1. Introduction
The skin serves as a vital barrier that shields the body from external influences, including physical, chemical, and microbiological challenges, while maintaining hydration by preventing water loss. However, being the body’s outermost layer, it is constantly exposed to environmental factors that accelerate its natural aging process. Among these, prolonged exposure to ultraviolet (UV) radiation from sunlight is the most significant contributor to extrinsic aging [
1], a process referred to as photoaging [
2,
3,
4]. Clinically, photoaged skin manifests as dryness, roughness, deep wrinkles, sagging, loss of elasticity, and hyperpigmentation [
5]. This appearance results from tissue-level structural changes involving both cellular components and the extracellular matrix (ECM). Key alterations include reduced collagen levels, driven by increased degradation and mediated by matrix metalloproteinases (MMPs), and decreased biosynthesis of new collagen fibers [
6]. Additionally, there is an accumulation of abnormal elastin fibers, known as elastosis, and a significant reduction in glycosaminoglycans (GAGs) such as hyaluronic acid, which are critical for maintaining skin hydration and elasticity. At the cellular level, UV radiation impairs the function of keratinocytes, fibroblasts, and melanocytes, leading to compromised barrier integrity, diminished regenerative capacity, hyperpigmentation, and an overall decline in skin resilience. These changes contribute to the structural and functional decline observed in photoaged skin, increasing its susceptibility to skin disorders, including non-melanoma and melanoma skin cancers [
3,
7,
8].
The detrimental effects of photoaging result from complex biological mechanisms triggered by UV radiation, visible light, and infrared radiation, all of which penetrate the skin at varying depths. A primary mechanism is the generation of reactive oxygen species (ROS), which directly damage cellular and extracellular biomolecules, including DNA, proteins, lipids, and polysaccharides [
9]. These oxidative events destabilize cellular components, impair repair processes, and activate signaling pathways, including the mitogen-activated protein kinase (MAPK) cascade [
10]. This activation leads to the nuclear translocation of transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1), which promote the expression of inflammatory mediators, such as interleukin-6 (IL-6) and interleukin-8 (IL-8), and matrix metalloproteinases (MMPs). MMPs are responsible for ECM degradation, particularly of collagen and elastin, which are vital for maintaining the skin’s structural integrity and elasticity. In addition to ECM remodeling, UV radiation induces premature cellular senescence, limiting the replicative capacity of fibroblasts, keratinocytes, and melanocytes, and promoting a senescence-associated secretory phenotype (SASP) characterized by the release of inflammatory cytokines and proteases. Furthermore, chronic low-grade inflammation, termed “inflammaging,” perpetuates oxidative damage and ECM degradation, creating a self-sustaining cycle of tissue damage [
11]. Chronic UV exposure also disrupts the skin microbiota [
12]. The microbiota play a vital role in maintaining skin health, including protection against harmful pathogens, modulation of immune responses, and enhancement of skin’s barrier function. UV radiation alters this microbial balance, reducing the skin’s natural defenses and exacerbating inflammation.
In response to this need, we have designed a reparative product that incorporates our novel OxIR PhotoRescue Technology® to target and counteract the primary mechanisms of photodamage. This advanced formulation combines antioxidants such as encapsulated beta-carotene, a corticosteroid-like anti-inflammatory agent that mimics hydrocortisone’s effects without associated adverse reactions, and a unique blend of plant-derived extracts with potent regenerative effects. When synergized with specially selected hyaluronic acid, an advanced combination of natural sugars comprising natural moisturizing factors (NMF) and physiomoisturizers, and a product that enhances skin microbiota protection, this formula is intended to improve signs of aging while providing robust protection against sun exposure-associated skin disorders. In order to validate the combined effects of the formulation in mitigating skin photodamage, a series of in vitro, ex vivo, and in vivo studies have been conducted in which antioxidant and anti-inflammatory capacity, its ability to counteract cellular damage and the effects of sun exposure on the extracellular matrix, as well as its impact on visible signs of skin aging have been evaluated.
2. Materials and Methods
2.1. Investigational Product
The product to be evaluated is a multi-active formula with antioxidant, anti-inflammatory, and regenerative ingredients combined in the novel technology OxIR PhotoRescue Technology®, manufactured by Unikare Bioscience SL (Vitoria-Gasteiz, Spain), part of i+Med Cooperative of Scientists group, for Sibari Republic® brand and commercialized under the trademark name of Cellular Rescue.
The specific ingredients of the product, listed according to the INCI (International Nomenclature of Cosmetic Ingredients), are as follows: AQUA, GLYCERIN, ISOTRIDECYL ISONONANOATE, ETHYL OLEATE, BATYL ALCOHOL, STEARIC ACID, GOSSYPIUM HERBACEUM SEED EXTRACT, PUNICA GRANATUM SEED EXTRACT, HYLOCEREUS UNDATUS FRUIT EXTRACT, KRAMERIA TRIANDRA ROOT EXTRACT, CAPRYLIC/CAPRIC TRIGLYCERIDE, SODIUM ACRYLATES COPOLYMER, PENTYLENE GLYCOL, PHYSALIS ANGULATA EXTRACT, HYDROXYACETOPHENONE, GLYCERYL BEHENATE, HYDROGENATED POLYDECENE, TREHALOSE, 1,2-HEXANEDIOL, CAPRYLYL GLYCOL, FRUCTOSE, UREA, CITRIC ACID, SODIUM HYDROXIDE, LECITHIN, PHOSPHOLIPIDS, POLYGLYCERYL-10 STEARATE, HELIANTHUS ANNUUS SEED OIL, SODIUM HYALURONATE, HYDROGENATED PHOSPHATIDYLCHOLINE, LYSOLECITHIN, MALTOSE, SODIUM PCA, SODIUM CHLORIDE, SODIUM LACTATE, TREHALOSE, ALLANTOIN, POTASSIUM SORBATE, FRUCTOOLIGOSACCHARIDES, TOCOPHEROL, PHYTIC ACID, GLUCOSE, DAUCUS CAROTA SATIVA ROOT EXTRACT, BETA-CAROTENE.
2.2. Reagents
Ascorbic acid (A4544), hydrogen peroxide solution (H2O2, H1009), and Hank’s Balanced Salts Solution (H-2387) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). 5 (and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate (carboxy-H2DFFDA) (C-13293) was sourced from Invitrogen (Waltham, MA, USA), while PBS (10×) was obtained from Roche (11 666 789001) (Basel, Switzerland). The IL-8 Human ELISA Kit (DY208) and IL-6 Human ELISA Kit (DY206) were provided by R&D Systems (Minneapolis, MN, USA). The Collagen Assay Kit (S1000) was acquired from Biocolor (Belfast, UK), and the CytoTox 96 kit (G1780) was supplied by Promega (Madison, WI, USA). Skin culture medium without animal components with Pen/Strep was supplied by Biopredic International MIL215 (Saint-Grégoire, France).
2.3. Measurement of Antioxidant Capacity
The antioxidant activity of the testing product was determined by using the in-tube DFFDA assay. This assay measures the capacity of the testing product to block the oxidation of DFFDA by H
2O
2. Different concentrations of the product (10%, 1%, and 0.1%
v/
v, diluted in assay buffer) were incubated in a solution with 5 µM DFFDA and 4 mM H
2O
2. After 1 h, fluorescence was measured at Ex/Em = 485/535 nm. Ascorbic acid (50 mM) was used as reference material (positive control). The percentage of antioxidant activity (AA%) was calculated by comparing the fluorescence values of the control (negative control) and test samples by applying the following formula:
F 485/535 CX: fluorescence at Ex/Em = 485/535 nm of the product at a given concentration in DFFDA and H2O2 presence.
F 485/535 B − CX: fluorescence at Ex/Em = 485/535 nm of the product at a given concentration (Blank).
F 485/535 Control: fluorescence Ex/Em = 485/535 nm of the assay buffer in DFFDA and H2O2 presence (C−).
