Abstract
The tap water that we normally use contains certain concentrations of free residual chlorine to kill microorganisms and viruses and make it safe for use. Water containing free residual chlorine not only dries out our hair and skin but can also cause irritation and itching in some people—especially those with sensitive skin or reduced skin barrier function. We investigated the effects of free residual chlorine on cultured dorsal root ganglion neurons and cultured epidermal keratinocytes. First, we measured neurite length in cultured rat dorsal root ganglion neurons. Next, to evaluate the effects of chlorine on semaphorin 3A (Sema3A) and nerve growth factor (NGF) levels in cultured human epidermal keratinocytes, we used an enzyme-linked immunosorbent assay to measure NGF in the supernatant and polymerase chain reaction and Western blot to determine Sema3A and NGF levels. Chlorine elongated the neurite length and increased the number of projections in cultured rat dorsal root ganglion neurons. Although there were no changes in NGF mRNA or protein levels in the supernatant of cultured human epidermal keratinocytes in the presence of chlorine, Sema3A mRNA and protein levels decreased, and the ratio of Sema3A to NGF was also reduced.
1. Introduction
Chlorine is used to disinfect the water that we use in our daily lives. Chlorine kills pathogenic organisms such as Escherichia coli and viruses that are often present in water; the Tokyo Metropolitan Government started disinfection with chlorine in 1922 [1]. In tap water, free residual chlorine is required to ensure good hygiene. The chlorine concentration standard is set by law to maintain a free residual chlorine level of 0.1 mg/L or more. To prevent the chalky odor caused by residual chlorine, the Tokyo Metropolitan Government’s Bureau of Waterworks sets its own target—a residual chlorine concentration of 0.1 to 0.4 mg/L—and the residual chlorine concentration in Tokyo is generally between 0.3 and 0.5 mg/L. In Japan as a whole, the Waterworks Law stipulates that a chlorine concentration of 0.1 mg/L or more is added, meaning that 10 to 15 times the global standard of chlorine is added in some areas. In addition, the Japanese hygiene standard for swimming pools (from the Ministry of Health, Labor, and Welfare) states that “the concentration of free residual chlorine shall be 0.4 mg/L or more. It should be less than 1.0 mg/L” [2]. It has been reported that more than 15 min of exposure to pool water with a chlorine concentration of 2 mg/L increases the risk of skin diseases [3]. Moreover, hair exposed to 10 mg/L chlorinated water has been reported to lose its cuticles after 30 washes and become dry at neutral pH [4]. Notably, although a residual chlorine concentration of 0.8 ± 0.1 mg/L is not high enough to increase the risk of skin diseases, it has been reported that long-term continuous exposure to low residual chlorine concentrations may affect the water retention function of the stratum corneum [5]. Furthermore, the free residual chlorine that is necessary for the safe use of water can have adverse effects on skin. People with compromised skin barrier function, such as those with sensitive skin or skin diseases, reportedly have irritation and reduced water retention when using water containing free residual chlorine [5]. Efforts should, therefore, be made on a daily basis to minimize personal exposure to chlorine.
When chlorine is dissolved in water, it reacts with the water to produce hypochlorous acid (HOCl) and its ionized form, hypochlorite ion (OCl−). These substances have a strong oxidizing effect that destroys bacterial membranes, inactivates enzymes, and exhibits bactericidal actions. However, because the concentration at which chlorine exhibits bactericidal action is relatively low (less than 1/1000 of the concentration that adversely affects human health), the oxidizing effect of chlorine is considered unlikely to have an adverse effect on human health.
In the case of tap water, various treatments are performed at the water purification plant just before the water is sent to each household. From there, the water must remain disinfected all the way to the tap in each household. Japanese law, therefore, requires that the free residual chlorine (HOCl; OCl−) concentration is maintained at 0.1 mg/L or higher (or 0.4 mg/L or higher for bound residual chlorine [chloramines]) at the end of the water pipe (i.e., when the water comes out of the tap). The high reactivity of chlorine makes it a highly reactive bactericide. However, various byproducts are also formed when chlorine reacts with components in water [6,7,8]. Of these, trihalomethanes (such as chloroform) are compounds in which three hydrogen atoms of methane are replaced by halogens, some of which are carcinogenic [9]. Because high trihalomethane concentrations are problematic, the World Health Organization has established guidelines that are based on animal studies [10]. On the basis of these guidelines, Japan has set standards stating that total trihalomethanes need to be below 0.1 mg/L [11]. Given that these concentrations are considerably lower than those reported to be carcinogenic in animal experiments, it is unlikely that continuously drinking tap water will cause cancer.
