Adult Female Acne: Recent Advances in Pathophysiology and Therapeutic Approaches
Abstract
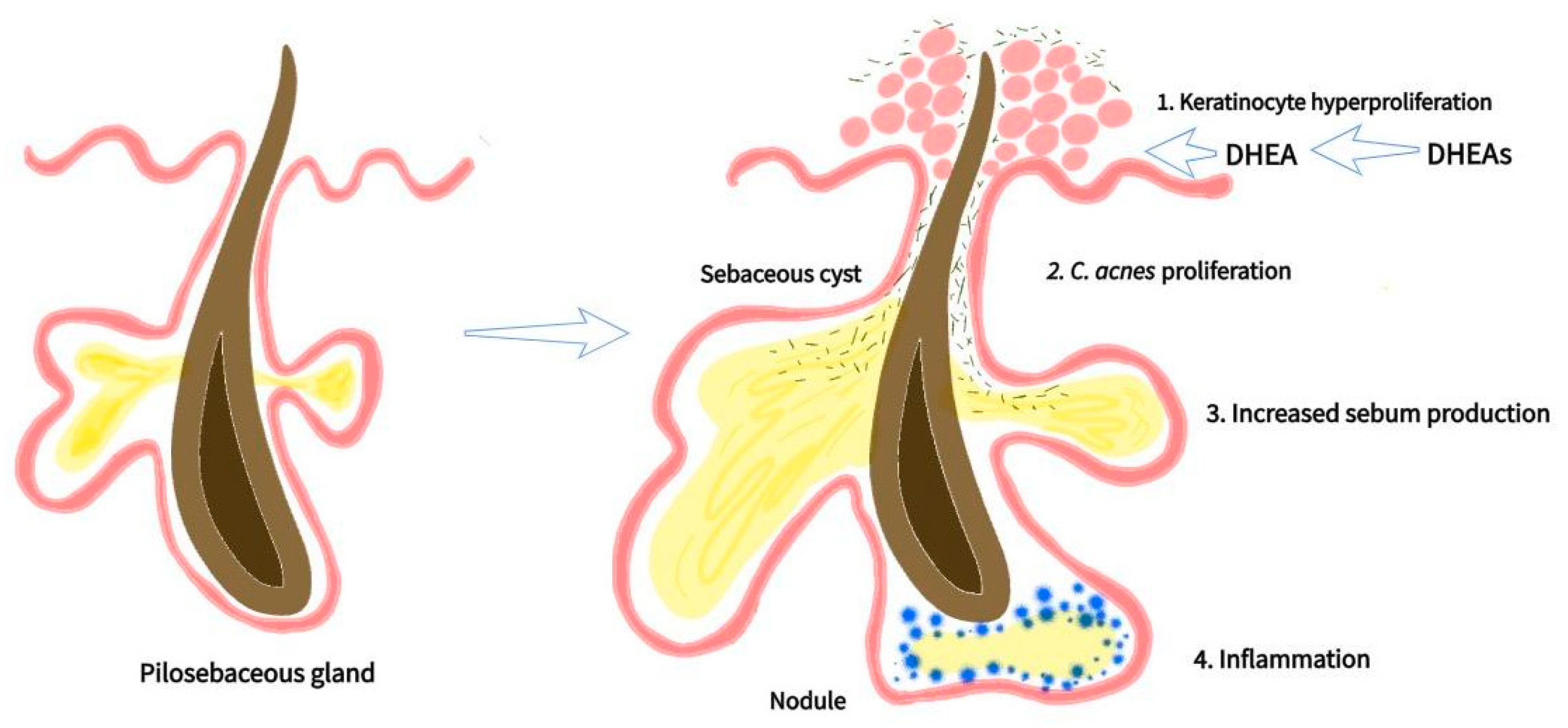
1. Introduction
2. New Insights into the Pathophysiology of AFA and PCOS
2.1. Hyperandrogenism and Increased AR Sensitivity
2.2. Endocrine Disturbances Affecting Follicle Maturation and Ovulation
2.3. Genetic Insights in AFA and PCOS
3. Classical and Novel Therapeutic Approaches in AFA
3.1. Topical Therapy
3.2. Systemic Therapy
3.3. Therapeutic Guidelines for Acne
3.4. Alternative Holistic Theraphy (Herbal Medicines)
3.5. Adjunctive Therapy
3.6. Lifestyle (Diet and Exercise)
3.7. Novel Therapeutic Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Branisteanu, D.E.; Toader, M.P.; Porumb, E.A.; Serban, I.L.; Pinzariu, A.C.; Branisteanu, C.I.; Vicovan, A.; Dimitriu, A.; Fartusnic, I.A.; Boda, D.; et al. Adult female acne: Clinical and therapeutic particularities (Review). Exp. Ther. Med. 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Q.; Liu, Y.; Liu, T.; Tang, W.; Li, S. The prevalence of acne in Mainland China: A systematic review and meta-analysis. BMJ Open 2017, 7, e015354. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Carmina, E.; Dreno, B.; Lucky, W.A.; Agak, W.G.; Dokras, A.; Kim, J.J.; Lobo, R.A.; Ramezani Tehrani, F.; Dumesic, D. Female Adult Acne and Androgen Excess: A Report from the Multidisciplinary Androgen Excess and PCOS Committee. J. Endocr. Soc. 2022, 6, bvac003. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.C.; Cheng, C.E.; Hillebrand, G.G.; Miyamoto, K.; Kimball, A.B. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Cannavò, S.; Guarneri, F.; Cannavò, S.P.; Vaccaro, M.; Guarneri, B. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm. Venereol. 2004, 84, 201–204. [Google Scholar] [PubMed]
- da Cunha, M.G.; Fonseca, F.L.; Machado, C.D. Androgenic hormone profile of adult women with acne. Dermatology 2013, 226, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Lobo, R.A. Evidence for increased androsterone metabolism in some normoandrogenic women with acne. J. Clin. Endocrinol. Metab. 1993, 76, 1111–1114. [Google Scholar] [PubMed]
- Bagatin, E.; Freitas, T.H.P.; Rivitti-Machado, M.C.; Machado, M.C.R.; Ribeiro, B.M.; Nunes, S.; Rocha, M. Adult female acne: A guide to clinical practice. An. Bras. Dermatol. 2019, 94, 62–75. [Google Scholar] [CrossRef]
- Preneau, S.; Dreno, B. Female acne—A different subtype of teenager acne? J. Eur. Acad. Dermatol. Venereol. 2012, 26, 277–282. [Google Scholar] [CrossRef]
- Crespo, R.P.; Bachega, T.; Mendonça, B.B.; Gomes, L.G. An update of genetic basis of PCOS pathogenesis. Arch. Endocrinol. Metab. 2018, 62, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Graber, E.M.; Thiboutot, D.M. Acne Vulgaris and Acneiform Eruptions. In Fitzpatrick’s Dermatology in General Medicine, 8th ed.; Goldsmith, L.A., Katz, S.I., Gilchrest, B.A., Paller, A.S., Leffell, D.J., Wolff, K., Eds.; McGraw-Hill: New York, NY, USA, 2012; Volume 1, pp. 897–917. [Google Scholar]
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.M.; Gallo, R.L. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome 2018, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Gonzalez, M.; Porter, R. Pathways to inflammation: Acne pathophysiology. Eur. J. Dermatol. 2011, 21, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, S.; Yu, X.; Fang, F.; Zhu, L.; Wang, L.; Zhang, X.; Yang, C.; Qian, Q.; Zhu, T. Association of different cell types and inflammation in early acne vulgaris. Front. Immunol. 2024, 15, 1275269. [Google Scholar] [CrossRef]