2.4. Ex Vivo Model of Photoaging
Human organotypic skin explant cultures (hOSECs) were obtained from healthy donors undergoing plastic surgery (authorization granted by the French government’s ethical committee according to French law L.1245 CSP). Up to 2 h from the surgery, the skin was cut into 0.8 cm2 pieces and shipped in transport medium. Upon receipt, samples were placed with dermis facing down and epidermis facing up in culture plates containing skin culture medium without animal components supplemented with Pen/Strep (1%). Tissue cultures were incubated for at least 48 h at 37 °C under 5% CO2 for recovery prior to study initiation.
In order to mimic skin photoaging, UV-Vis (ultraviolet-visible) light irradiation (5 J/cm
2) was applied daily to the hOSEC by using the SOL 500 Solar simulator (Dr. Hönle, Gräfelfing, Germany), based on previously published studies using similar doses and exposure times [
13,
14]. At the same time, the product was administered topically at 2 mg/cm
2. The product was in contact with the hOSEC throughout this study, for a total of 9 days. Four replicates of each experimental group (control, photoaged skin, and photoaged skin + product) were carried out.
2.5. Cytokine Quantification
The IL-6 and IL-8 cytokine quantification assays were performed in the supernatants of the skin culture. Culture supernatants were collected, clarified by centrifugation, aliquoted, and stored at −70 °C until analysis. Once thawed, cytokine levels were determined by typical sandwich ELISA assay kits according to the manufacturer’s recommendations.
2.6. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
The LDH Cytotoxicity test is a colorimetric assay that quantitatively measures lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released into the culture medium supernatant upon damage to the cytoplasmic membrane. The released LDH in culture medium supernatants can be measured by a 30 min coupled enzymatic reaction; LDH oxidizes lactate to pyruvate, which then reacts with the tetrazolium salt WST-1 to form formazan. The increase in the amount of formazan measured in the culture supernatant directly correlates to the increase in the number of damage cells in the skin explant. 50 µL of supernatant were removed from each sample and transferred into a 96-well microplate. After that, 50 µL of formazan dye was added to each sample, and, following a 30-min incubation period, absorbance was read using a standard ELISA plate reader at 490 nm.
2.7. Collagen Content Analysis
The soluble collagen content was determined using the collagen kit from Biocolor Ltd. (Carrickfergus, UK). The collagen assay is a dye-binding method for the analysis of acid and pepsin-soluble collagens. hOSECs were incubated with a solution of pepsin concentration at 0.1 mg/mL and acetic acid at 0.5 mM at 4 °C overnight. Collagen Dye Reagent (Sircol Dye Reagent) (1 mL) was added to each supernatant and shaken for 30 min. Subsequently, 750 μL ice-cold Acid-Salt Wash Reagent was added to the collagen-dye pellet to remove unbound dye from the surface of the pellet and centrifuged at 13,200× g for 10 min. Finally, 250 μL of alkali reagent was added. The dissolved dye (200 μL of each sample in 96 microwell plates) was measured using a standard ELISA plate reader at 556 nm. The reading for the samples was compared against a standard curve to obtain semi-quantitative information and made relative to mg of fresh dermal tissue.
2.8. Anti-Aging Efficacy Test Under Dermatological Control
The anti-aging efficacy of the product was evaluated in 20 volunteers with sensitive and photoaged skin for 56 days. During the study days, facial photographs and biometrical measurements were taken before the application of the product and after its continued use (images were processed using Adobe Lightroom software version 8). In addition, after 56 days of product use, the volunteers filled out the subjective evaluation questionnaire, with questions regarding the efficacy of the product. At the end of this study, the dermatologist also evaluated the tolerance of the product.
2.9. Volunteer Recruitment
Each volunteer participating in this study was previously informed about the type and procedures of the study, signing an informed consent before the start of the study. The volunteers participating in this study met the inclusion and exclusion criteria, verified through a recruitment questionnaire (
Appendix A).
2.10. Product Application Criteria
The application area was the face, and the product was applied to clean, dry skin morning and evening or after prolonged exposure to the sun by gently massaging until complete absorption. Volunteers were instructed not to use any other cosmetic products of the same type in the experimental areas during this study and not to change their usual hygiene habits.
One week before starting to use the product, the volunteers were instructed to carry out a correct pre-wash phase. This phase was performed to standardize the skin conditions of the volunteers. The conditions in the week before the examination were to avoid applying any skin care products to the experimental areas and to avoid the application of topical drugs in experimental areas.
2.11. Biometric Evaluation of Efficacy
On the measurement days, the volunteers participating in this study remained for 10 min in an acclimatized room at temperature 20 °C ± 2 °C and a relative humidity of 40–60%. The experimental area needed to be clean, without any product applied.
Moisturizing efficacy was determined by measuring the distribution of near-surface hydration and the microtopography of the skin with MoistureMap MM 100 (Courage & Khazaka electronic, Köln, Germany) the initial day (D0) and after 30 min (T30MIN), 2 h (T2H), 24 h (T24H), 28 days (T28D), and 56 days (T56D) of product use.
Efficacy in improving the barrier function was determined by measuring the water-evaporation density gradient of the skin with the Tewameter® TM 300 probe (Courage & Khazaka electronic, Köln, Germany) on the initial day (D0) and after 2 h (T2H), 24 h (T24H), 28 days (T28D), and 56 days (T56D) of product use.
Luminosity was determined by colorimetric image analysis, measuring the difference between lightness and darkness with the VisioFace® 1000D (Courage & Khazaka electronic, Köln, Germany) on the initial day (D0), at 28 days (T28D), and after 56 days (T56D) of product use.
Depigmenting efficacy was evaluated by measuring the light reflected by the skin, using the Mexameter® MX 18 probe (Courage & Khazaka electronic, Köln, Germany) on the initial day (D0), at 28 days (T28D), and after 56 days (T56D) of product use.
Firming and elasticity efficacy were determined by measuring the mechanical deformation of the skin with the Cutometer® dual MPA 580 probe (Courage & Khazaka electronic, Köln, Germany) on the initial day (D0) and at 30 min (T30MIN), 2 h (T2H), 24 h (T24H), and 28 days (T28D) of product use.
Anti-wrinkle efficacy was determined by measuring volume and density of wrinkles using AEVA3D-HE2 VisioHop (Eotech, MI, USA) on the initial day (D0), at 28 days (T28D), and after 56 days (T56D) of product use.
The time points selected were chosen based on their common use in evaluating the parameters described in our study and are typically employed in efficacy studies of similar products [
13,
14,
15].
Extended information on biometric measurements can be found in
Appendix B.
2.12. Dermatological Efficacy Evaluation
At the initial visit and after 28 days and 56 days of product application, the dermatologist evaluated the severity of volunteers’ wrinkles from two facial regions: forehead and frown lines. Wrinkles were classified using a scale from 0 to 5, being: 0 none, 1 noticeable, 2 shallow, 3 moderately deep, 4 deep with well-defined borders, and 5 very deep, redundant fold.
2.13. Subjective Evaluation
The volunteers, on their visit to the center at the end, filled out a questionnaire answering questions related to the efficacy (
Appendix C).
2.14. Tolerance Evaluation
At the end of this study, the dermatologist assessed the skin tolerance of the product. The alterations to be evaluated were erythema, xerosis/desquamation, edema, exudation, comedogenicity, and pigmentation alterations on a four-point scale (absence, slight/moderate/intense). It was also indicated whether the changes observed could be related to the use of the product, according to the scale: not related, improbable, possible, probable, certain, or not valuable.
2.15. Data Presentation
Data from in vivo experiments are presented in tables as the average percentage of variation from initial day (T0) across all volunteers (% variation from T0) and the percentage of volunteers showing improvement (% responders) at the indicated times: 30 min (T30MIN), 2 h (T2H), 24 h (T24H), 28 days (T28D), and/or 56 days (T56D) of product use.