Neuronal growth factors include nerve growth factor (NGF), amphiregulin (of the epidermal growth factor family), and artemin (of the glial cell line-derived neurotrophic factor family). Neurorepulsive factors include semaphorin 3A (Sema3A) and anosmin-1, an extracellular matrix glycoprotein encoded by the Kallmann syndrome 1 sequence gene [12,13]. These neuronal elongation and repulsive factors—for example, the nerve growth balance between NGF and Sema3A—are involved in itching. In healthy skin, epidermal Sema3A levels are higher than epidermal NGF levels; this inhibits the penetration and elongation of nerve fibers into the skin. By contrast, in the skin of patients with atopic dermatitis, epidermal NGF levels are higher than those of epidermal Sema3A [13]. These findings suggest that NGF and Sema3A may be involved in the induction of nerve fiber growth into normal epidermis. Furthermore, increased protein and mRNA levels of amphiregulin have been reported in a dry skin model (acetone-treated mouse back skin), indicating that amphiregulin is upregulated in dry skin [14]. Furthermore, although anosmin-1 is strongly expressed in the basal cell layer of healthy skin, its expression is lower in the skin of patients with atopic dermatitis and is accompanied by increased epidermal nerve density [13].
The NTRK1 (neurotrophic receptor tyrosine kinase 1, also known as tropomyosin receptor kinase A [TRKA]) gene encodes an NGF receptor (NGFR) and affects NGF mRNA levels in cultured epidermal keratinocytes. TRKA binds to ligand neurotrophic factors (NGF, brain-derived neurotrophic factor, and neurotrophin) and transduces intracellular signals to regulate neuronal differentiation and survival [15,16], pain [17], and thermoregulation [18] in the central and peripheral nervous systems.
To date, there is no knowledge of the effects of chlorine on the length of dorsal root ganglion cell neurites and the amount of Sema3A and NGF in epidermal keratinocytes. We, therefore, examined the effects of chlorine on cultured dorsal root ganglion cells and epidermal keratinocytes.
2. Materials and Methods
2.1. Preparation of Chlorinated Water
Because it has been reported that exposure to pool water with a chlorine concentration of 2 mg/L for more than 15 min increases the risk of skin diseases [3], we set the chlorine concentrations to 1 mg/L less and 1 mg/L more (i.e., to 1 mg/mL and 3 mg/L). Sodium hypochlorite solution (approximately 12%) was added to ion-exchanged pure water, and the pH was adjusted to 7.5 ± 0.1 using hydrochloric acid and sodium hydroxide. Three types of chlorinated water were prepared, with free residual chlorine concentrations of 292 mg/L (pH 7.59; electrical conductivity, 0.708 mS/cm), 95 mg/L (pH 7.58; electrical conductivity, 0.259 mS/cm), and 0 mg/mL (pH 7.41; electrical conductivity, 0.259 mS/cm). By measuring the concentration of residual chlorine over time, we confirmed that more than 90% of the concentration was retained for 10 days in an unopened container stored in a refrigerator (at approximately 5 °C).
2.2. Effects of Chlorinated Water on Neurite Length and Neuronal Cell Culture
2.2.1. Coatings
A laminin/poly-D-lysine solution of 2 μg/mL laminin (Sigma-Aldrich Co., LLC, St. Louis, MO, USA) and 30 μg/mL of poly-D-lysine (Sigma-Aldrich Co., LLC) dissolved in phosphate-buffered saline (PBS) was made. We added 200 μL of this solution to the center of a 35 mmφ dish (No.1S [0.16–0.19] 14 mmφ polylysine-coated; Matsunami Glass Ind., Ltd., Osaka, Japan) and placed the dish on a clean bench for 1 h. The dishes were then washed twice with sterile water and dried.
2.2.2. Neuronal Cell Culture
Primary Neuron Growth Medium (PNGM™) was prepared with PNBM™ Primary Neuron Basal Medium (CC-3256; Lonza Group AG, Basel, Switzerland) and the Primary Neuron Addition Factor Set (PNGM SingleQuots™ CC-4462; Lonza Group AG). Frozen rat dorsal root ganglion neurons (R-Drg-505; Lonza Group AG) were pipetted with 2 mL of PNGM medium, and 100 µL was added to the center of each 35 mmφ coated dish. The cells were cultured in an incubator; after cell adhesion, 10 mg/mL of NGF was added to a final concentration of 10 ng/mL, and 2 mL of filtered PNGM medium was added. Cells were then cultured for 7 days.
2.2.3. Chlorinated Water
We added 20 μL of free residual chlorine (at a concentration of 0, 100, or 300 mg/L) sterilized on a cellulose acetate membrane sterile filter (DISMIC-13cp 0.2 μm; Advantech Japan Co., Ltd., Tokyo, Japan) to 1.98 mL of PNBM Basic Medium. Cells were then incubated for 3 days, observed under an inverted microscope (Olympus IX70, Objective Olympus DP72; Olympus Corporation, Tokyo, Japan), and photographed using a digital camera (Olympus DP72). Neurite length was measured using ImageJ, and significant differences were analyzed using the two-tailed t-test in Microsoft Excel 2016.
