Abstract
Sunlight radiation is a fundamental component of our daily lives. Specifically, blue light as well as UV light appear to play a role in the development of oxidative stress, DNA damage, photoaging, and pigmentation through the chromophores in skin tissues. However, several skin problems like psoriasis, eczema, and atopic dermatitis can be avoided with short-duration exposures to low-energy blue light radiation or UV radiation. In addition, exploring the effects of blue light as well as UV radiation on skin is quite essential for the development of minimally invasive antiaging strategies and for the design of innovative cosmetic formulations in modern aesthetics and cosmetology. Thus, in this review, we present the advantages as well as the disadvantages of light radiation, with a special focus on blue light and UV radiation activity on the human skin. We also discuss the molecular action of blue light and UV radiation on human skin. Other types of light radiation are included to holistically approach the effect of light on human skin.
1. Introduction
Daily exposure to several environmental stresses and insults occurs on the skin [1]. Indeed, air pollutants, both indoors and outdoors, affect the environment and pose an increasing global health risk to people. Since these pollutants enter the skin through transdermal and systemic pathways, the skin is one of their primary targets as they may penetrate both its superficial and deeper layers [2].
The sun is the most important source of ultraviolet, visible, and infrared radiation [3]. Solar radiation, and especially ultraviolet radiation (UV), is thought to be the primary factor causing photoaging [4,5]. In addition, blue light is a form of radiation that causes a series of damages to the skin as we are exposed to it daily due to the increasing use of electronic devices.
Thus, this review’s objective is to provide an overview of the most recent findings on the impact of sunlight irradiation on skin function, with a special focus on blue and UV light, as these two types of sunlight are currently being scrutinized by many researchers. For this, recent studies based on different sources of blue light or UV light exposure are presented, focusing on their effects on the human skin. The publications were selected according to a search on PubMed using the terms “light radiation”, “blue light”, and “UV light” and filtering only for human skin during the last five years. The search yielded 119 publications that were manually filtered to select only the most relevant publications, which are presented in this review. Of note, we did not intend to perform systematic research here. We therefore intentionally limited our literature search to PubMed and the term “human skin cells”. In addition, the molecular mechanisms underlying the observed biological skin changes, such as the signs of skin aging, are also described. Specifically, in this review, the subcategories of solar radiation and the common sources of visible light and blue light are presented. This is followed by a section highlighting the advantages of UV light but also the unpleasant effects of exposure to it due to the molecular changes that are induced in cells. The role of matrix metalloproteases, the concept of photoaging, and the process of pigmentation are analyzed to comprehend the phenotypic changes in the skin. In the following sections, the known biological effects of the individual colors of visible radiation are discussed, focusing especially on blue light, its mechanism of action, its skin penetration depth, and its similarities and differences with UV radiation. In addition, the therapeutic effects of blue light and UV light are presented, whether they concern skin conditions or other diseases such as cancer. Finally, the damaging effects of blue light and UV light on human skin are highlighted.
2. Sunlight
The general composition of sunlight is as follows: 44% visible light (400–780 nm), 53% infrared radiation (700–1440 nm), and 3–7% ultraviolet (UV) energy [3]. Ultraviolet radiation consists of three zones of different energies known as UVA, UVB, and UVC, with different impacts on human health (Figure 1). Of note, ozone has an excellent function in mitigating hazardous UVC rays that pass through the stratosphere [3]. Numerous positive impacts of solar radiation are present in our lives, including its crucial role in the photosynthesis of microbial algae and plants, the control of circadian rhythms, and the sensation of sight [3].
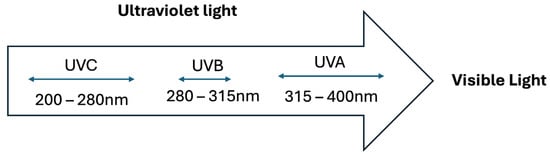
Figure 1.
Three zones of ultraviolet light.
2.1. Visible Light
About half of the solar energy that reaches the Earth’s surface at sea level is in the form of visible light, which has a wavelength between 400 and 700 nm [6,7,8]. A reference color is assigned to each range of wavelengths [9,10] (Table 1).

Table 1.
Light wavelengths with the reference color.
2.1.1. Visible Light Sources
Sunlight is the primary natural source of visible light [11], but artificial sources include fluorescent lamps, lasers, LED (light-emitting diode) lights, cell phones, television or computer monitors, and contemporary smart watches, all of which emit a full visible light spectrum [12].
2.1.2. Blue Light Sources
The sun, which also gives a blue hue to the sky, and all digital screens found in electronic devices are the two primary sources of blue light radiation [13].
In order to enhance the brightness and clarity of the electronic display, a large number of widely used digital devices produce blue light (400–480 nm) from their back-illuminated light-emitting diode (LED) display [14]. It is noteworthy that blue light is emitted by specialized medical equipment, such as incubators, to cure jaundice [15]. Currently, many researchers are very interested in studying the effects of blue light on the skin since its wavelength spectrum is quite close to UVA radiation.
Moreover, LED lights are present in a plethora of products that come into contact with the skin, including lamps for indoor and outdoor illumination, medical indications, and lamps used in extremity care facilities [11,16,17,18]. In addition, the maximum emission range for televisions and computer monitors is between 400 and 490 nm [19], whereas the range for tablets and smartphones is between 452 and 456 nm [20].
2.2. Recent Data on Light Exposure
Although artificial blue light emission from electronics is significantly lower than that of natural blue light—at least two to three times lower—the biological effects of this light on skin are still not well known [21].
The ratio of the solar spectrum’s irradiance to that of digital displays is predicted to be less than 1/200, although the average irradiance of the blue portion of the solar spectrum is 8 mW/cm2 [22]. As a result, blue light (420–490 nm) from screens on computers, TVs, and cell phones has a relative intensity that is 99–1000 times lower than sunlight. In addition, Rascalou et al. [22] found that the irradiance of a number of digital gadgets was 36 μW/cm2. The most powerful television displays have a blue light irradiance of 30 μW/cm2, according to a report by Passeron’s group [23]. In their work, Jo et al. [20] calculated the daily dose of blue light exposure using measurements of sunshine taken in March 2019 and August 2019, and the result was 38,790 J/cm2. The computed dose of blue light exposure indoors, excluding sunlight, was 6993 J/cm2.
More specifically, the dose of fluorescent lamps was calculated at 6.529 J/cm2/day, from computer screens at 0.383 J/cm2, from televisions at 0.022 J/cm2, from tablets at 0.031 J/cm2, and from mobile cell phones at 0.029 J/cm2. Based on the above data, Jo et al. concluded that individuals are frequently subjected to blue light, mainly from sunlight during outdoor activities but also from digital devices during indoor activities [20].
Nowadays, incandescent lighting is being phased out in favor of so-called low-energy technologies like light-emitting diodes (LEDs) and compact fluorescent lamps (CFLs) as a result of the current requirement to reduce energy usage [24]. However, it seems that 90% of human life involves activities in an indoor environment [13]. The recent increase in the use of modern technology has led to various types of electrical devices emitting electromagnetic fields in both the home environment and the workplace [3]. It has been reported that an office worker’s typical exposure is 1597 J/cm2 over approximately 27 days, 958 J/cm2 over approximately 16 days, and 447 J/cm2 over approximately 8 days [20]. Recent reports have shown a rapid increase in the use of smartphones and electronic devices in general during the COVID-19 pandemic [25]. In addition, according to recent research, 60% of adults use digital devices for up to six hours every day [14]. Indeed, nowadays, people are busy browsing the Internet, using social media, watching TV programs, playing online games, chatting with friends or even family, etc. Therefore, the rise in the use of electronic devices has led people to be more and more exposed to blue light, to which the eyes and facial skin are particularly exposed [19]. Depending on the source, this blue light usually has a peak emission between 440 and 460 nm, covering a spectrum from 420 to 490 nm [26].
Another survey based on blue light exposure to workers aged between 20 and 49 years has shown that the average time of smartphone use per day is 118.97 min, of tablet use is 63.61 min, of television use is 182.58 min, and of fluorescent light exposure is 1046.34 min, while on weekends, the average computer time is 579.6 min and sunlight exposure is 165.39 min [20]. According to this, the amount of time spent using electronic devices is constantly rising. The idea of healthy aging is being shaken by this modern phenomenon known as “digital pollution”, as numerous studies have shown ailments linked to it [27,28].
3. Effects of UV Irradiation on the Skin
3.1. UVA Radiation (320–380 nm)
Chronic UVA exposure causes epidermal hyperplasia by changing the thickness of the stratum corneum [29,30]. Specifically, UVA causes DNA damage indirectly through the formation of melanin and its byproducts. UVA2 wavelengths between 320 and 340 nm cause the distribution of melanin granules in the epidermis’s superficial layers, whilst UVA1 wavelengths between 340 and 400 nm increase melanin density in the basal layer [31,32,33]. UVA may act indirectly through photon absorption by endogenous photosensitizers, which subsequently trigger photo-oxidation processes, resulting in ROS [34,35]. Furthermore, it has been claimed that UVA radiation penetrates the skin 3 to 4 mm and directly damages cellular components, potentially contributing to skin aging and carcinogenesis [35].
3.2. UVB Radiation (280–320 nm)
UVB induces direct damage to cellular DNA by generating thymine dimers [33,36], which increases melanin synthesis [37]. It also interacts with skin cells, as opposed to UVA, which penetrates the skin’s deeper layers and impacts immune cells [38,39]. UVB exposure stimulates proinflammatory markers, including TNF-α, IL-6, iNOS, and COX-2 [40]. It has been observed that these proinflammatory and inflammatory molecules generate a variety of pathophysiological changes that worsen skin damage by increasing ROS generation [40,41].