- Jin, Z.; Song, Y.; He, L. A review of skin immune processes in acne. Front. Immunol. 2023, 14, 1324930. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Morohashi, M. Pathogenesis of acne. Med. Electron. Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef]
- Peigné, M.; Villers-Capelle, A.; Robin, G.; Dewailly, D. Hyperandrogenism in women. Presse Med. 2013, 42, 1487–1499. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE; ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Franks, S.; McCarthy, M.I.; Hardy, K. Development of polycystic ovary syndrome: Involvement of genetic and environmental factors. Int. J. Androl. 2006, 29, 278–285; discussion 286–290. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Nelson, V.L.; Legro, R.S.; Strauss, J.F., III; McAllister, J.M. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol. Endocrinol. 1999, 13, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Pastor, C.L.; Griffin-Korf, M.L.; Aloi, J.A.; Evans, W.S.; Marshall, J.C. Polycystic ovary syndrome: Evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J. Clin. Endocrinol. Metab. 1998, 83, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Kalro, B.N.; Loucks, T.L.; Berga, S.L. Neuromodulation in polycystic ovary syndrome. Obstet. Gynecol. Clin. N. Am. 2001, 28, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Stark, J.; Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update 2008, 14, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Abbott, D.H. Implications of polycystic ovary syndrome on oocyte development. Semin. Reprod. Med. 2008, 26, 53–61. [Google Scholar] [CrossRef][Green Version]
- La Marca, A.; Sighinolfi, G.; Radi, D.; Argento, C.; Baraldi, E.; Artenisio, A.C.; Stabile, G.; Volpe, A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update 2010, 16, 113–130. [Google Scholar] [CrossRef]
- De Leo, V.; la Marca, A.; Petraglia, F. Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr. Rev. 2003, 24, 633–667. [Google Scholar] [CrossRef]
- Bremer, A.A.; Miller, W.L. The serine phosphorylation hypothesis of polycystic ovary syndrome: A unifying mechanism for hyperandrogenemia and insulin resistance. Fertil. Steril. 2008, 89, 1039–1048. [Google Scholar] [CrossRef]
- Baillargeon, J.P.; Nestler, J.E.; Ostlund, R.E.; Apridonidze, T.; Diamanti-Kandarakis, E. Greek hyperinsulinemic women, with or without polycystic ovary syndrome, display altered inositols metabolism. Hum. Reprod. 2008, 23, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Rabson, S.M.; Mendenhall, E.N. Familial hypertrophy of pineal body, hyperplasia of adrenal cortex and diabetes mellitus; report of 3 cases. Am. J. Clin. Pathol. 1956, 26, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Flier, J.S.; Bar, R.S.; Archer, J.A.; Gorden, P.; Martin, M.M.; Roth, J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N. Engl. J. Med. 1976, 294, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.; Cochran, E.; Moran, S.A.; Skarulis, M.C.; Oral, E.A.; Taylor, S.; Gorden, P. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): A 30-year prospective. Medicine 2004, 83, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Burghen, G.A.; Givens, J.R.; Kitabchi, A.E. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J. Clin. Endocrinol. Metab. 