2.3. mRNA Levels of NGFR and NTRK1 in Cultured Dorsal Root Ganglion Neurons
We added 20 μL of free residual chlorine (at a concentration of 0, 100, or 300 mg/L) sterilized on a cellulose acetate membrane sterile filter (DISMIC-13cp 0.2 μm) to 1.98 mL of PNBM Basic Medium. Cells were then incubated for 3 days before an RNeasy Protect Mini One Step TB Green® PrimeScript™ RT-PCR Kit II (TaKaRa Bio Inc., Shiga, Japan) was used to evaluate the mRNA levels of NGFR and NTRK1 using real-time polymerase chain reaction (RT-PCR; QuantStudio® 5 Real-Time PCR System; Thermo Fisher Scientific Inc., Waltham, MA, USA). β-actin (ACTB) was used as the housekeeping gene. All primers were purchased from QIAGEN. Significant differences were analyzed using an unresponsive t-test (two-tailed) in Microsoft Excel 2016.
2.4. Culture of Human Epidermal Keratinocytes and Measurement of NGF and Sema3A mRNA Levels
Normal human neonatal epidermal keratinocytes (Kurabo Industries Ltd., Osaka, Japan) were seeded in 35 mmφ dishes (BioCoat™ poly-L-lysine; Corning Inc., Corning, NY, USA) and cultured until subconfluent in serum-free normal human epidermal keratinocyte growth liquid medium (DermaLife® K Comp kit; Kurabo Industries Ltd.). We then added 20 μL of chlorinated water (at a concentration of 0, 100, or 300 mg/L) sterilized with a cellulose acetate membrane sterile filter (DISMIC-13cp 0.2 μm) to 1.98 mL of serum-free basic medium for normal human epidermal keratinocyte proliferation for 3 days. An RNeasy Protect Mini kit (QIAGEN) was used to extract RNA, and a One Step TB Green PrimeScript RT-PCR Kit II was used to evaluate the NGF and Sema3A mRNA levels by real-time PCR (QuantStudio 5 Real-Time PCR System). ACTB was used as the housekeeping gene. All primers were purchased from QIAGEN. Significant differences were analyzed using an unresponsive t-test (two-tailed) in Microsoft Excel 2016.
2.5. Supernatant Protein Content of Cultured Human Epidermal Keratinocytes
Human epidermal growth medium was used to grow the cells. First, human epidermal keratinocytes were seeded at 2.0 × 104 cells/mL in 96-well plates, at 100 μL each. After 2 days of incubation in growth medium, the medium was removed, and 1 μL of chlorinated water (with a free residual chlorine concentration of 0, 100, or 300 mg/L, making a final concentration of 0, 1, or 3 mg/L in the medium) was added to 100 μL of basic medium for 3 days before the supernatant was collected.
The amount of protein in the culture supernatant was then measured using a BCA™ Protein Assay Kit—Reducing Agent Compatible. First, 5 mL of albumin standard and the sample were added to a 96-well plate for an enzyme-linked immunosorbent assay (ELISA), and 95 mL of working reagent was added. The absorbance at 562 nm was measured immediately after the addition of the working reagent (i.e., at 0 min), as well as at 20, 40, and 60 min.
2.6. NGF Measurement in the Supernatant of Cultured Human Epidermal Cells
The amount of NGF in the culture supernatant (collected as in Section 2.5) was measured by ELISA using a DuoSet® Human β-NGF kit (R&D Systems, Minneapolis, MN, USA). First, 100 μL of capture antibody diluted in PBS was added to a 96-well plate for ELISA, and the plate was sealed and incubated at room temperature overnight. The capture antibody was then removed, and the plate was washed three times with wash buffer. Next, the plate was sealed and incubated at room temperature for at least 1 h, and the reagent diluent was removed. The plate was then washed three times with wash buffer. After the β-NGF standard and samples were removed and washed three times with wash buffer, 100 μL of detection antibody diluted in reagent buffer was added, and the plate was sealed and incubated at room temperature for at least 1 h. After the detection antibody was removed and washed three times with wash buffer, the plate was incubated at room temperature for 20 min in 100 μL of streptavidin–horseradish peroxidase (HRP) diluted in reagent diluent, shielded from light. After removing the streptavidin–HRP and washing three times with wash buffer, 100 μL of substrate solution was added. Absorbances at 450 nm and 570 nm were measured immediately after the addition of the substrate solution (i.e., at 0 min) as well as at 20, 40, and 60 min.