3.3. Positive Effects of UV Radiation
Human skin benefits greatly from UV light in several ways, including antibacterial action, wound healing promotion, prevention of jaundice, and the creation of active vitamin D [40,42,43,44]. Moreover, a low UVR dose of 1.5 kJ/m2 on 4 consecutive days may find therapeutic use in individuals in need of local immunosuppressive treatment, such as contact hypersensitivity [45]. In addition, psoriasis and vitiligo may benefit from the UVB rays at 311–312 nm [46,47], and a moderate dose of UVA1 (340–400 nm, 50 J/cm2) can be helpful in treating atopic dermatitis and scleroderma [48,49]. Although the detailed mechanisms underlying the successful treatment of these refractory skin diseases remain unclear, the effectiveness of these phototherapies is thought to depend on their respective action spectra. These findings suggest that wavelengths greater than 296 nm are effective for the phototherapy of skin conditions [10].
3.4. Negative Effects of UV Radiation
Long-term exposure to UV radiation poses the danger of skin damage, accelerated aging of the skin, wrinkles, sagging, sunburn, inflammation, and even mutations that lead to the development of various types of skin cancer [3]. It must be emphasized that large dosages of UV radiation cause changes in the cellular redox balance and, as a result, oxidative alterations of lipids, nucleic acids, and proteins, which interfere with cellular metabolism by triggering photoreaction processes [50]. Regular and often unavoidable exposure to solar ultraviolet (UV) radiation makes it one of the most critical factors that cause DNA damage in human cells [51]. Recent studies have also demonstrated that skin exposure to UV radiation causes collagen damage and elastin degradation due to induced oxidative stress [35,52,53]. Indeed, oxidative stress plays an essential role in skin aging, and induced ROS also have a major role in stimulating skin pigmentation. Finally, in vitro reports have shown that UVA radiation demonstrates a more substantial effect than UVB radiation on collagen destruction at 330 nm [54].
3.5. Physiological Function of UV Radiation
The main negative effects of UV radiation on skin have been documented to include photoaging, inflammation, sunburn, and skin cancer [55,56]. According to Reichrath and Rass (2014), prolonged exposure to UVA rays causes epidermal hyperplasia and modifies the stratum corneum’s thickness [57]. Reactive oxygen species (ROS) and DNA damage are two negative consequences of UVB exposure [29,30]. Numerous investigations have shown that UVR typically stimulates activator protein-1 (AP-1)-mediated transcription in the skin and activates a number of kinases, including p38 MAP kinase and c-Jun N-terminal kinase (JNK) [58]. As a result, shielding the skin from UV radiation can prevent skin aging. UVR, however, is not limited to its detrimental effects on skin. According to Piotrowska et al., UVR interacts with 7-dehydrocholesterol, a cholesterol precursor in the skin that is eventually transformed into vitamin D [43,44]. In order to form bones, vitamin D helps with calcium absorption. Since food only provides 50% of the necessary amount of vitamin D, getting enough UVR exposure is crucial for good health. Furthermore, for patients requiring local immunosuppression therapy, such as in contact hypersensitivity, a low dose of UVR (1.5 kJ/m2) may have therapeutic uses [45]. Psoriasis and vitiligo can be improved by narrowband UVB (311–312 nm) [46].
According to Rodenbeck et al., atopic dermatitis and scleroderma, which cause the skin to tighten and harden, may benefit from a medium dosage of UVA1 (340–400 nm, 50 J/cm2) [48]. According to these findings, skin-related illnesses may benefit from therapeutic UVR exposure.
4. Skin Molecular Biology Mode of Action under UV Radiation
UV photons damage the skin through two mechanisms: direct absorption and photosensitization. In the direct absorption method, cellular chromophores absorb UV radiation and transform the absorbed energy into a biochemical signal, activating subsequent biological responses [59]. Cellular chromophores that absorb UVB radiation are nucleic acids, amino acids (such as tryptophan and tyrosine), kinins, flavins, porphyrins, and urocanic acid (UCA), while UCA absorbs UVA radiation [59]. The primary UV-induced damages are cyclobutane pyrimidine dimers (CPDs) and 6-4 pyrimidine pyrimidine (6-4PP). These photoproducts impede RNA transcription, activating the p53 gene and causing apoptosis in irradiated keratinocytes [60].
However, the second pathway, photosensitization, is indirect. Endogenous or exogenous sensitizers absorb UV radiation and trigger reactions that result in the creation of ROS and reactive nitrogen species (RNS), which can reach the nucleus and cause oxidative DNA alterations and strand breaks [3,10,18,56]. The primary ROS produced by UV light are the hydroxyl radical, superoxide anion, peroxyl radical, and their active precursors, which include singlet oxygen, hydrogen peroxide, and ozone. The primary RNS generated by UV radiation are nitric oxide and nitrogen dioxide. Excess free radicals cause DNA alteration and aberrant gene expression, resulting in the loss of cellular integrity [61].
In response to ROS accumulation, keratinocytes release melanocyte-activating factors, resulting in pigmentation [3]. After UVA and UVB exposure, the intracellular generation of ROS and inflammatory mediators is significantly increased, resulting in oxidative stress and an imbalance in the antioxidant defense system. These modifications induce structural and functional changes such as breakdown of extracellular matrix (ECM) proteins and overexpression of MMPs, which promote skin photoaging, sunburn development, inflammatory cell activation, and melanogenesis [62,63]. UV exposure also damages other essential macromolecules in the skin, such as proteins and lipids [64,65]. It is also responsible for causing phototoxic reactions associated with certain pharmaceutical preparations [64,65].
4.1. Matrix Metalloproteinases (MMPs)
MMPs are a category of zinc- and calcium-dependent endopeptidases that degrade extracellular matrix (ECM) components such as collagen, elastin, and fibrillin-1 [66]. Increased MMP and decreased collagen production cause a loss of collagen content in the skin, which eventually causes wrinkle formation. MMPs are induced by severe oxidative stress and inflammatory reactions [66,67] and break down different types of collagen (Figure 2).
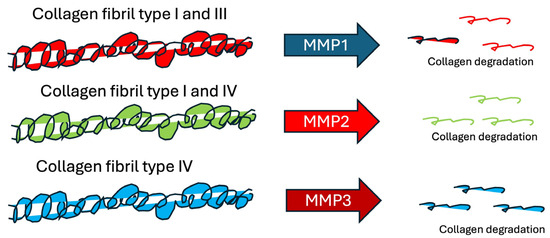
Figure 2.
Collagen tbreakdown by different matrix metalloproteinases.
4.2. Photoaging
Skin photoaging has phenotypic traits such as wrinkle formation and roughness. Furthermore, a recent study revealed other epidermal characteristics related to skin photoaging, such as epidermal hyperplasia and damage or disruption of collagen fibers [3]. Skin photoaging is also linked to an increase in metalloproteinases (MMPs) in dermal fibroblasts in response to UV exposure [67,68].
4.3. Pigmentation Process
ROS are produced by a wide range of stimuli and metabolic processes, and they consume electrons from other molecules (oxidation) to form additional ROS in a chain reaction. The excessive synthesis of oxidizing chemicals is known as oxidative stress, and it destroys lipids, proteins, and nucleic acids. ROS and oxidative stress play a role in sunburn, carcinogenesis, inflammatory disorders, aging, and pigmentation [69,70]. Both visible and UVA1 radiation cause pigment alterations in three phases: Immediate Pigment Darkening (IPD), which lasts up to 2 h after irradiation; Persistent Pigment Darkening (PPD), which lasts for 24 h; and Delayed tanning (DT), which begins about 5–7 days after irradiation and can extend for weeks to months. Specifically, UVR and visible radiation cause IPD, PPD, and DT, especially in the darkest FST phototypes (III–VI). Visible light-induced melanogenesis is most evident in phototypes IV to VI, with reports of both transitory and long-lasting (up to 8 weeks) pigmentation in human skin, depending on the total dose. It has been proposed that the creation of rapid pigmentation after exposure to visible light is photochemical in origin, while delayed pigmentation arises from neomelanogenesis [6]. The major stimulant of melanogenesis, the alpha-melanocyte-stimulating hormone (a-MSH), lowers UV-induced hydrogen peroxide levels in irradiated melanocytes, indicating the relationship between pigmentation and oxidative stress. Furthermore, short-wavelength visible light stimulates melanogenesis via the G protein-coupled membrane receptor opsin-3 [29]. Furthermore, a recent study found that oxidation of pre-existing melanin appears to contribute to enhanced pigmentation after UVA exposure; blue light may have a similar impact [70].
5. Effects of Visible and Near-Infrared Irradiation on Skin Cells
5.1. Violet Light (400–450 nm)
According to a recent study, UV irradiation (410 nm, 10–50 J/cm2) dramatically decreases the expression of cell differentiation factors [71]. Specifically, at 410 nm and at 30 J/cm2, it lowers the mRNA expression levels of antimicrobial peptides (AMPs), which are important for epithelial defense. Furthermore, UV irradiation stimulates ROS production, inflammatory cytokine release, and cellular DNA damage, and it also prevents early keloid formation [72].
