Nicotinamide Riboside Ameliorates Hyperpigmentation on Photo-Irradiated Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Chemical Treatments
2.3. UVB Exposure
2.4. Cell Viability Assay (CCK-8)
2.5. Cell Sulforhodamine B (SRB) Assay
2.6. Melanin Contents
2.7. Mushroom Tyrosinase Inhibition Assay
2.8. Quantitative Polymerase Chain Reaction (QPCR)
2.9. Statistical Analysis
3. Results
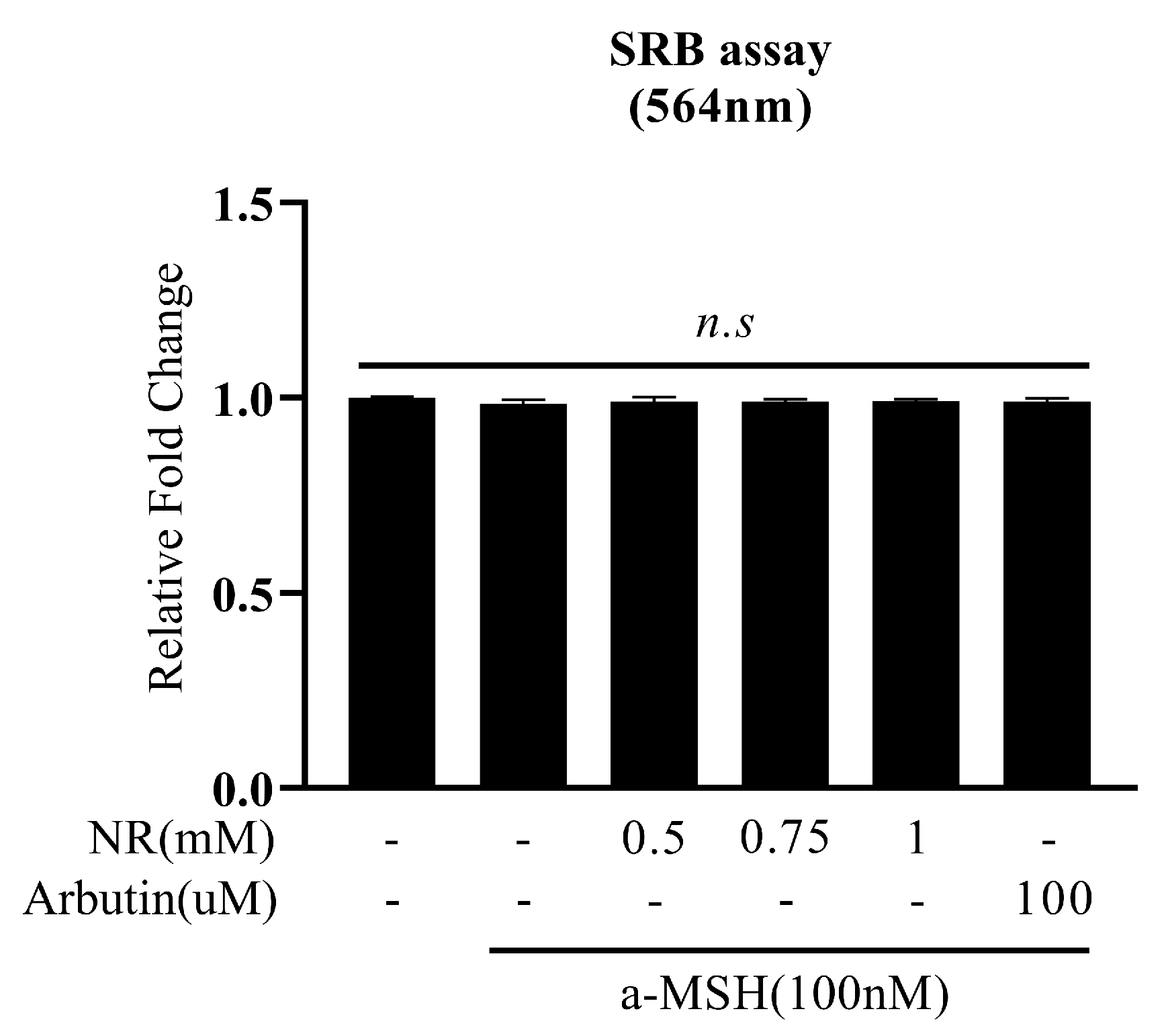
3.1. Effect of NR on Cell Survival
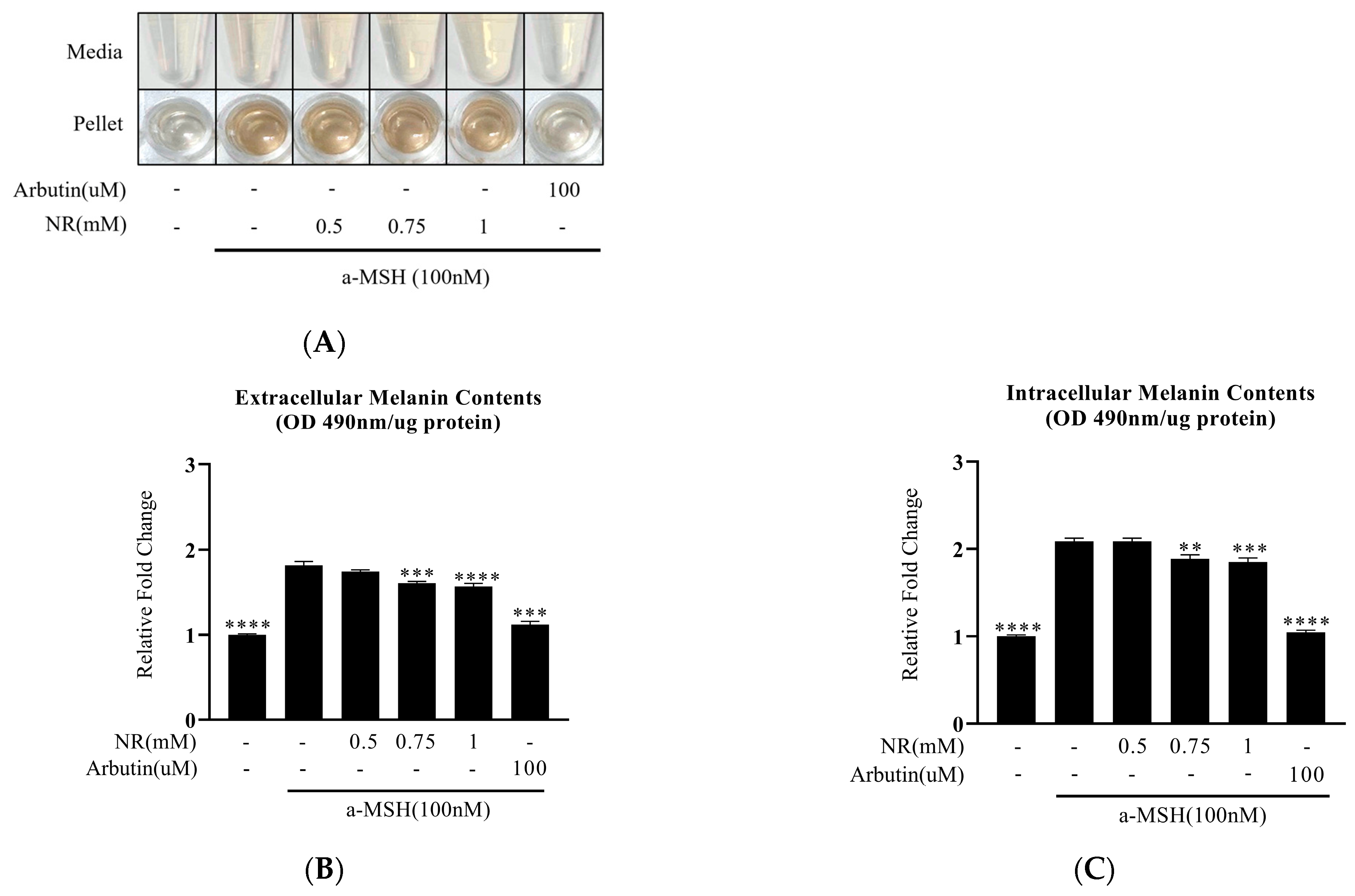
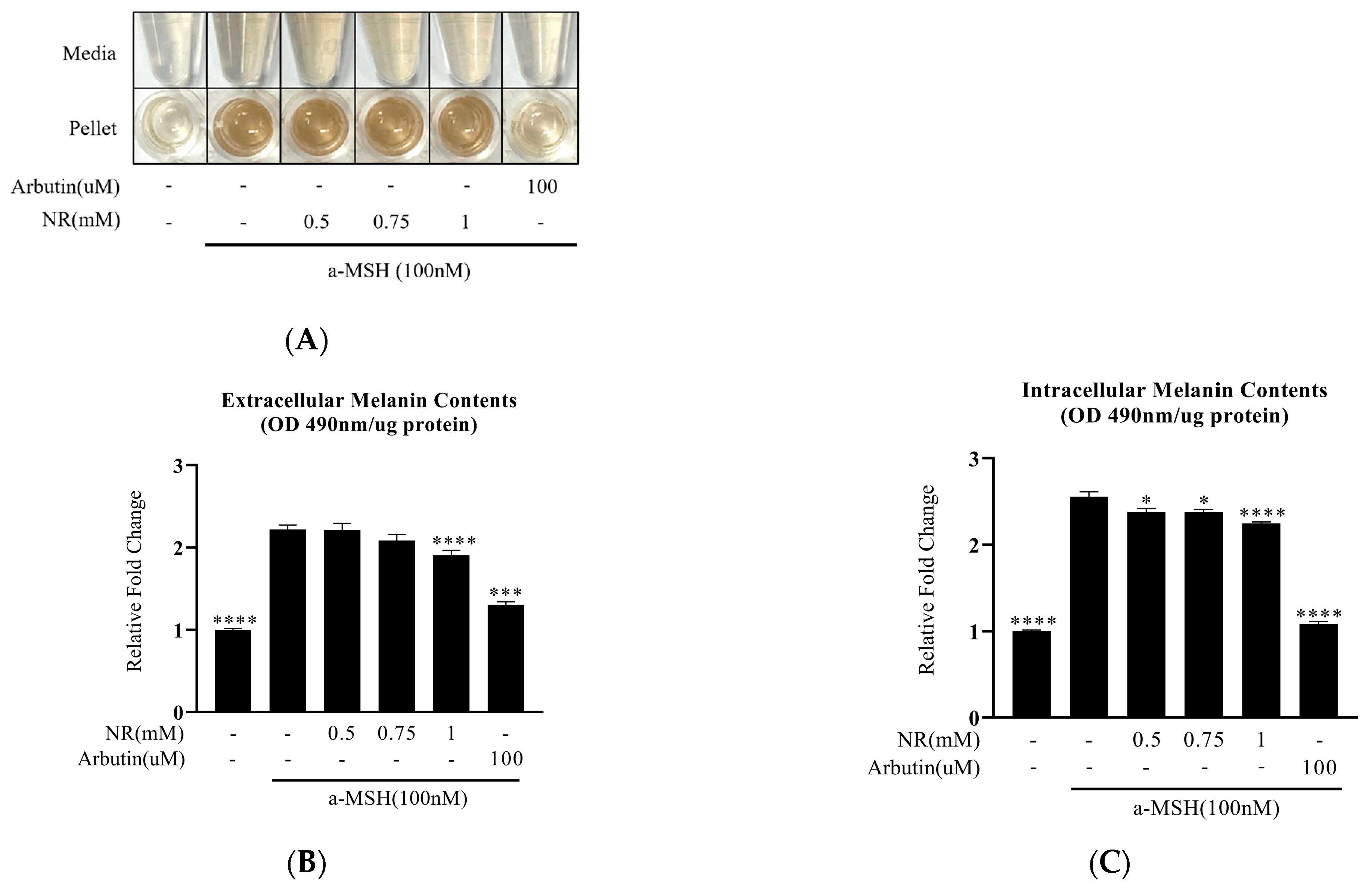
3.2. Decrease in α-MSH-Induced Melanin Content by NR
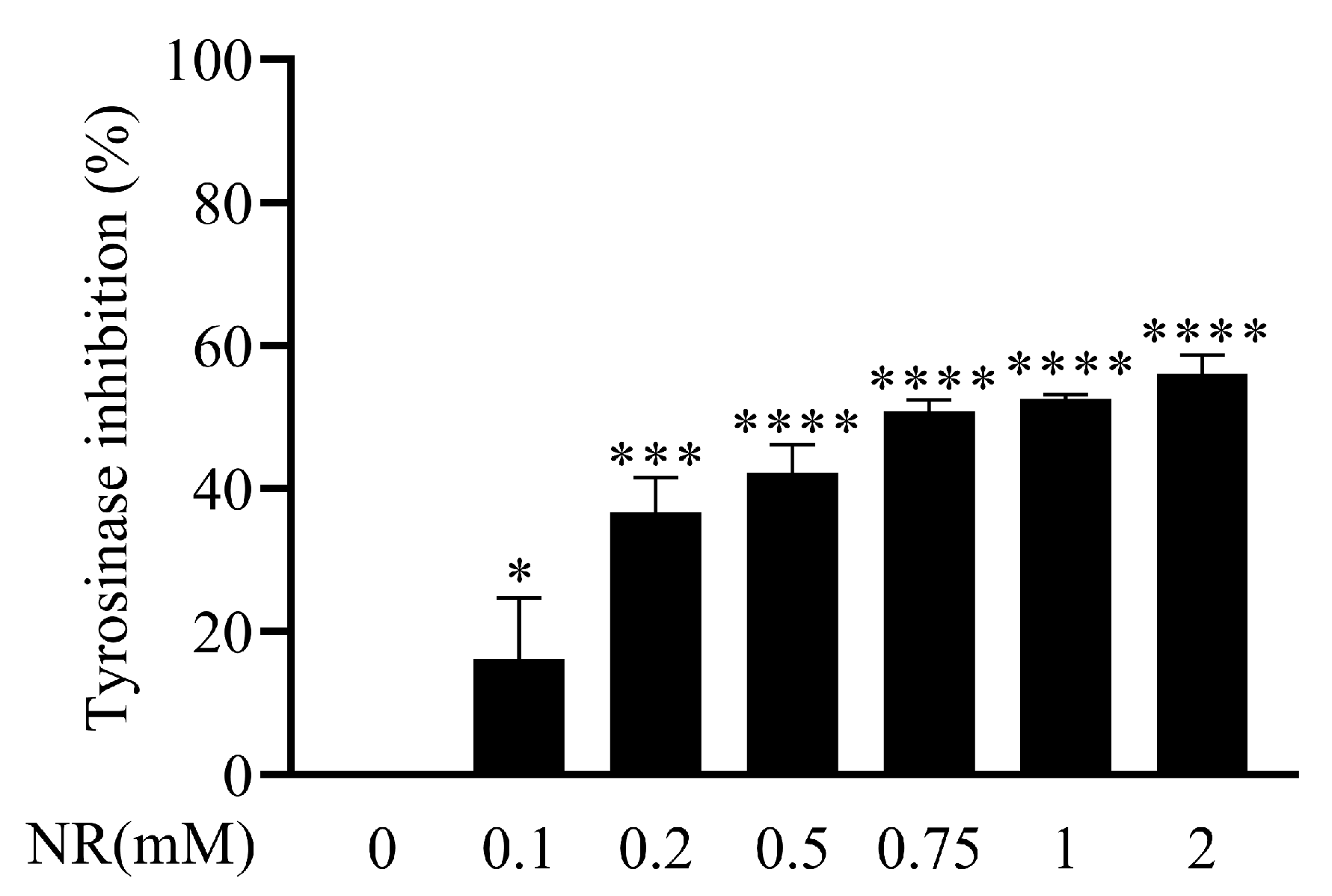
3.3. Direct Inhibition of Tyrosinase Activity by NR
3.4. Regulation of Melanogenic Genes by NR
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Plensdorf, S.; Livieratos, M.; Dada, N. Pigmentation Disorders: Diagnosis and Management. Am. Fam. Physician 2017, 96, 797–804. [Google Scholar] [PubMed]
- Bolognia, J.; Murray, M.; Pawelek, J. UVB-induced melanogenesis may be mediated through the MSH-receptor system. J. Investig. Dermatol. 1989, 92, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Prota, G. Recent advances in the chemistry of melanogenesis in mammals. J. Investig. Dermatol. 1980, 75, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Jangda, A.; Voloshyna, D.; Ramesh, K.; Bseiso, A.; Shaik, T.A.; Al Barznji, S.; Usama, M.; Saleem, F.; Ghaffari, M.A.Z. Hyperpigmentation as a Primary Symptom of Vitamin B12 Deficiency: A Case Report. Cureus 2022, 14, e29008. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Ng, M.J. Cutaneous lesions and vitamin B12 deficiency: An often-forgotten link. Can. Fam. Physician 2008, 54, 529–532. [Google Scholar] [PubMed]
- Cohen, P.R. Linea Nigra: Case Report of a Woman With a Pregnancy-Associated Linear Streak of Cutaneous Hyperpigmentation on Her Abdomen From the Umbilicus to the Pubic Symphysis. Cureus 2023, 15, e48408. [Google Scholar] [CrossRef]