2.7. Detection of Sema3A in Cultured Human Epidermal Cells
Human epidermal cells were seeded onto six-well plates (Corning; 300,000 cells/well) and cultured in 2 mL of medium for 1 day. Next, 20 μL of chlorine solution (final concentration 0, 1, or 3 mg/L) was added to 2 mL of basic medium for 3 days. We then added 100 μL of 2% weight/volume sodium dodecyl sulfate, 10% glycerol, 50 mmol/L of dithiothreitol, 0.01% bromophenol blue, and 1/7 cOmplete™ (EDTA-free protease inhibitor cocktail) to the sample buffer (62.5 mmol/L Tris-HCl; pH 6.8 at 25 °C). The cells were lysed, passed five times through a 21-gauge needle, and heated at 95 °C for 3 min. Next, we added 15 µL of each protein extract and molecular weight marker to a 4–20% sodium dodecyl sulfate–polyacrylamide gel mini 1.5 mm 15 well (TEFCO) and electrophoresised the gels for 1 h at 54 mA current. The pad and filter paper were soaked in blotting buffer, and the membrane was soaked in methanol for 10 s before soaking in blotting buffer. To transfer the proteins to the membrane, the gel was removed and blotted for 1 h at a current of 180 mA using a wet method. The membrane was washed with Tris-buffered saline containing 0.05% Tween 20 (TBST; pH 7.6) incubated in TBST solution with 5% skim milk for 30 min, washed twice with TBST, and stored in a refrigerator. Subsequently, the membrane was placed in an open Unipac with 5 mL of TBST containing 0.5% bovine serum albumin and the primary antibody (anti-β-actin antibody, 1:10,000; Proteintech Group, Inc. (Rosemont, IL, USA); anti-Sema3A antibody, 1:4000, GeneTex, Inc. (Alton Pkwy Irvine, CA, USA)), sealed with a sealer to remove air, and shaken at 190 rpm for 1 h on a horizontal seesaw shaker. The membrane was removed and washed with TBST; the first wash was immediately discarded and new TBST was added. The membrane was then shaken on a horizontal seesaw shaker at 360 rpm for 3 min, the TBST was changed again to new TBST, and the membrane was shaken for 5 min.
After the membrane was placed in a new Unipac, 5 mL of secondary antibody (peroxidase-labeled anti-mouse or -rabbit antibody, 1:5000 dilution in TBST) was placed in the Unipac. The foam was drained, the pack was sealed with a sealer, and the membrane was shaken at 190 rpm for 30 min on a horizontal seesaw shaker. After 30 min, the membrane was removed and washed with TBST; the first wash was immediately discarded and repeated with new TBST (every 5–10 min) for 1 h. The membrane was again placed in a Unipac, stained with Western BLoT Hyper HRP Substrate (Takara Bio Inc.), and visualized using LuminoGlaph I (ATTO Corporation, Tokyo, Japan).
3. Results
3.1. Effects of Chlorinated Water on Neurite Length in Cultured Dorsal Root Ganglion Neurons
Figure 1 shows brightfield micrographs of dorsal root ganglion neurons cultured for 3 days in media with various free residual chlorine concentrations (0, 1, or 3 mg/L).
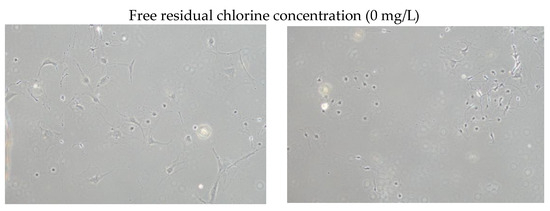
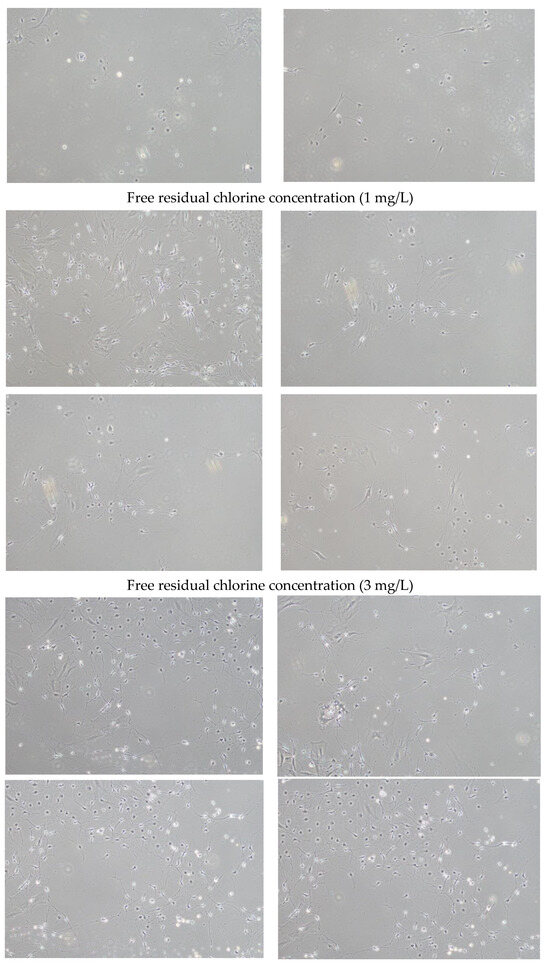
Figure 1.
Brightfield micrographs (10× objective lens) of dorsal root ganglion neurons cultured for 3 days in medium with a free residual chlorine concentration of 0, 1, or 3 mg/L.
A comparison of the neurite lengths of 30 dorsal root ganglion neurons using image analysis revealed that neurite lengths were significantly longer when cultured in medium with a free residual chlorine concentration of 1 or 3 mg/L than when cultured with a free residual chlorine concentration of 0 mg/L (Figure 2).
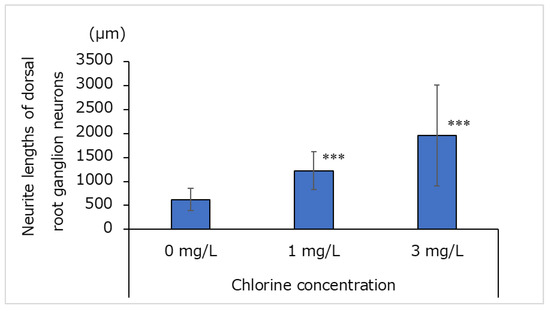
Figure 2.