1980, 50, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Tee, M.K. The post-translational regulation of 17,20 lyase activity. Mol. Cell. Endocrinol. 2015, 408, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. An overview of acne. J. Investig. Dermatol. 1974, 62, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, S.Z.; Orawa, H.; Zouboulis, C.C. Prevalence, severity, and severity risk factors of acne in high school pupils: A community-based study. J. Investig. Dermatol. 2009, 129, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Pang, Y.; Zhu, H.; Qu, L.; Xiao, T.; Wei, H.C.; Chen, H.D.; He, C.D. The epidemiology of adolescent acne in North East China. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Bataille, V.; Snieder, H.; MacGregor, A.J.; Sasieni, P.; Spector, T.D. The influence of genetics and environmental factors in the pathogenesis of acne: A twin study of acne in women. J. Investig. Dermatol. 2002, 119, 1317–1322. [Google Scholar] [CrossRef][Green Version]
- Pang, Y.; He, C.D.; Liu, Y.; Wang, K.B.; Xiao, T.; Wang, Y.K.; Zhu, H.; Wei, B.; Zhao, N.; Jiang, Y.; et al. Combination of short CAG and GGN repeats in the androgen receptor gene is associated with acne risk in North East China. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1445–1451. [Google Scholar] [CrossRef]
- Yang, J.K.; Wu, W.J.; Qi, J.; He, L.; Zhang, Y.P. TNF-308 G/A polymorphism and risk of acne vulgaris: A meta-analysis. PLoS ONE 2014, 9, e87806. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.M.; Xie, H.F.; Yang, T.; Hu, Y.H.; Li, J.; Wang, W.Z. Association study of tumor necrosis factor receptor type 2 M196R and toll-like receptor 2 Arg753Gln polymorphisms with acne vulgaris in a Chinese Han ethnic group. Dermatology 2010, 221, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Szabó, K.; Tax, G.; Kis, K.; Szegedi, K.; Teodorescu-Brinzeu, D.G.; Diószegi, C.; Koreck, A.; Széll, M.; Kemény, L. Interleukin-1A +4845(G> T) polymorphism is a factor predisposing to acne vulgaris. Tissue Antigens 2010, 76, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z. Genetic Variants Associated with Acne Vulgaris. Int. J. Gen. Med. 2023, 16, 3843–3856. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, Z.; Yu, H.; Cheng, B.; Tang, W.; Dong, Y.; Xiao, C. The relationship between CYP17 -34T/C polymorphism and acne in Chinese subjects revealed by sequencing. Dermatology 2006, 212, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Chamaie-Nejad, F.; Saeidi, S.; Najafi, F.; Ebrahimi, A.; Rahimi, Z.; Shakiba, E.; Rahimi, Z. Association of the CYP17 MSP AI (T-34C) and CYP19 codon 39 (Trp/Arg) polymorphisms with susceptibility to acne vulgaris. Clin. Exp. Dermatol. 2018, 43, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ostlere, L.S.; Rumsby, G.; Holownia, P.; Jacobs, H.S.; Rustin, M.H.; Honour, J.W. Carrier status for steroid 21-hydroxylase deficiency is only one factor in the variable phenotype of acne. Clin. Endocrinol. 1998, 48, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidis, A.; Drakoulis, N.; Roots, I.; Orfanos, C.E.; Zouboulis, C.C. Polymorphisms in the human cytochrome P-450 1A1 gene (CYP1A1) as a factor for developing acne. Dermatology 1998, 196, 171–175. [Google Scholar] [CrossRef]
- Blanché, H.; Vexiau, P.; Clauin, S.; Le Gall, I.; Fiet, J.; Mornet, E.; Dausset, J.; Bellanné-Chantelot, C. Exhaustive screening of the 21-hydroxylase gene in a population of hyperandrogenic women. Hum. Genet. 1997, 101, 56–60. [Google Scholar] [CrossRef]
