methods. Indeed, many monograph tests designed for whole plants, such as microscopy, macroscopy, and organoleptic analyses, cannot be applied to PRMs. Moreover, chemical tests relying on markers that are not extracted during processing may yield false negative results.
A single thin-layer chromatography (TLC) analysis is often used to verify compliance with monographs. While each test contributes to overall authentication, relying solely on one is insufficient in detecting subtle forms of adulteration in complex raw materials. This ambiguity increases the risk of adulteration and unfounded claims, for example, about extraction ratios, the concentration of dominant constituents, or their purity.
Similarly, in the cosmetic active ingredient industry, verifying the absence of adulteration (in the BRM or the processed products) is also performed by using diverse and mostly proprietary approaches, which has hampered the development of universal, reliable methods. While the benefits of standardized and harmonized testing methods are often discussed, their actual impact relies more on the robustness and thoroughness of the methods themselves than on the fact that they are harmonized. For example, the ability to anticipate the possibility of regulatory non-compliance, detect contaminants, or address product safety concerns (e.g., unexpected substances, toxicity) requires rigorous and well-designed methods, irrespective of whether they are universally harmonized. Nevertheless, such methods can still contribute to improving consistency and reliability, ultimately protecting brand reputation. However, the diversity of possible products derived from the same raw material makes it challenging to develop universally applicable methods. The only truly universal method would be to analyze all the constituents of a mixture in order to reach the most relevant conclusions possible.
Within the domain of phytochemistry and botanical extraction of active ingredients, adulteration can manifest in various forms (Table 2; Figure 1). These include: (1) the deliberate addition of another plant part from the same plant species (intentional adulteration), often in significant quantities, to meet specific content specifications such as achieving a required level of polyphenols; (2) the confusion with a closely related species (unintentional adulteration, such as a traceability error involving another batch from a closely related species or even a different one), or the intentional substitution (e.g., same or similar genus considered as an equivalent), sometimes as a result of fraudulent intent; (3) the utilization of a substantially different species (substitution), an adulteration that is either possibly intentional or the result of traditional usage (deliberate substitution), a case sometimes observed in regions where plants are not utilized based on their species names but rather on their functional properties or based on vernacular appellations that can apply to alternative species [36]; (4) the inadvertent inclusion of an inappropriate, possibly toxic, species (accidental contamination); (5) enrichment practices that may involve either the undeclared addition of known endogenous substances (most of the time it is an analyte that can serve as a marker) that allows the dilution of the original extract; (6) the incorporation of exogenous substances to potentially enhance the expected traditional activity (e.g., addition of phosphodiesterase type 5 inhibitor or Sildenafil in Ashwagandha extracts [37]); (7) excessive dilution using bulking agents, which lower the concentration of active components and compromise the quality of the extract [38]. In certain cases, reconstitution may occur, whereby the plant material is entirely absent and replaced with substituted chemicals, leading to an incorrect analytic profile characterized by isolated compounds and poor overall composition. Microbial or phytosanitary (pesticides, herbicides…) adulteration can result in the presence of additional metabolites or chemicals. While these may not significantly alter the overall analytic profile, their presence could indicate contamination that warrants further investigation.

Table 2.
Hallmarks of raw material and extract adulteration. “Expected species” refers to the botanically identified plant species that should be present in the raw material or extract. “✓” indicates the presence of the expected species, “ ” represents its absence.
” represents its absence.
 ” represents its absence.
” represents its absence.
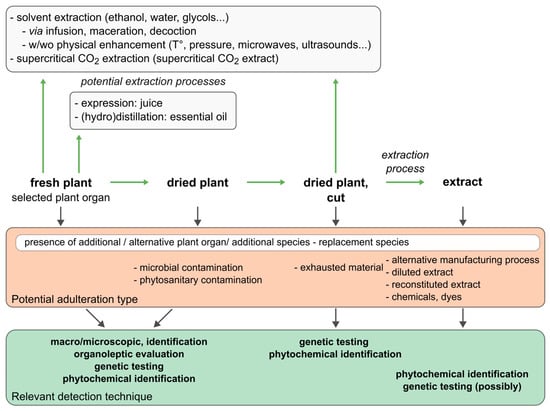
Figure 1.
Relevant detection techniques for the authentication of botanical raw materials and subsequent extracts.
Hence, different scenarios of using alternative plant materials exist: (1) “substitutes” which refers to alternative herbal raw material chosen based on their similar properties, as illustrated by the example of Tribulus terrestris fruits, known as Gokshura in Ayurvedic medicine, which is sometimes confused or substituted with the fruits of Pedalium murex, also known as Brihat Gokshura [39]; (2) “permissible interchangeable use” means materials officially recognized and sanctioned, as is the case for Crataegus or Salix spp.; (3) “equivalents”, which are botanically similar materials, often from the same genus, used for their comparable properties but not officially recognized as interchangeable; (4) “adulterants”, involving the addition of inferior quality or foreign substances, either unintentionally or deliberately, degrade the quality, biological properties, and efficacy of the original material [40].
As described by the FDA, fraudulent adulteration or “economically motivated adulteration” (which is intentional and motivated by reducing production costs and maximizing economic gain) can be basically the result of additions, omissions, or substitutions.
The hallmarks of raw material and extract adulteration, i.e., their various potential forms of adulteration, are detailed in Table 2 as an attempt at classification.
4. Overview of the Problems and Risks of Adulterated vs. Benefits of Genuine Materials
Certifying the absence of adulteration in botanical raw materials and plant extracts begin at the source itself—the cultivation and harvesting of the plants. Ensuring that the botanical raw materials are of the correct species, are free from chemical/dye contamination, and have been cultivated and harvested following good agricultural and collection practices (GACP) are measures that maintain the quality and integrity of the botanical source. Additionally, traceability documentation and certification of the botanical supply chain, from cultivation to extraction, help detect and prevent economically motivated adulteration or contamination. This transparent supply chain information will build trust and confidence in the authenticity of the botanical ingredients. While it is preferable to ensure proper authenticity as early as possible in the manufacturing chain, this is often not feasible. Therefore, good traceability is important but is clearly not sufficient. Controls at critical stages are necessary to ensure a sufficient level of quality.
As the botanical raw materials move through the extraction and processing stages, certification of the absence of adulteration guarantees the consistent composition and potency of the extracted active compounds. This consistency in quality and efficacy of the plant extract is crucial for use in cosmetic formulations. Furthermore, adulteration-free botanical extracts eliminate the risk of introducing contaminants, undeclared synthetic ingredients, or compounds that may cause skin irritation or other adverse or toxic effects, helping to maintain the safety and regulatory compliance of the final cosmetic products.
Finally, the benefits of certifying the absence of adulteration extend to the cosmetic formulations themselves. The consistent quality and potency of the unadulterated botanical extracts allow the cosmetic products to deliver the intended skin benefits reliably, building consumer trust and confidence in the brand’s authenticity and transparency. In the increasingly competitive natural and organic cosmetics market, this certification of botanical purity can provide a significant competitive advantage by demonstrating the brand’s commitment to quality, safety, and transparency—attributes that are highly valued by conscious consumers [21,25,32,41].
5. Authentication Methods for Botanical Raw Material and for Processed Products
Authentication methods encompass various orthogonal approaches, each providing a specific set of information, with complementary data reinforcing one another [21]. These methods can be classified in three main categories: (1) determination of the taxonomic identity by macroscopic botanical identification, botanical microscopy, and organoleptic evaluation; (2) determination of the genetic identity (genetic testing); (3) phytochemical identification and metabolomic profiling. It is worth noting that the technical requirement related to some approaches (e.g., metabolomic profiling) and the lack of a unified framework for evaluation methods may be a limitation to the certification of botanical quality and authenticity. Table 3 summarizes the analytical approaches that are relevant according to the adulteration type.

Table 3.
Relevance of approaches according to the adulteration type for (a) BRMs versus PRMs, and (b) PRMs. NF: not feasible. These are general guidelines; some specific cases exist in which the methods may be appropriate despite “NF”. “EO”: essential oils. “Specification”: quantitative evaluation of one or several phytocompounds. Botanical (green), genetic (orange) and phytochemical (blue) approaches are detailed.