5.2. Blue Light (450–490 nm)
Blue light (450–490 nm) has both beneficial and detrimental effects on the skin. Mignon et al. found that human dermal fibroblasts exhibit inhibitory effects on metabolic activity when exposed to 450 nm blue light at low intensities (<30 J/cm2), while cytotoxicity occurs at higher intensities (>30 J/cm2) [73]. Furthermore, blue light irradiation promotes oxidative stress in the mitochondria of cultured human keratinocytes, while from 430 nm to 510 nm, it slows epidermal barrier restoration following injury [74].
5.3. Green Light (490–560 nm)
Simoes et al. found that green light (520 ± 30 nm, 240 J/cm2) promotes angiogenesis and myofibroblast development, crucial for the repair of third-degree burns [75]. However, further research is needed into the biological impacts of green light.
5.4. Yellow/Orange Light (560–630 nm)
Yellow/orange light radiation at 590 nm lowers UVA-induced free radicals and MMP-1 expression in human fibroblasts, while at 595 ± 2 nm, it increases collagen type I and MMP-1 expression in human dermal fibroblasts [76,77]. Furthermore, yellow light at 590 nm dramatically decreases triglyceride levels via autophagy-related lysosomal degradation and may thus be therapeutically effective for lowering excess body fat [78].
5.5. Red Light (630–700 nm)
Red light radiation at 660 nm has been demonstrated to decrease MMP-1 expression, enhance collagen I expression in skin, expedite wound healing, protect against DNA damage induced by UVB radiation, and contribute to the protection of human dermal fibroblasts from UVB radiation [79,80,81,82]. These findings indicate that red light may be helpful to the skin and potentially useful in photomedical applications such as expediting the regeneration of wounded skin and antiaging treatments.
5.6. Near-Infrared Light (700–3000 nm)
According to a study by Akhalaya et al., near-infrared spectroscopy (NIRs) promotes the generation of oxygen free radicals (ROS) and destroys skin collagen in a manner similar to that seen with UVR [83]. However, a clinical investigation found that the majority of patients (around 51–75%) who received NIRs (830 nm) for approximately six months had better skin tone and reduced roughness [84]. Another study found that NIR (830 nm) significantly increases the amounts of collagen and elastin fibers in all experimental groups, and low-intensity NIR radiation (810 nm) enhances collagen accumulation and promotes cell proliferation and complete re-epithelialization, concluding that NIR can be used clinically to improve wound healing through photomodulation therapy [79].
6. Biological Effects of Visible Radiation
UVA and visible light radiation penetrate deeper into the dermis, affecting cellular and extracellular components. Until recently, visible light was assumed to be relatively innocuous because of its low energy [85] in comparison to ultraviolet and infrared radiation. Blue light is less hazardous than UV light due to changes in photon energies, yet it can trigger cell alterations [13].
Recent research and experimental approaches have revealed the ability of visible light to cause erythema in fair skin and pigmentary alterations in individuals with darker skin phototypes [12]. As a result, both the medical profession and social media users have been alarmed regarding its possible detrimental skin consequences [12].
Blue light radiation has the potential to generate biological effects similar to those of UVA radiation because of its proximity to the UVA spectrum. However, according to new studies, blue light has been linked to the effects of UVA radiation on photoaging and carcinogenic adverse effects in the long run [12,14]. The visible light spectrum accounts for more than half of solar radiation, and these wavelengths reach the lower dermis layer. Electron spin resonance spectroscopy has demonstrated that visible light and infrared radiation generate 50% of the skin’s total oxidant burden, contributing to accelerated skin aging via oxidative pathways [21]. Furthermore, another study indicated that skin fibroblasts are more harmed by exposure to this range of radiation than keratinocytes or melanocytes [86].
Interestingly, visible light radiation causes approximately 50% of the overall oxidative stress caused by sunshine; however, the oxidation efficacy of blue light is just a quarter of that of UVA radiation, implying a greater share of blue light emission in visible light [87,88,89].
6.1. Molecular Mechanism of Action of Visible Radiation in Skin
Photoreceptor chromophores, such as melanin, heme, and opsins, absorb photons from visible light, activating and transmitting energy to the chromophores and altering the function of the skin [90].
Enzymes, aromatic amino acids, urocanic acid, tryptophan, tyrosine, NADPH, NADH cofactors, riboflavins, porphyrins, melanin precursors, melanin, protoporphyrin IX, bilirubin, hemoglobin, carotene, or water molecules are examples of molecules that absorb light and are referred to as chromophores [91]. Thus, a variety of chromophores (photoreceptors) determine how blue light behaves. The most significant types of photoreceptors include nitrosated proteins (such as S-nitro-albumin), flavones, opsins, and porphyrins [92].
The main endogenous chromophore that is activated by blue light is flavins. In addition, the epidermis contains G protein-coupled receptors called opsins, which are light-sensitive [26,93]. Photoreceptors react to different wavelengths of sunlight; opsins (OPNs) are photoreceptors linked to the blue spectrum [26]. Opsins are classified into various types based on where they are expressed [93].
Human skin, melanocytes, and keratinocytes express short-wavelength OPN1, OPN2 (rhodopsin), and non-optical OPN3 (panopsin or encephalopsin) [94]. Human melanocytes contain OPN2, which is activated by UVA and leads to the synthesis of melanin. It has been discovered that both the anagen hair follicle and the skin express OPN2 (rhodopsin) and OPN3 (panopsin, encephalopsin). Buscone et al. have demonstrated that blue light radiation (3.2 J/cm, 2453 nm) can prolong the regenerative phase of hair follicles. The OPN3 sensor detects visible light with shorter wavelengths, such as blue light [95]. It works as a calcium-dependent melanocyte photoreceptor, activating the melanogenesis-related enzymes that cause hyperpigmentation [26]. It has been demonstrated that OPN4 is expressed in human fibroblasts, melanocytes, and keratinocytes. Blue light stimulates calcium entry and the phosphorylation of extracellular kinases in a dose-dependent manner [96].
Blue light has been shown to directly alter melanocytes and to influence melanogenesis via OPN3. Interestingly, the stimulation of other blue light photoreceptors causes the creation of ROS [92,97].
6.2. Penetration Depth of Visible Light Radiation
The depth of visible light penetration is determined by chromophores in the skin, the Fitzpatrick skin type (FST), reflection, scattering, and absorption, all of which are controlled by the skin’s natural barrier [6].
The principal components of visible light scattering and absorption in the skin include hemoglobin, melanin, keratin, bilirubin, carotene, lipids, and other structures such as filamentous proteins and cell nuclei. Melanin and keratins are the major pigments in the epidermis, scattering and absorbing light. In the epidermis, hemoglobin is the predominant absorber, and collagen is the primary scatterer of visible light, accounting for 18% to 30% of the layer volume [6,92].
Hemoglobin’s highest absorption occurs in blue radiation at 418 nm and in yellow/orange radiation at the 542 nm/577 nm wavelength bands; nevertheless, the two are highly connected with the concentration of erythrocytes [98].
Furthermore, various cell types in the epidermis and dermis, including melanocytes, keratinocytes, fibroblasts, and hair follicle stem cells, have been found to contain opsin receptors. Different spectrum sensitivities in the visible and UV light ranges are caused by differences in the amino acid sequences of opsins in the chromophore binding pocket, as well as the kinetics of binding to the chromophore [99]. Carotenoids, including α-carotene, γ-carotene, β-carotene, lycopene, lutein, and zeaxanthin, and their isomers absorb and scatter light in the skin. Carotenoids absorb light between 440 and 490 nm and reflect it between 458 and 472 nm. The distribution of carotenoids in human skin depends on the areas of skin studied, varies greatly between people, and is related to the skin’s antioxidant ability [100].
6.3. Positive Effects of Blue Light
Phototherapy is frequently used to treat a variety of common skin disorders. The effect of blue light radiation relies on its wavelength, frequency, and method of action, as well as the radiation period and dose [91]. In vitro data on cell survival, cell morphology, and mitochondrial function demonstrate that LED phototherapy is safe, with no negative consequences during or after treatment [12].
Photo-Bio-Modulation therapy (PBM) is a new and minimally invasive approach to the management of hypertrophic scars and keloids in combination with existing treatments [101]. It is a more recent term for low-level laser treatment (LLLT), which comprises applications utilizing visible, near-infrared, and infrared energy [8].
6.4. Blue Light for Clinical Applications
Blue light is characterized by its anti-inflammatory [102] and anti-proliferative effects [103]. Thus, it could be effective in different inflammation-related skin states, including psoriasis or eczema [104,105]. Other dermatological disorders treated with blue light include cutaneous T-cell lymphoma, rosacea, acne, and precancerous and cancerous skin lesions. Blue light radiation has also been found to alleviate itching and stimulate hair growth, and it can be utilized in acne therapy [11,30]. In addition, blue/red light combinations have been used to treat acne, scurvy, and seborrheic dermatitis [12].
Furthermore, blue light is utilized to treat neonatal jaundice, nonhyperkeratotic actinic keratosis, seasonal affective disorders, depression, and neurodermatitis [13,106]. Additionally, photodynamic therapy is utilized to treat cancer [107]. However, more research is needed to design protocols and monitor long-term safety [107].
6.5. Phototherapy
Phototherapy is the use of light for medical purposes. For safety reasons, light with a specific wavelength identified by the corresponding chromophore should be used. As such, it is critical to identify the photoreceptors in the skin and comprehend the fundamental mechanisms that underpin them [10].