2.16. Statistical Analysis
In the in vitro and ex vivo assays, values are given as mean ± SEM. The homogeneity of variance was confirmed by the Bartlett’s test, and the normality was confirmed by the Anderson–Darling test. One-factor analysis of variance (ANOVA) with Fisher’s LSD post-hoc tests were performed to assess differences between groups’ means. When homogeneity of variances could not be assumed, Welch’s correction was used, and when parametric assumptions were not met, Kruskal–Wallis with Dunn’s post-hoc test was used.
For in vivo study, a normality analysis (Shapiro–Wilk) and equal variance test (Brown–Forsythe) were conducted to determine the data distribution and choose the type of analysis: non-parametric tests (Kruskal–Wallis) or parametric tests (one-way repeated measures ANOVA + Holm–Sidak or Student–Newman–Keuls post hoc test). Asterisks indicate a statistically significant difference (* p < 0.05; ** p < 0.01; *** p < 0.001) compared to T0.
3. Results
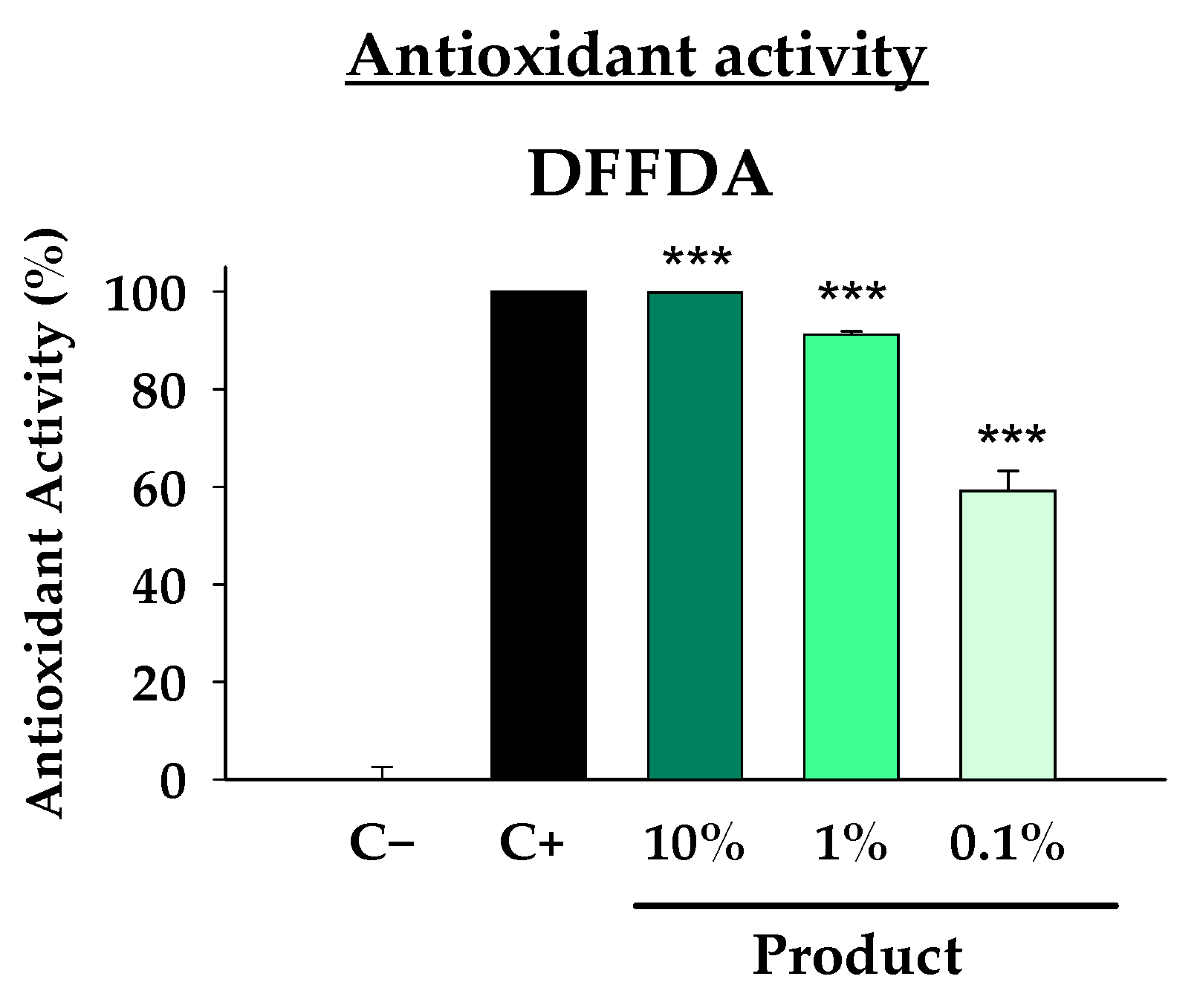
Firstly, given that oxidative stress is considered one of the central mechanisms of photodamage due to its direct and indirect effects on cells and tissues, in vitro studies were conducted to evaluate the antioxidant capacity of the formula. The DFFDA assay, which measures the capacity of the product to block the oxidation of DFFDA by H
2O
2, revealed that even at a 0.1% concentration, the product inhibited 60% of DFFDA oxidation (
Figure 1). This antioxidant capacity increased to over 90% at a 1% concentration, and a complete inhibition (100%) was observed at a 10% concentration, comparable to the antioxidant potential of the positive control. The results were statistically significant for all the analyzed doses. These results confirm the strong antioxidant potential of the formula.
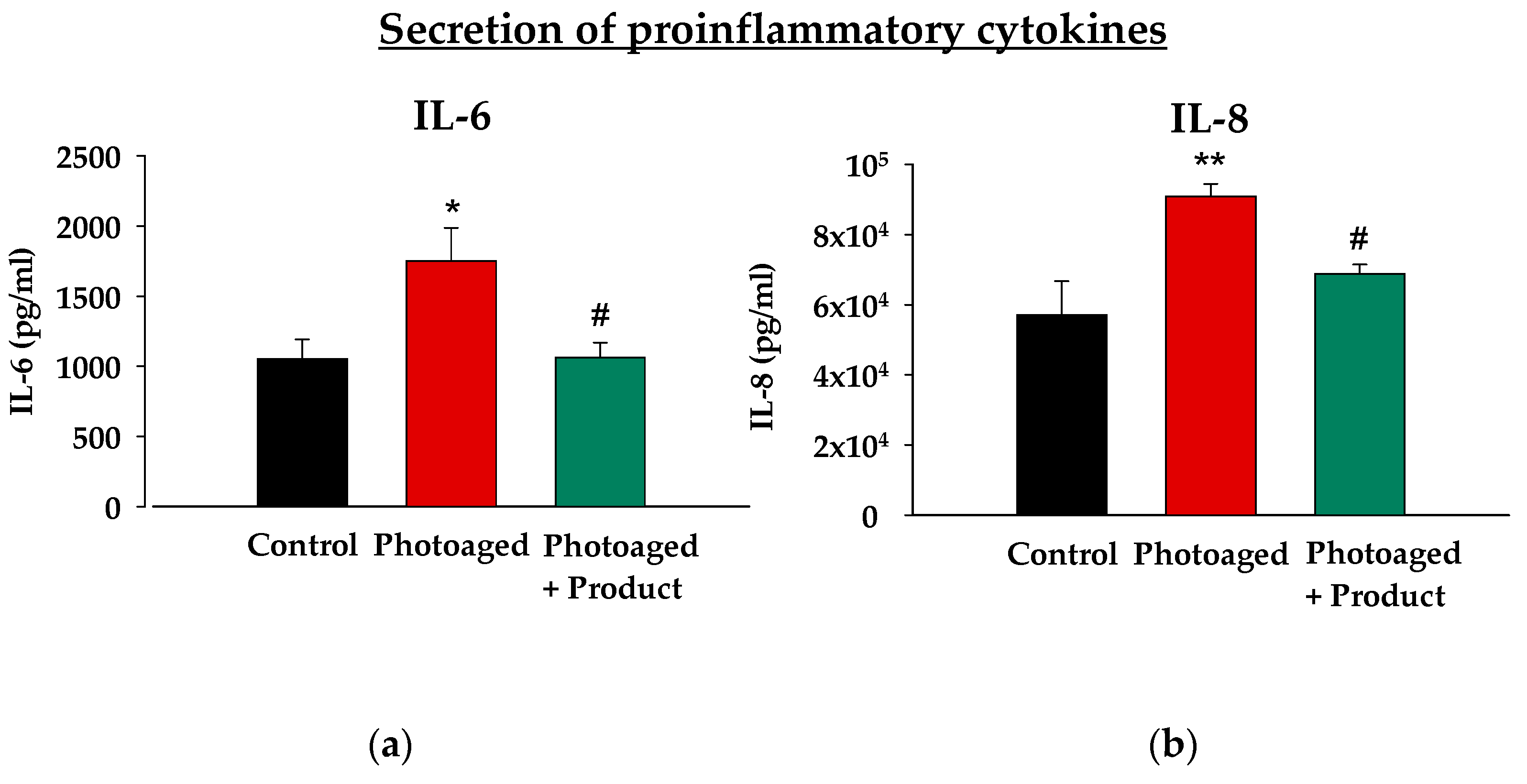
In addition, solar radiation can both directly and through generating oxidative stress create a pro-inflammatory environment that exacerbates tissue damage. Therefore, it is crucial for a reparative product to effectively reduce the levels of inflammatory mediators. For this reason, we evaluated the anti-inflammatory capacity of the product, using in this case an ex vivo model of photodamage. In this model, human skin explants (hOSECs) were exposed to UV-Vis (ultraviolet-visible) irradiation mimicking the harmful effects of sun exposure, and levels of two key pro-inflammatory cytokines, IL-6 and IL-8, commonly elevated in inflammatory conditions, were measured. As shown in
Figure 2, UV-Vis irradiation led to a statistically significant increase in the secretion of both IL-6 and IL-8 compared to the control, non-irradiated group. However, the topical application of the product on hOSECs resulted in an also statistically significant reduction in the levels of these cytokines compared to the photoaged group, reducing them to levels comparable to those of non-irradiated tissue. This result demonstrates the potent anti-inflammatory effect of the product, highlighting its potential to alleviate the inflammatory responses associated with photodamage and aging.