5.1. Macroscopic Botanical Identification
Macroscopic plant identification relies on the examination of specific taxonomic features (including traits such as color, size, shape, texture, organization, other unique characteristics…) and their comparison with other species. Indeed, taxonomic identification in the natural habitat, which is the basis for distinguishing among the various plant species, provides access to numerous distinguishing visual elements, allowing for the most accurate possible identification of a species according to the Cronquist system of species classification (a classification of angiosperms primarily based on morphological criteria), which remains widely used today [42]. Technically, this is the best identification technique, but the challenge lies in the fact that the further we progress in the stages of commercialization or processing, the fewer elements are available for identification.
The macroscopic approach, usually performed on cut plants or plant parts from plants not in the wild, can allow the determination of the presence of foreign matter, and is better suited for the whole plant (or roughly cut plant) than its finely ground or powdered form. Furthermore, certain aspects of macroscopic botanical observation will only be relevant to the plant in culture and not to a cut fresh/dried organ, as some distinctive features may be altered or lost during the drying process. This could be the case for plants with mucilaginous tissues (e.g., Malva), succulent plants (e.g., Aloe), or bryophytes, and certain flowers (color and scent are important), and leaf, stem, and bark features [43,44].
Moreover, deceptive practices can occur through the use of substitute materials with similar characteristics. For instance, seeds may be adulterated with similar-looking seeds, and various common flowers, leaves, and herbs may be mixed with or replace the organs of the expected plant, including the replacement of saffron (the stigmas of Crocus sativus flower) with corn stigmas, dried petals from Carthamus tinctorius, or other flowers [28]. Another deceptive practice involves selling pre-extracted plant materials from which valuable constituents have been removed; this may be the case for ginger (Zingiber officinale) extracts, for example [45]. This feature is however detectable through organoleptic assessment (with changes in color, texture, aroma, or taste), microscopic examination (with signs of extraction), or quantitative profiling analysis using techniques such as gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-mass spectrometry (LC-MS) [21]. Some adulterants or plant substitutions are easily detected visually (or microscopically), but in some cases, genetic or chromatographic methods may be necessary for accurate identification.
5.2. Botanical Microscopy
Microscopy analysis of botanical material (preferably whole, though sometimes cut or ground materials are involved) is a robust method used in quality control laboratories, which offers significant benefits in detecting adulteration, especially when closely related species are used for adulteration. However, the approach may prove more insightful with particular organs (flower) than others (stem, root). This effective approach allows assessment at the tissue level the specific and subtle botanical features that the eye alone cannot discriminate, as well as discern intricate taxonomic features, allowing microscopists to identify closely related species within the same genus or when the vernacular names encompass several species [46]. As an example, the adulteration with alternative species of plants (same or close genus) is well-documented for adaptogenic plants and depends on the plant and the region from where it is sourced. Notorious examples are the substitution of Asian ginseng (Panax ginseng) with American ginseng (P. quinquefolius) [29,47]; the replacement of roseroot (Rhodiola rosea) with other Rhodiola species (e.g., R. crenulata) [48]; the adulteration or replacement of “eleuthero” root (Eleutherococcus senticosus) with other species (E. giraldii, E. gracilistylus, E. nodiflorus, and E. sessiliflorus) or even plants of distinct genera (Aralia sp., Periploca sp.) [49]. Pollens, for example, are often highly distinctive and can, on their own, guide identification towards particularly precise taxa [50]. Finally, this approach is useful in the detection of certain diluents (e.g., maltodextrin, cellulose derivatives, starches…). It is of note that both macroscopic and microscopic identification cannot be of use regarding plant extracts (both liquid and dry forms).
5.3. Organoleptic Evaluation
Sensory assessment, known as organoleptic evaluation, plays a key role in the initial determination of authenticity of a botanical BRM. Indeed, among the three botanical analyses (microscopy, macroscopy, and organoleptic analysis), organoleptic analysis is often the only feasible method for processed raw materials (PRMs). In this context, taste and odor are the primary criteria providing useful information. More generally, it is the more apolar substances that are often responsible for the characteristically distinguishing taste or odor, making organoleptic analyses less effective on aqueous extracts than on more lipophilic ones. For example, an aqueous dry extract of lemon balm (Melissa officinalis) will no longer exhibit its characteristic lemon-like odor, as the key volatile compounds responsible for this aroma (e.g., citral and citronellal) are poorly extracted in water and are often lost during drying. In contrast, an alcoholic dry extract of ginger (Zingiber officinale) will retain its characteristic pungent aroma and taste, due to the presence of gingerols, which are phenolic compounds effectively extracted and preserved in alcohol.
Although replicating the precise tactile experience of authentic material proves difficult, the imitation of appearance, taste, and smell is prevalent in counterfeit products, particularly in powdered substances and extracts. Fraudulent imitation of appearance can be achieved by adding natural, synthetic, or food dyes. Taste can be mimicked by undeclared synthetic substances, while smell can be reproduced by substituting it with a mixture that mimics the original aroma. This is often the case with certain essential oils [51] and vegetal oils [52].
While organoleptic evaluation can provide initial insights for PRM, its subjective nature and variability necessitate its use alongside more objective techniques like GC-MS or HPTLC for reliable authentication.
5.4. Genetic Testing
Genetic approaches, particularly DNA barcoding, have emerged as powerful tools for the identification and authentication of botanical raw materials. DNA barcoding involves sequencing short, standardized gene regions from a plant genome, and comparing them to reference sequence databases to accurately identify the presence of the expected plant species, even when morphological features are not intact which is the case with grinded or powdered BRM [53].
Limitations may occur with BRM (1) when the resolution of the approach is not sufficient to differentiate closely related species or detect hybrid/cultivar adulteration; (2) when the initial raw material is degraded (for example, during the drying stage) or following the processing of the BRM, which may result in an extract with DNA of low quality; (3) sometimes when the nature of the adulterant species is unknown (although next-generation sequencing (NGS) can significantly aid in detecting and identifying multiple species simultaneously); (4) when reference databases are incomplete regarding certain lesser-known plant species [32]; (5) when DNA analysis cannot differentiate between plant parts, making it impossible to detect adulteration from specific plant parts; (6) if contamination occurs, even through cross-contamination during storage or by an operator, where foreign DNA strands can be amplified, resulting in false positives. This underscores the importance of extreme rigor in sampling for DNA analysis. These limitations highlight the importance of integrating DNA barcoding with metabolomics or phytochemical profiling, which can detect plant parts, added substances, or even geographical origins through chemical signatures.
In conclusion, DNA barcoding is a valuable complementary tool to other techniques, as it can confirm the presence or absence of genomic signatures specific to known or unknown botanical species. In fact, it is probably the most powerful technique currently available for accurately identifying the taxa of several non-mixed plants. However, this method has limitations. The inability to analyze metabolites means it cannot distinguish between plant parts or detect diluted material or adulteration involving chemical compounds. Moreover, it cannot determine the nature of added substances, geographical origin, or chemotype [54]. These limitations highlight the need for combining DNA barcoding with other analytical approaches to achieve comprehensive authentication.
5.5. Phytochemical/Metabolomic Identification
Once a BRM is processed, determining the specific composition of the resulting extract, in terms of secondary plant metabolites, requires a robust profiling approach. While phytochemical analysis serves as an excellent complementary method for BRM, it is, in theory, the most suitable technique for analyzing PRMs. This is because the extraction process primarily yields PRMs composed of secondary metabolites. Phytochemical analysis specifically targets these compounds, offering the potential to provide comprehensive information about the material. However, numerous techniques for analyzing chemical composition exist, and their levels of accuracy and verification vary significantly. Biases can arise when only a few selected metabolite markers are monitored to prove the absence of adulteration [55], potentially leading to false positives when alternative plant species share identical markers. To address this, only an extended metabolite profile can qualitatively ensure that the expected plant species or resulting botanical extracts comply with expectations. Untargeted profiling approaches, using metabolomics and advanced statistical tools, can mitigate these biases by analyzing broader chemical spectra, reducing the risk of false conclusions. Moreover, claims about specific compound concentrations may be exaggerated, either due to ignorance or deliberate attempts to mislead consumers, especially when irrelevant assay methods are employed.
The following sections will briefly review the various analytical techniques used in detecting adulteration (see also Table 4).

Table 4.
Advantages and disadvantages of analytical techniques for phytochemical analysis.