6.6. Photorejuvenation
When used correctly, photodynamic blue light treatment for photorejuvenation is thought to be both effective and safe. By maximizing absorption selectivity and limiting the duration and penetration depth of blue light, one can reduce injury to adjacent healthy tissues [108]. The most common adverse effects are erythema, edema, pruritus, epithelium desquamation, hyperpigmentation, and discomfort [109].
6.7. Psoriasis
A short clinical research study on psoriasis treatment revealed that blue light radiation at 420 nm and 453 nm was helpful and had low and infrequent adverse effects [110].
6.8. Eczema and Atopic Dermatitis
Studies by Keemss et al. [105] and Becker et al. [111] determined that using non-UV blue light with a peak emission of 453 nm was both safe and effective in reducing eczema and atopic dermatitis lesions. The authors also saw improvements in patients’ sleep and quality of life.
6.9. Acne
A rising number of research studies suggest that blue light can help treat moderate acne vulgaris [15]. According to Bonnans et al., exposure to a mixture of 415 nm and 470 nm LEDs had a long-lasting antibacterial effect, almost eliminating inflammatory nodules, pustules, and microcysts [8]. Furthermore, a study by Jung et al. demonstrated that red and blue light can interfere with sebocytes, preventing the production of sebum and enhancing the clinical appearance of acne [112].
6.10. Photodynamic Therapy (PDT)
The PDT method, which includes blue light, is mostly used to treat non-melanoma skin malignancies and actinic keratosis. It is important to apply a photosensitizer, followed by light radiation [106]. Target cells are destroyed when the photosensitizer and light react, producing cytotoxic reactive oxygen species (ROS) [113]. The most often employed topical compounds are 5-aminolevulinic acid (5-ALA) and methyl-aminolevulinate (MAL), which are then converted into protoporphyrin IX (PPIX) [114].
7. Negative Effects of Blue Light Radiation
Exposure to blue light radiation has been shown to improve a variety of skin problems. However, research has shown that extended exposure to high-energy blue light can increase the amount of DNA damage, cell and tissue death and injury, eye damage, skin barrier damage, and photoaging [115,116,117,118,119]. Liebmann et al. found that blue light radiation of 500 J/cm2 at 453 nm or above had no adverse impact on human skin keratinocytes and endothelial cells [115]. Similarly, Opländer et al. demonstrated that blue light radiation at 453 and 480 nm was not harmful to human fibroblasts. However, blue light exposure at 410 nm or 420 nm induced intracellular oxidative stress and harmful consequences in a dose- and wavelength-dependent manner [116]. In addition, other studies [13,120] have found that blue light radiation, like UVA radiation, contributes to skin aging. According to the aforementioned data, blue light radiation at different wavelengths can generate varied degrees of intracellular oxidative stress with diverse physiological effects, thereby contributing to premature skin photoaging.
7.1. Risks to Eyes and Oral Mucosa
Research on the toxic and genotoxic consequences of blue light has mostly focused on the therapeutic and ophthalmic risks. It was reported that blue light damages the retina, induces cell dysfunction and mortality in gingival and lens epithelial fibroblasts, and causes apoptosis in retinal pigment epithelium (RPE) cells [1,121].
7.2. Production of ROS
It has been observed that exposing human keratinocytes to 200 J/cm2 for 66 min results in a 147% increase in the formation of ROS [74,122]. Furthermore, dermal carotenoids are significantly reduced by blue/violet light radiation of 100 J/cm2 at 380–395 nm in human skin, as shown by Raman spectroscopy, indicating the generation of free radicals [7,123], leading to cell death [124].
7.3. Damage to Cellular DNA
Blue light radiation has a dose-dependent genotoxic effect on skin cells, as demonstrated by the study of Chamayou-Robert et al. [123]. Specifically, blue light radiation could interact with intracellular porphyrins and flavoproteins, leading to the production of ROS such as peroxide, nitric oxide, and peroxynitrite, causing damage to cellular DNA [1,117]. Furthermore, other research has shown that visible light damages DNA, induces oxidative stress, and alters the molecular structure of the skin, all of which have genotoxic effects on human keratinocytes that are comparable to those of UV radiation [119,125]. Also, it was reported that the reaction of nitric oxide (NO) induced by blue light produces peroxynitrites, which may be responsible for damaging cellular DNA [126].
7.4. Photoaging
HEV has been demonstrated to cause skin photoaging in vitro, ex vivo, and in vivo with respect to the photoaging effect of blue light radiation [119].
Specifically, it has been observed that keratinocytes exposed to UV and visible radiation contain carbonylated proteins (CPs), which are formed through lipid peroxidation and cause damage to DNA. When CPs are exposed to blue light radiation, they produce additional oxygen free radicals (O2−) in the skin, which use lipid peroxidation to resynthesize more CPs [127].
7.5. Oxidative Stress and Hyperpigmentation
According to Mamalis, blue light radiation at 30, 45, and 80 J/cm2 greatly boosts ROS production [128]. In Mahmoud’s study, people with darker skin types exposed to daily amounts of visible light developed more continuous dark staining than those with lighter skin types [129]. The lowest PPD dose in participants with Fitzpatrick skin types V and VI was 80–120 J/cm2, and induced pigmentation was visible for at least two weeks. When exposed to blue light radiation, darker skin showed greater molecular-size protein complexes, but types I and II did not [119]. This multimeric protein complex associated with tyrosinase is primarily generated in melanocytes in dark skin and generates sustained tyrosinase activity, explaining the long-term hyperpigmentation found only in skin types III and higher following exposure to blue light [26].
However, a recent study by Duteil et al. revealed that utilizing a computer screen for 8 h per day for 5 days at a distance of 20 cm (about 8 inches) did not aggravate melasma lesions [130]. In addition, subjecting HaCaT cells to blue light radiation at 41.35 J/cm2 and 453 nm caused a rapid increase in ROS after 1 h via photoreduction of intracellular flavins in normal human keratinocytes [131]. This oxidative stress leads to immediate and persistent hyperpigmentation.
7.6. Effect on Fibroblasts
Human dermal fibroblasts exposed to blue light at 450 nm at low intensities (<30 J/cm2) revealed inhibitory effects on metabolic activity and procollagen I synthesis, with cytotoxicity at higher intensities (>30 J/cm2) [73]. Furthermore, Austin et al.’s study found that merely one hour of exposure at a distance of 1 cm can promote the formation of ROS in fibroblasts, leading to premature aging of the skin [90]. Furthermore, it has been observed that fibroblasts are more sensitive to blue light radiation exposure (410–453 nm) at non-toxic dosages [116]. Furthermore, a recent study demonstrated that ROS production was identified between 400 and 500 nm but not at 582 nm in dermal fibroblasts treated with 150 J/cm2 at various wavelengths [98].
7.7. Effect on the Epidermal Barrier
Human skin exposure to blue light induces changes in stratum corneum lipids, causing damage to the skin barrier and delaying repair after injury [132], as demonstrated by tape stripping.
7.8. Effect on Antioxidants
ROS production harms healthy skin cells and alters the amounts of endogenous antioxidants, essential for skin protection, which are depleted after exposure to blue light radiation [6]. Endogenous recovery may take up to 24 h [99].
Researchers discovered that exposing the skin to blue light between 380 and 495 nm (with a maximum at 440 nm and 100 mW/cm2) reduced carotenoid levels. These antioxidants dropped by up to 20% with the highest dose but reverted to baseline levels after 1 h and 24 h with the half and high doses, respectively [74]. The degradation of dermal carotenoids shows the number of free radicals produced, particularly reactive oxygen radicals in the skin [74,100].
7.9. Effect on Collagen
The synthesis of collagen and elastin is negatively impacted by blue light exposure. This occurs when blue light or UV radiation is directly exposed to the skin because free radicals are produced. Blue light causes skin cells to produce matrix metalloproteinases (MMPs), which have been demonstrated to break down collagen and cause photoaging [133]. These MMPs destroy the existing collagen and impede new production [134]. Blue light radiation at different wavelengths can generate varied degrees of intracellular oxidative stress in dermal fibroblasts and decreased proliferation, resulting in premature skin photoaging [116]. A comparable study indicated that blue/violet light at lower wavelengths (410, 420 nm) demonstrated substantial toxicity to human skin fibroblasts, although blue light radiation at longer wavelengths (453, 480 nm) did not impair cell viability [135]. According to Solano, ROS caused inflammation and accelerated cellular aging by causing the breakdown of the main components of the dermis scaffold.
7.10. Changes in Pigmentation
According to recent studies, blue light irradiation produces skin pigmentation in the same way that UVA radiation does. This can cause hyperpigmentation, a visible symptom of photoaging, or age spots [3,85]. Blue light-induced skin pigmentation is more durable than UVA-induced pigmentation and is evident in skin phototypes IV–VI, but not phototype II [129].
7.11. Changes in Circadian Rhythm and Delay in Damage Repair
Blue light emission at 460 nm strongly inhibits melatonin, affecting circadian cycles and damaging skin cells [1]. Blue light radiation at 410 nm inhibits the transcription of the clock gene PER1 in keratinocytes, which is involved in the circadian rhythm. Skin cells can modulate PER1 expression based on their sensitivity to light. This excites the cells, especially their nocturnal cycle, which is crucial for regeneration and repair [1].
7.12. Effect on the Endoplasmic Reticulum and Autophagy
Lee et al. investigated the link between oxidative stress, endoplasmic reticulum (ER) stress, autophagy, and apoptosis in blue light-induced skin damage [136]. ER stress and autophagy are two systems that allow cells to withstand external interfering influences, preserve cell homeostasis, and maintain proper function, ultimately affecting apoptosis [136]. In addition, blue light radiation at 435–445 nm for 6–24 h induces autophagy in skin cells, hastening cell death. These findings show that blue light radiation activates the ROS-ER stress–autophagy–apoptosis axis signaling pathway, leading to skin damage and apoptosis.