- Schepsky, A.; Bruser, K.; Gunnarsson, G.J.; Goodall, J.; Hallsson, J.H.; Goding, C.R.; Steingrimsson, E.; Hecht, A. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol. Cell. Biol. 2006, 26, 8914–8927. [Google Scholar] [CrossRef]
- Shibahara, S.; Takeda, K.; Yasumoto, K.; Udono, T.; Watanabe, K.; Saito, H.; Takahashi, K. Microphthalmia-associated transcription factor (MITF): Multiplicity in structure, function, and regulation. J. Investig. Dermatol. Symp. Proc. 2001, 6, 99–104. [Google Scholar] [CrossRef]
- Hearing, V.J.; Jiménez, M. Mammalian tyrosinase--the critical regulatory control point in melanocyte pigmentation. Int. J. Biochem. 1987, 19, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Bieganowski, P.; Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 2004, 117, 495–502. [Google Scholar] [CrossRef]
- Guyton, J.R.; Bays, H.E. Safety considerations with niacin therapy. Am. J. Cardiol. 2007, 99, 22C–31C. [Google Scholar] [CrossRef] [PubMed]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. [Google Scholar] [CrossRef]
- Dollerup, O.L.; Christensen, B.; Svart, M.; Schmidt, M.S.; Sulek, K.; Ringgaard, S.; Stødkilde-Jørgensen, H.; Møller, N.; Brenner, C.; Treebak, J.T.; et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018, 108, 343–353. [Google Scholar] [CrossRef]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, B.C.; Gudmundsrud, R.; Frank, J.; Hov, A.; Lautrup, S.; Aman, Y.; Røsjø, H.; Brenner, C.; Ziegler, M.; Tysnes, O.B.; et al. Targeting NAD. Mech. Ageing Dev. 2020, 186, 111208. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Sharma, C.; Donu, D.; Cen, Y. Emerging Role of Nicotinamide Riboside in Health and Diseases. Nutrients 2022, 14, 3889. [Google Scholar] [CrossRef]