5.5.1. UV/VIS Spectrophotometry
UV/Visible spectrophotometry allows the detection of phytochemical compounds or classes when their structure bear chromophores absorbing visible light. Due to its ease of use, high throughput, and relatively low cost, the approach is widely used by botanical ingredient suppliers, dietary supplement manufacturers, and in the industry of active ingredients for cosmetics.
However, the lack of specificity in UV/VIS methods can be exploited by fraudsters attempting to partially or fully substitute the genuine botanical extract with lower-cost extracts with similar types of constituents (e.g., anthocyanin- or proanthocyanidin-rich extracts from other plant sources, which can be the case for grape seed extracts [56]), necessitating the use of more sophisticated analytical methods for a better discrimination of the constituents (e.g., HPTLC or HPLC-UV/Vis) to ensure botanical ingredient authenticity [21,32]).
5.5.2. TLC and HPTLC
Thin-layer chromatography (TLC) and high-performance TLC (HPTLC) which allows superior resolution and reproducibility, are routine chemical fingerprinting assays, often proposed in official pharmacopoeias for the authentication of botanical ingredients due to their ease of reproducibility and reliability. However, in some cases, adulteration can still remain undetectable after (HP)TLC analysis. This can occur by partially or entirely substituting the genuine botanical extract with exogenous extracts or purified fractions with a phytochemical composition behaving similarly under chromatographic conditions, sometimes to mask dilutions or the use of pre-extracted raw material. For example, Ginkgo biloba extracts can be adulterated with pure flavonols (e.g., quercetin, kaempferol) flavonol glycosides (e.g., rutin), or flavonol-rich extracts from Sophora japonica or Fagopyrum esculentum extracts [57], whereas oleogum resin extracts from Boswellia serrata or carterii can be replaced with extracts from other Boswellia species [58] and turmeric root extracts can be adulterated with synthetic curcumin [59]. These examples of commonly used botanical ingredients prone to adulteration highlight that HPTLC can reveal specific adulterations. However, by focusing on this precise verification, HPTLC examines only a small part of the composition, potentially missing other important components or even additional adulterations. This underscores the importance of using orthogonal analytical techniques to ensure a more exhaustive authentication. Furthermore, (HP)TLC analysis may prove inefficient in detecting oligo/polysaccharide, certain metabolite families, or the addition of food dyes, especially when there is uncertainty about the nature of adulteration, leading to the risk of overlooking it and failing to detect it. Despite these limitations, HPTLC remains a widely accepted method in pharmacopoeial standards due to its reproducibility, cost-effectiveness, and ease of use for initial screenings.
5.5.3. Gas Chromatography with Flame Ionization Detection (GC-FID) or Mass Spectrometric Detection (GC-MS)
Gas chromatography approaches are suited for the analysis of volatile botanical ingredients like essential oils, supercritical CO2 extracts, fatty acid-, fatty alcohols-, and sterols-containing extracts, as well as vegetal oils. Indeed, various adulteration techniques can occur for these categories of extracts, including the dilution with non-volatile materials, addition of isolates/fractions with similar composition (dilution with lower-cost material), or composing a product from isolated compounds with similar composition to the original ingredient. Advanced GC techniques like chiral separation (to detect the presence of synthetic or unnatural enantiomers) [60], multidimensional GC [61], and the estimation of isotopic ratios or 14C content [62], may also be required to help detect some adulteration and ensure authenticity for these extracts.
GC techniques focus only on volatile or potentially volatile compounds, meaning they can detect only a small portion of the existing substances in a defined BRM or extract. However, for these compounds, GC is clearly the most suitable technique. Its precision in analyzing volatiles ensures a detailed understanding of these specific components, making it indispensable for verifying the authenticity of essential oils, supercritical extracts, and other volatile-rich botanical products. To complement GC’s focus on volatiles, techniques such as LC-MS are essential for analyzing non-volatile components, ensuring a holistic profiling of the botanical sample.
5.5.4. HPLC and UHPLC, with UV/VIS, Mass Spectrometric, ELSD, or CAD Detection
High-Performance Liquid Chromatography (HPLC) and Ultra-High-Performance Liquid Chromatography (UHPLC) are indispensable techniques for quality control of botanical extract due to their robust separation capabilities and versatile detection options. The use of UV/VIS detector, mass spectrometric (MS) detector, Evaporative Light Scattering Detector (ELSD), or charged aerosol detectors (CADs), enables the comprehensive analysis of complex extracts, facilitating the identification and quantification of a wide array of molecules. The UV/VIS detector particularly excels in the quantification of phenolic compounds with strong chromophores, making it a cost-effective choice for routine quality control and pharmacopeial methods. In contrast, the MS detector provides high sensitivity and specificity, allowing for detailed structural elucidation and the detection of compounds in low concentrations, including those without strong chromophores. Additionally, the Charged Aerosol Detector (CAD) offers a unique capability in detecting virtually any non-volatile and many semi-volatile compounds. CAD is particularly valuable because its detection is largely independent of the chemical properties of the analytes, thus providing a more uniform response. This feature makes CAD highly effective for quantifying compounds that lack UV chromophores, such as sugars, lipids, and polymers, enhancing its utility in a broad range of applications where other detectors might miss non-UV absorbing substances. These methodologies also allow chromatographic fingerprinting [29,63], which is crucial for authenticating botanical ingredients by comparing the entire profile of detected compounds against the reference materials.
ELSD is required for certain pharmacopeial methods (e.g., ginkgo terpene lactones) due to its unique capability of detecting non-volatile and semi-volatile compounds that lack chromophores, which are otherwise undetectable by UV/VIS detectors. This makes ELSD particularly valuable for quantifying compounds such as terpenes, lipids, and certain sugars, providing a mass-based response that is independent of the chemical structure of the analytes, thereby enhancing its applicability in the analysis of complex botanical extracts.
(U)HPLC has significant potential for identifying a wide range of adulterations. However, it is extremely challenging to focus on the entire chemical diversity of plants within a single analysis. As such, a standard (U)HPLC-UV or (U)HPLC-MS analysis will not reveal many adulterations. On the contrary, the vast amount of information generated can be complex or even impossible to interpret, sometimes providing fewer conclusions than a simpler technique like HPTLC, which is easier to analyze.
In contrast, with a non-targeted analytical approach—performing correlations with associated databases and applying statistical analyses to process the results (metabolomics), this technique can fully exploit its potential. Statistical tools such as principal component analysis (PCA), hierarchical clustering, and orthogonal partial least squares discriminant analysis (OPLS-DA) are commonly employed to interpret complex datasets, enabling the detection of subtle adulteration patterns and providing deeper insights into the chemical diversity of botanical materials.
Nevertheless, certain limitations remain:
- -
- Despite the high separation power of UHPLC, some compound families are not better chromatographed compared to other chromatographic techniques, such as oligo/polysaccharides, condensed tannins like proanthocyanidins (PACs), or hydrolyzable tannins (gallic and ellagic derivatives). Indeed, the separation of larger molecular weight compounds is often inadequate even in reversed-phase chromatography, hindering the authentication of PAC-rich extracts. The reliance on specific monomers/oligomers for authentication makes these methods vulnerable to adulteration through the addition of extraneous materials with similar chemical profiles.
- -
- Despite the broad dynamic range of compounds that can be separated in a single analysis using a C18 column, the void volume (v0), which often represents a significant mass fraction of the sample, still contains numerous compounds that cannot be separated as they are. Adding a complementary HILIC mode to a reverse-phase system (like C18) is often necessary to drastically increase the percentage of detected compounds [64].
- -
- The detector can be a limiting factor. For example, with HPLC-DAD, all compounds lacking chromophores may go undetected, and some characteristic compounds might be missed. Some equipment manufacturers also offer solutions to achieve a more comprehensive overview with a DAD detector, enabling a closer approach to non-targeted analysis methods.
While standard (U)HPLC-UV may struggle to detect certain adulterations due to the complexity of plant matrices, method optimization with appropriate reference standards can improve its detection capabilities. For instance, targeting specific adulterants using (U)HPLC-MS or applying orthogonal detection techniques enhances its utility in routine analysis.
