Abstract
Background: Eyelid dermatitis is a common, multifactorial, chronic, and/or relapsing condition with a considerable impact on life quality that represents a diagnostic and treatment challenge. Methods: We carried out a single-blind, controlled, randomized, parallel-group study to evaluate the efficacy of two dermo-cosmetics (cream for the eyelids and eye contour area and cleansing face cream) in which the key ingredient was witch hazel extract, compared to generic cream, in the treatment of patients affected by eyelid dermatitis. Enrolled subjects were treated and followed-up for 4 weeks; dermatological evaluation was objectively performed using the DLQI, OSDI, NRS–itch, and EDSI indicators. Results: The products tested proved to be more effective than the placebo in ameliorating the dermatitis in the treated patients, based on all considered dermatological scores. In the treatment arm, we observed a reduction in both DLQI score and in the indicators related to the extension of dermatitis, greater than that observed in the control arm. Conclusions: This comparative study demonstrates the role of dermo-cosmetic products tested not only as a support treatment, but also as a first-choice clinical approach.
1. Introduction
Eyelid dermatitis is a common dermatologic discomfort condition with acute, chronic, or relapsing skin manifestations that can be due to different causes and have a considerable impact on patients’ quality of life. Moreover, eyelid dermatitis is often a challenge to diagnose and treat [1,2].
Indeed, the eyelids constitute one of the areas of thinnest skin (0.55 mm compared with about 2 mm in other facial areas) [3] and greatest abundance of sebaceous glands, and are continually exposed to external contaminants [1,4]. For this reason, eyelids are the elective site for both irritative (irritative contact dermatitis—DIC) and allergic phenomena. In particular, cosmetics and personal hygiene products, along with numerous airborne allergens, drugs for topical use, and eye drops frequently cause allergic contact dermatitis (ACD) in this specific location [5,6,7,8].
Furthermore, the eyelids represent the elective site of numerous inflammatory chronic relapsing dermatoses, such as psoriasis, seborrheic dermatitis, and, especially, atopic dermatitis [4,9]. In the latter condition, typical lesions appear on the eyelid(s), compatible with the clinical aspects of chronic eczema, especially in adolescent and adult patients, with an incidence that increases with the age of the affected subjects [9,10]. Also, ocular adverse events are reported in patients undergoing biologic systemic treatments, despite the good response of dermatitis in other anatomical sites [11]. The visual impact of skin changes (eyelid edema, erythema, weeping, and scale) and the itching and burning sensation that frequently accompany these pathological conditions have significant impact on the daily lives of affected patients [5,12]. Furthermore, the multifactorial nature of eyelid dermatitis and the need to reduce the use of corticosteroids in this site complicates the treatment [13].
In this context, the identification of topical products (emollients and cleanser) that allow a rapid regression of clinical manifestations and that can be well tolerated even by patients with polysensitization and/or inflammatory chronic skin diseases is essential.
In our dermatologic clinic in a Northern Italy hospital, we carried out a study to evaluate the efficacy of Rilastil Difesa® cream for the eyelids and eye contour area and Rilastil Difesa® cleansing face cream, compared to generic cream, in the treatment of adults with eyelid dermatitis of an irritant, allergic, or inflammatory nature in terms of reduction in dermatological score (clinical and related to quality of life).
2. Materials and Methods
2.1. Study Design
This is a single-blind, controlled, randomized, parallel group, single-center study. Subjects were enrolled (T0) and followed-up over time after two (T1) and four (T2) weeks.
The study was conducted between November 2022 and May 2023 in a dermatologic clinic of a Northern Italy hospital (AOU Maggiore della Carità, Novara, Italy); demographic and clinical characteristics of the patients involved were recorded. Participants were randomly assigned to receive treatment vs. control in a 1:1 ratio. To ensure blindness, an independent statistician generated a random allocation sequence with random block sizes of four and six patients for each disease. Then, during each follow-up visit, dermatological scores were calculated.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Comitato Etico Interaziendale A.O.U. “Maggiore della Carità”-ASL BI, ASL NO, ASL VCO (N° CE148/2022, 29 July 2022). All the patients enrolled in this study signed an informed consent form.
Inclusion and Exclusion Criteria
The inclusion and exclusion criteria for this study are listed below:
Inclusion criteria:
- Aged over 18 years.