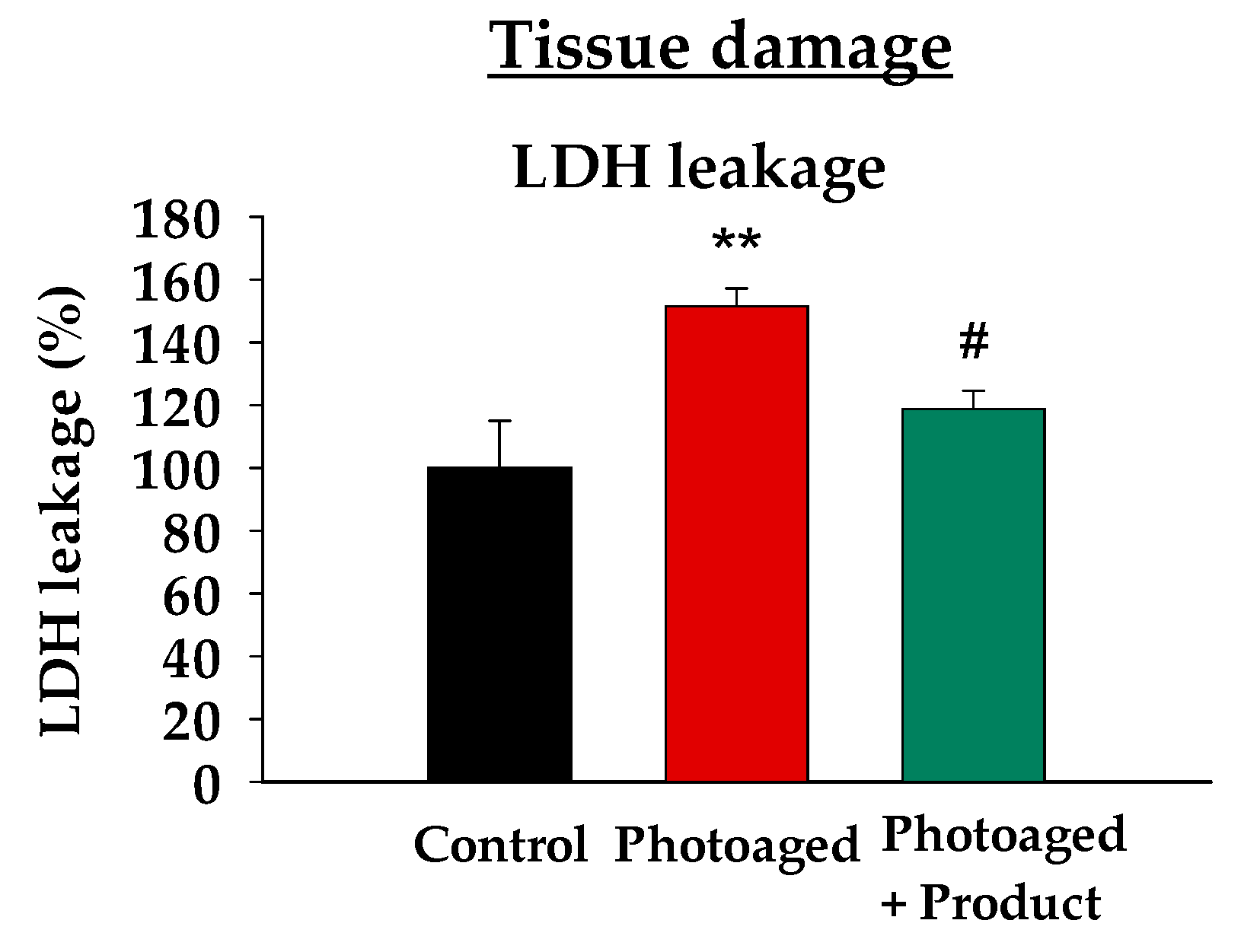
Following the previous observation of the product’s anti-inflammatory effects, we decided to study whether the product could also protect cells from damage caused by sun irradiation. To achieve this, we analyzed lactate dehydrogenase (LDH) leakage in the three experimental groups within the photoaged model. The LDH cytotoxicity test is a colorimetric assay that quantitatively measures lactate dehydrogenase, a stable cytosolic enzyme released into the culture medium upon cytoplasmic membrane damage. This assay is widely used as an indicator of cell viability in cytotoxicity studies. As expected, UV-Vis irradiation led to a 50% increase in LDH leakage compared to the non-irradiated group (statistically significant), indicating higher plasma membrane damage (
Figure 3). Remarkably, the topical application of the product reduced LDH leakage to levels comparable to those of the non-irradiated group, demonstrating its protective effect against UV-induced damage. This reduction was statistically significant compared to the photoaged condition.