In conclusion, (U)HPLC has very strong potential for detecting various types of adulterations, but only when using a non-targeted analytical methodology. However, these exhaustive compositional analyses require a high level of expertise that is often challenging for individual companies to internalize and maintain in-house.
5.5.5. Infrared, Near-Infrared, and Raman Spectroscopy
Infrared (IR), near-infrared (NIR), and Raman spectroscopy are valuable analytical techniques recognized for their cost-effectiveness and rapid sample preparation. Although these methods are not extensively applied in the cosmetic active ingredient industry, they are widely used in the food and spice industry for quality control of bulk botanical materials, such as leaves, roots, rhizomes, and seeds, and additionally to detect adulteration and confirm the geographic origin of the products, including vegetable oils (e.g., olive oil). However, these methods are more effective when applied to raw or minimally processed materials. Moreover, and despite their potential, IR and Raman spectroscopy are not commonly used in the course of specific adulteration strategies.
5.5.6. Mass Spectrometry
Mass spectrometry (MS) without prior HPLC separation, such as flow-injection mass spectrometry (FIMS) or direct analysis in real time (DART) MS, is a pivotal analytical tool that relies on comparing MS fingerprints with authenticated reference materials, often utilizing multivariate statistical analysis. MS is particularly effective for analyzing crude whole, cut, or powdered plants, as well as similarly processed batches of extracts which, due to its high sensitivity, enables the detection of adulterants at low concentrations [65]. However, MS methods can be misled by the addition of purified single compounds or mixtures that mimic the ingredient’s profile. Furthermore, MS may fail to detect adulterants with molecular weights outside the typical scan range (<150 Da or >1500 Da).
Despite these limitations, MS is underutilized in routine quality control processes by dietary supplements and botanical ingredient suppliers. Its precise and sensitive capabilities, however, make it a powerful tool for detecting adulteration, as it is challenging for fraudulent actors to bypass its scrutiny. This underscores the critical role of MS in ensuring the authenticity and integrity of botanical products.
5.5.7. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR-based methods, though offering significant potential for authenticating botanical ingredients, remain relatively underutilized in the industry. These methods involve comparing the NMR fingerprint of a sample with authenticated reference standards and performing adequate chemometric analysis. To cite an example, this approach can be used to detect saw palmetto extract adulteration [41,66]. While effective in detecting adulteration in commercial extracts, challenges arise from variations in manufacturing processes or excipients, which can complicate data interpretation. Moreover, unexpected highly purified constituents from natural or synthetic sources may evade detection through conventional NMR experiments, necessitating more specialized NMR approaches. For instance, specific NMR approaches have been developed to identify synthetic vanillin in vanilla extracts [62]. NMR is often perceived as being very expensive, but recent advancements, particularly in low magnetic field NMR technology, demonstrate that its costs can rival those of mass spectrometry (MS) instruments. Furthermore, NMR offers ease of sample preparation and instrument stability, enabling constituent quantification without the need for expensive reference compounds.
In summary, NMR-based methods offer several advantages to detecting adulteration in botanical extracts, including high detection rates, comprehensive analysis capabilities, minimal sample preparation, and versatility in identifying various types of adulterants. However, these techniques also have some drawbacks, such as the need for specialized equipment and expertise, dependence on comprehensive reference databases, potential for false positives, relatively high costs, and limitations in detecting trace-level or low-proton-density compounds. Despite these challenges, the recent developments in NMR technology further enhance its value as a reliable analytical tool. Overall, NMR-based approaches provide a powerful analytical tool for ensuring the authenticity and quality of botanical products, but their implementation requires careful consideration of the trade-offs and the specific requirements of the analysis.
5.5.8. Isotope Analysis
Isotope analysis is a precise tool for detecting the addition of synthetic compounds in processed raw materials (PRMs). By measuring stable isotope ratios (e.g., δ13C, δ2H, and δ18O), it identifies unique isotopic signatures influenced by photosynthetic pathways, geographical origin, and environmental conditions.
This method is particularly effective against fraudulent practices such as labeling synthetic vanillin as “natural vanilla extract”. Even when adulterators enrich synthetic vanillin with carbon-13 to mimic natural isotopic patterns, specific δ13C analyses can expose the deception [21]. Similarly, isotope analysis detects adulteration in essential oils, such as lavender or citrus oils, and reveals dilutions with materials from different regions through deviations in isotopic ratios.
However, robust isotope analysis has limitations. It may struggle with adulterants that closely match the isotopic profile of authentic materials or are present in trace amounts. Its complexity, cost, and need for expertise also limit its routine application [21].
When combined with complementary methods like GC-MS, isotope analysis enhances the detection of subtle adulterations, providing a critical tool for ensuring botanical authenticity and integrity in the cosmetics and dietary supplement industries.
6. Adulteration of Cosmetic Botanical Materials
Adulteration of botanical ingredients in the cosmetics industry, while less documented than in nutraceuticals, is a growing concern.
6.1. Plant Species Used to Develop Active Ingredients in the Cosmetics Industry
A wide range of extracts from various plant species are used as active ingredients in the cosmetics industry, targeting specific compounds within the three major classes: phenolic compounds, terpenoids, and alkaloids.
Phenolic compounds represent the major class of metabolites present in active ingredients. These phenolic compounds comprise phenolic acids (acids derived from hydroxybenzoic acid or hydroxycinnamic acid) and flavonoids [67]. Flavonoids can be sub-classified as: flavanols (e.g., catechin and epicatechin), flavanones (e.g., hesperetin and naringenin), flavones (e.g., apigenin, baicalein, and luteolin), flavonols (e.g., kaempferol, myricetin, and quercetin), anthocyanidins (e.g., cyanidin, delphinidin, peonidin, and petunidin), isoflavonoids or 3-benzopyrans (e.g., daidzein and genistein), neoflavonoids or 4-benzopyrans (e.g., dalbergin), aurones (e.g., hispidol), and chalcones (e.g., isoliquiritigenin and phloretin). Other notable classes of phenolic compounds, distinct from flavonoids, include: lignans (e.g., pinoresinol and secoisolariciresinol), tannins (classified as hydrolyzable tannins, and condensed tannins or proanthocyanidins), and stilbenes (e.g., trans-resveratrol and its glucoside, piceatannol).
In addition to phenolic compounds, several terpenoids, which are classified into mono-, sesqui-, di-, tri-, and tetraterpenes, may may serve as valuable compounds in botanical ingredients [68], along with various alkaloids, which also represent a diverse class of metabolites [69].
A selection of plants that are susceptible to adulteration within the industry is detailed (Table 5).

Table 5.
Selection of plant species used in the industry of cosmetic active ingredients and their potential adulteration. (Plant part): Major plant organs used in traditional medicine and phytotherapy. (IECIC): Major plant parts listed in the “Inventory of Existing Cosmetic Ingredients in China”, 2021 version, published by the “National Medical Products Administration”, NMPA, of the People’s Republic of China. “extract*” refers to the plant extract without mention of the plant part used.
6.2. Adaptogenic Plants Extracts Are Also of Use in the Cosmetics Industry
Adaptogenic plant extracts are widely used in the nutraceutical industry and have gained significant attention in the cosmetics sector due to their potential cutaneous benefits [35,105]. These extracts are valued as cosmetic active ingredients for their ability to enhance skin resilience and support skin health. The most relevant adaptogenic plants used as botanical actives in cosmetics include: Asian ginseng (Panax ginseng), American ginseng (Panax quinquefolius), schisandra (Schisandra chinensis), eleuthero (Eleutherococcus senticosus), rhodiola (Rhodiola rosea), astragalus (Astragalus membranaceus), Baikal skullcap (Scutellaria baicalensis), Ural licorice (Glycyrrhiza uralensis), ashwagandha (Withania somnifera), and suma (Pfaffia paniculata) (plants are cited in Table 5).