- Kahsar-Miller, M.D.; Nixon, C.; Boots, L.R.; Go, R.C.; Azziz, R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil. Steril. 2001, 75, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Louwers, Y.V.; de Jong, F.H.; van Herwaarden, N.A.; Stolk, L.; Fauser, B.C.; Uitterlinden, A.G.; Laven, J.S. Variants in SULT2A1 affect the DHEA sulphate to DHEA ratio in patients with polycystic ovary syndrome but not the hyperandrogenic phenotype. J. Clin. Endocrinol. Metab. 2013, 98, 3848–3855. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Carmina, E.; Azziz, R. DHEA, DHEAS and PCOS. J. Steroid Biochem. Mol. Biol. 2015, 145, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Draper, N.; Walker, E.A.; Bujalska, I.J.; Tomlinson, J.W.; Chalder, S.M.; Arlt, W.; Lavery, G.G.; Bedendo, O.; Ray, D.W.; Laing, I.; et al. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat. Genet. 2003, 34, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Gorsic, L.K.; Kosova, G.; Werstein, B.; Sisk, R.; Legro, R.S.; Hayes, M.G.; Teixeira, J.M.; Dunaif, A.; Urbanek, M. Pathogenic Anti-Müllerian Hormone Variants in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Pellatt, L.; Hanna, L.; Brincat, M.; Galea, R.; Brain, H.; Whitehead, S.; Mason, H. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J. Clin. Endocrinol. Metab. 2007, 92, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lv, Y.; Li, L.; Chen, Z.J. Genetic Studies on Polycystic Ovary Syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 56–65. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhao, H.; He, L.; Shi, Y.; Qin, Y.; Shi, Y.; Li, Z.; You, L.; Zhao, J.; Liu, J.; et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 2011, 43, 55–59. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Shi, Y.; Cao, Y.; Yang, D.; Li, Z.; Zhang, B.; Liang, X.; Li, T.; Chen, J.; et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 2012, 44, 1020–1025. [Google Scholar] [CrossRef]
- Lee, H.; Oh, J.Y.; Sung, Y.A.; Chung, H.; Kim, H.L.; Kim, G.S.; Cho, Y.S.; Kim, J.T. Genome-wide association study identified new susceptibility loci for polycystic ovary syndrome. Hum. Reprod. 2015, 30, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.G.; Urbanek, M.; Ehrmann, D.A.; Armstrong, L.L.; Lee, J.Y.; Sisk, R.; Karaderi, T.; Barber, T.M.; McCarthy, M.I.; Franks, S.; et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat. Commun. 2015, 6, 7502. [Google Scholar] [CrossRef] [PubMed]
- Day, F.R.; Hinds, D.A.; Tung, J.Y.; Stolk, L.; Styrkarsdottir, U.; Saxena, R.; Bjonnes, A.; Broer, L.; Dunger, D.B.; Halldorsson, B.V.; et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat. Commun. 2015, 6, 8464. [Google Scholar] [CrossRef]
- Heng, A.H.S.; Say, Y.H.; Sio, Y.Y.; Ng, Y.T.; Chew, F.T. Gene variants associated with acne vulgaris presentation and severity: A systematic review and meta-analysis. BMC Med. Genom. 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Baroud, S.; Wu, J.; Zouboulis, C.C. Acne Syndromes and Mosaicism. Biomedicines 2021, 9, 1735. [Google Scholar] [CrossRef]
- Xu, N.; Azziz, R.; Goodarzi, M.O. Epigenetics in polycystic ovary syndrome: A pilot study of global DNA methylation. Fertil. Steril. 2010, 94, 781–783.e1. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wang, F.F.; Yin, R.; Ding, G.L.; El-Prince, M.; Gao, Q.; Shi, B.W.; Pan, H.H.; Huang, Y.T.; Jin, M.; et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: Hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. 2012, 90, 911–923. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Trojanowski, S.; Kociszewska, A.; Szewczyk, G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)—Searching for Epigenetic Factors. Int. J. Mol. Sci. 2022, 23, 14663. [Google Scholar] [CrossRef]