- Signature of informed consent.
- Patients able to understand and respond to the questionnaires required by the study.
- Patients able to understand the application method of the cream (independently at their home) and who can guarantee the regularity of the checks.
Exclusion criteria:
- Absence of consent to participate.
- Absence of clinical signs compatible with eyelid dermatitis.
- Inability to understand the questionnaires and impossibility of autonomous application of the product.
- Failure to guarantee regularity in follow-up visits.
- Concomitant treatment with topical or systemic corticosteroids or other immunosuppressive drugs.
2.2. Treatment
The product tested in our study, i.e., Rilastil Difesa® cream for the eyelids and eye contour area, is a soothing and emollient cream indicated for subjects with sensitive, intolerant, and allergy-prone skin on eyelid and periocular areas.
Its key ingredient is witch hazel extract. This compound is extracted from the bark of witch hazel, is known to be rich in soluble tannins, and is a traditional remedy for some different inflammatory skin diseases. Witch hazel extract promotes the stabilization of keratinocyte cell membranes; hence, in in vitro models, it has been shown to reduce the production of proinflammatory cytokines [14]. Moreover, trehalose is a further active ingredient of the tested product. It is a sugar composed of two glucose units and its peculiarity is the capability to be more effective, when compared to other sugars, in preserving the functionality of biological molecules under stress conditions, thus avoiding their destabilization and denaturation. Trehalose has demonstrated anti-inflammatory and photoprotective activities in keratinocyte cultures submitted to UVB radiation through the induction of autophagy [15,16]. The cream was tested together with Rilastil Difesa® cleansing face cream, a cleanser containing the same active ingredient, suitable for delicately cleansing and removing make-up from sensitive, intolerant, and allergy-prone skin. The complete composition of both treatment and placebo products are listed below.
| Treatment Group | Placebo Group | |
| Cream | Cleanser | Cream |
| Aqua (Water) • Caprylic/capric Triglyceride • Glycerin • Pentylene glycol • Trehalose • Hydroxyethyl Cellulose • Plukenetia Volubilis Seed Oil • Methylpropanediol • Arachidyl alcohol • Hamamelis Virginiana (Witch Hazel) Bark/Twig Extract • Behenyl Alcohol • Arachidyl Glucoside. | Aqua (Water) • Glycerin • Pentaerythrityl Tetraethylhexanoate • Pentylene Glycol • Methyl Glucose Sesquistearate • Hydroxyethylcellulose •Xanthan Gum • Tetrasodium Glutamate Diacetate • Methylpropanediol • Lactic Acid • Hamamelis Virginiana (Witch Hazel) Bark/Twig Extract • Sodium Hydroxide. | Aqua (Water), Caprylic/Capric Triglyceride, Glycerin, Pentylene Glycol, Hydroxyethylcellulose, Arachidyl Alcohol, Behenyl Alcohol, Arachidyl Glucoside |
Patients were randomized 1:1 to receive the study products (treatment group) or a placebo (control group). The placebo administered to patients belonging to the control group was a product for topical use containing the same excipients as the Rilastil Difesa® cream for the eyelids, but without the active ingredients. The products have been packaged in identical tubes, so as not to make it possible for the patients to identify the product contained in them, and to ensure the blinding of the study. However, the patients belonging to the control group did not receive a specific detergent and were invited to use the usual one.
The cream and cleanser and the placebo were supplied free of charge by the cosmetic manufacturer.
Patients enrolled in both study arms were educated to cleanse once a day and apply the cream twice a day for four weeks.
A schematic flow-chart of the study protocol is reported below (Figure 1). The total number of enrolled patients in both arms is reported.
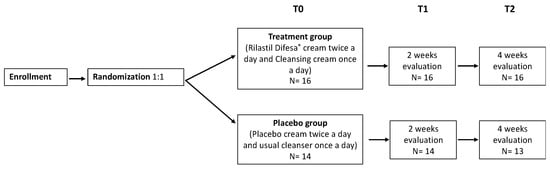
Figure 1.
Schematic flow-chart of the study protocol.
2.3. Dermatological Variables
Dermatological evaluations were performed by a group of experienced dermatologists, using the following indicators:
- -
- OSDI (Ocular surface disease index): evaluates the dry eye disease severity and effect on vision-related function, its scores vary from 0 to 100 (the higher scores representing greater disability) [17].