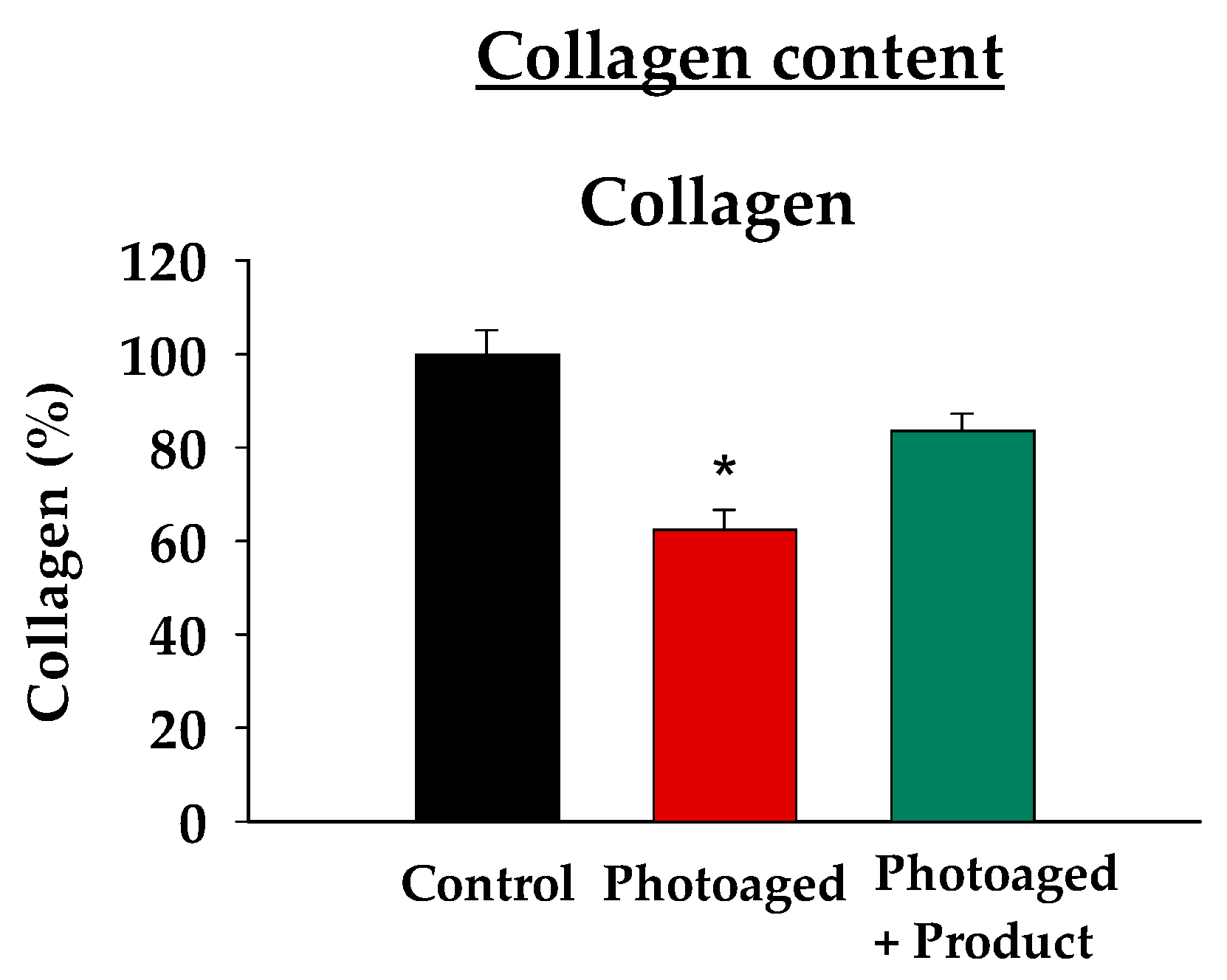
To assess if the observed improvements in cellular damage and inflammation led to better tissue function and extracellular matrix recovery, we measured tissue collagen levels. Using a dye-binding method that analyzes acid and pepsin-soluble collagens, which include the predominant types I and III that are crucial for maintaining dermal tissue structure and integrity, we found that, as expected, UV-Vis irradiated (Photoaged) skin explants exhibited a statistically significant reduction in soluble collagen content compared to the non-irradiated controls (
Figure 4). Remarkably, topical administration of the product was able to restore collagen levels to 80% of the control, indicating a recovery of 50% of the UV-induced loss. In fact, under the product treatment condition, collagen levels were no longer significantly different from the control group, suggesting that the product effectively restores the collagen loss observed in the absence of its application. These results demonstrate the product’s effectiveness in promoting collagen preservation and maintaining matrix integrity.
The model was found to be valid, as it effectively reproduced UV-Vis radiation-induced damage such as cellular damage, secretion of inflammatory molecules, and reduction in collagen levels. However, to further test the global anti-photoaging effect, we proceeded to perform a clinical evaluation of the efficacy in a group of volunteers.
A total of 20 volunteers with sensitive and photoaged skin participated in this study, and the product was used for 56 days. Anti-aging efficacy was assessed through biometric evaluations conducted by a dermatology specialist. Parameters such as hydration, barrier function, pigmentation, luminosity, firmness, elasticity, and wrinkles were measured at baseline, after 28 days, and at the end of the 56-day treatment period. At the conclusion of the trial, product tolerance and subjective efficacy through a participant questionnaire were also evaluated.
Regarding the tolerance evaluation, a dermatologist examined the possible alterations present in the volunteers in the experimental area: erythema, xerosis/desquamation, exudation, edema, comedogenicity, pigmentary alterations, and others. No volunteers presented adverse effects after continued use of the product.
The instrumental assessment of efficacy focused on several key parameters: moisturization, barrier function, luminosity, pigmentation, firmness, elasticity, and wrinkle severity.
Moisturizing efficacy was assessed by measuring near-surface hydration distribution and skin microtopography at 30 min, 2 h, and 24 h to evaluate immediate effects, as well as at 28 and 56 days to determine long-term benefits. Results, shown in
Table 1, indicated a 47% increase in hydration within the first 30 min, which remained high at 2 h (42%) and decreased only slightly at 24 h (26%). Improvements were sustained over time, with a 20% increase in hydration at 28 days, with 75% of volunteers showing improvement, and a 31% increase at 56 days, also with 75% of volunteers experiencing better hydration. The differences were statistically significant in all cases. This demonstrates a strong short-term and long-lasting moisturizing effect of the product. Barrier function was evaluated by measuring the water-evaporation density gradient of the skin. At 2 h post-application, a slight reduction (5%) in transepidermal water loss (TEWL) was observed, becoming more pronounced at 24 h (9%). Long-term assessments showed an 11% reduction at 28 days and a 20% reduction at 56 days, with 74% of volunteers demonstrating improvement at both time points, indicating a notable enhancement of the epidermal barrier function, especially after prolonged use (
Table 1).
Luminosity was measured using colorimetric image analysis, assessing the difference between lightness and darkness. The results showed that the average improvement among the volunteers that exhibited an enhanced luminosity was 59% and 69% for 28 and 56 days, respectively (
Table 2). On the other hand, the ability of the product to reduce existing pigmentation was evaluated by measuring the light reflected by the skin both in spot and control areas. At 28 and 56 days, 67% and 61% of volunteers showed a reduction in melanin content in spot areas, with statistically significant average reductions of 9% and 14% (negative results in the table indicate a reduction in melanin content) and maximum reductions of up to 19% and 53%, respectively (
Table 2).