Neurite lengths of dorsal root ganglion neurons cultured for 3 days in medium containing water with a free residual chlorine concentration of 0, 1, or 3 mg/L (n = 30; mean ± standard deviation; *** p < 0.001 vs. 0 mg/L).
3.2. NGFR and NTRK1 mRNA Levels in Cultured Dorsal Root Ganglion Neurons
Figure 3 shows the mRNA levels of NGFR and NTRK1 after the dorsal root ganglion neurons were cultured for 3 days in a medium with a free residual chlorine concentration of 0, 1, or 3 mg/L. Compared with the cells cultured with 0 mg/L residual chlorine, the mRNA levels of NGFR and NTRK1 were significantly higher in neurons cultured with 1 or 3 mg/L residual chlorine.
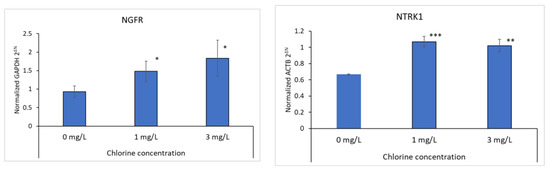
Figure 3.
mRNA levels of nerve growth factor receptor (NGFR) and neurotrophic receptor tyrosine kinase 1 (NTRK1) in dorsal root ganglion neurons cultured for 3 days in medium with a free residual chlorine concentration of 0, 1, or 3 mg/L (n = 3; mean ± standard deviation; * p < 0.05 vs. 0 mg/L; ** p < 0.01 vs. 0 mg/L; *** p < 0.001 vs. 0 mg/L).
3.3. Effects of Chlorinated Water on Cultured Human Epidermal Keratinocytes
The effects of chlorinated water on the proliferation of human epidermal keratinocytes are shown in Figure 4. The cells were cultured in a chlorinated medium for 3 days; there were no significant differences in cell counts at any concentrations compared with the control (0 mg/L).
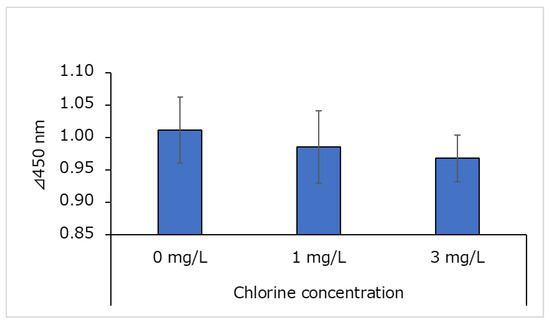
Figure 4.
Effects of chlorine on cell numbers of human epidermal keratinocytes (n = 4; mean ± standard deviation).
3.4. Effects of Chlorinated Water on NGF Levels in Human Epidermal Keratinocyte Supernatant
The NGF content of human epidermal keratinocyte supernatant is shown in Figure 5. The NGF content in the culture supernatant was divided by the total amount of protein in the culture supernatant to obtain the ratio of NGF to total protein. This ratio was not significantly different from that of the control (0 mg/L) at any chlorine concentration.
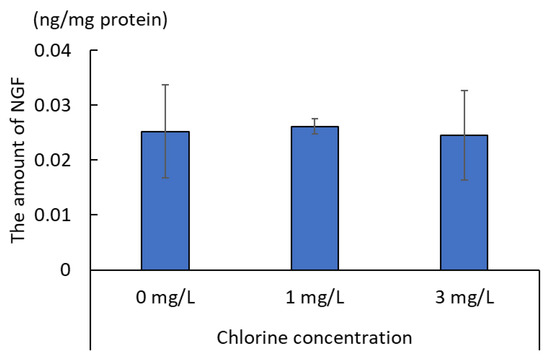
Figure 5.
Ratio of nerve growth factor (NGF) to total protein in cultured human epidermal keratinocyte supernatant (n = 3; mean ± standard deviation).
3.5. Effects of Chlorinated Water on NGF and Sema3A mRNA Levels in Cultured Human Epidermal Keratinocytes
The NGF and Sema3A mRNA levels in cultured human epidermal keratinocytes are shown in Figure 6a,b, respectively. The NGF mRNA levels were not significantly different between the control (0 mg/L) and chlorinated water (1 or 3 mg/L) conditions. However, Sema3A mRNA levels were significantly lower in chlorinated water conditions (1 or 3 mg/L) than in control conditions (0 mg/L), although there was no significant difference between the two chlorine concentrations (i.e., 1 and 3 mg/L).