- -
- NRS–itch (Numerical rating scale for pruritus): measures the intensity of itch using a scale from 0 (no itch) to 10 (worst imaginable itch) [18].
- -
- EDSI (Eyelid dermatitis severity index): evaluates eyelid dermatitis in terms of erythema, papules, and scaling. It ranges from 0 to 9 (modified by [19]).
Furthermore, since we decided to evaluate the impact of the dermatitis in the daily quality of life in both the groups, we used the following score:
- -
- DLQI (Dermatology life quality index): estimates patients’ perception of the impact of skin diseases in different aspects of their health-related quality of life in the last week. Score assumed values between 0 (no effect at all on patient’s life) and 30 (extremely large effect on patient’s life) [20].
2.4. Primary Outcome
The primary outcome of this study is represented by the evaluation of the effectiveness of the tested product in reducing the clinical signs and symptoms related to eyelid dermatitis and the possible impact on the quality of life of affected patients.
2.5. Sample Size
Sample size was estimated using a repeated measures model to evaluate the difference of dermatological score in time (one pre-randomization observation and 2 post-randomization observations); assuming an intercorrelation among subjects of 0.9 and an effect size of 0.4, with a I type error of 0.05 (two-tailed) and a power of 0.80, at least 15 subjects for groups are needed. So, we enrolled a total of 30 subjects.
2.6. Statistical Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at Università del Piemonte Orientale. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies.
A descriptive analysis was conducted considering subjects overall and separately for the treatment group. Absolute and relative percentages were reported for categorical variables while mean and standard deviation (SD) or median and interquartile range [IQR] were reported for numerical ones, as appropriate.
First, to assess if the randomization worked well, characteristics at baseline among treatment and the non-treatment group were compared using the chi-square or Fisher test and t-test or non-parametric alternative (Wilcoxon), without reporting the p-values.
Second, differences in time (T1 and T0 and T2 and T0) were calculated for dermatological scores, separately for the two groups. For categorical data, we counted the subjects who had an improvement (reduction in eczema, vesicles, extension, changes in the dermatitis extension from bilateral to unilateral, and/or changes in the position from under-over-palpebral to under/over-palpebral), while for continuous ones (DLQI, OSDI, NRS, and EDSI), the difference of score in time was calculated and graphical representations were performed. Appropriate tests (as described above) were used to compare the treatment and control groups in terms of clinical improvement or reduction in the dermatological score. Finally, repeated measures models were used to consider all time measurements together; each clinical score was used as outcome in a separate model and time and treatment were used as covariates. Beta and 95% confidence intervals [95% CI] were reported for all covariates.
A p-value of 0.05 (two tails) was considered statistically significant and all the analysis were conducted using the intention-to-treat approach. The software used was SAS 9.4 and R.4.3.2.
3. Results
3.1. Patients
Thirty subjects were enrolled in this randomized controlled trial: 16 in the treatment group and 14 as controls; only one patient (belonging to the control group) was lost to the closure follow-up visit (T2). Most patients were female (n = 20, 66.7%), and their age ranged from 19 to 84 years, with a median age of 44.2 [IQR 34.7; 59.1] years.
Most patients enrolled had a previous diagnosis of atopic dermatitis (AD) (21 patients, 72.41%) or irritant or allergic contact dermatitis (8 patients, 27.59%). Among the subjects affected by concomitant comorbidities, the majority suffered from allergies (14 patients 46.67%) and allergic rhinitis (10 patients, 33.33%); among allergies, 10 were caused by food, 10 were environmental-related, and 3 were by contact origin.
Twenty-two patients (78.57%) reported previous skin manifestations like those presented at the time of enrollment and 23 (76.67%) had been suffering from dermatitis for more than 6 months. Among the local treatments used before the enrollment, we noticed topical emollients (22), steroids (16), antibiotics plus steroids (5), delicate cleaners (5), and 1 other unspecified topic product. Most patients (25 patients, 83.33%) had previously undergone dermatologic examination for the same reason.
3.2. Clinical Characteristics at Baseline
Table 1 and Table 2 report the descriptive statistics of subjects enrolled in this study, at baseline.

Table 1.
Descriptive statistic of subjects, separately for treatment and control group.

Table 2.
Signs, symptoms, and scores.