- Li, F.; Wu, C.; Wang, G. Targeting NAD Metabolism for the Therapy of Age-Related Neurodegenerative Diseases. Neurosci. Bull. 2024, 40, 218–240. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Du, Z.; Liu, K.; Gong, S. Nicotinamide riboside protects noise-induced hearing loss by recovering the hair cell ribbon synapses. Neurosci. Lett. 2020, 725, 134910. [Google Scholar] [CrossRef] [PubMed]
- Lapatto, H.A.K.; Kuusela, M.; Heikkinen, A.; Muniandy, M.; van der Kolk, B.W.; Gopalakrishnan, S.; Pöllänen, N.; Sandvik, M.; Schmidt, M.S.; Heinonen, S.; et al. Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci. Adv. 2023, 9, eadd5163. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD. Nat. Commun. 2018, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, M.V.; Treebak, J.T. What is really known about the effects of nicotinamide riboside supplementation in humans. Sci. Adv. 2023, 9, eadi4862. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Su, X.; Quinn, W.J.; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E.; et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018, 27, 1067–1080.e5. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Lorz, L.R.; Yoo, B.C.; Kim, M.Y.; Cho, J.Y. Anti-Wrinkling and Anti-Melanogenic Effect of. Int. J. Mol. Sci. 2019, 20, 1043. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Park, S.H.; Kim, M.H.; Lee, S.; Cho, Y.K.; Kim, Y.A.; Park, B.J.; Lee, J.; Kang, H.; Cho, J.Y. Anti-Melanogenic Effects of Ethanol Extracts of the Leaves and Roots of. Molecules 2020, 25, 5375. [Google Scholar] [CrossRef]
- Kim, D.E.; Chang, B.Y.; Ham, S.O.; Kim, Y.C.; Kim, S.Y. Neobavaisoflavone Inhibits Melanogenesis through the Regulation of Akt/GSK-3β and MEK/ERK Pathways in B16F10 Cells and a Reconstructed Human 3D Skin Model. Molecules 2020, 25, 2683. [Google Scholar] [CrossRef]