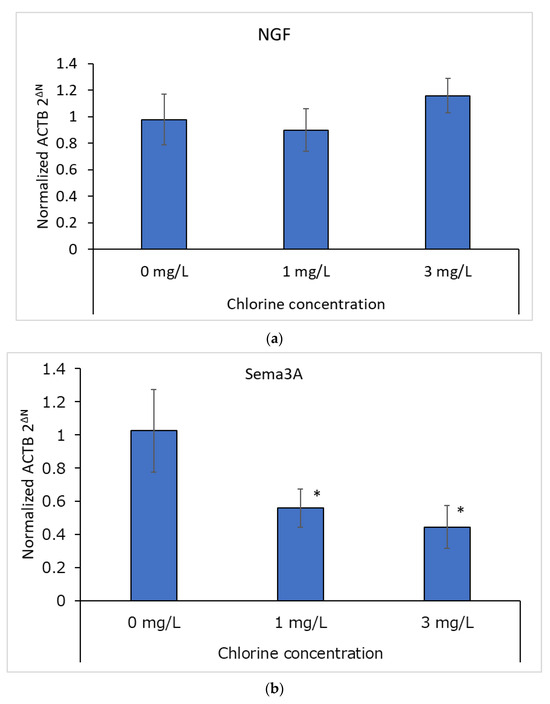
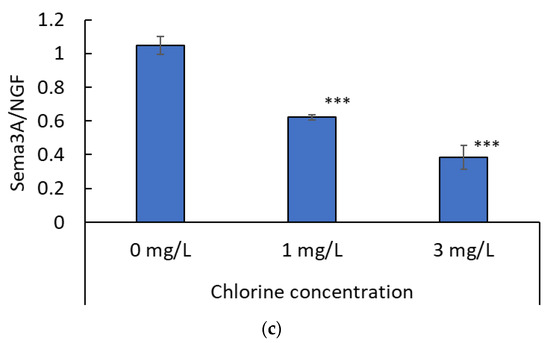
Figure 6.
(a) mRNA expression of NGF (n = 3; mean ± standard deviation). (b) mRNA expression of semaphorin-3A (Sema3A; n = 3; mean ± standard deviation; * p < 0.05 vs. 0 mg/L). (c) Mean Sema3A mRNA levels relative to NGF mRNA levels (n = 3; mean ± standard deviation; *** p < 0.0001 vs. 0 mg/L).
An analysis of Sema3A mRNA levels relative to NGF mRNA levels is shown in Figure 6c. The ratio of Sema3A to NGF mRNA was lower in the presence of chlorine than in the control (0 mg/L). Furthermore, this ratio was lower at a chlorine concentration of 3 mg/L than at a chlorine concentration of 1 mg/L.
3.6. Effects of Chlorinated Water on Sema3A Protein Levels in Cultured Human Epidermal Keratinocytes
In Western blotting, a β-actin band was detected at 42 kDa, and a Sema3A band was detected at 88 kDa. The concentration of Sema3A relative to that of β-actin was quantified using ImageJ, and the results are shown in Figure 7. Relative Sema3A protein concentrations decreased with increasing chlorine concentrations.
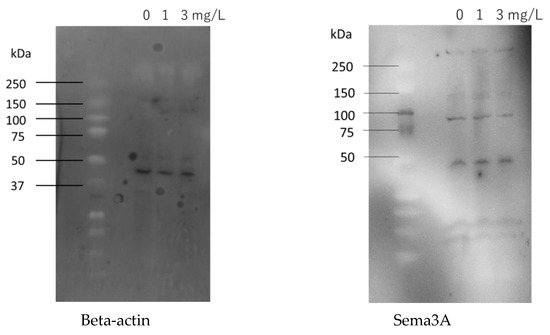
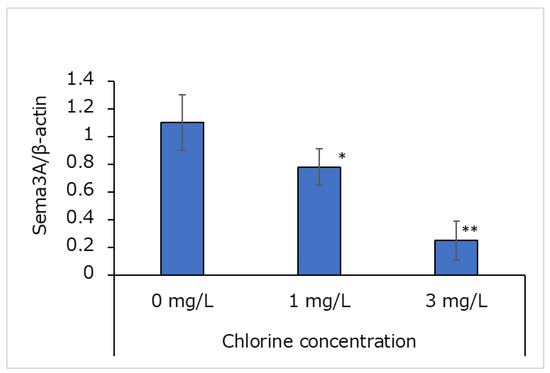
Figure 7.
Effects of chlorinated water on Sema3A protein concentrations in cultured human epidermal keratinocytes (n = 3; mean ± standard deviation; * p < 0.05; ** p < 0.001 vs. 0 mg/L).