7.13. Effect on the Structure and Elasticity of the Skin
Exposure to 415 nm for 18 min at 5 mW/cm2 produces fiber fragmentation in the upper dermis [8]. This result confirmed that blue light radiation at 415 nm penetrates the dermis and can harm its structure and flexibility, highlighting the need to create skin protection techniques, including shielding from visible light [74]. Furthermore, in another study, a light dose of blue light radiation between 180 and 269 J/cm2 was employed, corresponding to 38 days of exposure to indoor blue light, which confirmed skin change under these settings [20].
8. Comparison of Different Light Spectra
In general, visible radiation acts deeper in the dermis compared to ultraviolet radiation, while it is more superficial compared to infrared radiation. The maximum penetration of blue light radiation into the epidermis is 0.07–1 mm, while UVB shows low penetration in the skin, mainly in the epidermis [3]. In addition, the quanta of radiation in the visible spectrum carry more energy than infrared radiation, showing its thermal and chemical effects. Furthermore, the ratio of free radical formation in the skin during exposure to UV and visible light ranges from 67% to 33%, respectively [107]. Violet/blue light (400–500 nm), also known as HEV, has the capacity to penetrate deeper into the skin compared to UVA and UVB rays [126]. In fact, pigmentation generated by visible light is more durable than pigmentation caused by ultraviolet A radiation [129].
UV radiation is the principal cause of sun-exposed skin aging, but more emphasis is devoted to the detrimental effects of HEV light, as it has been demonstrated that up to half of the free radicals created in the skin are due to the visible parts of the spectrum [87]. In a study by Zastrow et al. [137], the free radical action spectrum encompassing UV and visible light (280–700 nm) was calculated for the first time, and it revealed that visible light accounted for 50% of the skin’s total oxidant burden. It has been shown in f skin graft investigations that visible light exposure accounts for half of the formation of reactive oxygen species (ROS), whereas UV-B exposure accounts for 4% and UV-A exposure for 46% [3]. Since UVB causes DNA damage, HEV’s effect on skin aging is similar to UVA’s, such as in the case of hyperpigmentation, because both contribute to the mechanical production of free radicals [19]. Table 2 depicts the most significant beneficial and harmful effects of different types of sunlight radiation.

Table 2.
Negative and positive effects of different types of sunlight radiation.
9. Conclusions
Due to its advantages and disadvantages, light of different wavelengths has both positive and negative effects on our daily lives (Table 1). Some studies suggest UV radiation is a major cause of skin aging but is effective in promoting vitamin D synthesis and alleviating skin diseases such as psoriasis. Blue light also causes oxidative stress, slows down the recovery of skin barriers, and, ultimately, has a negative effect on the skin; however, it also contributes positively to the elimination of P. acne. Therefore, in this review, we have provided an overview of the effects of different levels of solar radiation on human skin, as it is valuable to exploit the beneficial effects of each wavelength of light under the appropriate conditions (information that is currently missing in the literature). Additionally, many people continue to seek non-invasive procedures to improve medical and aesthetic skin conditions. Phototherapy is the use of non-thermal or non-invasive light to achieve therapeutic effects. Therefore, it is important to identify photoreceptors and elucidate their underlying mechanisms in the skin.
In addition, in combination with systems biology, we can gain insights into which wavelengths are most effective for specific skincare or the treatment of skin-related diseases. Strategies to detect each wavelength of light at a specific intensity can help improve skin health.
Author Contributions
Conceptualization, S.L., E.R. and V.K.; writing—original draft preparation, S.L.; writing—review and editing, S.L., A.B. and E.K.; supervision, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dong, K.; Goyarts, E.C.; Pelle, E.; Trivero, J.; Pernodet, N. Blue Light Disrupts the Circadian Rhythm and Create Damage in Skin Cells. Int. J. Cosmet. Sci. 2019, 41, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The Impact of Airborne Pollution on Skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV Radiation-Induced Inflammation and Immunosup-pression Accelerate the Aging Process in the Skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? In Sunlight, Vitamin D and Skin Cancer; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2020; pp. 19–36. [Google Scholar]
- Lim, H.W.; Kohli, I.; Ruvolo, E.; Kolbe, L.; Hamzavi, I.H. Impact of Visible Light on Skin Health: The Role of Antioxi-dants and Free Radical Quenchers in Skin Protection. J. Am. Acad. Dermatol. 2022, 86, S27–S37. [Google Scholar] [CrossRef] [PubMed]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation Effects of Blue Light Irradiation on Skin and How to Protect against Them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, M.; Fouque, L.; Pelletier, M.; Chabert, R.; Pinacolo, S.; Restellini, L.; Cucumel, K. Blue Light: Friend or Foe? J. Photochem. Photobiol. B 2020, 212, 112026. [Google Scholar] [CrossRef]
- Renard, G.; Leid, J. Les Dangers de La Lumière Bleue: La Vérité! J. Fr. Ophtalmol. 2016, 39, 483–488. [Google Scholar] [CrossRef]
- Shin, D.W. Various Biological Effects of Solar Radiation on Skin and Their Mechanisms: Implications for Phototherapy. Anim. Cells Syst. 2020, 24, 181–188. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of Skin with Visible Light Induces Reactive Oxygen Species and Matrix-Degrading Enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef]
- Cohen, L.; Brodsky, M.A.; Zubair, R.; Kohli, I.; Hamzavi, I.H.; Sadeghpour, M. Cutaneous Interaction with Visible Light: What Do We Know? J. Am. Acad. Dermatol. 2023, 89, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Wall, A.C.; Gius, J.P.; Buglewicz, D.J.; Banks, A.B.; Kato, T.A. Oxidative Stress and Endoreduplication Induced by Blue Light Exposure to CHO Cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 841, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from Olive Fruits Prevents Blue-light-induced Damage in Human Keratinocytes and Fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, F.; Madsen, P.H.; Vandborg, P.K.; Jakobsen, L.H.; Trydal, T.; Vreman, H.J. Bilirubin Isomer Distribution in Jaundiced Neonates during Phototherapy with LED Light Centered at 497 nm (Turquoise) vs. 459 nm (Blue). Pediatr. Res. 2016, 80, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.; Huang, A.; Adar, T.; Wang, E.; Jagdeo, J. Electronic Device Generated Light Increases Reactive Oxygen Species in Human Fibroblasts. Lasers Surg. Med. 2018, 50, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Mamalis, A.; Garcha, M.; Jagdeo, J. Light Emitting Diode-generated Blue Light Modulates Fibrosis Characteristics: Fi-broblast Proliferation, Migration Speed, and Reactive Oxygen Species Generation. Lasers Surg. Med. 2015, 47, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of Melanin Derivatives Induces DNA Photoproducts Long after UV Exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.F.; Sarkas, H.W.; Boland, P. Iron Oxides in Novel Skin Care Formulations Attenuate Blue Light for En-hanced Protection against Skin Damage. J. Cosmet. Dermatol. 2021, 20, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.L.; Jung, Y.; Kim, Y.K.; Kim, N.; Cho, E.; Han, J.; Hwang, Y.K.; Suh, B.; Kim, E. Efficacy of Ethyl Ascorbyl Ether–Containing Cosmetic Cream on Blue Light–Induced Skin Changes. J. Cosmet. Dermatol. 2022, 21, 1270–1279. [Google Scholar] [CrossRef]
- Bacqueville, D.; Jacques-Jamin, C.; Dromigny, H.; Boyer, F.; Brunel, Y.; Ferret, P.J.; Redoulès, D.; Douki, T.; Bes-sou-Touya, S.; Duplan, H. Phenylene Bis-Diphenyltriazine (TriAsorB), a New Sunfilter Protecting the Skin against Both UVB + UVA and Blue Light Radiations. Photochem. Photobiol. Sci. 2021, 20, 1475–1486. [Google Scholar] [CrossRef]