6.3. Vegetal Oils
Adulteration may also occur for vegetal oils used in the cosmetics industry and may involve mixing with other carrier oils or using lower-quality oils [52,106]. Many cosmetics rely heavily on vegetal oils for their moisturizing, nourishing, and protective properties, which makes the detection of adulteration crucial to ensure product efficacy and consumer safety. While vegetal oil adulteration is a complex topic on its own and will not be detailed here, it is important to note that ensuring the purity of these oils is crucial in maintaining product efficacy and safety. The vegetal oils commonly used in cosmetics are the following (the list includes also shea butter): almond oil (Prunus amygdalus dulcis), argan oil (Argania spinosa), avocado oil (Persea americana), baobab seed oil (Adansonia digitata), black cumin oil (Nigella sativa), chia seed oil (Salvia hispanica), clary sage seed oil (Salvia sclarea), coconut oil (Cocos nucifera), cottonseed oil (Gossypium spp.), evening primrose oil (Oenothera biennis), grapeseed oil (Vitis vinifera), hemp seed oil (Cannabis sativa), jojoba oil (Simmondsia chinensis), macadamia oil (Macadamia integrifolia), moringa oil (Moringa oleifera), olive oil (Olea europaea), pomegranate seed oil (Punica granatum), pumpkin seed oil (Cucurbita pepo), rosehip oil (Rosa spp.), safflower oil (Carthamus tinctorius), shea butter (Butyrospermum parkii), soybean oil (Glycine max), and sunflower seed oil (Helianthus annuus).
6.4. Essential Oils
Adulteration of essential oils, often via dilution with alternative oils or the addition of synthetic compounds, poses a significant issue in the cosmetics industry. Common types of adulteration include the use of another essential oil instead of the claimed one, reconstitution of the oil using only synthetic components, and dilution with small amounts of vegetable oil (e.g., 10–15%), which can evade detection in GC profiling. In addition, adulteration may involve the addition of exogenous substances to alter the chemical composition.
Given the widespread use of essential oils in skincare formulations (and in perfumes), ensuring their purity is crucial for maintaining product efficacy and safety. Detecting adulteration is challenging due to the complex chemical profiles of essential oils, but gas chromatography, often coupled with mass spectrometry (GC-MS), is considered the gold standard for identifying these adulterants. For essential oils derived from citrus fruits obtained through cold expression, the use of HPLC-UV or MS allows for the simultaneous detection of flavonoids, which serve as chemotaxonomic markers specific to citrus essences. This approach facilitates the identification of potential adulteration, such as the mixing of different citrus essential oils or the addition of limonene [107]. A selection of introductory scientific articles is cited hereafter to provide further insight into this topic [51,106,108].
7. Conclusions and Perspective
Botanical adulteration in the cosmetics industry presents a significant challenge that affects product quality, safety, and consumer trust. As the demand for natural and organic cosmetics continues to rise, the risk of economically motivated adulteration has grown, driven by market pressures and disrupted supply chains. The market dynamics surrounding popular adaptogenic plants, for example, have led to a higher prevalence of economically motivated adulteration (substitutions, additions of unexpected plant parts, dilutions) as suppliers strive to meet the soaring demand. The integrity of botanical raw materials and extracts is paramount for ensuring the efficacy and safety of cosmetic formulations.
This review has highlighted the multifaceted nature of botanical adulteration, encompassing misidentification, contamination, and the deliberate addition of synthetic or inferior materials. It has also outlined various analytical approaches—such as DNA barcoding, phytochemical profiling, and mass spectrometry—that are essential for authenticating botanical ingredients and detecting adulteration. While no single technique can address all aspects of botanical authentication, their combined use provides the most reliable results. For BRM, genetic identification is particularly effective, whereas PRM benefits most from exhaustive phytochemical analysis, given its focus on active compounds.
To summarize, for BRM, all identification techniques are possible and orthogonal, but genetic identification is generally more qualitatively effective and highly effective for taxonomic discrimination, even though the combined use of all three techniques will always provide the most accurate results. In contrast, for PRM, phytochemical analysis is the most suitable technique, both qualitatively and quantitatively, as the ingredients mainly contain active compounds, although other techniques can also prove useful.
To mitigate the risks associated with adulteration, the cosmetics industry must adopt robust, standardized authentication protocols. These should be integrated at every stage of the supply chain, from raw material sourcing to final product formulation. Furthermore, transparent traceability systems are crucial for building consumer trust and ensuring regulatory compliance. Additionally, monitoring the use of restricted species and adhering to CITES guidelines support conservation efforts and prevent biodiversity loss.
Ethical considerations are also paramount. Traditional knowledge, often rooted in the heritage of indigenous communities, plays a vital role in cosmetic formulations. Fair trade practices, benefit-sharing agreements, and compliance with frameworks like the Nagoya Protocol ensure that communities are recognized and compensated for their contributions. Adherence to the Nagoya Protocol not only supports equitable benefit-sharing but also helps mitigate the risk of unsustainable harvesting practices. Such practices can lead to adulteration driven by resource scarcity, as suppliers attempt to meet demand with substandard or substituted materials. Ethical sourcing and transparency not only foster equity but also strengthen consumer trust in products marketed as ‘natural’ and ‘sustainable’.
Guaranteeing the integrity of botanical ingredients in cosmetic formulations requires a proactive, multi-tiered strategy involving advanced authentication, supply chain transparency, and industry-wide awareness. First, stakeholders should implement a comprehensive and multi-level authentication system by incorporating robust testing methodologies, such as genetic identification, chromatographic analysis, and isotope ratio techniques, at multiple checkpoints from raw material sourcing to finished product evaluation. The integration of AI-driven detection tools and machine learning algorithms can further enhance vigilance by identifying complex adulteration patterns that may escape traditional testing methods.
Second, reinforcing supply chain transparency and regulatory compliance is essential to minimizing adulteration risks. Cosmetics manufacturers and suppliers should establish strict documentation requirements that trace ingredient origins, processing methods, and transportation conditions. Implementing supplier audits, requiring third-party certifications, and adhering to global regulatory standards, such as EU Cosmetics Regulation 1223/2009, can significantly reduce the prevalence of fraudulent botanical ingredients. Additionally, regulatory bodies and industry associations should work collaboratively to develop harmonized guidelines for botanical ingredient verification in cosmetics.
Finally, enhancing industry-wide awareness and consumer confidence is key to fostering a more transparent and responsible market. Educating industry professionals on adulteration risks through specialized training programs, conferences, and collaborative research initiatives will strengthen collective vigilance. Establishing a cosmetovigilance reporting framework to document and address cases of suspected adulteration will further support the industry’s integrity. At the consumer level, transparent labeling and scientifically validated claims regarding ingredient authenticity will build trust and empower buyers to make informed decisions.
Moving forward, the continuous evolution of authentication technologies will be essential to staying ahead of increasingly sophisticated adulteration techniques. Advancements in metabolomic and isotopic profiling, along with the refinement of non-targeted screening methods, will enhance the industry’s ability to detect even subtle discrepancies in botanical extracts. In parallel, the integration of blockchain technology into supply chain management holds promise for improving traceability, offering an immutable record of ingredient provenance from source to final formulation.
Moreover, fostering stronger collaboration between regulatory bodies, industry stakeholders, and scientific researchers will be key to establishing more effective global standards for botanical authentication. By embracing these innovations and reinforcing transparency at every stage of the supply chain, the cosmetics industry can not only mitigate the risks associated with adulteration but also promote sustainability, consumer trust, and long-term integrity in the marketplace.
In conclusion, ensuring the authenticity and ethical sourcing of botanical ingredients is a collective responsibility. By implementing comprehensive strategies for adulteration prevention, regulatory compliance, and ethical practices, the cosmetics industry can deliver high-quality products that inspire confidence while contributing to global conservation and equitable trade.
Funding
This research received no external funding.
Acknowledgments
We would like to express our gratitude to Rachel BAUWENS, Axel DECARLIS, and Julien DIAZ for their valuable scientific and technical insights that greatly enriched this work. Additionally, we extend our heartfelt thanks to Stefan GAFNER for kindly reviewing the manuscript and providing invaluable comments that significantly improved its quality.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Drouet, S.; Garros, L.; Hano, C.; Tungmunnithum, D.; Renouard, S.; Hagège, D.; Maunit, B.; Lainé, E. A Critical View of Different Botanical, Molecular, and Chemical Techniques Used in Authentication of Plant Materials for Cosmetic Applications. Cosmetics 2018, 5, 30. [Google Scholar] [CrossRef]
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P.P. Adulteration of Dietary Supplements by the Illegal Addition of Synthetic Drugs: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report On Traditional And Complementary Medicine. 2019. Available online: https://iris.who.int/handle/10665/312342 (accessed on 20 March 2025).