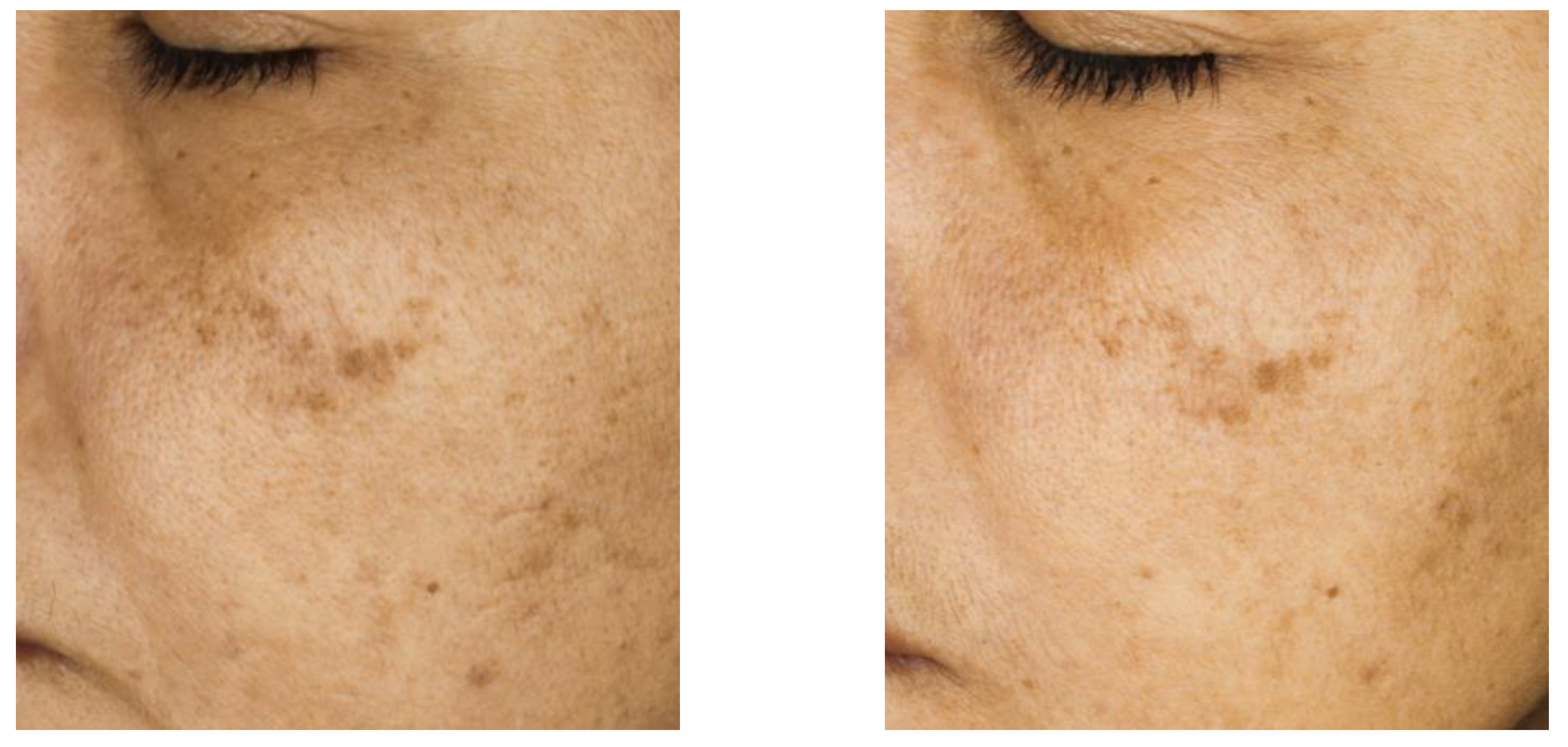
Figure 5 presents a representative image of one of the volunteers, highlighting a visible reduction in pigmentation of the larger spots, along with a diminished appearance of the smaller ones. Importantly, melanin content in control areas was not decreased (
Supplementary Information, Table S1), indicating that the product does not reduce basal skin tone. Thus, these results evidence the product’s ability to even out the skin tone, improving the uniformity and appearance of the skin.
Firming and elasticity efficacy were also analyzed, in this case by measuring the mechanical deformation of the skin under suction. Skin firmness was evaluated based on resistance to deformation, while elasticity was assessed by the skin’s ability to return to its original position. Firmness improved significantly within 30 min of product application (24%), with a further increase observed at 2 h (30%). Although there was a slight reduction at 24 h (14%), long-term treatment demonstrated renewed improvement, with a 21% increase at 28 days (
Table 3). Elasticity followed a similar pattern, showing a 24% improvement at 30 min, rising to 31% at 2 h, and slightly decreasing to 16% at 24 h before reaching 28% at 28 days. Notably, up to 88% of volunteers reported enhanced firmness and elasticity. All time points assessed showed statistically significant differences in both parameters.
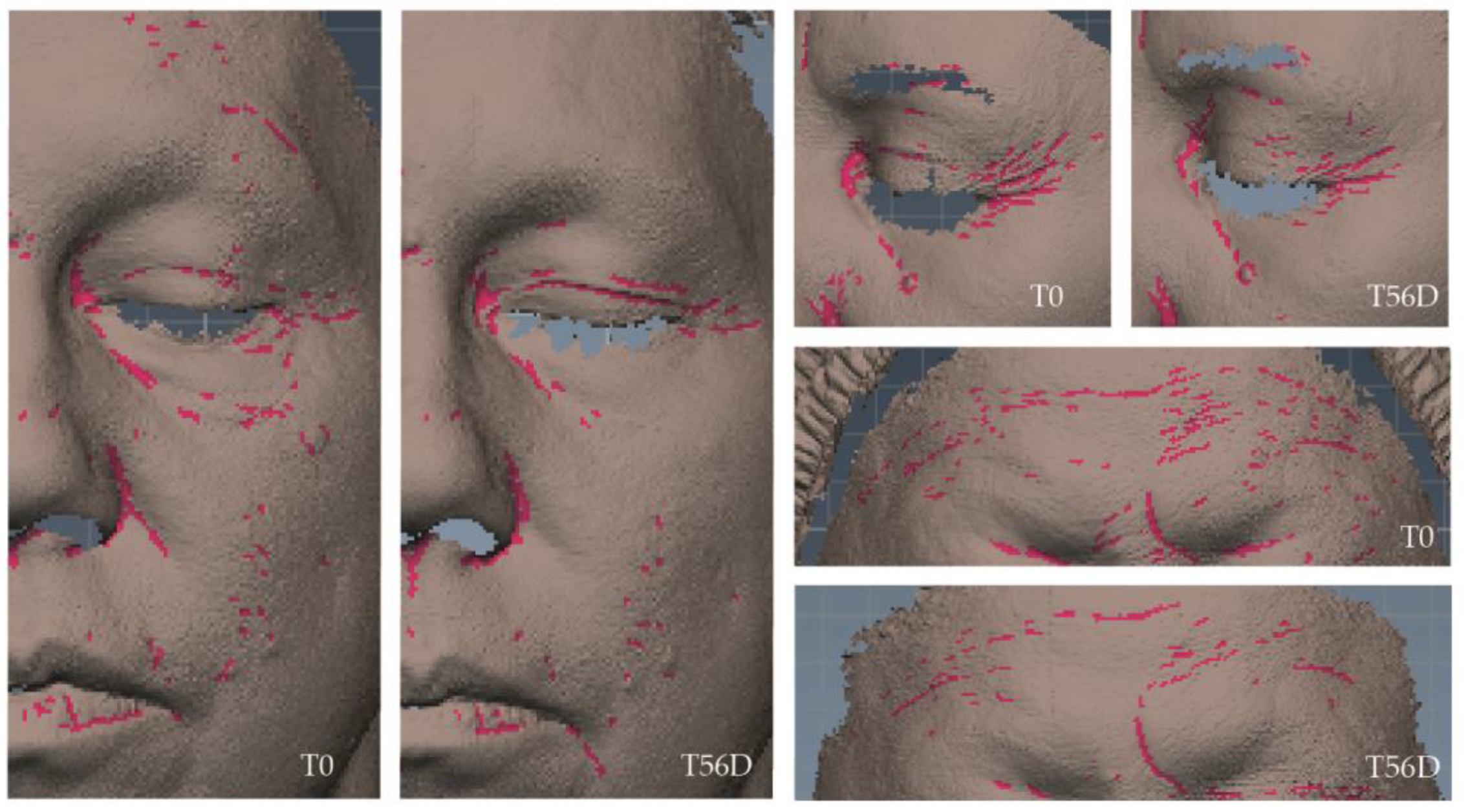
Wrinkle-improvement efficacy was assessed using both instrumental and dermatological evaluations. The instrumental assessment was conducted by a three-dimensional scanning sensor that measures skin topography based on fringe-projection technology combined with active stereometry, and both wrinkle volume and density were evaluated (
Table 4). Wrinkle volume decreased in 44% of volunteers after 28 days, with an average reduction of 27% among those showing improvement and maximum reductions reaching 40%. By day 56, 56% of volunteers exhibited positive changes, with an average reduction in volume of 29% and maximum reductions of 53%. Regarding wrinkle density, 47% and 53% of volunteers showed improvements at 28 and 56 days, respectively, with an average reduction of 8% but reaching maximum reductions of up to 19% in both cases (
Table 4). Variation percentages were statistically significant for all cases. Representative images of the three-dimensional scanning sensor AEVA-HE showing wrinkle density can be found in
Figure 6.