4. Discussion
The transduction of irritation and itching is initiated when the mediators of irritation and itching bind to receptors expressed on primary sensory nerve endings. Ligand-bound receptors open transient receptor potential (TRP) channels via their own intracellular signals, thus allowing Ca2+ to enter cells [19]. This results in action potentials that are transmitted via secondary sensory nerves to the brain, where irritation and itching are sensed [20]. For example, histamine binds to histamine H1 receptors on primary sensory nerves (mainly C fibers) and opens TRPV1, resulting in Ca2+ influx and action potentials [13]. Similarly, hypochlorite, an oxidative mediator of chlorine, activates Ca2+ influx and membrane currents in an oxidant-sensitive subpopulation of chemosensory neurons that have been identified as TRPA1-expressing cells [21], and NGF opens TRPM8 via NGFR and generates action potentials [22]. TRPM8 is mechanistically related to cold hypersensitivity [23], and cold sensitization by TRPM8 appears to be induced by neurotrophic factors such as artemin and NGF, which exert their effects via specific receptors that are co-expressed with TRPM8 in a subset of sensory neurons [24,25]. In the present study, compared with cells grown in a medium with a chlorine concentration of 0 mg/L, neurite length was significantly elongated with a chlorine concentration of 1 or 3 mg/L. Furthermore, this elongation was concentration-dependent, suggesting that higher chlorine concentrations lead to greater neurite elongation. This effect may be mediated by chlorine-induced increases in NGFR and NTRK1 mRNA.
We also examined the effects of chlorinated water on NGF and Sema3A mRNA levels in human epidermal keratinocytes. Sema3A mRNA levels were decreased at 1 and 3 mg/L compared with 0 mg/L chlorine concentrations; however, there were no significant differences in NGF mRNA levels. The amount of Sema3A protein was also decreased when chlorine was present. Together, these findings suggest that free residual chlorine decreases Sema3A mRNA levels, which may facilitate the extension of sensory nerves to the epidermis and lead to irritation and itch induction.
When the epidermis has an impaired barrier function, such as with dry skin or atopic dermatitis lesions, it is prone to intraepidermal nerve fiber elongation caused by the decreased expression of neurorepulsive factors in epidermal keratinocytes, which leads to a predominance of nerve elongation factors [12,26]. The elongation of nerve fibers within the epidermis is regulated by axon guidance molecules—such as nerve elongation or neurorepulsive factors—that are produced by epidermal keratinocytes [12]. Nerve elongation factors induce axon terminals, whereas neurorepulsive factors repel axon terminals; they are thus involved in axonal elongation and direction determination. Nerve elongation factors that are produced by epidermal keratinocytes include NGF, amphiregulin, and artemin; neurorepulsive factors include Sema3A and anosmin-1. Semaphorins act on growth cones at axon tips to inhibit the polymerization of cytoskeletal actin and alter microtubule dynamics, thus causing growth cones to collapse and axons to regress [26,27]. More than 20 such molecules have been identified and classified into eight subclasses [27,28]. Sema3A, a secreted semaphorin, is secreted extracellularly after its production by epidermal keratinocytes. The immunohistochemical staining of normal skin shows staining mainly in the intercellular spaces of epidermal keratinocytes, near the spinous layer [28]. Lesioned skin (e.g., dry skin or atopic dermatitis) has lower Sema3A expression than normal skin, suggesting that nerve fibers tend to elongate within the epidermis in these conditions [12,29]. Histamine is not involved in this mechanism of itching (as the result of nerve fiber elongation in the epidermis), meaning that antihistamines are not likely to be successful as a treatment [30]. However, ultraviolet (UV) therapy, such as with psoralen UV-A and narrowband UV-B [28], leads to the recovery of Sema3A expression and decreased numbers of intraepidermal nerve fibers in the lesions of patients with atopic dermatitis. UV exposure may, therefore, be effective for treating skin irritation and itching caused by chronic free residual chlorine exposure.
It has been reported that prolonged exposure to low concentrations of residual chlorine may affect the water retention function of the stratum corneum [5]; future studies should examine the effects of prolonged exposure to low concentrations of residual chlorine on neurons and skin keratinocytes.
5. Conclusions
The present results suggest that free residual chlorine may disrupt the balance between Sema3A and NGF expression to promote neurite outgrowth into the epidermis, thus making skin more sensitive and causing chronic irritation and itching.
Author Contributions
Conceptualization, K.M.; methodology, K.M. and H.T.; validation, K.M., N.O., A.O. and H.T.; formal analysis, K.M. and H.T.; investigation, K.M., N.O., A.O. and H.T.; resources, H.T.; data curation, K.M.; writing—original draft preparation, N.O. and A.O.; writing—review and editing, K.M.; visualization, N.O. and A.O.; supervision, K.M.; project administration, K.M.; funding acquisition, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Conflicts of Interest
Hatsumi Takeda was employed by the company Mitsubishi Chemical Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tokyo Metropolitan Waterworks Bureau. Available online: https://www.waterworks.metro.tokyo.lg.jp (accessed on 3 March 2024).
- Division of Public Health, Health Bureau, Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/public/kekka/p0724-1.html (accessed on 3 March 2024).