- Sen, S.; Chakraborty, R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J. Tradit. Complement. Med. 2016, 7, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Thas, J.J. Siddha Medicine—Background and principles and the application for skin diseases. Clin. Dermatol. 2008, 26, 62–78. [Google Scholar] [CrossRef]
- Wangyal, R.; Tidwell, T.; Dhondrup, W.; Yungdrung, T.; Dhondrup, G.; He, Q.; Zhang, Y. Dataset of materia medica in Sowa Rigpa: Tibetan medicine botanicals and Gawé Dorjé’s classification system. Data Brief 2020, 33, 106498. [Google Scholar] [PubMed]
- Singh, K.; Gupta, J.K.; Jain, D.; Kumar, S.; Singh, T.; Saha, S. Exploring the ancient wisdom and modern relevance of Chinese medicine: A comprehensive review. Pharmacol. Res. Mod. Chin. Med. 2024, 11, 100448. [Google Scholar]
- Sreedhar, R.; Watanabe, K.; Arumugam, S. Introduction to Japanese Kampo Medicines. In Japanese Kampo Medicines for the Treatment of Common Diseases: Focus on Inflammation; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 1. [Google Scholar]
- Han, S.Y.; Kim, H.Y.; Lim, J.H.; Cheon, J.; Kwon, Y.K.; Kim, H.; Yang, G.Y.; Chae, H. The past, present, and future of traditional medicine education in Korea. Integr. Med. Res. 2016, 5, 73–82. [Google Scholar] [CrossRef]
- Elfahmi; Woerdenbag, H.J.; Kayser, O. Jamu: Indonesian traditional herbal medicine towards rational phytopharmacological use. J. Herbal Med. 2014, 4, 51–73. [Google Scholar]
- Khan, A.A.; Zulkifle, M.; Ansari, A.H.; Khan, A.H.N.; Farooque, M. Persian contribution to Unani medicine a review. Hamdard Med. 2008, 51, 137–143. [Google Scholar]
- Redvers, N.; Blondin, B. Traditional Indigenous medicine in North America: A scoping review. PLoS ONE 2020, 15, e0237531. [Google Scholar] [CrossRef]
- Mendoza, R.G. Lords of the medicine bag: Medical science and traditional practice in ancient Peru and South America. In Medicine Across Cultures: History and Practice of Medicine in Non-Western Cultures; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 225–257. [Google Scholar]
- Ozioma, E.J.; Nwamaka Chinwe, O.A. Herbal Medicines in African Traditional Medicine. In Herbal Medicine; IntechOpen: London, UK, 2019; pp. 1–25. [Google Scholar]
- Turpin, G.; Ritmejerytė, E.; Jamie, J.; Crayn, D.; Wangchuk, P. Aboriginal medicinal plants of Queensland: Ethnopharmacological uses, species diversity, and biodiscovery pathways. J. Ethnobiol. Ethnomed. 2022, 18, 54. [Google Scholar] [CrossRef]
- Tyler, V.E. Herbal medicine: From the past to the future. Public Health Nutr. 2000, 3, 447–452. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 5th ed.; Lavoisier: Paris, France, 2016; 1504p. [Google Scholar]
- Najem, M.; Nassiri, L.; Ibijbijen, J. Vernacular names of plants between diversity and potential risks of confusion: Case of toxic plants used in medication in the central Middle Atlas, Morocco. J. Pharm. Pharmacogn. Res. 2021, 9, 222–250. [Google Scholar] [CrossRef]
- Dural, E. Investigation of the presence of sildenafil in herbal dietary supplements by validated HPLC method. Turk. J. Pharm. Sci. 2019, 17, 56. [Google Scholar] [CrossRef]
- Gafner, S.; Loffredo, L.; Kababick, J.; Wise, S.; Upton, R. The undisclosed presence of excipients and diluents in botanical extracts. HerbalGram 2024, 140, 44–51. [Google Scholar] [CrossRef]
- Jayanthy, A.; Deepak, M.; Remashree, A.B. Pharmacognostic characterization and comparison of fruits of Tribulus terrestris L. and Pedalium murex L. Int. J. Herb. Med. 2013, 1, 29–34. [Google Scholar]
- Moras, B.; Loffredo, L.; Rey, S. Quality assessment of saffron (Crocus sativus L.) extracts via UHPLC-DAD-MS analysis and detection of adulteration using gardenia fruit extract (Gardenia jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef]
- Simmler, C.; Graham, J.G.; Chen, S.N.; Pauli, G.F. Integrated analytical assets aid botanical authenticity and adulteration management. Fitoterapia 2018, 129, 401–414. [Google Scholar]
- Wallace, E.D.; Todd, D.A.; Harnly, J.M.; Cech, N.B.; Kellogg, J.J. Identification of adulteration in botanical samples with untargeted metabolomics. Anal. Bioanal. Chem. 2020, 412, 4273–4286. [Google Scholar] [CrossRef] [PubMed]
- Ichim, M.C.; Booker, A. Chemical Authentication of Botanical Ingredients: A Review of Commercial Herbal Products. Front. Pharmacol. 2021, 12, 666850. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Blumenthal, M.; Foster, S.; Cardellina, J.H., 2nd; Khan, I.A.; Upton, R. Botanical Ingredient Forensics: Detection of Attempts to Deceive Commonly Used Analytical Methods for Authenticating Herbal Dietary and Food Ingredients and Supplements. J. Nat. Prod. 2023, 86, 460–472. [Google Scholar] [CrossRef]
- Cronquist, A. The Evolution and Classification of Flowering Plants, 2nd ed.; New York Botanical Garden: New York, NY, USA, 1988. [Google Scholar]
- Smith, B.; Chinnappa, C. Plant Collection, Identification, and Herbarium Procedures. In Plant Microtechniques and Protocols; Springer: Berlin, Germany, 2015; pp. 541–572. [Google Scholar]
- Upton, R.; David, B.; Gafner, S.; Glasl, S. Botanical ingredient identification and quality assessment: Strengths and limitations of analytical techniques. Phytochem. Rev. 2019, 19, 1157–1177. [Google Scholar] [CrossRef]
- Han, Q.; Erasmus, S.W.; Elliott, C.T.; van Ruth, S.M. A sense of ginger fraud: Prevalence and deconstruction of the China-European union supply chain. npj Sci. Food 2022, 6, 51. [Google Scholar] [CrossRef]
- Joshi, V.; Khan, I. Microscopy Techniques for the Identification and Authentication of Botanicals. Acta Hortic. 2006, 720, 73–80. [Google Scholar] [CrossRef]
- Xie, P.; Chen, S.; Liang, Y.-Z.; Wang, X.; Tian, R.; Upton, R. Chromatographic fingerprint analysis—A rational approach for quality assessment of traditional Chinese herbal medicine. J. Chromatogr. A 2006, 1112, 171–180. [Google Scholar] [CrossRef]
- Ichim, M.C.; de Boer, H.J. A Review of Authenticity and Authentication of Commercial Ginseng Herbal Medicines and Food Supplements. Front. Pharmacol. 2021, 11, 612071. [Google Scholar] [CrossRef]
- Bejar, E.; Upton, R.; Cardellina, J.H., II. Adulteration of Rhodiola (Rhodiola rosea) Rhizome and Root and Extracts. Botanical Adulterants Prevention Bulletin, October 2017, pp. 1–8. Available online: https://umb.herbalgram.org/media/kvqiswnx/bap-babs-rhodiola-cc-v4-final.pdf (accessed on 20 March 2025).
- Hosbas Coskun, S.; Brinckmann, J. Adulteration of Eleuthero (Eleutherococcus senticosus) Root and Its Extracts. Botanical Adulterants Prevention Bulletin, January 2022, pp. 1–13. Available online: https://www.researchgate.net/publication/358063851_Adulteration_of_Eleuthero_Eleutherococcus_senticosus_Root_and_its_Extracts (accessed on 20 March 2025).
