Clinical Efficacy of Endocannabinoid-Mimetic Fatty Acid Amide as a Skin-Soothing Ingredient
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymatic Synthesis of Endocannabimimetics
2.2. In Vitro Cell Studies
2.3. Measurement of Cytokines
2.4. Ex Vivo Studies
2.5. Immunohistochemical Analysis
2.6. Clinical Efficacy Study
2.7. Protein Analysis
2.8. Statistical Analysis
3. Results
3.1. Enzymatic Synthesis of Fatty Amides from Sunflower Oil
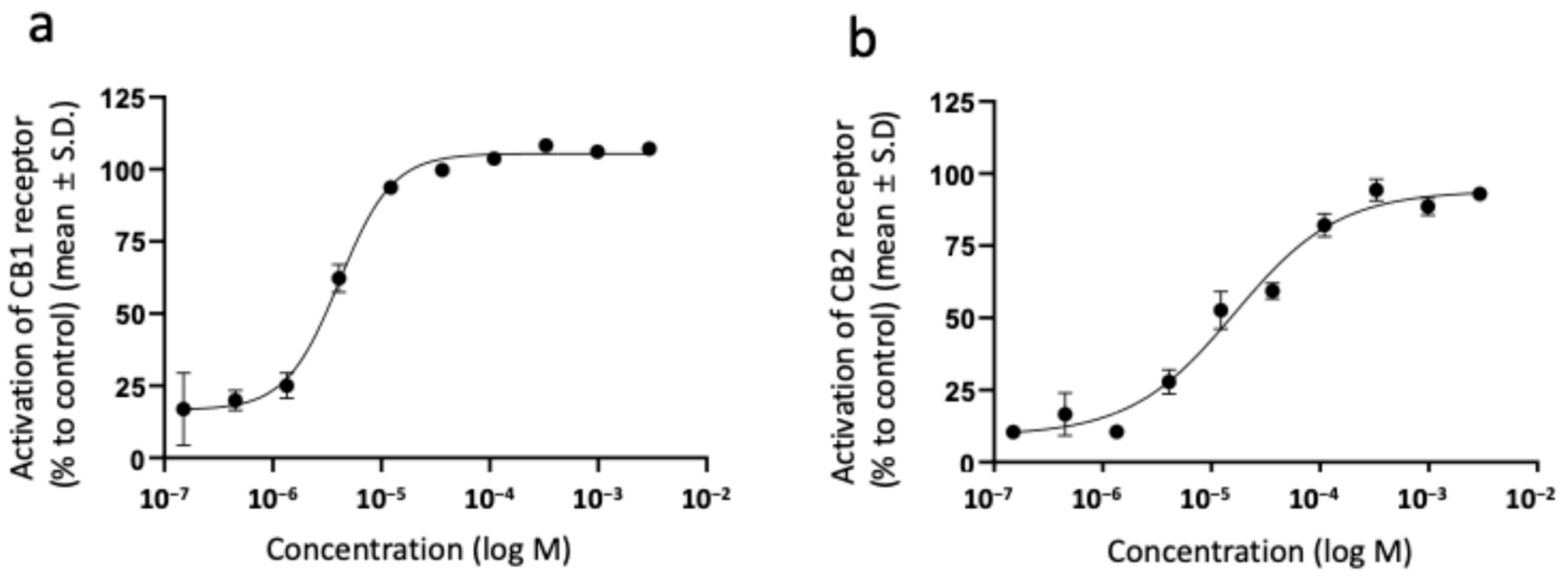
3.2. Activation of Cannabinoid Receptors by Sunflower Oil-Derived Endocannabimimetic Fatty Acid Amides
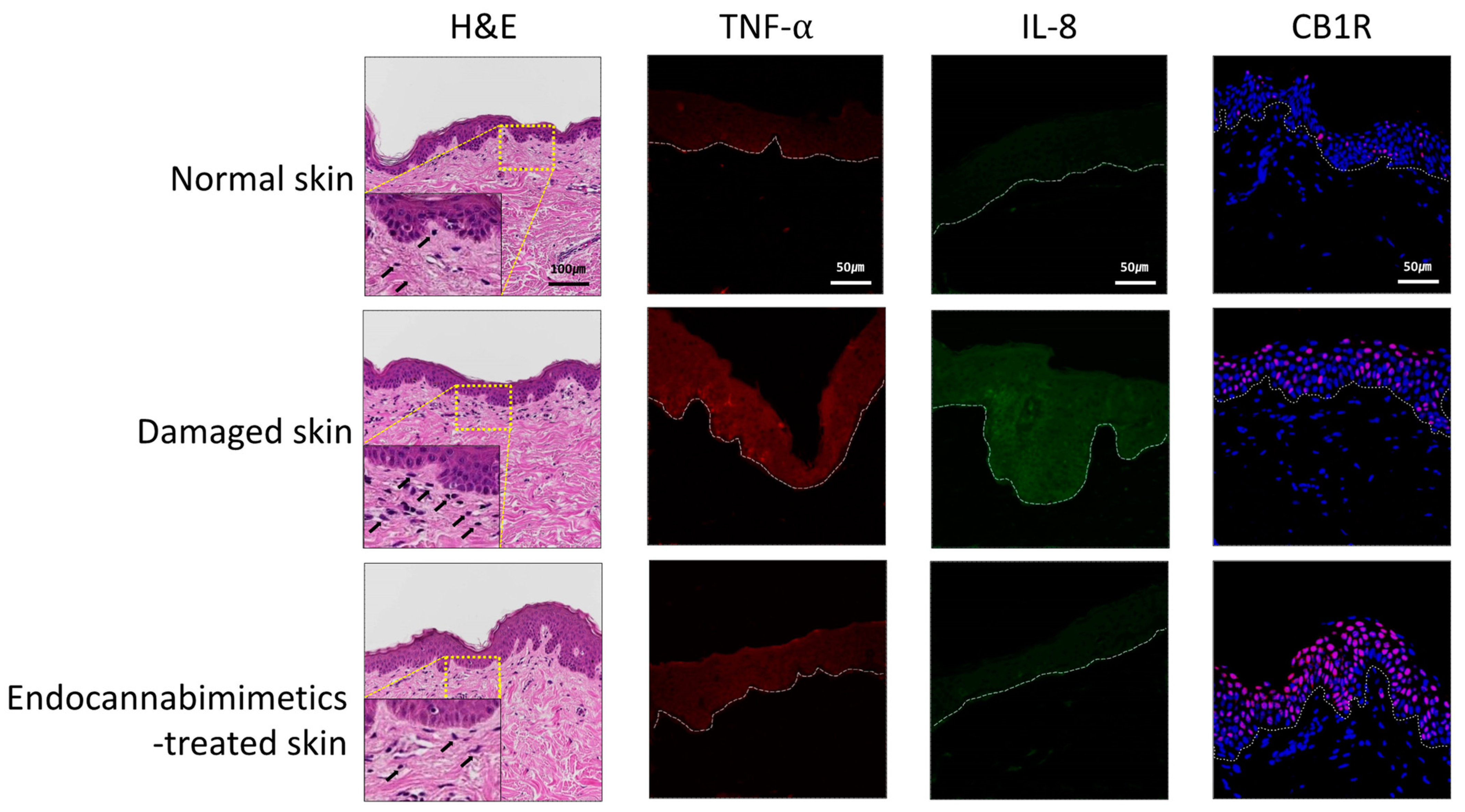
3.3. Anti-Inflammatory and Skin-Soothing Evaluation
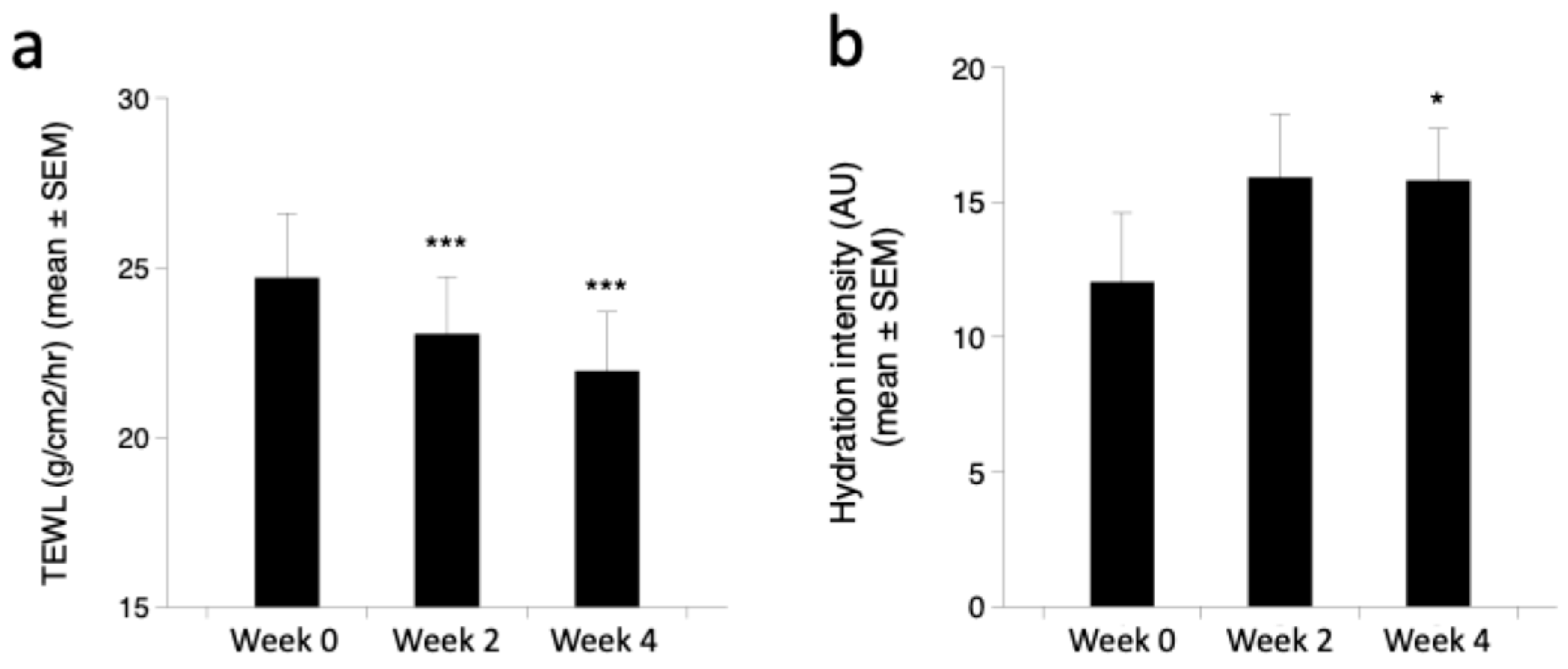
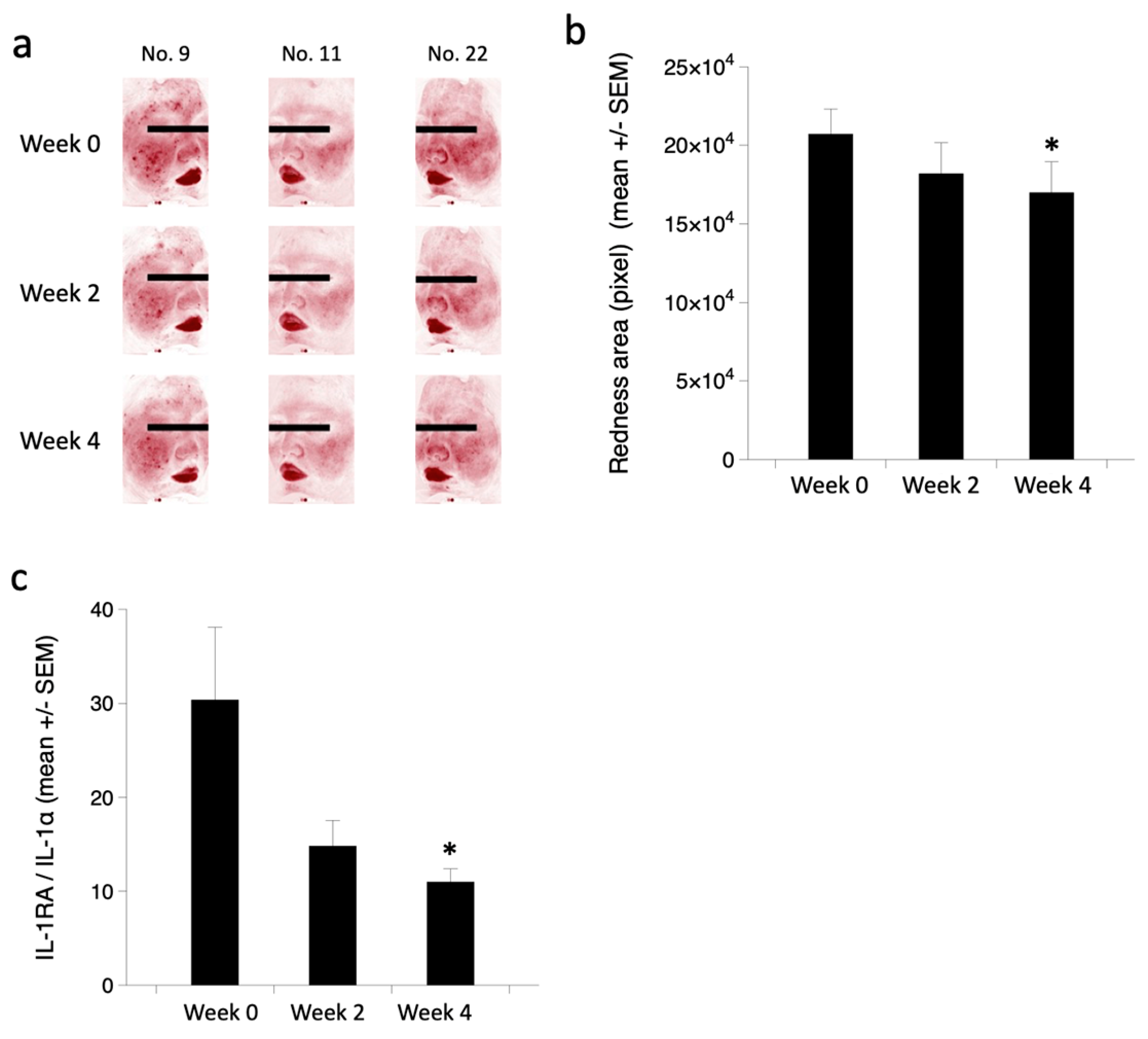
3.4. Clinical Efficacy of Endocannabimimetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosović, E.; Sýkora, D.; Kuchař, M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics 2021, 13, 412. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The Potential Role of Cannabinoids in Dermatology. J. Dermatol. Treat. 2019, 31, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Bojanowski, K.; Singh, P.; Randhawa, M.; Chaudhuri, R.K. Bakuchiol and Ethyl (Linoleate/Oleate) Synergistically Modulate Endocannabinoid Tone in Keratinocytes and Repress Inflammatory Pathway mRNAs. JID Innov. 2023, 3, 100178. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.; Alencar, A.K.N.; De Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Characteristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agrò, A.; Breton, L.; et al. Endocannabinoids Stimulate Human Melanogenesis via Type-1 Cannabinoid Receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef]