- Rascalou, A.; Lamartine, J.; Poydenot, P.; Demarne, F.; Bechetoille, N. Mitochondrial Damage and Cytoskeleton Re-organization in Human Dermal Fibroblasts Exposed to Artificial Visible Light Similar to Screen-Emitted Light. J. Dermatol. Sci. 2018, 91, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T. The Key Question of Irradiance When It Comes to the Effects of Visible Light in the Skin. J. Dermatol. Sci. 2019, 93, 69–70. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, J.B.; Khazova, M.; Price, L.L.A. Low-Energy Light Bulbs, Computers, Tablets and the Blue Light Hazard. Eye 2016, 30, 230–233. [Google Scholar] [CrossRef]
- Jakhar, D.; Kaul, S.; Kaur, I. Increased Usage of Smartphones during COVID-19: Is That Blue Light Causing Skin Damage? J. Cosmet. Dermatol. 2020, 19, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef]
- Flies, E.J.; Mavoa, S.; Zosky, G.R.; Mantzioris, E.; Williams, C.; Eri, R.; Brook, B.W.; Buettel, J.C. Urban-Associated Diseases: Candidate Diseases, Environmental Risk Factors, and a Path Forward. Environ. Int. 2019, 133, 105187. [Google Scholar] [CrossRef]
- Alaimo, A.; Liñares, G.G.; Bujjamer, J.M.; Gorojod, R.M.; Alcon, S.P.; Martínez, J.H.; Baldessari, A.; Grecco, H.E.; Kotler, M.L. Toxicity of Blue Led Light and A2E Is Associated to Mitochondrial Dynamics Impairment in ARPE-19 Cells: Im-plications for Age-Related Macular Degeneration. Arch. Toxicol. 2019, 93, 1401–1415. [Google Scholar] [CrossRef]
- Chung, Y.H.; Jeong, S.A.; Choi, H.S.; Ro, S.; Lee, J.S.; Park, J.K. Protective Effects of Ginsenoside Rg2 and Astaxanthin Mixture against UVB-Induced DNA Damage. Anim. Cells Syst. 2018, 22, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Widel, M.; Krzywon, A.; Gajda, K.; Skonieczna, M.; Rzeszowska-Wolny, J. Induction of Bystander Effects by UVA, UVB, and UVC Radiation in Human Fibroblasts and the Implication of Reactive Oxygen Species. Free Radic. Biol. Med. 2014, 68, 278–287. [Google Scholar] [CrossRef]
- Hönigsmann, H. Erythema and Pigmentation. Photodermatol. Photoimmunol. Photomed. 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Wallisch, S.; Diffey, B. A Library of Action Spectra for Erythema and Pigmentation. Photochem. Photobiol. Sci. 2012, 11, 251–268. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Short-Term and Long-Term Cellular and Molecular Events Following UV Irra-diation of Skin: Implications for Molecular Medicine. Expert. Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective Effect of Curcumin against Ultraviolet A Irradia-tion induced Photoaging in Human Dermal Fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef]
- Wang, X.; Heraud, S.; Thépot, A.; Dos Santos, M.; Luo, Z. The Whitening Properties of the Mixture Composed of Pomegranate, Osmanthus and Olive and the Protective Effects Against Ultraviolet Deleterious Effects. Clin. Cosmet. Investig. Dermatol. 2021, 14, 561–573. [Google Scholar] [CrossRef]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining Light on Skin Pigmentation: The Darker and the Brighter Side of Effects of UV Radiation †. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef]
- Schneider, L.A.; Hinrichs, R.; Scharffetter-Kochanek, K. Phototherapy and Photochemotherapy. Clin. Dermatol. 2008, 26, 464–476. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New Insights in Photoaging, UVA Induced Damage and Skin Types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. Beneficial Effects of UV Radiation Other than via Vitamin D Production. Dermato Endocrinol. 2012, 4, 109–117. [Google Scholar] [CrossRef]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of Ultraviolet B Exposure-Mediated Activation of NF-ΚB in Normal Human Keratinocytes by Resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D. Dermato Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the Skin Physiology and Pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef]
- Neale, R.E.; Khan, S.R.; Lucas, R.M.; Waterhouse, M.; Whiteman, D.C.; Olsen, C.M. The Effect of Sunscreen on Vitamin D: A Review. Br. J. Dermatol. 2019, 181, 907–915. [Google Scholar] [CrossRef]
- Schwarz, A.; Navid, F.; Sparwasser, T.; Clausen, B.E.; Schwarz, T. 1,25-Dihydroxyvitamin D Exerts Similar Immuno-suppressive Effects as UVR but Is Dispensable for Local UVR-Induced Immunosuppression. J. Investig. Dermatol. 2012, 132, 2762–2769. [Google Scholar] [CrossRef]
- Esmat, S.; Hegazy, R.A.; Shalaby, S.; Chu-Sung Hu, S.; Lan, C.-C.E. Phototherapy and Combination Therapies for Vitiligo. Dermatol. Clin. 2017, 35, 171–192. [Google Scholar] [CrossRef]
- Morita, A. Current Developments in Phototherapy for Psoriasis. J. Dermatol. 2018, 45, 287–292. [Google Scholar] [CrossRef]
- Rodenbeck, D.L.; Silverberg, J.I.; Silverberg, N.B. Phototherapy for Atopic Dermatitis. Clin. Dermatol. 2016, 34, 607–613. [Google Scholar] [CrossRef]
- Teske, N.M.; Jacobe, H.T. Phototherapy for Sclerosing Skin Conditions. Clin. Dermatol. 2016, 34, 614–622. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and Ascorbic Acid Coop-eration in Antioxidant and Antiapoptotic Effect on Human Skin Keratinocytes and Fibroblasts Exposed to UVA and UVB Radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight Damage to Cellular DNA: Focus on Oxidatively Generated Lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin Structure and Its Involvement in Skin Photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. In Ultraviolet Light in Human Health, Diseases and Environment; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; pp. 15–23. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J. Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical CAMP Manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Reichrath, J.; Rass, K. Ultraviolet Damage, DNA Repair and Vitamin D in Nonmelanoma Skin Cancer and in Malignant Melanoma. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2014; pp. 208–233. [Google Scholar]
- Xu, Q.; Hou, W.; Zheng, Y.; Liu, C.; Gong, Z.; Lu, C.; Lai, W.; Maibach, H.I. Ultraviolet A-Induced Cathepsin K Ex-pression Is Mediated via MAPK/AP-1 Pathway in Human Dermal Fibroblasts. PLoS ONE 2014, 9, e102732. [Google Scholar] [CrossRef]
- de Assis, L.V.M.; Moraes, M.N.; Magalhães-Marques, K.K.; de Castrucci, A.M.L. Melanopsin and Rhodopsin Mediate UVA-Induced Immediate Pigment Darkening: Unravelling the Photosensitive System of the Skin. Eur. J. Cell Biol. 2018, 97, 150–162. [Google Scholar] [CrossRef]
- Denda, M.; Fuziwara, S. Visible Radiation Affects Epidermal Permeability Barrier Recovery: Selective Effects of Red and Blue Light. J. Investig. Dermatol. 2008, 128, 1335–1336. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, B.R.; Kim, J.E.; Bae, S.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Lee, H.S.; Lee, C.Y.; Kim, B.-H.; et al. Thera-peutic Effects of Cold-Pressed Perilla Oil Mainly Consisting of Linolenic Acid, Oleic Acid and Linoleic Acid on UV-Induced Photoaging in NHDF Cells and SKH-1 Hairless Mice. Molecules 2020, 25, 989. [Google Scholar] [CrossRef]
- Her, Y.; Shin, B.-N.; Lee, Y.; Park, J.; Kim, D.; Kim, K.; Kim, H.; Song, M.; Kim, J.-D.; Won, M.-H.; et al. Oenanthe Ja-vanica Extract Protects Mouse Skin from UVB Radiation via Attenuating Collagen Disruption and Inflammation. Int. J. Mol. Sci. 2019, 20, 1435. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin Delivery of Epigallocatechin-3-Gallate (EGCG) and Hyaluronic Acid Loaded Nano-Transfersomes for Antioxidant and Anti-Aging Effects in UV Radiation Induced Skin Damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. Molecular and Biochemical Basis of Minocy-cline-Induced Hyperpigmentation—The Study on Normal Human Melanocytes Exposed to UVA and UVB Radiation. Int. J. Mol. Sci. 2021, 22, 3755. [Google Scholar] [CrossRef]
- Kwak, C.S.; Yang, J.; Shin, C.-Y.; Chung, J.H. Topical or Oral Treatment of Peach Flower Extract Attenuates UV-Induced Epidermal Thickening, Matrix Metalloproteinase-13 Expression and pro-Inflammatory Cytokine Pro-duction in Hairless Mice Skin. Nutr. Res. Pr. 2018, 12, 29. [Google Scholar] [CrossRef]
- Khan, A.; Bai, H.; Shu, M.; Chen, M.; Khan, A.; Bai, Z. Antioxidative and Antiphotoaging Activities of Neferine upon UV-A Irradiation in Human Dermal Fibroblasts. Biosci. Rep. 2018, 38, BSR20181414. [Google Scholar] [CrossRef]