- Lee, H.J.; An, S.; Bae, S.; Lee, J.H. Diarylpropionitrile inhibits melanogenesis via protein kinase A/cAMP-response element-binding protein/microphthalmia-associated transcription factor signaling pathway in α-MSH-stimulated B16F10 melanoma cells. Korean J. Physiol. Pharmacol. 2022, 26, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Liu, S.; Xu, S.Y.; Chen, L.; Shan, Y.H.; Wei, W.; Liang, W.Q.; Gao, J.Q. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine 2013, 20, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.W.; Jiang, Y.; Oh, J.Y.; Jeon, Y.J.; Ryu, B. Ishophloroglucin A Isolated from. Mar. Drugs 2020, 18, 470. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin Suppresses α-MSH-Induced Melanogenesis in B16F10 Mouse Melanoma Cells by Inhibiting Tyrosinase Activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, S.; Yasumoto, K.; Amae, S.; Udono, T.; Watanabe, K.; Saito, H.; Takeda, K. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Res. 2000, 13 (Suppl. S8), 98–102. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Bae, S.; Kim, T.J.; An, S.; Lee, J.H. Nodakenin Inhibits Melanogenesis Via the ERK/MSK1 Signaling Pathway. Pharmazie 2023, 78, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Ohnishi, T.; Mori, T. Melanin reduces ultraviolet-induced DNA damage formation and killing rate in cultured human melanoma cells. J. Investig. Dermatol. 1993, 101, 685–689. [Google Scholar] [CrossRef] [PubMed]
- GVR Report Cover Skin Lightening Products Market Size, Share & Trends Analysis Report by Product (Creams, Cleanser, Mask), by Nature, by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/skin-lightening-products-market (accessed on 1 March 2024).
- Degen, G.H.; SCCS. Opinion of the Scientific Committee on Consumer Safety (SCCS)--Opinion on the safety of the use of β-arbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2015, 73, 866–867. [Google Scholar] [CrossRef]