- Melnik, B.C. Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment. Cells 2023, 12, 2600. [Google Scholar] [CrossRef]
- Wang, H.; Dang, T.; Feng, J.; Wu, W.; He, L.; Yang, J. Identification of differentially methylated genes for severe acne by genome-wide DNA methylation and gene expression analysis. Epigenetics 2023, 18, 2199373. [Google Scholar] [CrossRef]
- Liu, L.; Xue, Y.; Chen, J.; Li, Y.; Chen, T.; Pan, X.; Zhong, J.; Shao, X.; Chen, Y.; Chen, J. DNA methylation profiling and integrative multi-omics analysis of skin samples reveal important contribution of epigenetics and immune response in the pathogenesis of acne vulgaris. Clin. Immunol. 2023, 255, 109773. [Google Scholar] [CrossRef] [PubMed]
- Goulden, V.; Clark, S.M.; Cunliffe, W.J. Post-adolescent acne: A review of clinical features. Br. J. Dermatol. 1997, 136, 66–70. [Google Scholar] [CrossRef]
- Dréno, B.; Layton, A.; Zouboulis, C.C.; López-Estebaranz, J.L.; Zalewska-Janowska, A.; Bagatin, E.; Zampeli, V.A.; Yutskovskaya, Y.; Harper, J.C. Adult female acne: A new paradigm. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1063–1070. [Google Scholar] [CrossRef]
- Gollnick, H.P.; Bettoli, V.; Lambert, J.; Araviiskaia, E.; Binic, I.; Dessinioti, C.; Galadari, I.; Ganceviciene, R.; Ilter, N.; Kaegi, M.; et al. A consensus-based practical and daily guide for the treatment of acne patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1480–1490. [Google Scholar] [CrossRef]
- Gollnick, H.P.; Graupe, K.; Zaumseil, R.P. Comparison of combined azelaic acid cream plus oral minocycline with oral isotretinoin in severe acne. Eur. J. Dermatol. 2001, 11, 538–544. [Google Scholar] [PubMed]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef]
- Zaenglein, A.L. Acne Vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Tanno, O.; Ota, Y.; Kitamura, N.; Katsube, T.; Inoue, S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br. J. Dermatol. 2000, 143, 524–531. [Google Scholar] [CrossRef]
- Kim, J.; Ochoa, M.T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety. Exp. Dermatol. 2019, 28 (Suppl. S1), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tempark, T.; Satapornpong, P.; Rerknimitr, P.; Nakkam, N.; Saksit, N.; Wattanakrai, P.; Jantararoungtong, T.; Koomdee, N.; Mahakkanukrauh, A.; Tassaneeyakul, W.; et al. Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13: 01 allele in the Thai population. Pharmacogenet. Genom. 2017, 27, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H. Androgens and acne: Perspectives on clascoterone, the first topical androgen receptor antagonist. Expert Opin. Pharmacother. 2021, 22, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Rosette, C.; Agan, F.J.; Mazzetti, A.; Moro, L.; Gerloni, M. Cortexolone 17α-propionate (Clascoterone) Is a Novel Androgen Receptor Antagonist that Inhibits Production of Lipids and Inflammatory Cytokines from Sebocytes In Vitro. J. Drugs Dermatol. 2019, 18, 412–418. [Google Scholar] [PubMed]