The eyelid dermatitis was bilateral in 76.67% of cases (23 patients) and in 83.33% of cases (25 patients) affected both the under- and the over-palpebral area. The lesions extended to more than 75% of the eyelid area in 11 subjects (36.67%).
Regarding clinical signs of eyelid dermatitis, we evaluated edema and vesiculation separately since these signs are not included in the EDSI score. At baseline, 40% of patients presented edema, and the EDSI score showed a mean of 2.17 (range 0–3) for erythema and a mean total score of 4.73 (range 0–9). These values were slightly higher in the treatment group compared to the control group. Other signs (i.e., vesiculation, papules, and scaling) were less represented.
No statistical difference (p-value > 0.10) among demographic and clinical characteristics between treatment and control groups was observed, indicating that randomization worked well (p-values are not reported in the tables).
3.3. Response to Treatment
As shown in Table 3, a reduction in the extension of dermatitis was observed in 13 patients (43.3%) at T1 vs. T0 and in 15 patients (53.6%) at T2 vs. T0, with overlapping results between the two groups (treatment and control).

Table 3.
Time variation. Absolute and relative percentage of improvement from T1 vs. T0 and T2 vs. T0. Results are reported overall and separately for the control and treatment groups, both with p-value obtained by Chi-square or Fisher tests.
Only a few patients had an improvement in terms of reduction in edema (2, 6.7% at T1 and 4, 13.8% at T2 vs. T0) and vesiculation (3, 10.3% at T1 and 3, 10.3% at T2 vs. T0); no differences were observed between the two groups for the parameter “edema”, whereas all the patients in which we observed a reduction in the parameter “vesiculation” belonged to the treatment group.
No patient ameliorated from bilateral to unilateral dermatitis; in 2 patients (6.9%) at T1 and 5 (15.2%) at T2 vs. T0, we observed a reduction in the extension of the areas involved (from both upper and lower eyelid to upper eyelid alone), with better results in the treatment group compared to the controls (18.75% vs. 15.38%), even if no statistically significant differences were observed among the two groups.
Finally, a reduction in the extension of dermatitis was observed in 13 (43.3%) and 15 (53.6%) patients at T1 and T2 vs. T0, respectively, with overlapping results between the two groups (treatment and control)
In Table 4 we show that overall, patients in both groups had an improvement in terms of dermatological score, as the differences among T1 and T0 and T2 and T0 were negatives (high scores indicate a worse situation). A slightly greater reduction in DLQI (−1.06 at T1 vs. T0, −2.75 at T2 vs. T0), disease severity (EDSI) (−1.03 at T1 vs. T0, 1.81 at T2 vs. T0), and itch (1.27 at T1 vs. T0 and −2.38 at T2 vs. T0) was observed in the treatment group than in control one (DLQI: −0.14 and −1.62, EDSI: −0.75 and −1.15, itch: −0.64 and −1.54, for T1 vs. T0 and T2 vs. T0, respectively). Particularly, the score in which we found the most significant reduction after treatment was OSDI (−6.20 and −4.51 in treatment group, and −1.24 and −2.94 in control group for T1 vs. T0 and T2 vs. T0, respectively). Despite this trend of improvement in treatment groups rather than control one, we were not able to reach the statistical significance as all the p-value were higher than 0.05 (Tables S1 and S2).

Table 4.
Time variation. Mean and standard deviation of score difference in time (T1 vs. T0 and T2 vs. T0). Results are reported overall and separately for the control and treatment groups, both with p-value obtained by Student t-test.
Generally, the reduction was clearer after 4 weeks than after 2 weeks (compared to baseline) (Figure 2). The results argue in favor of a major activity of the treatment compared to the control but in the absence of statistically significant results (Figure 3).
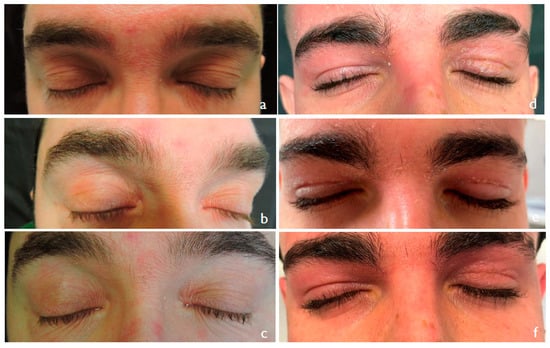
Figure 2.