- Cannarozzo, G.; Fazia, G.; Bennardo, L.; Tamburi, F.; Amoruso, G.F.; Del Duca, E.; Nisticò, S.P. A New 675 nm Laser Device in the Treatment of Facial Aging: A Prospective Observational Study. Photobiomodul. Photomed. Laser Surg. 2021, 39, 118–122. [Google Scholar] [CrossRef]
- Ruvolo, E.; Boothby-Shoemaker, W.; Kumar, N.; Hamzavi, I.H.; Lim, H.W.; Kohli, I. Evaluation of Efficacy of Antiox-idant-enriched Sunscreen Prodcuts against Long Wavelength Ultraviolet A1 and Visible Light. Int. J. Cosmet. Sci. 2022, 44, 394–402. [Google Scholar] [CrossRef]
- Portillo, M.; Mataix, M.; Alonso-Juarranz, M.; Lorrio, S.; Villalba, M.; Rodríguez-Luna, A.; González, S. The Aqueous Extract of Polypodium Leucotomos (Fernblock®) Regulates Opsin 3 and Prevents Photooxidation of Melanin Precursors on Skin Cells Exposed to Blue Light Emitted from Digital Devices. Antioxidants 2021, 10, 400. [Google Scholar] [CrossRef]
- Kim, H.-J.; Son, E.D.; Jung, J.-Y.; Choi, H.; Lee, T.R.; Shin, D.W. Violet Light Down-Regulates the Expression of Specific Differentiation Markers through Rhodopsin in Normal Human Epidermal Keratinocytes. PLoS ONE 2013, 8, e73678. [Google Scholar] [CrossRef]
- Lee, H.S.; Jung, S.-E.; Kim, S.K.; Kim, Y.-S.; Sohn, S.; Kim, Y.C. Low-Level Light Therapy with 410 nm Light Emitting Diode Suppresses Collagen Synthesis in Human Keloid Fibroblasts: An In Vitro Study. Ann. Dermatol. 2017, 29, 149. [Google Scholar] [CrossRef]
- Mignon, C.; Uzunbajakava, N.E.; Castellano-Pellicena, I.; Botchkareva, N.V.; Tobin, D.J. Differential Response of Human Dermal Fibroblast Subpopulations to Visible and Near-infrared Light: Potential of Photobiomodulation for Addressing Cutaneous Conditions. Lasers Surg. Med. 2018, 50, 859–882. [Google Scholar] [CrossRef]
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E. Blue-Violet Light Irradiation Dose Dependently Decreases Ca-rotenoids in Human Skin, Which Indicates the Generation of Free Radicals. Oxid. Med. Cell. Longev. 2015, 2015, 579675. [Google Scholar] [CrossRef]
- Simões, T.M.S.; de Fernandes Neto, J.A.; de Oliveira, T.K.B.; Nonaka, C.F.W.; de Catão, M.H.C.V. Photobiomodulation of Red and Green Lights in the Repair Process of Third-Degree Skin Burns. Lasers Med. Sci. 2020, 35, 51–61. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Ho, P.-Y.; Wu, C.-S.; Yang, R.-C.; Yu, H.-S. LED 590 nm Photomodulation Reduces UVA-Induced Met-alloproteinase-1 Expression via Upregulation of Antioxidant Enzyme Catalase. J. Dermatol. Sci. 2015, 78, 125–132. [Google Scholar] [CrossRef]
- Kim, H.; Choi, M.S.; Bae, I.-H.; Jung, J.; Son, E.D.; Lee, T.R.; Shin, D.W. Short Wavelength Visible Light Suppresses Innate Immunity-Related Responses by Modulating Protein S-Nitrosylation in Keratinocytes. J. Investig. Dermatol. 2016, 136, 727–731. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, H.-J.; Ham, M.; Choi, D.-H.; Lee, T.R.; Shin, D.W. Amber Light (590 nm) Induces the Breakdown of Lipid Droplets through Autophagy-Related Lysosomal Degradation in Differentiated Adipocytes. Sci. Rep. 2016, 6, 28476. [Google Scholar] [CrossRef]
- Gupta, A.; Dai, T.; Hamblin, M.R. Effect of Red and Near-Infrared Wavelengths on Low-Level Laser (Light) Thera-py-Induced Healing of Partial-Thickness Dermal Abrasion in Mice. Lasers Med. Sci. 2014, 29, 257–265. [Google Scholar] [CrossRef]
- Martignago, C.C.S.; Tim, C.R.; Assis, L.; Da Silva, V.R.; Dos Santos, E.C.B.; Vieira, F.N.; Parizotto, N.A.; Liebano, R.E. Effects of Red and Near-Infrared LED Light Therapy on Full-Thickness Skin Graft in Rats. Lasers Med. Sci. 2020, 35, 157–164. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.-J.; Kim, H.L.; Kim, H.J.; Kim, H.S.; Lee, T.R.; Shin, D.W.; Seo, Y.R. A Protective Mechanism of Visible Red Light in Normal Human Dermal Fibroblasts: Enhancement of GADD45A-Mediated DNA Repair Activity. J. Investig. Dermatol. 2017, 137, 466–474. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Kim, S.J.; Kang, D.S.; Lee, T.R.; Shin, D.W.; Kim, H.-J.; Seo, Y.R. Transcriptomic Analysis of Human Dermal Fibroblast Cells Reveals Potential Mechanisms Underlying the Protective Effects of Visible Red Light against Damage from Ultraviolet B Light. J. Dermatol. Sci. 2019, 94, 276–283. [Google Scholar] [CrossRef]
- Akhalaya, M.Y.; Maksimov, G.V.; Rubin, A.B.; Lademann, J.; Darvin, M.E. Molecular Action Mechanisms of Solar Infrared Radiation and Heat on Human Skin. Ageing Res. Rev. 2014, 16, 1–11. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, K.-H.; Choi, J.-W.; Kwon, J.-K.; Lee, D.R.; Shin, M.S.; Lee, J.S.; You, C.E.; Park, M.Y. A Prospective, Randomized, Placebo-Controlled, Double-Blinded, and Split-Face Clinical Study on LED Phototherapy for Skin Re-juvenation: Clinical, Profilometric, Histologic, Ultrastructural, and Biochemical Evaluations and Comparison of Three Different Tre. J. Photochem. Photobiol. B 2007, 88, 51–67. [Google Scholar] [CrossRef]
- Lorrio, S.; Rodríguez-Luna, A.; Delgado-Wicke, P.; Mascaraque, M.; Gallego, M.; Pérez-Davó, A.; González, S.; Juar-ranz, Á. Protective Effect of the Aqueous Extract of Deschampsia Antarctica (EDAFENCE®) on Skin Cells against Blue Light Emitted from Digital Devices. Int. J. Mol. Sci. 2020, 21, 988. [Google Scholar] [CrossRef]
- Bennet, D.; Viswanath, B.; Kim, S.; An, J.H. An Ultra-Sensitive Biophysical Risk Assessment of Light Effect on Skin Cells. Oncotarget 2017, 8, 47861–47875. [Google Scholar] [CrossRef][Green Version]
- Zastrow, L.; Meinke, M.C.; Albrecht, S.; Patzelt, A.; Lademann, J. From UV Protection to Protection in the Whole Spectral Range of the Solar Radiation: New Aspects of Sunscreen Development. In Ultraviolet Light in Human Health, Diseases and Environment; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; pp. 311–318. [Google Scholar] [CrossRef]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does Blue Light Restore Human Epidermal Barrier Function via Activation of Opsin during Cutaneous Wound Healing? Lasers Surg. Med. 2019, 51, 370–382. [Google Scholar] [CrossRef]
- Umino, Y.; Denda, M. Effect of Red Light on Epidermal Proliferation and Mitochondrial Activity. Skin Res. Technol. 2023, 29, e13447. [Google Scholar] [CrossRef]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible Light. Part I: Properties and Cutaneous Effects of Visible Light. J. Am. Acad. Dermatol. 2021, 84, 1219–1231. [Google Scholar] [CrossRef]
- Sowa, P.; Rutkowska-Talipska, J.; Rutkowski, K.; Kosztyła-Hojna, B.; Rutkowski, R. Optical Radiation in Modern Medicine. Adv. Dermatol. Allergol. 2013, 4, 246–251. [Google Scholar] [CrossRef]
- Garza, Z.C.F.; Born, M.; Hilbers, P.A.J.; van Riel, N.A.W.; Liebmann, J. Visible Blue Light Therapy: Molecular Mecha-nisms and Therapeutic Opportunities. Curr. Med. Chem. 2019, 25, 5564–5577. [Google Scholar] [CrossRef]
- Serrage, H.; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M.R. Under the Spotlight: Mechanisms of Photobiomodulation Concentrating on Blue and Green Light. Photochem. Photobiol. Sci. 2019, 18, 1877–1909. [Google Scholar] [CrossRef]
- Haltaufderhyde, K.; Ozdeslik, R.N.; Wicks, N.L.; Najera, J.A.; Oancea, E. Opsin Expression in Human Epidermal Skin. Photochem. Photobiol. 2015, 91, 117–123. [Google Scholar] [CrossRef]
- Buscone, S.; Mardaryev, A.N.; Raafs, B.; Bikker, J.W.; Sticht, C.; Gretz, N.; Farjo, N.; Uzunbajakava, N.E.; Botchkareva, N.V. A New Path in Defining Light Parameters for Hair Growth: Discovery and Modulation of Photoreceptors in Human Hair Follicle. Lasers Surg. Med. 2017, 49, 705–718. [Google Scholar] [CrossRef]
- Kusumoto, J.; Takeo, M.; Hashikawa, K.; Komori, T.; Tsuji, T.; Terashi, H.; Sakakibara, S. OPN4 Belongs to the Photo-sensitive System of the Human Skin. Genes Cells 2020, 25, 215–225. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue Light for Infectious Diseases: Propi-onibacterium Acnes, Helicobacter Pylori, and Beyond? Drug Resist. Updates 2012, 15, 223–236. [Google Scholar] [CrossRef]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical Properties of Human Skin. J. Biomed. Opt. 2012, 17, 0909011. [Google Scholar] [CrossRef]