- Tóth, B.I.; Dobrosi, N.; Dajnoki, A.; Czifra, G.; Oláh, A.; Szöllősi, A.G.; Juhász, I.; Sugawara, K.; Paus, R.; Bíró, T. Endocannabinoids Modulate Human Epidermal Keratinocyte Proliferation and Survival via the Sequential Engagement of Cannabinoid Receptor-1 and Transient Receptor Potential Vanilloid-1. J. Investig. Dermatol. 2011, 131, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Zákány, N.; Oláh, A.; Markovics, A.; Takács, E.; Aranyász, A.; Nicolussi, S.; Piscitelli, F.; Allarà, M.; Pór, Á.; Kovács, I.; et al. Endocannabinoid Tone Regulates Human Sebocyte Biology. J. Investig. Dermatol. 2018, 138, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Rapino, C.; Talamonti, E.; Leuti, A.; Lanuti, M.; Gueniche, A.; Jourdain, R.; Breton, L.; Maccarrone, M. Anandamide Suppresses Proinflammatory T Cell Responses In Vitro through Type-1 Cannabinoid Receptor–Mediated mTOR Inhibition in Human Keratinocytes. J. Immunol. 2016, 197, 3545–3553. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, M.; Lee, S.; Park, B. Epidermal Endocannabinoid System (EES) and Its Cosmetic Application. Cosmetics 2019, 6, 33. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Liu, Y.; Xu, Y.; Yang, B.; Li, H.; Chen, L. An Overview on Synthetic and Biological Activities of Cannabidiol (CBD) and Its Derivatives. Bioorganic Chem. 2023, 140, 106810. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhou, Y.-Q.; Yu, Z.-P.; Zhang, X.-Q.; Shi, J.; Shen, H.-W. Restoring Glutamate Homeostasis in the Nucleus Accumbens via Endocannabinoid-Mimetic Drug Prevents Relapse to Cocaine Seeking Behavior in Rats. Neuropsychopharmacology 2021, 46, 970–981. [Google Scholar] [CrossRef]
- Ko, H.; Kim, M.-J.; Kim, H.-J.; Kang, J.; Lee, H.-Y.; Lee, J.H.; Bae, J.-H.; Sung, B.H.; Sohn, J.-H. Efficient Valorization of Food Waste Oils to Renewable Biodiesel by a Candida Antarctica Lipase B Mutant That Catalyzes the Ester Synthesis Reaction in the Presence of Water. J. Clean. Prod. 2023, 428, 139336. [Google Scholar] [CrossRef]
- Terui, T.; Hirao, T.; Sato, Y.; Uesugi, T.; Honda, M.; Iguchi, M.; Matsumura, N.; Kudoh, K.; Aiba, S.; Tagami, H. An Increased Ratio of Interleukin-1 Receptor Antagonist to Interleukin-1? In Inflammatory Skin Diseases. Exp. Dermatol. 1998, 7, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Talukder, M. Cannabinoids for Skin Diseases and Hair Regrowth. J. Cosmet. Dermatol. 2021, 20, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, B.; Vadalà, M. A Therapeutic Effect of Cbd-Enriched Ointment in Inflammatory Skin Diseases and Cutaneous Scars. Clin. Ter. 2019, 170, 93–99. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Koseki, J.; Tsuchiya, K.; Matsubara, Y.; Iizuka, S.; Imamura, S.; Matsumoto, T.; Watanabe, J.; Kaneko, A.; Aiba, S.; et al. Suppression of Propionibacterium Acnes-Induced Dermatitis by a Traditional Japanese Medicine, Jumihaidokuto, Modifying Macrophage Functions. Evid. Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Anti-Inflammatory Effects of Cannabidiol (CBD) on Acne. J. Inflamm. Res. 2022, 15, 2795–2801. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Scheau, C.; Caruntu, C.; Badarau, I.A.; Scheau, A.-E.; Docea, A.O.; Calina, D.; Caruntu, A. Cannabinoids and Inflammations of the Gut-Lung-Skin Barrier. J. Pers. Med. 2021, 11, 494. [Google Scholar] [CrossRef]
- Roelandt, T.; Heughebaert, C.; Bredif, S.; Giddelo, C.; Baudouin, C.; Msika, P.; Roseeuw, D.; Uchida, Y.; Elias, P.M.; Hachem, J. Cannabinoid Receptors 1 and 2 Oppositely Regulate Epidermal Permeability Barrier Status and Differentiation. Exp. Dermatol. 2012, 21, 688–693. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, B.; Park, B.M.; Jeon, J.E.; Lee, S.H.; Mann, S.; Ahn, S.K.; Hong, S.; Jeong, S.K. Topical Cannabinoid Receptor 1 Agonist Attenuates the Cutaneous Inflammatory Responses in Oxazolone-induced Atopic Dermatitis Model. Int. J. Dermatol. 2015, 54, e401–e408. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol Exerts Sebostatic and Antiinflammatory Effects on Human Sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.-Y. The Ameliorative Effect of Hemp Seed Hexane Extracts on the Propionibacterium Acnes-Induced Inflammation and Lipogenesis in Sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef]