- Pospiech, M.; Javůrková, Z.; Hrabec, P.; Štarha, P.; Ljasovská, S.; Bednář, J.; Tremlová, B. Identification of pollen taxa by different microscopy techniques. PLoS ONE 2021, 16, e0256808. [Google Scholar] [CrossRef]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Tan, C.H.; Kong, I.; Irfan, U.; Solihin, M.I.; Pui, L.P. Edible Oils Adulteration: A Review on Regulatory Compliance and Its Detection Technologies. J. Oleo Sci. 2021, 70, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Nithaniyal, S.; Vassou, S.L.; Poovitha, S.; Raju, B.; Parani, M. Identification of species adulteration in traded medicinal plant raw drugs using DNA barcoding. Genome 2017, 60, 139–146. [Google Scholar] [CrossRef]
- Letsiou, S.; Madesis, P.; Vasdekis, E.; Montemurro, C.; Grigoriou, M.E.; Skavdis, G.; Moussis, V.; Koutelidakis, A.E.; Tzakos, A.G. DNA Barcoding as a Plant Identification Method. Appl. Sci. 2024, 14, 1415. [Google Scholar] [CrossRef]
- Kopka, J.; Fernie, A.; Weckwerth, W.; Gibon, Y.; Stitt, M. Metabolite profiling in plant biology: Platforms and destinations. Genome Biol. 2004, 5, 109. [Google Scholar] [CrossRef]
- Kupina, S.; Gafner, S. On adulteration of grape seed extract. Botanical Adulterants Bulletin, April 2016, pp. 1–5. Available online: https://www.polyphenolics.com/wp-content/uploads/2016/06/052015-BAP-BABs-GrapeSeedEx-CC-v2.pdf (accessed on 20 March 2025).
- Gafner, S. Adulteration of Ginkgo biloba Leaf Extract. Botanical Adulterants Bulletin, January 2018, pp. 1–8. Available online: https://www.cspinet.org/sites/default/files/botanical-adulterants-bulletin.pdf (accessed on 20 March 2025).
- McCutcheon, A. Adulteration of milk thistle (Silybum marianum). Botanical Adulterants Prevention Bulletin, October 2020, pp. 1–12. Available online: https://www.semanticscholar.org/paper/Adulteration-of-Milk-Thistle-%28Silybum-marianum%29-McCutcheon/75bba5e5ddb43a3a12e76863d3fa74ee03f54b89 (accessed on 20 March 2025).
- Bejar, E. Turmeric (Curcuma longa) Root and Rhizome, and Root and Rhizome Extracts. Botanical Adulterants Bulletin, May 2018, pp. 1–5. Available online: https://www.researchgate.net/publication/325617460_Adulteration_of_turmeric_Curcuma_longa_root_and_rhizome_and_root_and_rhizome_extracts (accessed on 20 March 2025).
- Bechis, G.; Raccary, B.; Sarrazin, E.; Corbi, E.; Peres, C.; David, N.; Bicchi, C.; Cagliero, C. Assessing the environmental and overall performance of gas chromatographic analyses. Development of a comprehensive evaluation framework and application to routine chiral analyses of fragrances as a case study. Sustain. Chem. Pharm. 2023, 35, 101217. [Google Scholar] [CrossRef]
- Nolvachai, Y.; Amaral, M.S.S.; Marriott, P.J. Foods and Contaminants Analysis Using Multidimensional Gas Chromatography: An Update of Recent Studies, Technology, and Applications. Anal. Chem. 2023, 95, 238–263. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.G.; Zapf, C.M. Flavor, Quality, and Authentication. In Handbook of Vanilla Science and Technology, 2nd ed.; Havkin-Frenkel, D., Belanger, F.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 261–284. [Google Scholar]
- Ranjan, S.; Adams, E.; Deconinck, E. Multidimensional Chromatographic Fingerprinting Combined with Chemometrics for the Identification of Regulated Plants in Suspicious Plant Food Supplements. Molecules 2023, 28, 3632. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J.; Wahman, R.; Schröder, P.; Letzel, T. Plant Metabolomic Workflows Using Reversed-Phase LC and HILIC with ESI-TOF-MS. LCGC Int. 2019, 37, 8–15. [Google Scholar]
- Satheeshkumar, N.; Paul, D.; Lingesh, A. Liquid Chromatography–Mass Spectrometry (LC–MS): Approaches to Adulterant Detection in Herbal Products. In Medicinal Plants—Recent Advances in Research and Development; Springer: Singapore, 2016. [Google Scholar]
- de Combarieu, E.; Martinelli, E.M.; Pace, R.; Sardone, N. Metabolomics study of Saw palmetto extracts based on 1H NMR spectroscopy. Fitoterapia 2015, 102, 56–60. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Botto, J.M. Les phytoadaptogènes: Une catégorie à part d’extraits de plantes aux propriétés pléiotropes d’adaptation à notre environnement. Phytothérapie 2023, 21, 75–86. [Google Scholar]
- Cavagnino, A.; Breton, L.; Ruaux, C.; Grossgold, C.; Levoy, S.; Abdayem, R.; Roumiguiere, R.; Cheilian, S.; Bouchara, A.; Baraibar, M.A.; et al. Adaptogen Technology for Skin Resilience Benefits. Cosmetics 2023, 10, 155. [Google Scholar] [CrossRef]
- Ali, S.; Ekbbal, R.; Salar, S.; Yasheshwar Ali, S.A.; Jaiswal, A.K.; Singh, M.; Yadav, D.K.; Kumar, S.; Gaurav. Quality Standards and Pharmacological Interventions of Natural Oils: Current Scenario and Future Perspectives. ACS Omega 2023, 8, 39945–39963. [Google Scholar]
- Masson, J.; Liberto, E.; Beolor, J.-C.; Brevard, H.; Bicchi, C.; Rubiolo, P. Oxygenated heterocyclic compounds to differentiate Citrus spp. essential oils through metabolomic strategies. Food Chem. 2016, 206, 223–233. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P. Detection of Essential Oils Adulteration: A Quick Overview and Current Challenges. Am. J. Biomed. Sci. Res. 2019, 4, 10–11. [Google Scholar]
- Silva, M.L.; Rita, K.; Bernardo, M.A.; de Mesquita, M.F.; Pintão, A.M.; Moncada, M. Adansonia digitata L. (Baobab) Bioactive Compounds, Biological Activities, and the Potential Effect on Glycemia: A Narrative Review. Nutrients 2023, 15, 2170. [Google Scholar] [CrossRef] [PubMed]
- Surjushe, A.; Vasani, R.; Saple, D. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci. 2002, 82, 1336–1345. [Google Scholar]
- Preethi, K.; Kuttan, R. Wound healing activity of flower extract of Calendula offlcinalis. J. Basic Clin. Physiol. Pharmacol. 2009, 20, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, J.; Pu, Y.; Wang, X. Tea (Camellia sinensis): A review of nutritional composition, potential applications, and Omics Research. Appl. Sci. 2022, 12, 5874. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar] [CrossRef]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Bommareddy, A.; Brozena, S.; Steigerwalt, J.; Landis, T.; Hughes, S.; Mabry, E.; Knopp, A.; VanWert, A.L.; Dwivedi, C. Medicinal properties of alpha-santalol, a naturally occurring constituent of sandalwood oil: Review. Nat. Prod. Res. 2017, 33, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Habashy, R.R.; Abdel-Naim, A.B.; Khalifa, A.E.; Al-Azizi, M.M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol. Res. 2005, 51, 95–105. [Google Scholar]
- Akter, S.; Addepalli, R.; Netzel, M.; Fletcher, M.; Sultanbawa, Y.; Osborne, S. Impact of polyphenol-rich extracts of Terminalia ferdinandiana fruits and seeds on viability of human intestinal and liver cells in vitro. Food Chem. Mol. Sci. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Kang, T.; Dou, D.; Xu, L. Establishment of a quality marker (Q-marker) system for Chinese herbal medicines using burdock as an example. Phytomedicine 2019, 54, 339–346. [Google Scholar] [CrossRef]
- Yang, J.; Yin, C.; Miao, X.; Meng, X.; Liu, Z.; Hu, L. Rapid discrimination of adulteration in Radix Astragali combining diffuse reflectance mid-infrared Fourier transform spectroscopy with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119251. [Google Scholar] [CrossRef]