- Matsumoto, Y.; Mori, H.; Hayakawa, A.; Ohashi, M. Influence of free residual chlorine on cultured human epidermal keratinocytes from normal skin and hypertrophic scars. J. Dermatol. Sci. 1995, 10, 1–7. [Google Scholar] [CrossRef]
- Fair, N.; Gupta, B.S. The chlorine-hair interaction. II: Effect of chlorination at varied pH levels on hair properties. J. Soc. Cosmet. Chem. 1987, 38, 371–384. [Google Scholar]
- Seki, T.; Morimatsu, S.; Nagahori, H.; Morohashi, M. Free residual chlorine in bathing water reduces the water-holding capacity of the stratum corneum in atopic skin. J. Dermatol. 2003, 30, 196–202. [Google Scholar] [CrossRef]
- Hang, C.; Zhang, B.; Gong, T.; Xian, Q. Occurrence and health risk assessment of halogenated disinfection byproducts in indoor swimming pool water. Sci. Total Environ. 2016, 543, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Genisoglu, M.; Minaz, M.; Tanacan, E.; Sofuoglu, S.C.; Kaplan-Bekaroglu, S.S.; Kanan, A.; Ates, N.; Sardohan-Koseoglu, T.; Yigit, N.Ö.; Harman, B.I. Halogenated by-products in chlorinated indoor swimming pools: A long-term monitoring and empirical modeling study. ACS Omega 2023, 8, 11364–11372. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, X.; Zhai, H.; Lo, I.M.; Tipoe, G.L.; Yang, M.; Pan, Y.; Chen, G. New halogenated disinfection byproducts in swimming pool water and their permeability across skin. Environ. Sci. Technol. 2012, 46, 7112–7119. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, W.; Han, F.; Geng, Y.; Yu, X.; Wang, H.; Kimura, S.Y.; Wei, X.; Kauffman, A.; Xiao, S.; et al. Precise exposure assessment revealed the cancer risk and disease burden caused by trihalomethanes and haloacetic acids in Shanghai indoor swimming pool water. J. Hazard. Mater. 2020, 388, 121810. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda. World Health Organization. 2022. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 3 March 2024).
- Water Supply in Japan, Ministry of Health, Labour and Welfare in Japan. Available online: https://www.mhlw.go.jp/english/policy/health/water_supply/menu.html (accessed on 3 March 2024).
- Fiore, R.; Püschel, A.W. The function of semaphorins during nervous system development. Front. Biosci. 2003, 8, s484–s499. [Google Scholar]
- Tominaga, M.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Ozawa, S.; Tengara, S.; Ogawa, H.; Takamori, K. Intraepidermal nerve fibers increase in dry skin of acetone-treated mice. J. Dermatol. Sci. 2007, 48, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Amatu, A.; Sartore-Bianchi, A.; Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016, 1, e000023. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.J.; Li, Z.; McKee, A.E. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin. Cancer Res. 2009, 15, 5962–5967. [Google Scholar] [CrossRef] [PubMed]
- Indo, Y. Nerve growth factor and the physiology of pain: The relationships among interoception, sympathetic neurons and the emotional response indicated by the molecular pathophysiology of congenital insensitivity to pain with anhidrosis. No Hattatsu 2015, 47, 173–180. (In Japanese) [Google Scholar]
- Loewenthal, N.; Levy, J.; Schreiber, R.; Pinsk, V.; Perry, Z.; Shorer, Z.; Hershkovitz, E. Nerve growth factor-tyrosine kinase A pathway is involved in thermoregulation and adaptation to stress: Studies on patients with hereditary sensory and autonomic neuropathy type IV. Pediatr. Res. 2005, 57, 587–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kashio, M. Thermosensation involving thermo-TRPs. Mol. Cell Endocrinol. 2021, 520, 111089. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of pain and itch by TRP channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Bessac, B.F.; Sivula, M.; von Hehn, C.A.; Escalera, J.; Cohn, L.; Jordt, S.-E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Investig. 2008, 118, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Shibata, M.; Takizawa, T.; Ibata, K.; Nakahara, J.; Shimizu, T.; Toriumi, H.; Yuzaki, M.; Suzuki, N. Signaling pathways relevant to nerve growth factor-induced upregulation of transient receptor potential M8 expression. Neuroscience 2017, 367, 178–188. [Google Scholar] [CrossRef]
- Allchorne, A.J.; Broom, D.C.; Woolf, C.J. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol. Pain 2005, 1, 36. [Google Scholar] [CrossRef]
- Lippoldt, E.K.; Elmes, R.R.; McCoy, D.D.; Knowlton, W.M.; McKemy, D.D. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J. Neurosci. 2013, 33, 12543–12552. [Google Scholar] [CrossRef]
- Lippoldt, E.K.; Ongun, S.; Kusaka, G.K.; McKemy, D.D. Inflammatory and neuropathic cold allodynia are selectively mediated by the neurotrophic factor receptor GFRalpha3. Proc. Natl. Acad. Sci. USA 2016, 113, 4506–4511. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Offermanns, S. Semaphorins and plexins as therapeutic targets. Nat. Rev. Drug Discov. 2014, 13, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.B.; Kolodkin, A.L.; Ginty, D.D.; Cloutier, J.F. Signaling at the growth cone: Ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003, 26, 509–563. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Tengara, S.; Kamo, A.; Ogawa, H.; Takamori, K. Psoralen-ultraviolet A therapy alters epidermal Sema3A and NGF levels and modulates epidermal innervation in atopic dermatitis. J. Dermatol. Sci. 2009, 55, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Ogawa, H.; Takamori, K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br. J. Dermatol. 2008, 158, 842–844. [Google Scholar] [CrossRef]
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).