In parallel, a dermatological assessment was conducted by a dermatologist who evaluated wrinkles on the forehead and frown lines, using a scoring scale from 0 (no wrinkles) to 5 (very deep, redundant folds). The evaluation revealed that wrinkles were generally classified as less severe after product use (
Figure 7). In particular, forehead wrinkles rated at the highest levels showed a 10% reduction at both 28 and 56 days, and frown lines exhibited a 10% reduction at 28 days and a 5% reduction at 56 days.
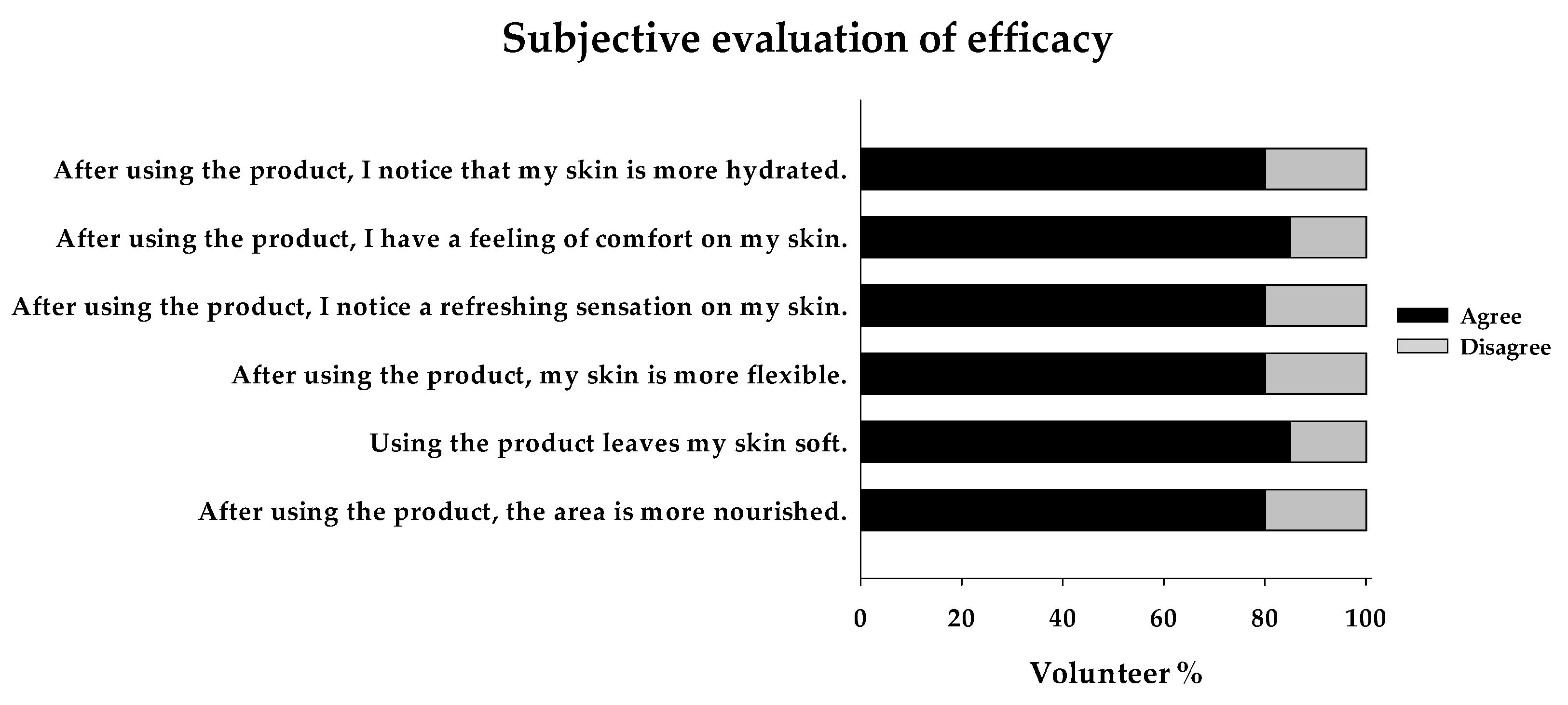
Lastly, the subjective evaluation revealed that at least 80% of the volunteers felt their skin was significantly more hydrated, nourished, soft, flexible, refreshed, and comfortable after using the product (
Figure 8).
In conclusion, both instrumental indicators and volunteer perceptions confirm that this product, due to its potent antioxidant, anti-inflammatory, protective, and moisturizing properties, effectively repairs sun-damaged skin, improving its appearance and functionality.
4. Discussion
The present work demonstrates, through a series of in vitro, ex vivo, and in vivo studies, that we have achieved a groundbreaking advancement in skincare with the development of a single formulation capable of addressing photodamage at multiple levels. By effectively counteracting key mechanisms such as oxidative stress, inflammation, and extracellular matrix degradation, our product provides a comprehensive solution to the complex challenges of photoaging. This includes restoring collagen levels, improving hydration, repairing the skin barrier, enhancing elasticity and firmness, reducing wrinkles, and correcting uneven skin tone. The results highlight the formulation’s ability to not only enhance the skin’s appearance but also restore its functionality, offering an advanced approach to the repair of sun-induced damage.
This remarkable achievement is the result of our specific approach that combines a deep understanding of the mechanisms underlying photodamage with the precise selection of scientifically validated ingredients and their integration using our advanced OxIR PhotoRescue Technology®.
Among these mechanisms, oxidative stress plays a critical role in photoaging. Since the skin’s natural antioxidants are insufficient to fully counteract the effects of sun exposure [
16], the addition of antioxidants to skincare formulations has proven to be an effective strategy in mitigating photoinduced damage [
17]. However, the challenge with using antioxidants in topical formulations lies in their low stability and the need for effective delivery into the deeper skin layers to be beneficial. Our product addresses these challenges by incorporating a delivery system that stabilizes and delivers beta-carotene into deep epidermal layers. Beta-carotene is the most abundant and efficient precursor of Vitamin A, also called Pro-Retinol, a natural sun protector and powerful antioxidant [
18,
19]. Experimental studies have consistently demonstrated its protective effects against UV-induced photodamage [
20], with topical applications of beta-carotene providing protective against acute and chronic manifestations of photodamage, including sunburn, premature aging, actinic keratosis, and other skin conditions [
21].
Another key active ingredient in our formulation is
Hylocereus undatus fruit extract, which offers not only hydrating, mineralizing, and vitaminizing properties but also has potent antioxidant activity due to its polyphenolic compounds, pigments, flavonoids, and vitamins B, C, and E [
22,
23]. These components have demonstrated strong free-radical scavenging activity in various studies related to photoaging [
24,
25,
26]. The inclusion of all these ingredients has yielded a product with powerful antioxidant activity, as evidenced by our data.
Our results also indicate that the product exerts a potent anti-inflammatory effect on radiation-induced damage due to the inclusion of strong anti-inflammatory agents such as
Physalis angulata extract, which has demonstrated efficacy as a topical anti-inflammatory and wound-healing agent [
27,
28]. Actually,
Physalis angulata extract exhibited corticosteroid-like effects comparable to hydrocortisone in a randomized double-blind placebo-controlled clinical trial [
29].
Additionally, our formulation includes a lipid complex composed primarily of batyl alcohol and soy lecithin, which possess anti-inflammatory and moisturizing properties. Batyl alcohol is known for its anti-inflammatory and DNA-protecting properties, while it has also demonstrated significant reparative effects on the epidermal barrier [
30,
31]. Soy lecithin, a phospholipid, is highly valued in cosmetic formulations due to its role as a key component of cell membranes, which gives it a natural affinity with the skin. This strong biocompatibility and biomimetic properties enhance the bioavailability of active ingredients, leading to more effective results [
32]. Additionally, soy lecithin has been found to improve skin hydration, reduce irritation, and promote wound healing [
33,
34]. The inclusion of
Krameria triandra root extract, rich in catechin tannins and other components, further contributes to the anti-inflammatory properties of the formulation [
35].