- Yadav, A.; Ahmad, J.; Chaudhary, A.A.; Ahmad, A. Development of Sequence Characterized Amplified Region (SCAR) Marker for the Authentication of Bacopa monnieri (L.) Wettst. Eur. J. Med. Plants 2012, 2, 186–198. [Google Scholar] [CrossRef]
- Püski, P.; Körmöczi, T.; Berkecz, R.; Barta, A.; Bajtel, Á.; Kiss, T. Rapid Detection of Adulteration in Boswellia Extracts with Citric Acid by UPLC-HRMS and 1H NMR. J. Diet. Suppl. 2024, 21, 462–477. [Google Scholar] [PubMed]
- Schmiderer, C.; Lukas, B.; Ruzicka, J.; Novak, J. DNA-based identification of Calendula officinalis (Asteraceae). Appl. Plant Sci. 2015, 3, apps.1500069. [Google Scholar] [CrossRef]
- Pereira, A.G.; Garcia-Perez, P.; Cassani, L.; Chamorro, F.; Cao, H.; Barba, F.J.; Simal-Gandara, J.; Prieto, M.A. Camellia japonica: A phytochemical perspective and current applications facing its industrial exploitation. Food Chem. X 2022, 13, 100258. [Google Scholar] [CrossRef]
- Song, S.Y.; Park, D.H.; An, K.W.; Cho, S.S. Process Optimization Based on Biological Effects and Biomarker Contents of Camellia japonica L. for the Development of Anti-Hyperuricemic and Anti-Wrinkle Source. Separations 2022, 9, 281. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Nakamura, S.; Muraoka, O.; Yoshikawa, M. New biofunctional effects of oleanane-type triterpene saponins. J. Nat. Med. 2023, 77, 644–664. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Mao, J.; Zhang, L.; Xie, H.; Chen, L.; Yu, L.; Ma, F.; Wang, X.; Zhang, Q.; Li, P. Multispecies Adulteration Detection of Camellia Oil by Chemical Markers. Molecules 2018, 23, 241. [Google Scholar] [CrossRef] [PubMed]
- Keshari, D.S.; Co Authors: Parwar, S.; Bishnupriya, M. Tea (Camellia sinensis Linn.)—The Pharmacological Activities, Substitutes and Adulterants. Int. J. Ayurveda Pharm. Chem. 2022, 18, 60–67. [Google Scholar]
- Li, Y.; Elliott, C.T.; Petchkongkaew, A.; Wu, D. The classification, detection and ‘SMART’control of the nine sins of tea fraud. Trends Food Sci. Technol. 2024, 149, 104565. [Google Scholar]
- Chen, D.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Physiological genetics, chemical composition, health benefits and toxicology of tea (Camellia sinensis L.) flower: A review. Food Res. Int. 2020, 137, 109584. [Google Scholar] [CrossRef]
- Alqahtani, A.; Cho, J.-L.; Wong, K.H.; Li, K.M.; Razmovski-Naumovski, V.; Li, G.Q. Differentiation of Three Centella Species in Australia as Inferred from Morphological Characteristics, ISSR Molecular Fingerprinting and Phytochemical Composition. Front. Plant Sci. 2017, 8, 1980. [Google Scholar] [CrossRef] [PubMed]
- Indhumathi, K.; Prabhu, M.; Vennila, M.A. Centella asiatica and its Adulterants. AgroSci. Today 2023, 4, 663–667. [Google Scholar]
- Masullo, M.; Montoro, P.; Mari, A.; Pizza, C.; Piacente, S. Medicinal plants in the treatment of women’s disorders: Analytical strategies to assure quality, safety and efficacy. J. Pharm. Biomed. Anal. 2015, 113, 189–211. [Google Scholar] [CrossRef]
- Viciano-Tudela, S.; Sendra, S.; Parra, L.; Jimenez, J.M.; Lloret, J. Proposal of a Gas Sensor-Based Device for Detecting Adulteration in Essential Oil of Cistus ladanifer. Sustainability 2023, 15, 3357. [Google Scholar] [CrossRef]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Canilli, L.; Terno, M.; Angarano, M.; et al. Anti-Inflammatory and Anti-Acne Effects of Hamamelis virginiana Bark in Human Keratinocytes. Antioxidants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, A.; Taglialatela-Scafati, O.; Minassi, A.; Pollastro, F.; Ballero, L.M.; Maxia, A.; Sanna, C. Helichrysum italicum: The Sleeping Giant of Mediterranean Herbal Medicine. HerbalGram 2015, 105, 34–45. [Google Scholar]
- Safer, S.; Cicek, S.S.; Pieri, V.; Schwaiger, S.; Schneider, P.; Wissemann, V.; Stuppner, H. Metabolic fingerprinting of Leontopodium species (Asteraceae) by means of 1H NMR and HPLC–ESI-MS. Phytochemistry 2011, 72, 1379–1389. [Google Scholar] [CrossRef]
- Mahgoub, Y.A.; Shawky, E.; Eldakak, M.; Bahey-El-Din, M.; Darwish, F.A.; El Sebakhy, N.A.; El-Hawiet, A. Plant DNA barcoding and metabolomics for comprehensive discrimination of German Chamomile from its poisonous adulterants for food safety. Food Control 2022, 136, 108840. [Google Scholar] [CrossRef]
- Patora, J.; Majda, T.; Góra, J.; Klimek, B. Variability in the content and composition of essential oil from lemon balm (Melissa officinalis L.) cultivated in Poland. Acta Pol. Pharm. 2004, 60, 395–400. [Google Scholar]
- Pawar, R.S.; Tamta, H.; Ma, J.; Krynitsky, A.J.; Grundel, E.; Wamer, W.G.; Rader, J.I. Updates on chemical and biological research on botanical ingredients in dietary supplements. Anal. Bioanal. Chem. 2013, 405, 4373–4384. [Google Scholar] [CrossRef]
- de Almeida, F.A.N.; Oliveira, P.V.; Matos, N.S.; Fialho, V.L.S.; Lugon, M.D.; Vieira, M.C.; Ferreira, M.F.S.; Paneto, G.G. Authentication of Brazilian Ginseng using Bar-HRM analysis. Braz. J. Pharm. Sci. 2023, 59, e21179. [Google Scholar] [CrossRef]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.H., II; Gafner, S. Adulteration of Pomegranate Ingredients and Products. Botanical Adulterants Prevention Bulletin, June 2021, pp. 1–9. Available online: https://www.stauberusa.com/wp-content/uploads/2021/09/ABC-2021-Pomegranate-adulteration-Bulletin.pdf (accessed on 20 March 2025).
- Booker, A.; Jalil, B.; Frommenwiler, D.; Reich, E.; Zhai, L.; Kulic, Z.; Heinrich, M. The authenticity and quality of Rhodiola rosea products. Phytomedicine 2016, 23, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Jariani, P.; Shahnejat-Bushehri, A.-A.; Naderi, R.; Zargar, M.; Naghavi, M.R. Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.). Molecules 2024, 29, 3145. [Google Scholar] [CrossRef] [PubMed]
- Saint-Lary, L. Évaluation de L’approche Métabolomique pour L’authentification des Extraits Naturels Utilisés dans le Secteur Arômes et Parfums. Ph.D. Thesis, Autre. Université Nice Sophia Antipolis, Nice, France, September 2015. [Google Scholar]
- Mykhailenko, O. Adulteration of Rose (Rosa × damascena) Essential Oil. Botanical Adulterants Prevention Bulletin, June 2024, pp. 1–20. Available online: https://umb.herbalgram.org/media/swpjd2yl/bapp-babs-roseoil-062024-final.pdf (accessed on 20 March 2025).
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- Reddy, V.V.V.V.; Johar, V. Whispers of the sacred wood: A comprehensive review of Santalum album. Int. J. Phytol. Res. 2024, 4, 1–7. [Google Scholar]
- Jiang, P.; Lu, Y.; Chen, D. Authentication of Schisandra chinensis and Schisandra sphenanthera in Chinese patent medicines. J. Pharm. Biomed. Anal. 2016, 131, 263–271. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Singh, V.K.; Mundkinajeddu, D.; Agarwal, A.; Nguyen, J.; Sudberg, S.; Gafner, S.; Blumenthal, M. Adulteration of Ashwagandha (Withania somnifera) Roots and Extracts. Botanical Adulterants Prevention Bulletin, January 2019, pp. 1–7. Available online: https://www.researchgate.net/publication/343862896_Adulteration_of_Ashwagandha_Withania_somnifera_Roots_and_Extracts (accessed on 20 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