- Jeon, J.S.; Kim, B.H.; Lee, S.H.; Kwon, H.J.; Bae, H.J.; Kim, S.K.; Park, J.A.; Shim, J.H.; Abd El-Aty, A.M.; Shin, H.C. Simultaneous determination of arbutin and its decomposed product hydroquinone in whitening creams using high-performance liquid chromatography with photodiode array detection: Effect of temperature and pH on decomposition. Int. J. Cosmet. Sci. 2015, 37, 567–573. [Google Scholar] [CrossRef]
- Yang, C.H.; Chang, N.F.; Chen, Y.S.; Lee, S.M.; Lin, P.J.; Lin, C.C. Comparative study on the photostability of arbutin and deoxy arbutin: Sensitivity to ultraviolet radiation and enhanced photostability by the water-soluble sunscreen, benzophenone-4. Biosci. Biotechnol. Biochem. 2013, 77, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [PubMed]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.; Hayashi, N.; Maizumi, H.; Huff, J.; Barrett, J.C. Benzene-, catechol-, hydroquinone- and phenol-induced cell transformation, gene mutations, chromosome aberrations, aneuploidy, sister chromatid exchanges and unscheduled DNA synthesis in Syrian hamster embryo cells. Mutat. Res. 1997, 373, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Marrazzini, A.; Chelotti, L.; Barrai, I.; Loprieno, N.; Barale, R. In vivo genotoxic interactions among three phenolic benzene metabolites. Mutat. Res. 1994, 341, 29–46. [Google Scholar] [CrossRef]
- Tse, T.W. Hydroquinone for skin lightening: Safety profile, duration of use and when should we stop? J. Dermatolog. Treat. 2010, 21, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells. Chem. Biol. Interact. 2008, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Shariff, R.; Du, Y.; Dutta, M.; Kumar, S.; Thimmaiah, S.; Doraiswamy, C.; Kumari, A.; Kale, V.; Nair, N.; Zhang, S.; et al. Superior even skin tone and anti-ageing benefit of a combination of 4-hexylresorcinol and niacinamide. Int. J. Cosmet. Sci. 2022, 44, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Byun, K.A.; Oh, S.; Yang, J.Y.