Eyelid dermatitis at baseline (a,d) and after 2 weeks (b,e) and 4 weeks (c,d) of treatment. Left side (a–c) treatment arm; right side (d–f) control arm.
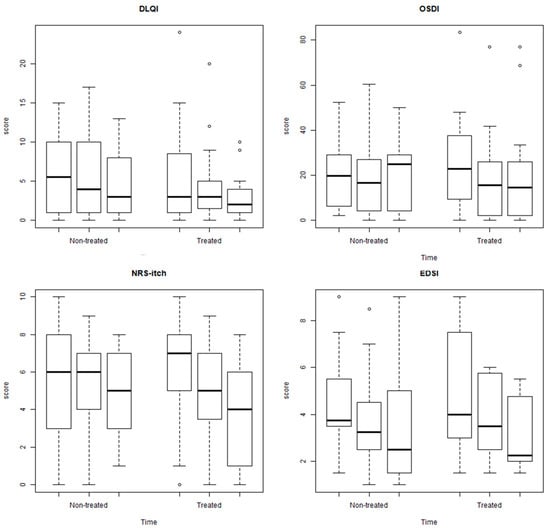
Figure 3.
Box plots of DLQI, OSDI, NRS–itch, and EDS separately for non-treated and treated in different time points. The ° indicates the outliers.
Finally, repeated measurement models were performed considering as outcome the dermatological scores (DLQI, OSDI, NRS–itch, and EDSI) and as covariate the time and the treatment. The results are reported in Table 5. First, we observe that time had a significant impact in terms of score reduction and the difference is more marked after 4 weeks than 2 weeks. The impact of treatment, adjusted for time, did not have a statistically significant effect.

Table 5.
Repeated measures models.
4. Discussion
This study aims to provide evidence of the effectiveness of two dermo-cosmetic products (Rilastil Difesa® cream for the eyelids and eye contour area and Rilastil Difesa® cleansing face cream) in subjects suffering from various types of eyelid dermatitis. The patients enrolled in the treatment arm were compared with a control group, which received a placebo consisting of only the excipients and continued cleansing with products already in use.
Eyelid dermatitis has always been a diagnostic and therapeutic challenge for the dermatologist, as the presence of skin lesions in a visible location has a significant impact on the patient’s quality of life and because exposure can lead to continuous interaction with irritating and/or allergenic agents, that contribute to the maintenance of dermatitis [21,22,23,24]. Furthermore, the distinguishing structure of this anatomical region [3], and the thinness of the skin, increases the risk of side effects caused by topical treatments, including poor tolerability and steroid-induced atrophy [25,26]. So, it is of utmost importance to identify topical products capable of inducing rapid regression of clinical manifestations and symptoms, and that can be well tolerated by patients, with the slightest adverse effects.
Few studies are published in the literature comparing, in patients affected by eyelid dermatitis, emollient medical devices with topical steroids or topical calcineurin inhibitors [27], such as the product’s characteristics and the efficacy of different dermo-cosmetics or “dermatologist-recommended” moisturizers [28,29,30]. To the best of our knowledge, this is the first study which highlights the results obtained in the treatment of eyelid eczema with specific dermo-cosmetic products, in comparison with a placebo.
The products tested (Rilastil Difesa® cream for the eyelids and eye contour area and Rilastil Difesa® cleansing face cream) have proven to be more effective than the placebo in determining an improvement in the treated patients, based on all considered dermatological scores. In detail, regarding the general impact of dermatitis on the patient’s quality of life, we observed a greater reduction in scores obtained by the DLQI questionnaire in the treatment than in the control group, both for the single time intervals considered (treatment: −1.06 at T1 and −2.75 at T2; control: −0.14 at T1 and −1.62 at T2) and through the repeated measurement model (treatment vs. control: −1.42). These results are relevant, considering the high burden on life-quality caused by chronic eyelid dermatitis [12].