- Savage, L.J.; Layton, A.M. Treating acne vulgaris: Systemic, local and combination therapy. Expert Rev. Clin. Pharmacol. 2010, 3, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Garner, S.E.; Eady, A.; Bennett, C.; Newton, J.N.; Thomas, K.; Popescu, C.M. Minocycline for acne vulgaris: Efficacy and safety. Cochrane Database Syst. Rev. 2012, 2012, CD002086. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Nunley, J.R. Use of systemic agents in the treatment of acne vulgaris. Am. Fam. Physician 2000, 62, 1823–1830. [Google Scholar] [PubMed]
- Ochsendorf, F. Systemic antibiotic therapy of acne vulgaris. J. Dtsch. Dermatol. Ges. 2006, 4, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.D.; Bowe, W.P.; Heughebaert, C.; Shalita, A.R. Therapeutic considerations for severe nodular acne. Am. J. Clin. Dermatol. 2011, 12, 7–14. [Google Scholar] [CrossRef]
- Tzellos, T.; Zampeli, V.; Makrantonaki, E.; Zouboulis, C.C. Treating acne with antibiotic-resistant bacterial colonization. Expert Opin. Pharmacother. 2011, 12, 1233–1247. [Google Scholar] [CrossRef]
- Thiboutot, D.M.; Dréno, B.; Abanmi, A.; Alexis, A.F.; Araviiskaia, E.; Barona Cabal, M.I.; Bettoli, V.; Casintahan, F.; Chow, S.; da Costa, A.; et al. Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2018, 78, S1–S23.e1. [Google Scholar] [CrossRef]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef]
- Carmina, E. Cutaneous manifestations of polycystic ovary syndrome. Curr. Opin. Endocrinol. Metab. Res. 2020, 12, 49–52. [Google Scholar] [CrossRef]
- Patiyasikunt, M.; Chancheewa, B.; Asawanonda, P.; Noppakun, N.; Kumtornrut, C. Efficacy and tolerability of low-dose spironolactone and topical benzoyl peroxide in adult female acne: A randomized, double-blind, placebo-controlled trial. J. Dermatol. 2020, 47, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Arowojolu, A.O.; Gallo, M.F.; Lopez, L.M.; Grimes, D.A.; Garner, S.E. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst. Rev. 2009, 8, CD004425. [Google Scholar]
- Koo, E.B.; Petersen, T.D.; Kimball, A.B. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J. Am. Acad. Dermatol. 2014, 71, 450–459. [Google Scholar] [CrossRef]
- Carmina, E.; Lobo, R.A. A comparison of the relative efficacy of antiandrogens for the treatment of acne in hyperandrogenic women. Clin. Endocrinol. 2002, 57, 231–234. [Google Scholar] [CrossRef]
- Dreno, B.; Moyse, D.; Alirezai, M.; Amblard, P.; Auffret, N.; Beylot, C.; Bodokh, I.; Chivot, M.; Daniel, F.; Humbert, P.; et al. Multicenter randomized comparative double-blind controlled clinical trial of the safety and efficacy of zinc gluconate versus minocycline hydrochloride in the treatment of inflammatory acne vulgaris. Dermatology 2001, 203, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis—Back to the future? Gut Pathog. 2011, 3, 1. [Google Scholar] [CrossRef]
- Kazandjieva, J.; Dimitrova, J.; Sankeva, M.; Yankov, D.; Bocheva, V.; Kircheva, K.; Gincheva, V.; Gospodinova, K.; Andasorova, R.; Milanova, M.; et al. Efficacy of a retinoid complex plus anti-inflammatory component cream alone or in combination with prebiotic food supplement in adult acne: A randomized, assessor-blinded, parallel-group, multicenter trial on 184 women. J. Cosmet. Dermatol. 2022, 21, 5716–5722. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Grant, P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother. Res. 2010, 24, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Akdoğan, M.; Tamer, M.N.; Cüre, E.; Cüre, M.C.; Köroğlu, B.K.; Delibaş, N. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother. Res. 2007, 21, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh Bashtian, M.; Emami, S.A.; Mousavifar, N.; Esmaily, H.A.; Mahmoudi, M.; Mohammad Poor, A.H. Evaluation of Fenugreek (Trigonella foenum-graceum L.), Effects Seeds Extract on Insulin Resistance in Women with Polycystic Ovarian Syndrome. Iran. J. Pharm. Res. 2013, 12, 475–481. [Google Scholar] [PubMed]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Hwang, J.; Nasreen, I.; Sicignano, D.; Pasupuleti, V.; Snow-Caroti, K.; White, C.M. Impact of Berberine or Berberine Combination Products on Lipoprotein, Triglyceride and Biological Safety Marker Concentrations in Patients with Hyperlipidemia: A Systematic Review and Meta-Analysis. J. Diet. Suppl. 2024, 21, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Giacosa, A.; Fazia, T.; Bernardinelli, L.; Gasparri, C.; Peroni, G.; Perna, S. Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest-Post-Test Explanatory Study. Nutrients 2021, 13, 3665. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.J.; Pyun, B.J.; Lee, H.W. Licorice ethanol extract improves symptoms of polycytic ovary syndrome in Letrozole-induced female rats. Integr. Med. Res. 2018, 7, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef]
- Fox, L.; Csongradi, C.; Aucamp, M.; du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef]
- Haedersdal, M.; Togsverd-Bo, K.; Wulf, H.C. Evidence-based review of lasers, light sources and photodynamic therapy in the treatment of acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wu, Y.; Xu, X.; Gao, X.; Chen, H.D.; Li, Y. Evidence-based review of photodynamic therapy in the treatment of acne. Eur. J. Dermatol. 2014, 24, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Pathmarajah, P.; Peterknecht, E.; Cheung, K.; Elyoussfi, S.; Muralidharan, V.; Bewley, A. Acne Vulgaris in Skin of Color: A Systematic Review of the Effectiveness and Tolerability of Current Treatments. J. Clin. Aesthet. Dermatol. 2022, 15, 43–68. [Google Scholar]
- Melnik, B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 371–388. [Google Scholar] [CrossRef]
- Lynn, D.D.; Umari, T.; Dunnick, C.A.; Dellavalle, R.P. The epidemiology of acne vulgaris in late adolescence. Adolesc. Health Med. Ther. 2016, 7, 13–25. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 527–532. [Google Scholar] [CrossRef]
- Chilicka, K.; Rusztowicz, M.; Rogowska, A.M.; Szyguła, R.; Asanova, B.; Nowicka, D. Efficacy of Hydrogen Purification and Cosmetic Acids in the Treatment of Acne Vulgaris: A Preliminary Report. J. Clin. Med. 2022, 11, 6269. [Google Scholar] [CrossRef]
- Hajam, I.A.; Katiki, M.; McNally, R.; Lázaro-Díez, M.; Kolar, S.; Chatterjee, A.; Gonzalez, C.; Paulchakrabarti, M.; Choudhury, B.; Caldera, J.R.; et al. Functional divergence of a bacterial enzyme promotes healthy or acneic skin. Nat. Commun. 2023, 14, 8061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, H.L.; Bu, X.L.; Zhang, J.B.; Lu, Y.G. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann. Transl. Med. 2022, 10, 645. [Google Scholar] [CrossRef]