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2. Molecules 2022, 27, 1276. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.A.; Jacobson, E.L. Niacin restriction upregulates NADPH oxidase and reactive oxygen species (ROS) in human keratinocytes. Free Radic. Biol. Med. 2008, 44, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Brito, S.; Baek, J.M.; Cha, B.; Heo, H.; Lee, S.H.; Lei, L.; Jung, S.Y.; Lee, S.M.; Kwak, B.M.; Chae, S.; et al. Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling. J. Dermatol. Sci. 2022, 106, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Cercillieux, A.; Ciarlo, E.; Canto, C. Balancing NAD. Cell. Mol. Life Sci. 2022, 79, 463. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hong, Y.S.; Jun, W.; Yang, S.J. Nicotinamide Riboside Ameliorates Hepatic Metaflammation by Modulating NLRP3 Inflammasome in a Rodent Model of Type 2 Diabetes. J. Med. Food 2015, 18, 1207–1213. [Google Scholar] [CrossRef]
- Heer, C.D.; Sanderson, D.J.; Voth, L.S.; Alhammad, Y.M.O.; Schmidt, M.S.; Trammell, S.A.J.; Perlman, S.; Cohen, M.S.; Fehr, A.R.; Brenner, C. Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity. J. Biol. Chem. 2020, 295, 17986–17996. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Cabanes, J.; García-Cánovas, F.; Lozano, J.A.; García-Carmona, F. A kinetic study of the melanization pathway between L-tyrosine and dopachrome. Biochim. Biophys. Acta 1987, 923, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Bajpai, V.K.; Lee, J.; Zhao, P.; Byeon, J.H.; Ra, J.S.; Majumder, R.; Lee, J.S.; Yoon, J.I.; Rather, I.A.; et al. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-Kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci. Rep. 2017, 7, 45858. [Google Scholar] [CrossRef] [PubMed]