- Fukunaga, A.; Fukushima, S.; Iwata, H.; Nakahara, M.; Sasaki, R.; Baba, N.; Matsunaka, H.; Murakami, Y.; Furue, M.; Nishigori, C. Bioactive Substances in the Stratum Corneum of the Epidermis Found as Indicators of Skin Damage Due to Sun Exposure. Photodermatol. Photoimmunol. Photomed. 2022, 38, 241–249. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Rienzo, M.; Battista, N.; Gasperi, V.; Guerrieri, P.; Rossi, A.; Finazzi-Agrò, A. The Endocannabinoid System in Human Keratinocytes. J. Biol. Chem. 2003, 278, 33896–33903. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Ando, H.; Unno, S.; Kitagawa, J. Targeting Peripherally Restricted Cannabinoid Receptor 1, Cannabinoid Receptor 2, and Endocannabinoid-Degrading Enzymes for the Treatment of Neuropathic Pain Including Neuropathic Orofacial Pain. Int. J. Mol. Sci. 2020, 21, 1423. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.P.; Costa, G.; Mascarenhas-Melo, F.; Pires, P.C.; Heidarizadeh, F.; Giram, P.S.; Mazzola, P.G.; Cabral, C.; Veiga, F.; Paiva-Santos, A.C. Skin Applications of Cannabidiol: Sources, Effects, Delivery Systems, Marketed Formulations and Safety. Phytochem. Rev. 2023, 22, 781–828. [Google Scholar] [CrossRef]
- Few, J.; Lee, M.J.; Semersky, A.; Mariscal, E.; Vachon, G. A Single-Center Study Evaluating the Effects of a Novel Retinol and Cannabidiol Combination Topical on Facial Skin. Aesthetic Surg. J. Open Forum 2022, 4, ojac002. [Google Scholar] [CrossRef] [PubMed]


| Aqua |
|---|
| Dimethicone (AND) Dimethicone/Vinyldimethicone Crosspolymer Vinyldimethicone Crosspolymer |