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Picardo, M.; Cardinali, C.; La Placa, M.; Lewartowska-Białek, A.; Lora, V.; Micali, G.; Montisci, R.; Morbelli, L.; Nova, A.; Parodi, A.; et al. Efficacy and safety of N-acetyl-GED-0507-34-LEVO gel in patients with moderate-to severe facial acne vulgaris: A phase IIb randomized double-blind, vehicle-controlled trial. Br. J. Dermatol. 2022, 187, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Firlej, E.; Kowalska, W.; Szymaszek, K.; Roliński, J.; Bartosińska, J. The Role of Skin Immune System in Acne. J. Clin. Med. 2022, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, C.; Wang, J.; Yang, L.; Lv, Z.; An, Q.; Wang, Y.; Shao, X.; Wang, F.; Huo, T.; et al. Rhizoma Paridis saponins attenuate Gram-negative bacteria-induced inflammatory acne by binding to KEAP1 and modulating Nrf2 and MAPK pathways. J. Cell. Mol. Med. 2024, 28, e18146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhen, N.; Liao, D.; Niu, J.; Liu, R.; Li, Z.; Lei, Z.; Yang, Z. Application of bacteriophage φPaP11-13 attenuates rat Cutibacterium acnes infection lesions by promoting keratinocytes apoptosis via inhibiting PI3K/Akt pathway. Microbiol. Spectr. 2024, 12, e0283823. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xie, M.; Zhang, X.; Qiu, Z.; Pu, Z.; Huang, S.; Li, B. Meconopsis quintuplinervia Regel Improves Cutibacterium acnes-Induced Inflammatory Responses in a Mouse Ear Edema Model and Suppresses Pro-Inflammatory Chemokine Production via the MAPK and NF-κB Pathways in RAW264.7 Cells. Ann. Dermatol. 2023, 35, 408–416. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Zhan, Y.; Yi, Y.; Jiang, Q.; Zhang, Q.; Wu, Y.; Wu, M. Neutrophil extracellular trap-related mechanisms in acne vulgaris inspire a novel treatment strategy with adipose-derived stem cells. Sci. Rep. 2024, 14, 1521. [Google Scholar] [CrossRef]


| Name | Gene ID | Description | Location | Aliases | MIM |
|---|---|---|---|---|---|
| FST | 10468 | Follistatin (Homo sapiens) | Chromosome 12, NC_000012.12 (102395874..102481839, complement) | IGF, IGF-I, IGFI, MGF | 14,440 |
| IL1B | 3553 | Interleukin 1 beta (Homo sapiens) | Chromosome 2, NC_000002.12 (112829751..112836779, complement) | IL-1, IL1-BETA, IL1F2, IL1beta | 147,720 |
| AR | 367 | Androgen receptor (Homo sapiens) | Chromosome X, NC_000023.11 (67544021..67730619) | AIS8, DHTR, HUMARA, HYSP1, KD, NR3C4, SBMA, SMAX1, TFM, AR | 313,700 |
| VDR | 7421 | Vitamin D receptor (Homo sapiens) | Chromosome 12, NC_000012.12 (47841537..47904994, complement) | NR1I1, PPP1R163 | 601,769 |
| CYP17A1 | 1586 | Cytochrome P450 family 17 subfamily A member 1 (Homo sapiens) | Chromosome 10, NC_000010.11 (102830531..102837413, complement) | CPT7, CYP17, P450C17, S17AH | 609,300 |
| IGF1 | 24482 | Insulin-like growth factor 1 (Rattus norvegicus) | Chromosome 7, NC_086025.1 (24169608..24249446) | IGF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amuzescu, A.; Tampa, M.; Matei, C.; Georgescu, S.R. Adult Female Acne: Recent Advances in Pathophysiology and Therapeutic Approaches. Cosmetics 2024, 11, 74. https://doi.org/10.3390/cosmetics11030074
Amuzescu A, Tampa M, Matei C, Georgescu SR. Adult Female Acne: Recent Advances in Pathophysiology and Therapeutic Approaches. Cosmetics. 2024; 11(3):74. https://doi.org/10.3390/cosmetics11030074
Chicago/Turabian StyleAmuzescu, Andreea, Mircea Tampa, Clara Matei, and Simona Roxana Georgescu. 2024. "Adult Female Acne: Recent Advances in Pathophysiology and Therapeutic Approaches" Cosmetics 11, no. 3: 74. https://doi.org/10.3390/cosmetics11030074
APA StyleAmuzescu, A., Tampa, M., Matei, C., & Georgescu, S. R. (2024). Adult Female Acne: Recent Advances in Pathophysiology and Therapeutic Approaches. Cosmetics, 11(3), 74. https://doi.org/10.3390/cosmetics11030074