As previously mentioned, sun radiation-induced oxidative stress and inflammation lead to cellular damage, induction of senescence, and the degradation of the extracellular matrix. Given the potent ability of our product to mitigate these harmful processes, we could expect a protective effect on cellular and tissue levels in a photodamage model. Indeed, our studies demonstrate that the application of the product significantly reduced cellular damage, while it preserved collagen levels within the tissue.
This protective effect was not only observed in ex vivo models but was also confirmed through instrumental measurements in an in vivo study involving volunteers who applied the product for 56 days. The results demonstrated significant improvements in skin firmness as well as in elasticity, suggesting that the product also protects against degradation and modification of other extracellular matrix components, such as elastin. This beneficial impact on the extracellular matrix explains the reductions in wrinkle density and volume observed in the volunteers. This reduction levels, although they may seem subtle in terms of average numerical improvement, are of considerable significance, since the results are comparable to those obtained with collagen-based products. However, while these products improve signs of aging by providing an external source of collagen, our product is able to act on pre-existing wrinkles by stimulating endogenous extracellular matrix production, thereby facilitating the recovery of collagen levels within the skin.
Another hallmark of photoaging is the loss of hydration due to impaired barrier function and the degradation of natural moisturizing factors caused by sun damage. To restore hydration and maintain the skin’s structural integrity and protective function, potent hydrating and nourishing agents were included in our formulation.
Hyaluronic acid (HA), a naturally occurring polysaccharide in the skin, plays a crucial role in maintaining dermal and epidermal hydration due to its hygroscopic nature. Beyond providing hydration, HA is crucial for skin integrity as it influences cell-to-cell and cell-to-matrix interactions [
36] and regulates keratinocyte differentiation and the formation of extracellular lipids in the stratum corneum, which are essential for the integrity and functionality of the epidermal barrier [
37], thereby contributing to skin resilience and promoting regeneration [
38]. Due to its unique viscoelasticity, biocompatibility, biodegradability, and non-immunogenicity properties, HA has been extensively used in dermal applications for skin rejuvenation [
39], among multiple other applications [
40]. To date, numerous clinical studies have demonstrated the effectiveness and tolerability of HA [
39,
41]. In cosmetics, the trend has increasingly favored the use of sodium hyaluronate, the salt form of hyaluronic acid, due to its enhanced water solubility and stability, which allows for deeper skin penetration. In addition, it is especially suitable for sensitive and, hence, photodamaged skin.
Another potent hydrating component of the formulation is a complex blend of natural sugars, natural moisturizing factors (NMFs), and physiomoisturizers. This mixture has been specifically designed to deliver both immediate and long-lasting hydration to the skin, acting through a dual mechanism; hygroscopic molecules restore moisture to the skin layers, while filmogenic polymers form a protective barrier to prevent water evaporation [
42]. These sugars have low molecular weights, allowing them to penetrate the skin effectively and restore hydration. Together, these components help to maintain the skin’s moisture balance, improving its overall resilience and comfort.
The formula also contains a component with phospholipids, which support the regeneration of the skin barrier, thereby reducing transepidermal water loss. Additionally, it enhances the anti-inflammatory and antioxidant effects of the formula due to its content of
Helianthus annuus (sunflower) seed oil, rich in vitamin E [
43,
44]. This combination of agents is intended to reinforce skin’s protective barrier while improving hydration and provide added protection against oxidative stress and inflammation. Indeed, our TEWL evaluation results reveal that the product significantly repairs the skin’s barrier function, restoring its ability to protect against external aggressors and maintaining adequate hydration levels. Coupled with the potent moisturizing agents included in the formulation, the product demonstrates both immediate and long-lasting hydration benefits, which not only improves the skin’s appearance but also facilitates better absorption of active ingredients, thereby increasing the overall efficacy of the product.
The product also enhanced facial luminosity and reduced dark spot pigmentation without altering the natural skin tone, thereby promoting a more even skin appearance. This effect can be attributed to an improved cellular function of melanocytes, as well as to the properties of additional active ingredients in the formulation, which reduce excessive melanogenesis and promote the production of protective molecules by the skin microbiota [
45,
46,
47,
48,
49]. Due to recent evidence highlighting the importance of the sun–microbiota–skin axis, incorporating a product that supports and enhances the skin microbiota is crucial for enhancing the effectiveness of a reparative cream. The skin microbiota plays a key role in protecting against solar radiation by producing beneficial compounds, named as solar postbiotics, which contribute to UV defense, reduce inflammation, and help maintain overall skin homeostasis. However, prolonged sun exposure can disrupt this protective function, leading to increased oxidative stress and inflammation, which accelerates photoaging. By incorporating ingredients that protect and maintain healthy skin microbiota, the product not only reinforces the skin’s natural defenses but also mitigates the harmful effects of solar exposure, thus offering a complete protection and repair. In particular, this formulation includes
Gossypium herbaceum seed extract, a cellular lysate rich in protective factors consisting on a mix of plant chromophores, polyphenolic antioxidants, anti-inflammatory agents, photoprotective compounds [
50],
Punica granatum seed extract, rich in selective membrane lipids and prebiotic polyphenols with anti-inflammatory and antioxidant properties [
51,
52,
53], and other plant-derived components such as fructooligosaccharides and trehalose. These ingredients enhance the resistance of microbial cell membranes to solar radiation, thereby preventing the formation of harmful molecules produced by prolonged sun exposure while they promote the secretion of postbiotic solar molecules, which act as natural photoprotective agents. As a result, the product restores microbiota balance and, consequently, supports overall skin homeostasis.
While studies on the individual active ingredients explain why the product is effective in addressing multiple aspects of photoaging, it is also important to highlight that our product has delivered better results than similar products. We have found several studies assessing the efficacy of different products. However, these are often less comprehensive, typically targeting specific aspects of photoaging separately. Furthermore, many of these studies demonstrate effects using cellular systems, with far fewer utilizing tissue-based explants, and even fewer conducting clinical trials. Among the studies we reviewed, one investigating the effect of natural retinol analogs [
13] reported a 5.6% increase in firmness, a 14% improvement in elasticity, and a 3.8% reduction in wrinkle volume after 28 days, results that fall significantly short of those achieved with our product. Another study on a biorevitalizing facial serum [
15] demonstrated improvements in elasticity (10%) and hydration (13%) after 28 days, but, once again, these results are notably less substantial compared to ours. Even a product claiming to repair sun damage [
14] showed only a 14.1% improvement in firmness, which is still much lower than the results achieved with our product. Based on these findings, we can confidently assert that our carefully designed strategy, involving the meticulous selection and combination of active ingredients, has proven to be highly effective, resulting in a product that delivers remarkable improvements across multiple parameters of photoaging.
We believe it is crucial to emphasize that, while this product also offers protective benefits, its primary strength lies in its ability to repair the damage caused by cumulative sun exposure. For many years, the focus on UV protection, while essential, has overlooked the harm already caused on the skin, which retains a ‘memory’ of past exposure with long-lasting effects on its structure and function. This damage is not merely an aesthetic concern but also a matter of long-term skin health. As such, the development of products capable of repairing existing damage is essential, and our product stands out for addressing both the functional and aesthetic dimensions of photoaging, providing protection while effectively restoring the skin’s integrity.
While our findings are robust, certain limitations should be considered. First, the in vivo study included a limited number of volunteers, which may affect the statistical robustness of the results. Expanding the sample size in future studies will improve the generalizability of these findings. Second, while significant improvements were observed after 56 days, long-term studies would provide additional insights into the product’s sustained effects on deeper dermal remodeling and pigmentation normalization. Furthermore, while our studies focused on key processes relevant to photodamage, additional mechanisms or alternative techniques could have been explored to provide a more comprehensive understanding of the formulation’s effects.