| Polysorbate 60 |
| Cety Eethylhexanoate |
| Dimethicone |
| Panthenol |
| 1,2-Hexanediol |
| Butylene Glycol |
| Glycerin |
| Xanthan Gum |
| Ammonium Acryloyldimethyltaurate/VP Copolymer |
| PEG-240/HDI copolymer bis-decyltetradeceth-20 ether |
| Hydroxyethylacrylate/Sodium Acryloyldimethyltaurate Copolymer &Squalane& Polysorbate 60 |
| Sunflower oil-derived endocannabimimetics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, Y.; Jung, J.; Kim, H.-J.; Jeong, S.; Shin, H.; Ryu, W.-S.; Sohn, J.-H.; Nam, G. Clinical Efficacy of Endocannabinoid-Mimetic Fatty Acid Amide as a Skin-Soothing Ingredient. Cosmetics 2024, 11, 225. https://doi.org/10.3390/cosmetics11060225
Kim S, Kim Y, Jung J, Kim H-J, Jeong S, Shin H, Ryu W-S, Sohn J-H, Nam G. Clinical Efficacy of Endocannabinoid-Mimetic Fatty Acid Amide as a Skin-Soothing Ingredient. Cosmetics. 2024; 11(6):225. https://doi.org/10.3390/cosmetics11060225
Chicago/Turabian StyleKim, Sungwoo, Yeonjae Kim, Juyeon Jung, Hyun-Jung Kim, Sekyoo Jeong, Heesung Shin, Wuk-Sang Ryu, Jung-Hoon Sohn, and Gaewon Nam. 2024. "Clinical Efficacy of Endocannabinoid-Mimetic Fatty Acid Amide as a Skin-Soothing Ingredient" Cosmetics 11, no. 6: 225. https://doi.org/10.3390/cosmetics11060225
APA StyleKim, S., Kim, Y., Jung, J., Kim, H.-J., Jeong, S., Shin, H., Ryu, W.-S., Sohn, J.-H., & Nam, G. (2024). Clinical Efficacy of Endocannabinoid-Mimetic Fatty Acid Amide as a Skin-Soothing Ingredient. Cosmetics, 11(6), 225. https://doi.org/10.3390/cosmetics11060225