- Gonçalez, M.L.; Corrêa, M.A.; Chorilli, M. Skin delivery of kojic acid-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. Biomed. Res. Int. 2013, 2013, 271276. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Funasaka, Y.; Komoto, M.; Ichihashi, M. Effect of arbutin on melanogenic proteins in human melanocytes. Pigment. Cell Res. 1998, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim. Biophys. Acta 1991, 1073, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Shin, C.M.; Park, C.H.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J. Lipid Res. 2005, 46, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Shin, J.M.; Eun, H.C.; Chung, J.H. The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PLoS ONE 2009, 4, e4864. [Google Scholar] [CrossRef] [PubMed]
- Koźák, I.; Klisenbauer, D.; Juhás, T. UV-B induced production of MMP-2 and MMP-9 in human corneal cells. Physiol. Res. 2003, 52, 229–234. [Google Scholar] [CrossRef]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR- and SP1-dependent manner. JCI Insight 2022, 7, e156344. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Jang, S.G.; Ryu, M.J.; Choi, S.H. Nicotinamide Riboside Ameliorates Hyperpigmentation on Photo-Irradiated Skin. Cosmetics 2024, 11, 73. https://doi.org/10.3390/cosmetics11030073
Lee YJ, Jang SG, Ryu MJ, Choi SH. Nicotinamide Riboside Ameliorates Hyperpigmentation on Photo-Irradiated Skin. Cosmetics. 2024; 11(3):73. https://doi.org/10.3390/cosmetics11030073
Chicago/Turabian StyleLee, Yeon Jae, Seul Gi Jang, Min Jeong Ryu, and Seung Hee Choi. 2024. "Nicotinamide Riboside Ameliorates Hyperpigmentation on Photo-Irradiated Skin" Cosmetics 11, no. 3: 73. https://doi.org/10.3390/cosmetics11030073
APA StyleLee, Y. J., Jang, S. G., Ryu, M. J., & Choi, S. H. (2024). Nicotinamide Riboside Ameliorates Hyperpigmentation on Photo-Irradiated Skin. Cosmetics, 11(3), 73. https://doi.org/10.3390/cosmetics11030073