Also, we observed in the treatment arm a greater improvement in the scores related to the extension of dermatitis (treatment: EDSI: −1.03 at T1 and −1.81 at T2; OSDI: −6.2 at T1 and −4.51 at T2; control: EDSI −0.75 at T1 and −1.15 at T2; OSDI: −1.24 at T1 and −2.94 at T2), and for the intensity of itching (NRS–itch: treatment 1.27 at T1, 2.38 at T2; control: −0.64 at T1 and −1.54 at T2). Similarly, all patients (3.18, 75%) in whom a reduction in the vesiculation parameter was observed at T2 belonged to the treatment arm. These parameters, such as the “edema” parameter, were evaluated separately, as they are not included as an integral part in the ESDI and OSDI scores. Regarding edema, no changes were evident between T0, T1, and T2, neither in the treatment arm nor in the control arm; however, this parameter has proven to be more difficult to modify with the use of dermo-cosmetics, even when used in association with corticosteroids [29]. It should also be noted that for all the considered parameters, the improvement in the treatment arm was achieved more quickly than in the control arm, with clinical and symptomatic improvement evident only 2 weeks after starting. This is a critical point, as it facilitates the patient’s adherence to the treatment, guaranteeing its continuation and therefore the possibility of obtaining even better results. The comparison of the scores obtained at T1 and T2 versus the baseline values, considered separately for the treatment group and the control group, allowed us, in fact, to achieve statistically significant results both with regards to the means and the medians for the OSDI, NRS, and ESDI parameters.
In the treatment group, we also observed a greater reduction in the extent of the affected areas (18.75% in the treatment vs. 15.38% in the control group), although these differences did not reach statistical significance. These patients presented sub- and supra-palpebral dermatitis at T0 and a single supra-palpebral localization at T2, while the dermatitis did not change from bilateral to unilateral in any patients.
To reinforce the relevance of our results, it should be noted that most patients included in this study were suffering from chronic (76.67%) and/or recurrent dermatitis (78.57%), predominantly associated with allergic comorbidities (46.67%). Furthermore, 83.33% had already received at least one previous dermatological evaluation and had undergone at least one line of treatment before study inclusion, to which they were unresponsive or refractory. Among these, 21 patients had used a topical steroid (alone or in combination with antibiotics) before enrollment in our study; despite the planned two week wash-out period, a possible “rebound” effect deriving from the suspension of these treatments, with a consequent worsening of dermatitis during the observation period, must be taken into consideration, as well as the “trigger” effect on dermatitis carried out by the possible contact, even during the study, by airborne and/or ingested allergens [6,31,32,33]. These confounders may have contributed to reducing the significance of the observed differences.
One of the main strengths of this study is represented by the standardized and validated dermatological scores used for the evaluation of the clinical progress of dermatitis, its symptoms, and its relative impact on the patient’s quality of life [17,18,19,20]; these scores allow the response to treatment to be objectively assessed. Another strong point is represented by the independent randomization of the patients enrolled in the study into the two treatment/control arms. The descriptive statistics of the population at baseline demonstrate the absence of statistically significant differences both in terms of demographic aspects and clinical aspects between the two groups, proving the correct randomization process. To the best of our knowledge, this is also the first comparison study on the effectiveness of a dermo-cosmetic on eyelid dermatitis.
A possible limitation is represented by the relatively low sample size; the number of patients eligible for inclusion in the study was lower than expected, perhaps because of the unusual climatic conditions of recent seasons, which have reduced the incidence of “seasonal” dermatitis (seborrheic dermatitis, irritant contact dermatitis, etc.). Even though we applied corrections in the statistical processing of the results, as explained in the “Sample size” section, this could have influenced the statistical significance of the results. Another limitation is represented by the risk that some patients have not correctly applied the provided topical products. Indeed, it should also be remembered that the first draft of the study envisaged that the patients enrolled in the control group would also receive the cleansing cream, in addition to the topical placebo product. This draft was subsequently modified, for the fear that even the detergent alone could have a specific effect in the control group; thus, we evaluated the synergistic effect of both treatment and the cleansing cream in the treatment group. However, the indication for patients enrolled in the control group to use the habitual cleanser could have effectively led to an “opening” of the study, making it easier for patients to recognize that they had received a placebo rather than the product under study, and increasing the risk of interference with self-medication (always a risk for studies conducted on products for topical use, as well as that of inappropriate or insufficient application of the topical itself).