- Olinski, L.E.; Lin, E.M.; Oancea, E. Illuminating Insights into Opsin 3 Function in the Skin. Adv. Biol. Regul. 2020, 75, 100668. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Magni, G.; Banchelli, M.; Cherchi, F.; Coppi, E.; Fraccalvieri, M.; Rossi, M.; Tatini, F.; Pugliese, A.M.; Rossi Degl’Innocenti, D.; Alfieri, D.; et al. Experimental Study on Blue Light Interaction with Human Keloid-Derived Fibro-blasts. Biomedicines 2020, 8, 573. [Google Scholar] [CrossRef]
- Fischer, M.R.; Abel, M.; Lopez Kostka, S.; Rudolph, B.; Becker, D.; von Stebut, E. Blue Light Irradiation Suppresses Dendritic Cells Activation In Vitro. Exp. Dermatol. 2013, 22, 558–560. [Google Scholar] [CrossRef]
- Yoo, J.A.; Yu, E.; Park, S.-H.; Oh, S.W.; Kwon, K.; Park, S.J.; Kim, H.; Yang, S.; Park, J.Y.; Cho, J.Y.; et al. Blue Light Irradiation Induces Human Keratinocyte Cell Damage via Transient Receptor Potential Vanilloid 1 (TRPV1) Regulation. Oxid. Med. Cell. Longev. 2020, 2020, 8871745. [Google Scholar] [CrossRef]
- Lesiak, A.; Bednarski, I.; Narbutt, J. Prospective 3-Month Study on the Efficacy of UV-Free Blue Light in Mild Psoriasis Vulgaris Treatment. Adv. Dermatol. Allergol. 2021, 38, 446–449. [Google Scholar] [CrossRef]
- Keemss, K.; Pfaff, S.C.; Born, M.; Liebmann, J.; Merk, H.F.; von Felbert, V. Prospective, Randomized Study on the Efficacy and Safety of Local UV-Free Blue Light Treatment of Eczema. Dermatology 2016, 232, 496–502. [Google Scholar] [CrossRef]
- Queirós, C.; Garrido, P.M.; Maia Silva, J.; Filipe, P. Photodynamic Therapy in Dermatology: Beyond Current Indications. Dermatol. Ther. 2020, 33, e13997. [Google Scholar] [CrossRef]
- Tsibadze, A.; Chikvaidze, E.; Katsitadze, A.; Kvachadze, I.; Tskhvediani, N.; Chikviladze, A. Visible Light and Human Skin (Review). Georgian Med. News 2015, 46–53. [Google Scholar]
- Lin, J.; Wan, M.T. Current Evidence and Applications of Photodynamic Therapy in Dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar] [CrossRef]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef]
- Weinstabl, A.; Hoff-Lesch, S.; Merk, H.F.; von Felbert, V. Prospective Randomized Study on the Efficacy of Blue Light in the Treatment of Psoriasis Vulgaris. Dermatology 2011, 223, 251–259. [Google Scholar] [CrossRef]
- Becker, D.; Langer, E.; Seemann, M.; Seemann, G.; Fell, I.; Saloga, J.; Grabbe, S.; von Stebut, E. Clinical Efficacy of Blue Light Full Body Irradiation as Treatment Option for Severe Atopic Dermatitis. PLoS ONE 2011, 6, e20566. [Google Scholar] [CrossRef]
- Jung, Y.R.; Kim, S.J.; Sohn, K.C.; Lee, Y.; Seo, Y.J.; Lee, Y.H.; Whang, K.U.; Kim, C.D.; Lee, J.H.; Im, M. Regulation of Lipid Production by Light-Emitting Diodes in Human Sebocytes. Arch. Dermatol. Res. 2015, 307, 265–273. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two—Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Kostović, K.; Pastar, Z.; Ceović, R.; Mokos, Z.B.; Buzina, D.S.; Stanimirović, A. Photodynamic Therapy in Dermatology: Current Treatments and Implications. Coll. Antropol. 2012, 36, 1477–1481. [Google Scholar]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-Light Irradiation Regulates Proliferation and Differentiation in Human Skin Cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of Blue Light Irradiation on Human Dermal Fibroblasts. J. Photochem. Photobiol. B 2011, 103, 118–125. [Google Scholar] [CrossRef]
- Opländer, C.; Deck, A.; Volkmar, C.M.; Kirsch, M.; Liebmann, J.; Born, M.; van Abeelen, F.; van Faassen, E.E.; Kröncke, K.-D.; Windolf, J.; et al. Mechanism and Biological Relevance of Blue-Light (420–453 nm)-Induced Nonenzymatic Nitric Oxide Generation from Photolabile Nitric Oxide Derivates in Human Skin In Vitro and In Vivo. Free Radic. Biol. Med. 2013, 65, 1363–1377. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Coats, J.G.; Maktabi, B.; Abou-Dahech, M.S.; Baki, G. Blue Light Protection, Part I—Effects of Blue Light on the Skin. J. Cosmet. Dermatol. 2021, 20, 714–717. [Google Scholar] [CrossRef]
- Kohli, I.; Braunberger, T.L.; Nahhas, A.F.; Mirza, F.N.; Mokhtari, M.; Lyons, A.B.; Kollias, N.; Ruvolo, E.; Lim, H.W.; Hamzavi, I.H. Long-wavelength Ultraviolet A1 and Visible Light Photoprotection: A Multimodality Assessment of Dose and Response. Photochem. Photobiol. 2020, 96, 208–214. [Google Scholar] [CrossRef]
- Hu, L.; Xu, G. Potential Protective Role of TRPM7 and Involvement of PKC/ERK Pathway in Blue Light–Induced Apoptosis in Retinal Pigment Epithelium Cells In Vitro. Asia-Pac. J. Ophthalmol. 2021, 10, 572–578. [Google Scholar] [CrossRef]
- Sadowska, M.; Narbutt, J.; Lesiak, A. Blue Light in Dermatology. Life 2021, 11, 670. [Google Scholar] [CrossRef]
- Chamayou-Robert, C.; DiGiorgio, C.; Brack, O.; Doucet, O. Blue Light Induces DNA Damage in Normal Human Skin Keratinocytes. Photodermatol. Photoimmunol. Photomed. 2022, 38, 69–75. [Google Scholar] [CrossRef]
- Zamarrón, A.; Lorrio, S.; González, S.; Juarranz, Á. Fernblock Prevents Dermal Cell Damage Induced by Visible and Infrared A Radiation. Int. J. Mol. Sci. 2018, 19, 2250. [Google Scholar] [CrossRef]
- Sekhon, B.S. Surfactants: Pharmaceutical and Medicinal Aspects. J. Pharm. Technol. Res. Manag. 2013, 1, 43–68. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue Light-Induced Oxidative Stress in Live Skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Mizutani, T.; Okano, Y.; Masaki, H. The Impact of Carbonylated Proteins on the Skin and Potential Agents to Block Their Effects. Exp. Dermatol. 2019, 28, 32–37. [Google Scholar] [CrossRef]
- Mamalis, A.; Koo, E.; Jagdeo, J. Resveratrol Prevents Reactive Oxygen Species–Induced Effects of Light-Emitting Diode–Generated Blue Light in Human Skin Fibroblasts. Dermatol. Surg. 2016, 42, 727–732. [Google Scholar] [CrossRef]
- Mahmoud, B.H.; Ruvolo, E.; Hexsel, C.L.; Liu, Y.; Owen, M.R.; Kollias, N.; Lim, H.W.; Hamzavi, I.H. Impact of Long-Wavelength UVA and Visible Light on Melanocompetent Skin. J. Investig. Dermatol. 2010, 130, 2092–2097. [Google Scholar] [CrossRef]
- Duteil, L.; Queille-Roussel, C.; Lacour, J.-P.; Montaudié, H.; Passeron, T. Short-Term Exposure to Blue Light Emitted by Electronic Devices Does Not Worsen Melasma. J. Am. Acad. Dermatol. 2020, 83, 913–914. [Google Scholar] [CrossRef]
- Becker, A.; Klapczynski, A.; Kuch, N.; Arpino, F.; Simon-Keller, K.; De La Torre, C.; Sticht, C.; van Abeelen, F.A.; Oversluizen, G.; Gretz, N. Gene Expression Profiling Reveals Aryl Hydrocarbon Receptor as a Possible Target for Photobiomodulation When Using Blue Light. Sci. Rep. 2016, 6, 33847. [Google Scholar] [CrossRef]
- Falcone, D.; Uzunbajakava, N.E.; van Abeelen, F.; Oversluizen, G.; Peppelman, M.; van Erp, P.E.J.; van de Kerkhof, P.C.M. Effects of Blue Light on Inflammation and Skin Barrier Recovery Following Acute Perturbation. Pilot Study Results in Healthy Human Subjects. Photodermatol. Photoimmunol. Photomed. 2018, 34, 184–193. [Google Scholar] [CrossRef]
- Dumbuya, H.; Grimes, P.; Lynch, S.; Ji, K.; Brahmachary, M.; Zheng, Q.; Bouez, C.; Wangari-Talbot, J. Impact of Iron-Oxide Containing Formulations against Visible Light-Induced Skin Pigmentation in Skin of Color Individuals. J. Drugs Dermatol. 2020, 19, 712–717. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Krassovka, J.M.; Suschek, C.V.; Prost, M.; Grotheer, V.; Schiefer, J.L.; Demir, E.; Fuchs, P.C.; Windolf, J.; Stürmer, E.K.; Opländer, C. The Impact of Non-Toxic Blue Light (453 nm) on Cellular Antioxidative Capacity, TGF-Β1 Signaling, and Myofibrogenesis of Human Skin Fibroblasts. J. Photochem. Photobiol. B 2020, 209, 111952. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Lee, D.-H.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-Induced Endoplasmic Reticulum Stress: Cross-Talk between Ferroptosis and Apoptosis. Mol. Cancer Res. 2018, 16, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Renneberg, R.; Ferrero, L. The Missing Link—Light-Induced (280–1600 nm) Free Radical Formation in Human Skin. Skin Pharmacol. Physiol. 2009, 22, 31–44. [Google Scholar] [CrossRef]
- Barolet, D.; Roberge, C.J.; Auger, F.A.; Boucher, A.; Germain, L. Regulation of Skin Collagen Metabolism In Vitro Using a Pulsed 660 nm LED Light Source: Clinical Correlation with a Single-Blinded Study. J. Investig. Dermatol. 2009, 129, 2751–2759. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).