5. Conclusions
Our experience confirms the deep impact of chronic eyelid dermatitis on the quality of life of affected patients and the need to identify targeted therapeutic approaches. This comparative study demonstrates the role of dermo-cosmetic products tested, not only as a support treatment, but also as a first-choice clinical approach.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cosmetics11030083/s1: Table S1. p-value obtained comparing mean values for T1 vs. T0 and T2 vs. T0, separately for treatment and controls using paired t-test. Table S2. p-value obtained comparing median values for T1 vs. T0 and T2 vs. T0, separately for treatment and controls using Wilcoxon signed rank.
Author Contributions
Conceptualization, F.V., E.Z. and P.S.; methodology, C.A.; software, C.A.; validation, F.V., E.E. and P.S.; formal analysis, C.A.; investigation, F.V., E.E., N.D.C., P.P. and E.Z.; data curation, P.S.; writing—original draft preparation, F.V.; writing—review and editing, C.A., E.Z. and P.S.; visualization, E.E., N.D.C., P.P. and C.A.; supervision, P.S.; project administration, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
We received non-conditional financial support from Istituto Ganassini S.p.A., Milano, Italy, for the completion of this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee) of Comitato Etico Interaziendale A.O.U. “Maggiore della Carità”-ASL BI, ASL NO, ASL VCO (N° CE148/2022, 29 July 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
The dataset presented in this study is available on request from the corresponding author.
Acknowledgments
We are indebted to patients who participated in this study. We also thank the Istituto Ganassini S.p.A. for donating the study product and for guaranteeing open access of the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Warshaw, E.M.; Voller, L.M.; Maibach, H.I.; Zug, K.A.; DeKoven, J.G.; Atwater, A.R.; Reeder, M.J.; Sasseville, D.; Taylor, J.S.; Fowler, J.F., Jr.; et al. Eyelid dermatitis in patients referred for patch testing: Retrospective analysis of North American Contact Dermatitis Group data, 1994–2016. J. Am. Acad. Dermatol. 2021, 84, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Orion, E.; Tüzün, Y. Periorbital (eyelid) dermatides. Clin. Dermatol. 2014, 32, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cochran, M.L.; Lopez, M.L.; Czyz, C.N. Anatomy, Head and Neck: Eyelid; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Temesvári, E.; Pónyai, G.; Németh, I.; Hidvégi, B.; Sas, A.; Kárpáti, S. Periocular dermatitis: A report of 401 patients. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Doan, S.; Zagórski, Z.; Palmares, J.; Yağmur, M.; Kaercher, T.; Benítez-Del-Castillo, J.M.; Van Dooren, B.; Jonckheere, P.; Jensen, P.K.; Maychuk, D.Y.; et al. Eyelid Disorders in Ophthalmology Practice: Results from a Large International Epidemiological Study in Eleven Countries. Ophthalmol. Ther. 2020, 9, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Feser, A.; Plaza, T.; Vogelgsang, L.; Mahler, V. Periorbital dermatitis—A recalcitrant disease: Causes and differential diagnoses. Br. J. Dermatol. 2008, 159, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Belsito, D.V. The aetiology of eyelid dermatitis: A 10-year retrospective analysis. Contact Dermat. 2006, 55, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Hine, A.M.; Waldman, R.A.; Grzybowski, A.; Grant-Kels, J.M. Allergic disorders of the eyelid. Clin. Dermatol. 2023, 41, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Julián-Gónzalez, R.E.; Orozco-Covarrubias, L.; Durán-McKinster, C.; Palacios-Lopez, C.; Ruiz-Maldonado, R.; Sáez-de-Ocariz, M. Less common clinical manifestations of atopic dermatitis: Prevalence by age. Pediatr. Dermatol. 2012, 29, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Rayala, B.Z.; Morrell, D.S. Common Skin Conditions in Children: Noninfectious Rashes. FP Essent. 2017, 453, 8–25. [Google Scholar]
- Touhouche, A.T.; Cassagne, M.; Bérard, E.; Giordano-Labadie, F.; Didier, A.; Fournié, P.; Paul, C.; Tauber, M. Incidence and risk factors for dupilumab associated ocular adverse events: A real-life prospective study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 172–179. [Google Scholar] [CrossRef]
- Beltrami, E.J.; Grzybowski, A.; Grant-Kels, J.M. Chronic eyelid and ocular itch. Clin. Dermatol. 2023, 41, 509–514. [Google Scholar] [CrossRef]
- Kim, D.; Bautista, S.; Meer, E.; McGeehan, B.; Maguire, M.; Briceño, C.J. Changes in Intraocular Pressure with Use of Periocular Triamcinolone Cream. Ophthalmic Vis. Res. 2022, 17, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Pozzoli, C.; Terno, M.; Canilli, L.; Angarano, M.; Maranta, N.; Dell’Agli, M.; et al. Unveiling the Ability of Witch Hazel (Hamamelis virginiana L.) Bark Extract to Impair Keratinocyte Inflammatory Cascade Typical of Atopic Eczema. Int. J. Mol. Sci. 2022, 23, 9279. [Google Scholar] [CrossRef]
- Xie, G.; Timasheff, S.N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 1997, 64, 25–43. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Chen, X.; Chen, S.; Gu, H. Trehalose Protects Keratinocytes against Ultraviolet B Radiation by Activating Autophagy via Regulating TIMP3 and ATG9A. Oxid. Med. Cell Longev. 2022, 2022, 9366494. [Google Scholar] [CrossRef]
- Schiffman, R.M. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Storck, M.; Sandmann, S.; Bruland, P.; Pereira, M.P.; Steinke, S.; Riepe, C.; Soto-Rey, I.; Garcovich, S.; Augustin, M.; Blome, C.; et al. Pruritus Intensity Scales across Europe: A Prospective Validation Study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1176–1185. [Google Scholar] [CrossRef]
- Wollenberg, A.; Oppel, T. Scoring of skin lesions with the perioral dermatitis severity index (PODSI). Acta Derm. Venereol. 2006, 86, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Derm. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Chisholm, S.A.M.; Couch, S.M.; Custer, P.L. Etiology and Management of Allergic Eyelid Dermatitis. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 248–250. [Google Scholar] [CrossRef]
- Pandit, S.A.; Glass, L.R.D. Non-glaucoma periocular allergic, atopic, and irritant dermatitis at an academic institution: A retrospective review. Orbit 2019, 38, 112–118. [Google Scholar] [CrossRef]
- Zirwas, M.J. Contact Dermatitis to Cosmetics. Clin. Rev. Allergy Immunol. 2019, 56, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, M.; Shah, A.; Yang, Y.W.; Mangold, A.; Shen, J. Allergic contact dermatitis of the eyelids: An interdisciplinary review. J. Ocul. Surf. 2023, 28, 124–130. [Google Scholar] [CrossRef]
- Phua, V.M.; Hakin, K.N. Eyelid skin atrophy associated with chronic usage of ophthalmic steroid ointment, and its successful treatment with the Versapulse laser. Eye 2001, 15 Pt 5, 659–660. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Yasuoka, N.; Ueta, M.; Katoh, N. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol. Int. 2018, 67, 388–391. [Google Scholar] [CrossRef]
- Frankel, A.; Sohn, A.; Patel, R.V.; Lebwohl, M.J. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. Drugs Dermato. 2011, 10, 666–672. [Google Scholar]
- Bergera-Virassamynaïk, S.; Ardiet, N.; Sayag, M. Evaluation of the Efficacy of an Ecobiological Dermo-Cosmetic Product to Help Manage and Prevent Relapses of Eyelid Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2023, 16, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Kwa, M.; Lohman, M.E.; Evers-Meltzer, R.; Silverberg, J.I. Consumer Preferences, Product Characteristics, and Potentially Allergenic Ingredients in Best-selling Moisturizers. JAMA Dermatol. 2017, 153, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Demessant, A.; Le Dantec, G.; Le Floc’h, C.; Kerob, D. Tolerance and Efficacy of a Dermocosmetic Containing Neurosensine® in Subjects with Eyelid Eczema. Clin. Cosmet. Investig. Dermatol. 2023, 16, 161–165. [Google Scholar] [CrossRef]
- Rapaport, M.J. Eyelid dermatitis. Dermatol. Nurs. 2000, 12, 352–354. [Google Scholar]
- Sergoynne, L.; Mertens, M.; Dendooven, E.; Leysen, J.; Aerts, O. Allergic contact dermatitis, mimicking atopic dermatitis, associated with the use of essential oils in “home-made” cosmetics and aromatherapy diffusers. Contact Dermat. 2020, 83, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Reeder, M.; Atwater, A.R. Systemic contact dermatitis: Sometimes it is the food. Cutis 2019, 104, 337–340. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).